Abstract
In several recent conferences, the principal questions have been whether Xenotransplantation technology should be encouraged and, if so, how it should be regulated. Because the prospect of successful transplantation of animal organs into humans is still remote, the rush to achieve consensus about clinical application1,2 would be inexplicable were it not for two ostensibly unrelated issues. The first is the small but undeniable theoretical hazard of causing new human infections with the intermingling of tissues from different species. The second, advanced by animal-rights advocates, concerns the spiritual and ethical relationship of humans to animals.
CLOSELY RELATED SPECIES
The rhetoric of these discussions has been heightened by the fact that all clinical organ xenotransplantations attempted since 1963 have failed, including >25 involving subhuman primate donors (19 reported or unreported chimpanzees, 10 baboons, and 1 [or 2] Rhesus monkeys). This experience began with Reemtsma and associates,3 who proved in 1963 that kidneys from at least two subhuman primate donor species (Rhesus and chimpanzee) would not be rejected hyperacutely by humans. In fact, one of his chimpanzee xenografts functioned for 9 months. However, Reemtsma was the first to recognize that the humanoid qualities and threatened extinction of the chimpanzee would prevent its widespread use as a donor.
Later in 1963, a team at the University of Colorado/Minnesota showed that baboon kidneys also would escape hyperacute rejection. In contrast to the chimpanzee, baboons flourished, and still do, in southern and central Africa. The six baboon renal xenografts of 1963 supported dialysis-free life for 6–60 days before undergoing fierce cellular rejection.4
In addition, all of the baboon kidneys had occlusive endotheliolitis that was severe enough to cause regional parenchymal infarcts and islands of gangrene. We concluded by early 1964 that this humoral component of xenograft rejection could not be controlled with cell-directed immune suppression (azathioprine and prednisone at the time). A moratorium was self-imposed on further attempts.
This hiatus lasted for 28 years, until investigations by Murase and colleagues5 with the hamster-to-rat model appeared to justify a further trial. In this strain combination, in which the immune barrier resembles that between the baboon and human,6 the combination of T-cell–specific immune suppression with tacrolimus plus B-cell–directed cyclophosphamide permitted the unprecedented routine survival of heart and liver xenografts for >100 days.
In June 1992 and January 1993, two baboon-to-human orthotopic liver transplantations were performed in patients with chronic end-stage organ failure caused by hepatitis B virus infection. Two factors in addition to proximity to death influenced their candidacy. First, it was thought (and subsequently confirmed) that baboon hepatocytes were not subject to infection by the human hepatitis B virus,7 presumably because they do not have appropriate viral receptors. Second, there were medical contraindications to conventional allotransplant candidacy (eg, human immunodeficiency virus, older age, cardiac disease). Although it was anticipated that the xenografts would provide definitive, as opposed to bridge, function, both grafts failed after 70 and 26 days despite heavy immunosuppression with tacrolimus, cyclophosphamide, prostaglandin E1, and prednisone.8,9
The liver xenografts showed no trace of the occlusive endotheliolitis that had been responsible for patchy gangrene of the 1963 kidney xenografts and in the baboon-heart xenograft of Leonard Bailey’s Baby Fae case.10 Cellular rejection was found in only 1 of the 14 biopsies or autopsy specimens from the two xenografts (n = 7 each). The only positive biopsy specimen, on day 12 in the first case, showed only mild cellular rejection.
Although the two livers were not rejected hyperacutely or by the usual delayed mechanisms of allografts, red blood cell sludging and a few polymorphonuclear leukocytes were seen in the sinusoids of the xenograft biopsy specimens 1 hour after revascularization. Upon close examination, a very fine microsteatosis was seen in the hepatocytes of both xenografts, which dramatically worsened over the next few days, especially in the second case.8,9
The grafts had survived an aborted hyperacute rejection. Although IgM found in the 1-hour biopsy specimen had largely cleared at 12 days, significant amounts of IgG remained in the xenografts throughout the survival periods of both patients. Total complement remained depleted for the first 2 weeks after transplantation, while complement components C3, C4, and C5 became undetectable. Circulating immune complexes appeared early, receded, and reappeared sporadically until the time of death.8,9
Good control of adaptive immunity had unmasked a low-grade innate immune response which, we concluded, could not be treated safely, if at all, with any combination of agents currently available. Consequently, we canceled the last two patients in our series of four approved by the Institutional Review Board. One of the unanswered questions was whether the species restriction of complement described in 1994 by Valdivia and coauthors9,11 played a role in the failures. Because the liver is the principal or sole source of most complement components, the complement was eventually transformed to potentially protective baboon phenotype in both cases,9 but because this change required several days, it may have been too late to be advantageous.
DISTANTLY RELATED SPECIES
In the pessimistic climate that followed the liver xenotransplant failures, it seemed inconceivable that the use of even more discordant donors could ever be seriously entertained. This period of pessimism, however, preceded insight into the xenogeneic barrier that has brought transplantation immunity onto common ground with infectious immunity, with particular reference to graft acceptance and acquired tolerance.12
Cytopathic parasites
From the perspective of infection, the immune system makes an immediate strategic decision, based on differentiation of cytopathic parasites from the less dangerous noncytopathic parasites (ie, viruses, bacteria, or protozoans). The antigenic signal of “anger” issued by a cytopathic invader may come from its rigid, densely arranged, and ordered repetitive epitopes, aided by lipopolysaccharides and other unknown means.13–15
The host immune armamentarium is mobilized to eliminate the pathogens quickly and completely, with little regard for damage to infected host cells. The first line of defense is dominated by interferons, macrophages, γ/δ T cells, natural killer cells, and by B cells, which recognize suspect antigen patterns and may be activated without T-cell help. In addition, nonspecific or less specific effector mechanisms such as complement, interleukins, and phagocytes are promptly involved13–15 (Table 1).
Table 1.
Effectors Involved in Response to Cytopathic Parasites and Discordant Xenografts*
| The first line of defense |
| Interferons |
| Macrophages |
| γ/δ T cells |
| Natural killer cells |
| B cells |
| Nonspecific or less specific effectors |
| Complement |
| Early interleukins |
| Phagocytes |
The transplant analogy
These are the same mechanisms, predominantly those of innate immunity, that are responsible for the hyperacute rejection of discordant xenografts and also of allografts transplanted to ABO-incompatible or highly sensitized recipients.9 The best-characterized signal on the cells of discordant xenografts is the terminal residue Gal-α (1,3) Gal.16,17 This antigen is chemically similar to ABO antigens and is found on numerous bacteria, protozoa, and viruses.18
In an effort to prevent clinical hyperacute xenograft rejection, investigators have transfected human complement regulatory proteins into pigs.19–21 This results only in temporary delay of xenograft destruction.22,23 The reason is that the other mechanisms of innate immunity (shown in Table 1) promptly cause inexorable rejection. To avoid the devastating consequences of these effectors, additional genetic manipulation will be required, whereby antigens are eliminated or equivalent human genes are introduced.
Gene knockout procedures have not yet been done in the pig. Using molecular technologies, though, some of which already have been shown to be applicable in pigs, the team of Osman and co-workers24 in Australia has been able to reduce cell-surface expression of the Gal-α-Gal gene product in cultured African green monkey fibroblasts (so-called COS cells) to negligible levels. The experiments were staged. As a first step, the COS cells, which normally do not express the Gal-α-Gal epitope, were transfected with the Gal cDNA. Because these transfected COS cells now presented a Gal-α-Gal target, they were vigorously lysed by the antibodies in human serum (Fig. 1).
Figure 1.
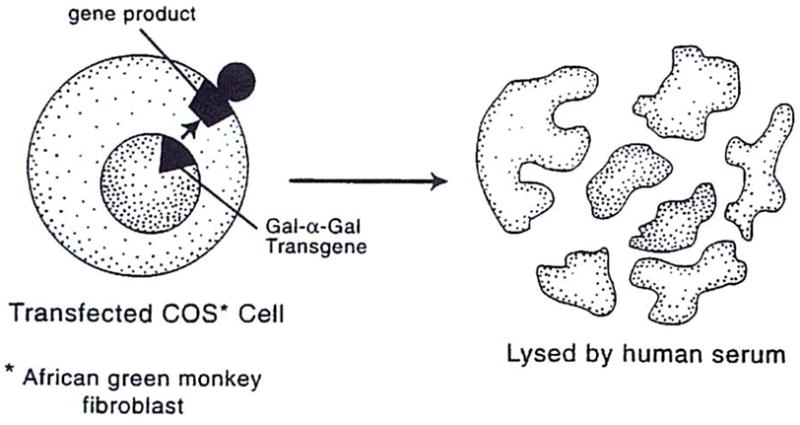
COS cells are lysed by human serum after their transfection with the Gal-α (1,3) Gal gene (see text). COS, cultured African green monkey fibroblasts.
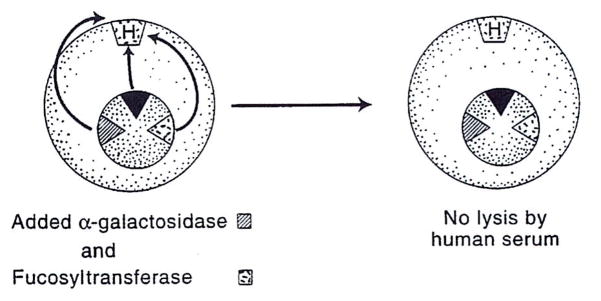
The anti-Gal lysis was reduced, but not eliminated, by transfection of the altered COS cell with human α-galactosidase, which cleaves off α-linked galactosyl residues of the target epitope (Fig. 2). Because this exposes subterminal saccharides (ie, N-acetyl lactosamine), to which there also are “natural” human antibodies, lysis is only reduced. The additional insertion of an α(1,2)fucosyltransferase gene, however, resulted in the substitution of Gal-α Gal with the nonimmunogenic H substance (ie, the universally tolerated O blood-group antigen). The double transfection (galactosidase plus fucosyltransferase) completely eliminated complement-mediated lysis of the COS cells (Fig. 3).
Figure 2.
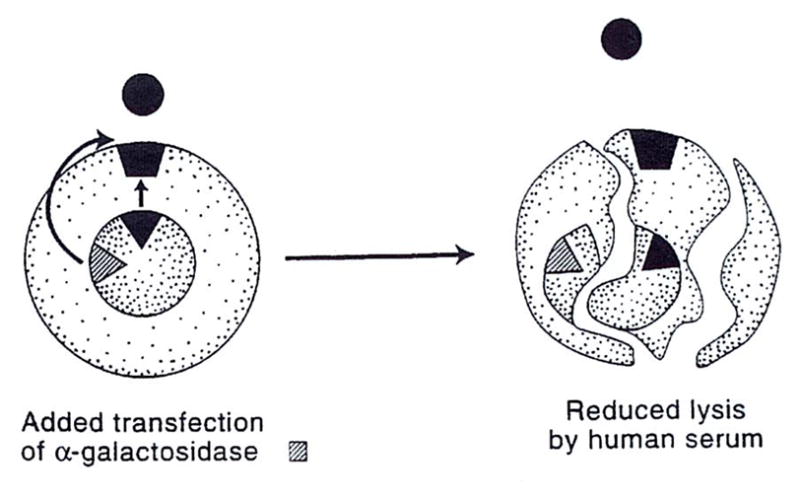
Insertion of the α-galactosidase gene diminishes, but does not eliminate, the lysis shown in Figure 1 (see text).
Figure 3.
Additional insertion of the α(1,2)fucosyltransferase gene converts the xenogeneic Gal-α(1,3) Gal antigen to the H (O, or universal donor) antigen and eliminates lysis (see text).
The α-galactosidase gene has not yet been transfected in pigs, but this has been accomplished with the α-fucosyltransferase gene by John Logan and associates of the Nextran Corporation in collaboration with Sharma and associates at Duke University.25 Stable double transfection in pigs would seem to be only a matter of time.
WHAT LIES BEYOND?
There has been much speculation about what unanticipated obstacles will arise when, and if, the first barrier of hyperacute rejection is broken down.26,27 With an understanding of the commonality of infectious and transplantation immunity,12 the answer already is obvious, and not alarming. In contrast to the cytopathic parasites, whose antigens are mimicked by discordant xenografts, the noncytopathic microorganisms are routinely accommodated by the host in ways made possible by the major histocompatibility complex restriction, which allows both host and invader to survive.13–15–28 For this fundamental discovery,29,30 Doherty and Zinkernagel were awarded the 1996 Nobel Prize. In the noncytopathic scenario, the highest priority is not elimination of the pathogens, but avoidance of damage to host tissues.
The recognition and effector mechanisms that evolved to deal with noncytopathic infections are the same as those that have been subverted successfully for organ allotransplantation with the aid of immunosuppression.12 In both transplant and infectious circumstances, the immune response is governed by the antigen’s migration and localization. The traffic is oriented selectively at first to lymphoid organs (Fig. 4A), where activation-associated clonal exhaustion/deletion occurs.12 Ultimately, the antigen escapes to ubiquitous nonlymphoid areas (Fig. 4B), where a secondary tolerance mechanism of immune indifference may contribute in the transplant setting to maintenance of the chimerism-dependent deletion/exhaustion that takes place in organized lymphoid collections. No other mechanisms of tolerance, including negative regulation, are essential to explain graft acceptance.12
Figure 4.
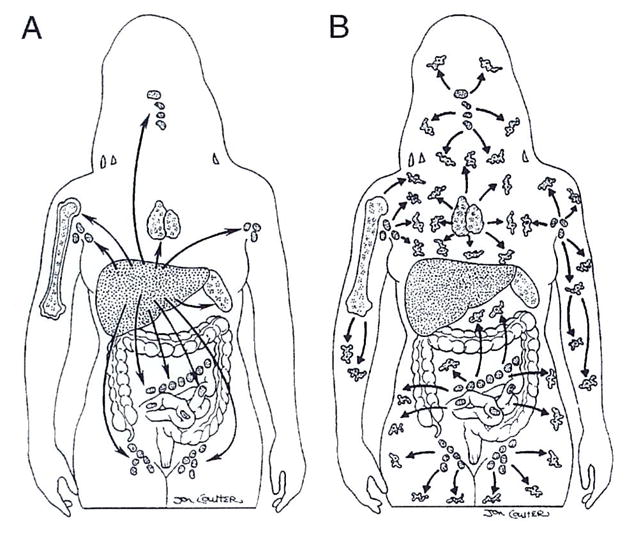
(A) Similar selective migration to lymphoid organs by invasive noncytopathic microorganisms and passenger leukocytes from organ grafts. (B) Ubiquitous antigen spread after a pause at the locations shown in A.
The rules for transgenic xenografts, whose anti-genic epitopes are altered enough to avoid evoking innate immunity, should be the same as those that permit the characteristic immunologic confrontation and resolution first identified in kidney allograft recipients receiving azathioprine and dose-maneuverable prednisone.31 It has been possible since then to exploit this discovery with progressively greater efficiency using increasingly potent baseline immunosuppressants, but the essential pattern has remained the same. The only difference in principle from response to a noncytopathic infection is that a double immune reaction is involved after transplantation, in which responses of coexisting donor and recipient immune cells, each to the other, cause reciprocal clonal expansion, followed by variable clonal exhaustion and deletion, which are maintained by persistent microchimerism.12,32–34
CONCLUSIONS
Far from being bleak, the future of xenotransplantation is brighter than at any previous time because what must be done to succeed has become remarkably clear. Although nature did not evolve defensive barriers to frustrate transplant surgeons, rules laid down by coevolution of the host-parasite relationship must be followed. First, we have already learned empirically with allotransplantation how to work around, or, more accurately, work with, the defense mechanisms developed by nature to control noncytopathic infections.
Second, because this approach will not work for the xenogeneic antigens that resemble cytopathic microorganisms, these antigens in animal donors will have to be deleted or changed. It remains to be seen whether species restriction of complement 9,11,26,27 will necessitate transfection of complement regulatory proteins to prevent continuous complement activation. If so, strategies for xenotransplantation of the liver will be more complex because this organ is the source of most complement.
Finally, bridge trials with xenografts to provide desperately needed temporary organ function for candidates waiting for allografts may be the best way, consistent with ethical patient care, to obtain information about the efficacy of donor species alteration. If it is emphasized that these efforts are being made with patient benefit foremost in mind, the public will support such trials. If we do not, or if we indulge in exaggerated claims, xenotransplantation will be shut down.
Acknowledgments
Aided by Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland.
Footnotes
Presented at the American College of Surgeons 83rd Annual Clinical Congress, Chicago, October 1997.
References
- 1.Institute of Medicine. Xenotransplantation: Science, Ethics, and Public Policy. Washington, DC: National Academy Press; 1996. [PubMed] [Google Scholar]
- 2.Nuffield Council on Bioethics. Animal to Human Transplants: The Ethics of Xenotransplantation. London: KKS Printing; 1996. [Google Scholar]
- 3.Reemtsma K, McCracken BH, Schlegel JU, et al. Renal heterotransplantation in man. Ann Surg. 1964;160:384–410. doi: 10.1097/00000658-196409000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Marchioro TL, Peters GN, et al. Renal heterotransplantation from baboon to man: experience with 6 cases. Transplantation. 1964;2:752–776. doi: 10.1097/00007890-196411000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murase N, Starzl TE, Demetris AJ, et al. Hamster-to-rat heart and liver xenotransplantation with FK506 plus antiproliferative drugs. Transplantation. 1993;55:701–708. doi: 10.1097/00007890-199304000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker CF, Billingham RE. Histocompatibility requirements of heart and skin grafts in rats. Transplant Proc. 1971;3:172–175. [PubMed] [Google Scholar]
- 7.Michaels MG, Lanford R, Demetris AJ, et al. Lack of susceptibility of baboons to infection with hepatitis B virus. Transplantation. 1996;61:350–351. doi: 10.1097/00007890-199602150-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Fung J, Tzakis A, et al. Baboon-to-human liver transplantation. Lancet. 1993;341:65–71. doi: 10.1016/0140-6736(93)92553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Valdivia LA, Murase N, et al. The biologic basis of and strategies for clinical xenotransplantation. Immunol Rev. 1994;141:213–244. doi: 10.1111/j.1600-065x.1994.tb00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey L, Nehlsen-Cannarella S, Concepcion W, Jolley W. Baboon to human cardiac xenotransplantation in a neonate. JAMA. 1985;254:3321–3329. [PubMed] [Google Scholar]
- 11.Valdivia LA, Fung JJ, Demetris AJ, et al. Donor species complement after liver xenotransplantation: mechanism of protection from hyperacute rejection. Transplantation. 1994;57:918–922. doi: 10.1097/00007890-199403270-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Zinkernagel R. The regulation of immune reactivity by antigen migration and localization: a comparison of “tolerance” to infectious agents and allografts. N Engl J Med. in press, provisional. [Google Scholar]
- 13.Zinkernagel RM. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- 14.Janeway CA, Travers P. Immunobiology: The Immune System in Health and Disease. 2. New York: Current Biology Ltd./Garland Publishing Inc; 1996. [Google Scholar]
- 15.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 16.Galili U, Clark MR, Shohet SB, et al. Evolutionary relationship between the natural anti-Gal antibody and the Galα (1,3) Gal epitope in primates. Proc Natl Acad Sci USA. 1987;84:1369–1373. doi: 10.1073/pnas.84.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper DKC, Koren E, Oriol R. Oligosaccharides and discordant xenotransplantation. Immunol Rev. 1994;141:31–58. doi: 10.1111/j.1600-065x.1994.tb00871.x. [DOI] [PubMed] [Google Scholar]
- 18.Galili U. Evolution and pathophysiology of the human natural anti-α-galactosyl IgG (anti-Gal) antibody. Springer Semin Immunopathol. 1993;15:155–171. doi: 10.1007/BF00201098. [DOI] [PubMed] [Google Scholar]
- 19.White DJG, Oglesbay T, Liszewski MK, et al. Expression of human decay accelerating factor of membrane cofactor protein genes on mouse cells inhibits lysis by human complement. Transplant Int. 1992;5:648–650. doi: 10.1007/978-3-642-77423-2_190. [DOI] [PubMed] [Google Scholar]
- 20.Cozzi E, Yannoutsos EC, Langford GA, et al. Effect of transgenic expression of human decay-accelerating factor on the inhibition of hyperacute rejection of pig organs. In: Cooper DKC, Kemp E, Plate JL, White DJ, editors. Xenotransplantation. The Transplantation of Organs and Tissues Between Species. 2. Heidelberg; Verlag: 1997. pp. 665–682. [Google Scholar]
- 21.McCurry KR, Kooyman DL, Alvarado CG, et al. Human complement regulatory proteins protect swine-to-primate cardiac xenografts from humoral injury. Nat Med. 1995;1:423–427. doi: 10.1038/nm0595-423. [DOI] [PubMed] [Google Scholar]
- 22.Bach FH, Winkler H, Ferran C, et al. Delayed xenograft rejection. Immunol Today. 1996;17:379–384. doi: 10.1016/0167-5699(96)10024-4. [DOI] [PubMed] [Google Scholar]
- 23.Galili U, Minanov OP, Michler RE, Stone KR. High affinity anti-Gal IgG in chronic rejection in xenografts. Xenotransplantation. 1997;4:127–131. [Google Scholar]
- 24.Osman N, McKenzie IFC, Ostenried K, et al. Combined transgenic expression of α-galactosidase and α-1,2-fucosyltransferase leads to optimal reduction in the major xenoepitope Gal-α (1,3) GAL. PNAS. 1997;94:14677–14682. doi: 10.1073/pnas.94.26.14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma A, Okabe J, Birch P, et al. Reduction in the level of Gal (α1, 3) Gal in transgenic mice and pigs by the expression of an α (1, 2)fucosyltransferase. Proc Natl Acad Sci USA. 1996;93:7190–7195. doi: 10.1073/pnas.93.14.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker P, Saadi S, Lin SS, et al. Transplantation of discordant xenografts: a challenge revisited. Immunol Today. 1996;17:373–378. doi: 10.1016/0167-5699(96)10028-1. [DOI] [PubMed] [Google Scholar]
- 27.Platt JL. Xenotransplantation: recent progress and current perspectives. Curr Opin Immunol. 1996;8:721–728. doi: 10.1016/s0952-7915(96)80091-4. [DOI] [PubMed] [Google Scholar]
- 28.Zinkernagel RM, Ehl S, Aichele P, et al. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol Rev. 1997;156:199–209. doi: 10.1111/j.1600-065x.1997.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 29.Doherty PC, Zinkernagel RM. A biological role for the major histocompatibiliry antigens. Lancet. 1975;i:1406–l409. doi: 10.1016/s0140-6736(75)92610-0. [DOI] [PubMed] [Google Scholar]
- 30.Landmarks. Genetic control of the antibody response in inbred mice. J NIH Res. 1997;9:43–50. [Google Scholar]
- 31.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 32.Starzl TE, Demetris AJ, Murase N, et al. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 34.Starzl TE, Demetris AJ, Murase N, et al. The lost chord: microchimerism. Immunol Today. 1996;17:577–584. doi: 10.1016/s0167-5699(96)10070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]