Abstract
Type 5 adenylyl cyclase (AC5) is highly concentrated in the dorsal striatum and nucleus accumbens (NAc), two brain areas which have been implicated in motor function, reward, and emotion. Here we demonstrate that mice lacking AC5 (AC5−/−) display strong reductions in anxiety-like behavior in several paradigms. This anxiolytic behavior in AC5−/− mice was reduced by the D1 receptor antagonist SCH23390 and enhanced by the D1 dopamine receptor agonist, dihydrexidine (DHX). DHX-stimulated c-fos induction in AC5−/− mice was blunted in the dorso-lateral striatum, but it was overactivated in the dorso-medial striatum and NAc. The siRNA-mediated inhibition of AC5 levels within the NAc was sufficient to produce an anxiolytic-like response. Microarray and RT-PCR analyses revealed an up-regulation of prodynorphin and down-regulation of cholecystokinin (CCK) in the NAc of AC5−/− mice. Administration of nor-binaltorphimine (a kappa opioid receptor antagonist) or CCK-8s (a CCK receptor agonist) reversed the anxiolytic-like behavior exhibited by AC5−/− mutants. Taken together, these results suggest an essential role of AC5 in the NAc for maintaining normal levels of anxiety.
Keywords: anxiety, dopamine receptor, striatum, type 5 adenylyl cyclase
Animals that are exposed to a tangible external threat display a fear response characterized by one or more signs of sympathetic autonomic activation. This is an adaptive emotional response designed to allow the animal to remain attentive and assess the risks of a variety of escape options. When such emotions occur in response to an intangible, vague, or internal stressor, it is termed anxiety and is often associated with irritability and restlessness (Kaplan and Sadock 2007). Several psychiatric syndromes are associated with pathologically enhanced anxiety, where individuals respond to ambiguous or non-harmful stimuli in an exaggerated fashion as well as suffer from a variety of somatic complaints related to heightened autonomic activation (Martis et al. 2002; Fuchs and Flugge 2004). However, the neurobiological mechanisms and molecular mediators of anxiety-related behaviors are poorly understood.
An increasing body of evidence implicates mesolimbic dopamine signaling in the neurobiology of mood and anxiety-related disorders (Millan 2003; Nestler and Carlezon 2006). The mesolimbic dopamine circuit consists, in part, of dopaminergic neurons of the ventral tegmental area (VTA) that project to the nucleus accumbens (NAc), a forebrain structure involved in responses to emotional and rewarding stimuli (Morgane et al. 2005). In rodents, stressful stimuli activate VTA dopamine neurons, and this phenomenon is thought to contribute to the heightened arousal states that allow for better coping strategies during adverse experiences (Tidey and Miczek 1996; Krishnan et al. 2007). Additionally, the positive emotional effects associated with mesolimbic activation are thought to outweigh the negative impact of stressors (Millan 2003). Stimuli that elicit increases in dopamine signaling within the NAc such as chronic stresses or drugs of abuse lead to the induction of cAMP-response element binding protein, a neuroadaptation that serves to reduce anxiety-related behavior in rodent models (Barrot et al. 2002, 2005).
Adenylyl cyclases (AC) are a family of enzymes that are responsible for the conversion of ATP to cAMP. Of the nine known transmembrane AC isoforms, AC5 is particularly enriched within the NAc and dorsal striatum (caudate/putamen complex) (Glatt and Snyder 1993; Lee et al. 2002). We have previously shown that the genetic elimination of AC5 (AC5−/−) leads to a significant impairment in the pharmaco-behavioral responses to D2 receptor agonists and antagonists (Lee et al. 2002), consistent with a critical role for AC5 in D2 receptor signal transduction. However, while striatal tissues from AC5−/− mice display strong reductions in basal and D1-stimulated AC activity, their behavioral responses to pharmacologic manipulations of D1 signaling are relatively intact (Lee et al. 2002), suggesting that D1 dopamine receptors can signal through other ACs or non-AC mechanisms.
Given the contribution of mesolimbic dopamine signaling to anxiety-related processes, and to further delineate the interactions between AC5 and D1 receptor signaling, the current study was undertaken. Through a combination of behavioral, pharmacologic, and molecular techniques, we demonstrate for the first time that AC5 elimination produces a robust anxiolytic phenotype that is mediated through enhanced D1 receptor-mediated signaling. We discuss the therapeutic implications of these findings.
Materials and methods
Animals
AC5−/− mice, which have been described in previous studies (Lee et al. 2002; Kim et al. 2006), were backcrossed to the C57BL/6J strain for 9 or 10 generations to obtain heterozygote N9 or N10 mice. Intercrossing between N9 or N10 heterozygotes produced homozygote (AC5−/−), heterozygote (AC5+/−), and wild-type (AC5+/+) littermates. Mice were housed in clear plastic cages in a temperature- and humidity-controlled environment with a 12 h light/dark cycle (lights ON at 7:00 AM), and were maintained on an ad libitum diet of lab chow and water. All experiments were performed in accordance with The Guideline of Animal Care at Ewha Womans University School of Medicine.
Drug administration
Dihydrexidine (DHX), SCH23390, cholecystokinin (CCK) octapeptide (sulfated), and nor-binaltorphimine (BNI) dihydrochloride were purchased from Tocris (Bristol, UK). Haloperidol and diazepam were from Sigma (St Louis, MO, USA) and Daewon Pharm. Co. (Seoul, Korea), respectively. All drugs were administered intraperitoneally and were dissolved in 0.9% saline, except for haloperidol which was dissolved in dimethylsulfoxide, then diluted in saline to a final concentration.
Immunohistochemistry
Mice were transcardially perfused with a solution of 4% parafor-maldehyde in 0.1 M phosphate buffer (pH 7.4), and isolated brains were post-fixed in the same solution overnight at 4°C. Brain sections were prepared by cutting the brain at 40-μm intervals using a vibratome as previously described (Lee et al. 2006). The primary antibody for c-fos was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
RT-PCR analyses
Total RNA was purified from tissue samples of 4–5 animals for each group with TRI reagent (cat #T9424, Sigma). RNA from each group was treated with DNase I to avoid genomic contamination. Conventional RT-PCR analysis used in Figs 2 and 5 was carried out using the following primer sets: c-fos (5′-TACTACCATTCCCCAGCCG-3′and 5′-TTGGCAATCTCGGTCTGCAA-3′), egr-1 (5′-AGATGATG-CTGCTGAGCAAC-3′and 5′-TACTGCAAGGCTGTGCCTGC-3′), junB (5′-CCGGATGTGCACGAAAATGGAACAG-3′and 5′-ACC-GTCCGCAAAGCCCTCCTG-3′), prodynorphin (5′-GTGCAGTG-AGGATTCAGGATGGG-3′, and 5′-GAGCTTGGCTAGTGCAC-TGTAGC-3′), and glyceraldehyde-3-phosphate dehydrogenase (5′-ACCACAGTCCATGCCATCAC-3′and 5′-TCCACCACCCT-GTTGCTGTA-3′). SYBR Green-based real-time RT-PCR analysis applied in Fig. 4 was performed using the Mini-Opticon Real-time PCR System Detector (Bio-Rad, Richmond, CA, USA) as described in a previous study (Ha et al. 2008). The primer sets of AC5 (5′-GGGAGAACCAGCAACAGG-3′and 5′-CATCTCCATGGC-AACATGAC-3′) and L32 (5′AGGCACCAGTCAGACCGATATG-3′and 5′-ACCTTCTCCGCACCCTGTTG-3′) were used. Expression levels of the RT-PCR data were quantified using a gel documentation system (Bio-Rad) and expressed relative to expression levels in vehicle-treated wild-type mice.
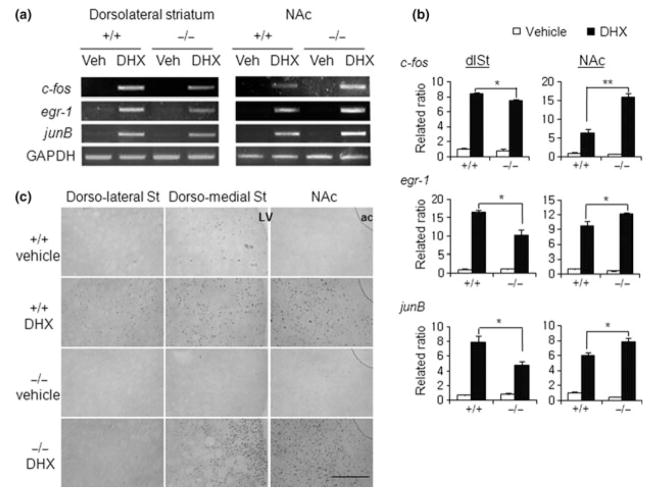
Fig. 2.
Dihydrexidine (DHX)-dependent induction of genes in the striatum of AC5−/− mice. (a, b) RT-PCR analysis of the induction of the immediately early genes, c-fos, egr-1, and junB in the dorso-lateral striatum and nucleus accumberns (NAc) of AC5+/+ and AC5−/− mice 45 min after the administration of the D1 dopamine receptor agonist, DHX (30 mg/kg) (a). Relative expression levels quantified with respect to the paired vehicle-treated wild-type condition are presented (b). (c) The expression of c-fos in the dorsal striatum and NAc of AC5+/+ and AC5−/− mice 45 min after the administration of the D1 dopamine receptor agonist, DHX (30 mg/kg). Note that the DHX-dependent induction of c-fos in AC5−/− mice occurred in the dorso-medial striatum and NAc, but was barely detectable in the dorso-lateral striatum. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Veh, vehicle (0.9% NaCl); St, striatum; LV, lateral ventricle; ac, anterior commissure. Scale Bar, 200 μm.
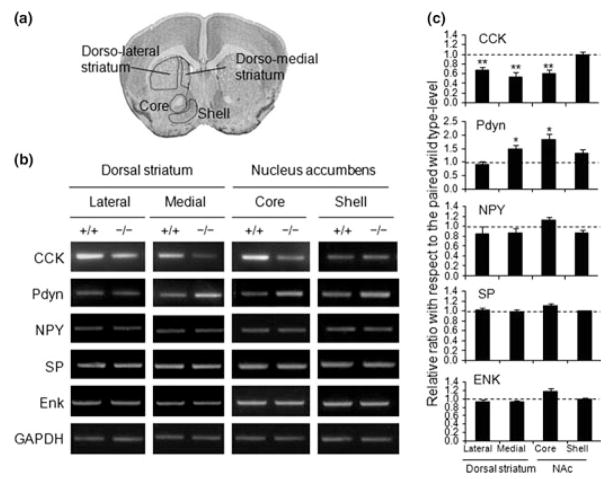
Fig. 5.
Altered expression of prodynorphin (Pdyn) and cholecystokinin (CCK) in the dorso-medial striatum and nucleus accumbens (NAc). (a–c) RT-PCR data showing the up-regulation of prodynorphin and the down-regulation of CCK in the NAc and dorsal striatum of AC5−/− mice (b, c). Several other neuropeptides, including neuropeptide Y (NPY), substance P (SP), and enkephalin (Enk), were not changed in their expression levels. Areas of tissue dissection for each brain region are depicted in (a) Coronal image taken from The Mouse Brain in Stereotaxic Coordinates by Keith B. J. Franklin, Academic press. All data were prepared using 4–5 animals with 3–5 PCR repeats for each genotype. Means ± SEM are presented. * and ** denote differences at the p < 0.05 and p < 0.01 levels, respectively, compared with the wild-type control. +/+, AC5−/−; −/−, AC5−/− Veh, vehicle; GAPDH, glyc-eraldehyde-3-phosphate dehydrogenase.
Fig. 4.
Inhibition of AC5 expression in the NAc by siRNA-AC5 induces anxiolytic-like behavior. (a) The experimental schedule used. Three days after the infusion of siRNA-AC5 or siRNA control into the each nucleus accumberns (NAc) or dorso-lateral striatum, elevated plus maze (EPM) tests were performed (upper panel). Infusion areas are indicated by arrows in lower panel. (b) Representative photomicrograph depicting the native fluorescence of siRNA control anatomically localized to the NAc. After behavioral analysis on day 3, injection sites in the brain were histologically examined for their anatomical localization under a microscope by aid of the native fluorescence emitted from siRNA control and/or of the needle tract. Mice with mislocalized injections were excluded from behavioral analysis. Scale Bar, 250 μm. (c) RT-PCR data showing the knock-down of AC5 mRNA levels in the NAc of heterozygote ((AC5+/−) mice 3 days after injection with siRNA-AC5 (n = 4–5). (d–g) Behavioral responses in the EPM test. (AC5+/−) mice infused with siRNA-AC5 into the NAc (d, e), but not into the dorsal striatum (f, g) showed increases in the number of entries (d) and the time (e) spent in the open arm of the EPM. Total number of arm entry was slightly enhanced for the group injected into the NAc, but were alike for the group injected into the dorso-lateral striatum, compared with the respective control groups (data not shown). The numbers of animals included and excluded were 13–14 and 3–4, respectively. (h-j) AC5+/+ mice infused with siRNA-AC5 into the NAc showed a strong tendency of increases in the number of entries (h) and the time (i) spent in the open arm of the EPM. Total arm entry numbers were not different between the two groups (j). The numbers of animals examined were 7–9. Data are presented as means ± SEM. *and ** denote the difference between the control and indicated group at p < 0.05 and p < 0.01, respectively. dlSt, dorso-lateral striatum.
Microarray analyses
The NAc from five animals for each genotype was pooled and used in microarray analysis. Total RNA was purified with TRI reagent (cat #T9424, Sigma) and subsequently with RNeasy column (Qiagen, Hilden, Germany). Total RNA was amplified and purified using the Ambion Illumina RNA amplification kit (Austin, TX, USA) to yield biotinylated cRNA according to manufacturer instructions. After purification, the cRNA was quantified using an ND-1000 Spectrophotometer (NanoDrop, Wilmington, DE, USA). Labeled cRNA samples of 750 ng were hybridized to each Sentrix MouseRef-8 Expression BeadChip (Illumina Inc., San Diego, CA, USA) which contained 25 600 transcripts (http://www.switchtoi.com/annotationfiles.ilmn) for 16–18 h at 58°C. Hybridization was performed in duplicate for each genotype. Detection of array signals was carried out by aid of Amersham fluorolink streptavidin-Cy3 (GE Healthcare Bio Sciences, Little Chalfont, UK) following the BeadChip manual. Arrays were scanned with an Illumina Bead Array Reader Confocal Scanner. Hybridization, scanning of signals, and statistical analysis were performed by Macrogen Inc. (Seoul, Korea). Array data processing and analysis were performed using Illumina BeadStudio. The quality of hybridization and overall chip performance were monitored by visual inspection of both internal quality control checks and the raw scanned data. Data were extracted using the software provided by the manufacturer (Bead-Studio v. 1.0.0.5, Illumina Inc, San Diego, CA, USA) and normalized by Quantile normalization. One-way ANOVA and local-pooled-error test (LPE) test (http://bioinformatics.oxfordjournals.org/cgi/reprint/19/15/1945) were applied to determine differentially expressed sets of genes across three experimental groups. Statistically significant differences were adjusted by the false discovery rate multiple testing suggested by Benjamini and Hochberg (1995).
Stereotaxic injection of siRNA
Animals were anesthesized with ketamine hydrochloride and xylazine hydrochloride as described in a previous study (Kim et al. 2006). siRNA in a volume of 0.5 μL was injected into the each NAc or dorso-lateral striatum. The stereotaxic coordinates were anterior-posterior, + 1.2; midlateral, ± 1.5; dorsal-ventral, )4.9 (mm) for the NAc and anterior-posterior, + 1.0; midlateral, ± 1.5; dorsal-ventral, −3.6 (mm) for the dorsal striatum. Surgically manipulated mice awakened from anesthesia were returned to their home cages until use. After 72 h of injection, behavioral tests were performed. siRNA controls (6-carboxyfluorescein fluorophore-labeled oligonucleotide siGLO Green [D-001630-0105] and non-targeting siRNA control [D-001210-0205]) and siRNA-AC5 (M-051739-00-0010, XM_156060) were purchased from Dharmacon Inc. (Chicago, IL, USA). The purchased siRNA-AC5 is a mixture of siRNA with five different sequences. They were diluted to 20 μM as a stock solution and then to 50 ng/μL. One volume of diluted siRNA control or siRNA-AC5 + siRNA control (10 : 1 ratio) was mixed with 2.5 volume of oligofectamine (Invitrogen, CA, USA) and incubated for 20 min before stereotaxic injections. Each NAc or dorsal striatum was injected with 0.5 μL that carried 5 ng of siRNA control or siRNA-AC5 + siRNA control mix.
Behavioral assessments
Behavioral assessments were carried out using a computerized video-tracking system, SMART (Panlab S.I., Barcelona, Spain) as described in previous studies (Kim and Han 2006; Lee et al. 2006). For the open-field test, locomotor activity was measured in a white Foamex chamber (45 cm× 45 cm× 40 cm). Each mouse was placed at the center of the open-field and horizontal locomotor activity was recorded for the indicated period. The inner rectangular area that constituted 30% of the open field was defined as the center. For the ‘novel object’ open-field test, a black cylinder [8.5 cm (diameter) × 20 cm (height)] was placed at the center of each chamber. For this test, the central area was defined as a circle, with 22 cm diameter, surrounding the novel object. The elevated plus maze (EPM) was made of gray Foamex. The apparatus consisted of four arms (30 cm × 7 cm) which were elevated 50 cm above the floor and placed at right angles to each other. Two of the arms had 20-cm high walls (enclosed arms), while the other two had no walls (open arms). Unless otherwise noted, EPM testing was carried out under a lighting condition of 70 lux. Each mouse was placed at the center of the platform and left to explore the arms for 5 min. The number of entries into the open and enclosed arms and the time spent in each arm were recorded. Entry into each arm was scored as an event if the animal placed all paws into the corresponding arm.
The light-dark box test was performed as previously described (Costall et al. 1989). The light box (20 cm × 20 cm × 20 cm) and dark box (10 cm × 20 cm × 20 cm) were made of white Foamex, with a shuttle door (5 cm × 7 cm) between the two at floor level. The light box was open at the top and illuminated with an 800 lux light at the bottom. The dark box was painted black and had a removable black lid. The test started by placing a mouse into the dark box, with the shuttle door closed initially. After 10 s, the shuttle door was opened. The total numbers of transitions between the dark and light compartments, crossings, and the time spent in the light box during a 5-min period were measured.
Statistical analysis
Two-sample comparisons were carried out using the Student’s t-test, while multiple comparisons were made using one-way ANOVA followed by the Newman–Keuls multiple range test. All data were presented as means ± SEM and statistical difference was accepted at the 5% level unless otherwise indicated.
Results
AC5−/− mice display reduced anxiety
In open-field testing, AC5−/− mice displayed significantly increased locomotor activity. Unlike the typical habituation response displayed by AC5+/+ mice, the activity of AC5−/− mice was persistently enhanced for the 2 h of the testing period (Fig. 1a). AC5−/− mice also spent significantly more time in the center, and their ambulatory activity in the center increased over time, in stark contrast to their wild-type littermates (Fig. 1b). In a modified open-field test, where a novel object was placed at the center (see Materials and Methods), AC5−/− mice explored the object more than AC5+/+ mice and the exploratory activity of AC5−/− mice in the object area increased over time (Fig. 1c and d). In the EPM test, AC5+/+ mice showed a typical preference for the enclosed arm over the open arm in terms of the number of entries and the time spent on each arm. This preference was abolished in AC5−/− mice, which explored the open and closed arms of the maze equally (Fig. 1e and f). The total arm entry numbers on the EPM were comparable between AC5+/+ and AC5−/− mice (Fig. 1g). In the light-dark box test, another paradigm widely used to measure anxiety-like behavior in rodents, AC5−/− mice spent more time in the light compartment than the AC5+/+ mice, while the number of crossings between the light and dark compartments in the two genotypes was not different (Figs 1h and i). These results demonstrate a strong anxiolyic phenotype in AC5−/− mice.
Fig. 1.
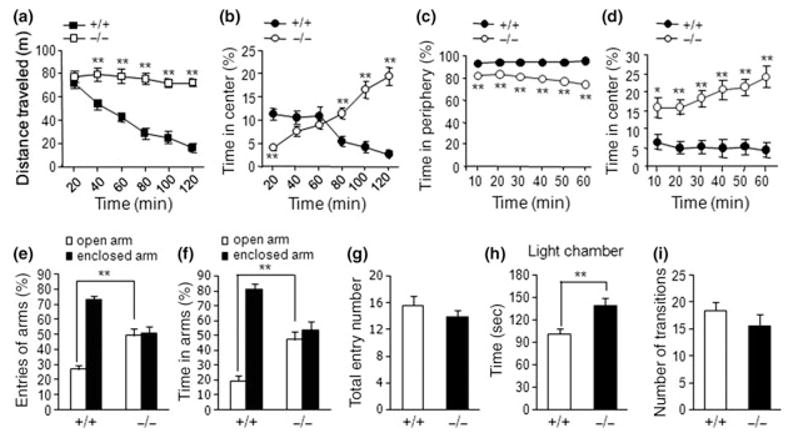
AC5−/− mice display strong reductions in anxiety-like behavior. (a, b) The total distance traveled for each 20-minute block in the open field (a, squares) and the percentage of time spent in the center (b) are presented (n = 14–15). The center was defined as the central 30% of the open field. (c, d) The percentage of time spent in the periphery (c) or in the center (d) of the open field with a novel object placed in the center (n = 14–15). (e–g) The percentages of the number of entries (e) and the time spent (f) in the open and enclosed arms of the elevated plus maze are presented (n = 10–13). Total arm entries were not different between the two genotypes (g). (h, i) The time spent in the light versus dark compartment of the light-dark box test and the number of crossings between the two compartments were presented (n = 6). Data are presented as means ± SEM. * and ** denote the difference between control and indicated data points at p < 0.05 and p < 0.01, respectively. +/+, AC5+/+; −/−, AC5−/−.
Benzodiazepines produce anxiolytic-like responses in animal models (Corbett et al. 1991). The GABAA receptor allosteric activator, diazepam (at 1 mg/kg), increased the numbers of entries and the time spent in the open arm of the EPM in AC5+/+ mice (Fig. S1a and b). The same dose of diazepam in AC5−/− mice similarly enhanced the numbers of entries and the time spent in the open arm (Fig. S1a and b). These results suggest that the increased exploratory behavior in AC5−/− mice does not represent a ceiling effect, as it can be further enhanced through a GABAergic mechanism.
The DHX-stimulated c-fos induction was enhanced in the dorso-medial striatum and NAc in AC5−/− mice
In a previous study, we demonstrated that the D1 dopamine receptor system regulating locomotion is functional in AC5−/− mice and over-activated in response to the D1 agonist DHX partly because the D2 dopamine receptor system is impaired (Lee et al. 2002). To examine the functional state of striatal neurons in response to D1 receptor activation, we injected AC5+/+ and AC5−/− mice with the D1 agonist DHX (30 mg/kg, i.p.) and obtained the dorso-lateral striatum and NAc, separately, 45 min later for RT-PCR analysis. These experiments revealed that DHX-triggered induction of the immediate early genes, c-fos, egr-1, and junB, in the NAc was markedly enhanced in AC5−/− mice compared with that in AC5+/+ mice, while the induction in the dorso-lateral striatum was suppressed in AC5−/− mice (Fig. 2a and b).
Analysis of region-specific c-fos induction serves to track the effects of D1-dopamine receptor activation in the brain as demonstrated by previous studies (Moratalla et al. 1996; Rohrer and Kobilka 1998; Zhang et al. 2002). Therefore, we applied immunohistochemical techniques to visualize DHX-induced c-fos expression across several striatal subregions. DHX administration in AC5+/+ mice induced c-fos expression moderately in the dorso-lateral and dorso-medial striatum and strongly in the NAc as measured 60 min after DHX administration. In AC5−/− mice, the DHX-stimulated c-fos induction was blunted in the dorso-lateral striatum, but it was overactivated in the dorso-medial striatum and NAc (Fig. 2c).
Anxiolytic-like behavioral responses of AC5−/− mice are modulated by D1 dopamine receptor activation
The fact that the striatal subregions in AC5+/+ mice are differentially activated by the D1 agonist DHX led us to examine whether anxiolytic-like behavior of AC5−/− mice can be modulated by the D1 receptor system. In the EPM test, while administration of the D1 agonist DHX (30 mg/kg) increased open arm exploration in AC5+/+ mice, this effect was not observed in AC5−/− mice (Fig. 3a and b). To test whether this blunted response in AC5−/− mice was because of a ceiling effect, we repeated the EPM test under brighter illumination conditions (200–250 lux instead of 70 lux), which provides more anxious environment. Under this configuration, DHX (30 mg/kg) administration in AC5−/− mice enhanced the numbers of entries and the time spent in the open arm (Fig. 3c and d), strongly implicating that a DHX-responsive anxiolytic pathway is functional in AC5−/− mice. In line with this result, the administration of a D1 receptor antagonist, SCH23390 (0.01–0.02 mg/kg), in a dose-dependent manner reduced open-arm exploration in AC5−/− mice (Fig. 3e and f). Together, these results support that anxiolytic-like behavioral responses of AC5−/− mice can be reversed by D1 dopamine receptor inhibition.
Fig. 3.
Anxiolytic-like behavior of AC5−/− mice is modulated by D1 dopamine receptor activity. (a, b) The number of entries (a) and the time (b) spent in the open arm of the elevated plus maze (EPM) with a brightness of 70 lux, presented as percent dihydrexidine (DHX, 30 mg/kg), was anxiolytic in AC5+/+ mice, but barely changed the anxiety-related behavior of AC5−/− mice in this condition. (c, d) DHX (30 mg/kg) increased the number of entries (c) and the time (d) spent by AC5−/− mice in the open arm (200 lux brightness). (e, f) SCH23390 (0.02 mg/kg) in a dose-dependent manner reduced open-arm entries (e) and open-arm time (f) in AC5−/− mice (70 lux). The total horizontal locomotion in the EPM displayed by mice treated with 0.02 mg/kg of SCH23390 was slightly reduced, but it was statistically insignificant (data not shown). (g, h) The number of entries (g) and the time spent (h) by AC5+/+ and AC5−/− mice treated with haloperidol (0.3 mg/kg) in the open and closed arms of the EPM with a brightness of 70 lux are presented as percentage. Haloperidol (0.3 mg/kg) did not change the anxiety-related behavior of AC5−/− mice. The EPM test was carried out 30 min after the administration of DHX, SCH23390 (SCH), or haloperidol (Hal). Data are presented as means ± SEM (n = 5–14). (+/+), AC5+/+; (−/−), AC5−/−. * and ** denote the difference between vehicle-treated control and indicated data points (comparison between the open arms in the EPM test) at p < 0.05 and p < 0.01, respectively. Veh, vehicle.
Haloperidol, a D2 receptor antagonist, functions as a potent neuroleptic in AC5+/+ mice, whereby low doses of haloperidol (ex, 0.3 mg/kg) produces a profound reduction in locomotor activity (Lee et al. 2002). However, haloperidol administration at the dose of 0.3 mg/kg in AC5−/− mice produces a paradoxical hyperlocomotor effect in the open field, and this phenotype is abolished by the inhibition of D1 receptor with SCH23390 (Lee et al. 2002). Consistent with this previous study, haloperidol (0.3 mg/kg) in AC5−/− mice induced hyperlocomotion (Fig. S2a). However, haloperidol administration in AC5−/− mice did not alter the percentage of the time spent in the center of the open field (Fig. S2b). On the EPM test, 0.3 mg/kg of haloperidol did not change the open-arm entry number and time, in either 70 or 200 lux illumination conditions (Fig. 3g and h). Collectively, these results suggest that the anxiolytic-like behavioral responses of AC5 mice cannot be ascribed to increased locomotor activity per se.
Inhibition of AC5 activity within the NAc is sufficient to produce anxiolytic-like behavior
We next tested whether the inhibition of AC5 activity in the NAc or dorsal striatum of adult animals produces anxiolytic-like behavior. To address this, we stereotaxically infused siRNA-AC5 (or a siRNA-control) into the striatum of wild-type (AC5+/+) or heterozygote (AC5+/−) mice (Fig. 4a and b). (AC5+/−) mice displayed normal locomotor activity and EPM behavior (data not shown). Given that the siRNA-mediated inhibition of AC5 levels within the injection site favors an advantage of using (AC5+/−) mice over AC5+/+ mice with respect to the fact that AC5 activity in the brain of (AC5+/−) mice has been abolished by 50% of the wild-type level and siRNA-AC5 infusion will inhibit remaining AC5 activity further. Three days after siRNA-AC5 infusion, the suppression of AC5 transcript levels in the NAc was confirmed by RT-PCR (Fig. 4c). At this timepoint, the siRNA-AC5 injected mice had markedly enhanced numbers of entries and time spent in the open arm of the EPM (Fig. 4d and e). On the contrary, siRNA-AC5 infusion into the dorso-lateral striatum of (AC5+/−) mice did not affect their anxiety-like behavior (Fig. 4a, f and g). The siRNA-mediated inhibition of AC5 levels within the NAc in AC5+/+ mice also produced an anxiolytic-like response (Fig. 4h and i). Collectively, these results suggest that down-regulation of AC5 activity in the NAc of adult animals is sufficient to produce anxiolytic-like behavior, partly recapitulating the anxiolytic phenotype of AC5−/− mice.
Altered expression of CCK and prodynorphin in the dorso-medial striatum and NAc of AC5−/− mice
To examine global transcriptional changes induced by the constitutive loss of AC5 within the NAc, we compared striatal gene expression patterns between AC5+/+ and AC5−/− mice through microarray and RT-PCR analyses. A summary of gene expression changes by microarray analysis is provided in Table 1 and Table S1). Among the genes whose expression is highly affected by loss of AC5, were prodynorphin (significantly up-regulated) and CCK (significantly down-regulated). RT-PCR analyses on striatal subregions showed that prodynorphin and CCK were, respectively, up- and down-regulated in the dorso-medial striatum and NAc (Fig. 5; Table 1).
Table 1.
A summary of selected genes that were up- or down-regulated in the nucleus accumbens of AC5−/− mice greater than approximately 1.2-fold in expression levels
| Accession # | Symbol | Gene names | Fold change | |
|---|---|---|---|---|
| 1 | NM_031161.1 | Cck | Cholecystokinin | −1.642 |
| 2 | NM_007470.1 | Apod | Apolipoprotein D | −1.496 |
| 3 | NM_008525 3 | Alad | Aminolevulinate delta-dehydratase | −1.469 |
| 4 | NM_182993.1 | Slc17a7 | Solute carrier family 17 | −1.450 |
| 5 | NM_009349 | Temt | Thioether S-methyltransferase | −1.436 |
| 6 | NM_153529.1 | Nm1 | Neuritin 1 | −1.431 |
| 7 | NM_010787.1 | Mea1 | Male enhanced antigen 1 | −1.396 |
| 8 | NM_009109 | Ryr1 | Ryanodine receptor 1 skeletal muscle | −1.390 |
| 9 | NM_153151 | Acat3 | Acetyl-coenzyme A acetyltransferase 3 | −1.369 |
| 10 | NM_175177.3 | Bdh | 3-Hydroxybutyrate dehydrogenase | −1.357 |
| 1 | NM_010234.2 | Fos | FBJ osteosarcoma oncogene | 1.541 |
| 2 | NM_133362 | Erdrl | Erythroid differentiation regulator 1 | 1.535 |
| 3 | NM_010731.1 | Zbtb7 | Zinc finger and BTB domain containing 7 | 1.530 |
| 4 | NM_011599.2 | Tle1 | Transducin-like enhancer of split 1 homolog of Drosophila E | 1.512 |
| 5 | NM_010118.1 | Egr2 | Early growth response 2 | 1.446 |
| 6 | NM_016789.2 | Nptx2 | Neuronal pentraxin 2 | 1.424 |
| 7 | XM_148699.3 | Crebbp | CREB binding protein | 1.414 |
| 8 | NM_177751.2 | Cnksr2 | Connector enhancer of kinase suppressor of Ras 2 | 1.412 |
| 9 | NM_023422 | Hist1h2bc | Histone l H2bc | 1.406 |
| 10 | NM_018863.2 | Pdyn | Prodynorphin | 1.222 |
CREB, cAMP-response element binding protein.
A full list is presented in Table S1 in supporting information
Previous studies have reported that CCK receptor agonists (e.g., CCK-8s) and CCK receptor antagonists (e.g., LY225910) are anxiogenic and anxiolytic, respectively (Biro et al. 1997; Zanoveli et al. 2004; Wang et al. 2005). Prodynorphin is processed into several smaller peptides which act on kappa opioid receptors. Inhibition of kappa receptors by the kappa receptor antagonist, nor-BNI, does not affect anxiety state in wild-type mice, but attenuates diazepam- or nicotine-induced anxiolytic responses (Tsuda et al. 1996; Balerio et al. 2005). In the present study, the activation of CCK receptors by administration of CCK-8s (3 or 30 μg/kg, i.p.) in AC5−/− mice produced a dose-dependent reduction in the number of entries and the time spent in the open arm of the EPM (Fig. 6a and b). Conversely, the inhibition of kappa receptors by administration of nor-BNI (3 or 10 mg/kg, i.p.) in AC5−/− mice reduced the number of entries and the time spent in the open arm of the EPM (Fig. 6c and d). These findings provide direct evidence that altered expression of prodynorphin and CCK in AC5−/− mice contributes to their anxiolytic phenotype.
Fig. 6.
Anxiolytic-like behavior of AC5−/− mice is reversed by a cholecystokinin (CCK) receptor agonist (CCK-8s) or by a kappa opioid receptor antagonist nor-binaltorphimine (nor-BNI) dihydrochloride in the elevated plus maze (EPM) test. (a, b) Administration of CCK-8s (3 or 30 μg/kg, i.p.) decreased the percentages of the number of entries (a) and the time (b) spent in the open arm of the EPM in the AC5−/− mice. The EPM test was performed 1 h after CCK-8s injection (n = 8–12). (c, d) Administration of nor-BNI (3 or 10 mg/kg, i.p.) decreased the percentages of the number of entries (c) and the time (d) spent in the open arm of the EPM in the AC5−/− mice. The EPM test was performed 2 days after nor-BNI injection (n = 7–10). Data are presented as means ± SEM. *and ** denote the difference between the open arm of vehicle group and the indicated drug-treated groups at p < 0.05 and p < 0.01, respectively.
Discussion
The present study demonstrates that the striatally enriched AC isoform, AC5, plays a key role in anxiety-related processes. Using four different measures of exploration and anxiety-like behaviors in rodents, we show that the genetic elimination of AC5 produces a strong anxiolytic phenotype. While AC5 is very highly expressed in the striatum, it is expressed at more moderate levels in other brain areas (Matsuoka et al. 1997). Consistently, forskolin-stimulated AC activity was reduced by more than 80% in the striatum of AC5−/− mice, with much smaller decrements in the prefrontal cortex and cerebellum (Lee et al. 2002). The anxiolytic phenotype of AC5−/− mice were sensitive to D1 dopamine receptor modulation: DHX (a D1 agonist) produced an anxiolytic effect and SCH23390 (a D1 antagonist) produced an anxiogenic effect. Collectively, these results provide a novel role for AC5 as a physiologically pertinent mediator of anxiety-related behavior, and further implicate the dopaminergic circuit as a key neural substrate within the broad list of neuroanatomic regions that are involved in anxiety-related processes.
Based on our c-fos induction studies, we speculate that D1 receptor signaling in the dorso-lateral striatum is blunted in AC5−/− mice and is therefore AC5-dependent. On the other hand, D1 signaling in the dorso-medial striatum and NAc is likely to involve other isoforms of AC or non-AC effectors. Together, these results suggest that both the dorsal striatum and NAc sites of D1 receptor signaling comprise candidate sites regulating anxiety-related behavior, although their contributions may not necessarily function in the same direction. Nevertheless, our siRNA experiments clearly illustrated that loss of AC5-mediated signaling in the NAc per se promotes anxiolytic-like behavior.
There are several possible mechanisms by which the loss of AC5 signaling may lead to an anxiolytic phenotype. First, several lines of evidence point to the cAMP second messenger system as a potential mechanism in anxiety. Many drugs that stimulate monoamine receptors such as the 5-HT1A and 5-HT1B/D (serotonin) receptors that are coupled via Gi to AC (Lanfumey and Hamon 2004) and noradrenergic receptors such as α2-ARs adrenoceptors that are coupled via Gs to AC (Millan 2003) produce anxiolytic effects. Thus, the cAMP system that is coupled to these neurotransmitter receptors, possibly in the striatum, might be important for anxiety. Because AC5 is the major AC isoform in striatum (Lee et al. 2002), defects in these signaling pathways might lead to changes in anxiety state.
Second, the loss of AC5 altered expression levels of prodynorphin and CCK in the NAc, and these two molecules have been extensively examined in rodent models of anxiety (e.g., Tsuda et al. 1996; Biró et al. 1997; Wang et al. 2005). Prodynorphin and CCK had opposite effect by AC5 knockout: prodynorphin was up-regulated while CCK was down-regulated in the NAc of AC5−/− mice (Figs 2b and 5). Moreover, administration of nor-BNI (a kappa opioid receptor antagonist) or CCK-8s (a CCK receptor agonist) reversed the anxiolytic-like behavior displayed by AC5−/− mice (Fig. 6). These results suggest that the normal expression of these two peptide systems is regulated by AC5 and AC5-related intracellular signaling pathways. Therefore, we further speculate that the anxiolytic behavior exhibited by AC5−/− mice is mediated in part via the altered expressions of these neuropeptides.
Third, altered neuronal interactions between the dorsal striatum and NAc in AC5−/− mice might lead to a failure to maintain normal levels of anxiety, thus resulting in increased exploration. This possibility is supported by the findings that the D1 dopamine receptor system in the NAc was superactivated in AC5−/− mice (Fig. 2) and SCH23390 completely reversed the anxiolytic-like behavior of these mutants (Fig. 3). We envision the presence of potential antagonistic interactions between the D1 receptor system in the dorso-lateral striatum and that in the NAc. The dorsal and ventral striata are composed of distinct neural circuits, but are highly interrelated via dopamine itself and via direct and indirect circuits (Haber 2003; Ikemoto 2007). The dorsal striatum is innervated by the nigrostriatal dopaminergic system that originates from the substantia nigra, while the NAc in the ventral striatum receives input of the mesolimbic dopaminergic pathway, which originates in the VTA. Recent reviews summarize various levels of interactions between dorsal and ventral subdivisions of the striatum (Haber 2003; Ikemoto 2007). Further studies, including electrophysiological analysis or tissue-specific genetic, pharmacologic, and molecular manipulation of AC5- or D1-expressed neural systems would provide needed insight into the role of these striatal subdivisions in the control of anxiety.
AC5−/− mice showed indefatigable locomotor activity in the open-field test throughout the 2-hour testing period (Fig. 1a). This behavioral phenotype of AC5−/− mice is distinct from that of mice deficient in D1 or D2 dopamine receptors: Mice lacking either D1 or D2 receptors showed, respectively, normal and reduced locomotion in the open-field test (Kelly et al. 1998; Clifford et al. 2000; K. S. Kim and P. L. Han unpublished data). Unlike that in AC5+/+ mice, the antipsychotic drug, haloperidol (a potent D2 antagonist), dramatically enhanced the locomotion of AC5−/− mice in the open-field test. Furthermore, the hyperlocomotion of AC5−/− mice was reversed by the suppression of D1 dopamine receptor activity with SCH23390 (Lee et al. 2002). Also, in the open-field test, the percentage of time spent in the center was similar to that of the vehicle-treated AC5−/− mice (Fig. S2a and b). Therefore, the long-lasting locomotor activity of AC5−/− mice in the open field cannot be ascribed to the same property as that which produces the enhanced locomotion, and raises the possibility that hyperactivity is not a prerequisite for anxiolytic states. Given that chronic cocaine exposure sensitizes animals in the open-field test, these complex phenotypes of AC5−/− mice suggest that the hyperlocomotion of AC5−/− mice could reflect a hyper- dopaminergic state or a state of impaired D2 suppression in the dorsal striatum (Lee et al. 2002). It will be interesting to investigate in future studies whether the enhanced locomotion in the open field originates primarily from other psychiatric alterations in AC5−/− mice such as manic-like behavioral responses as we recently demonstrated (Krishnan et al. 2007).
In summary, the present study shows a dramatic anxiolytic-like state in AC5 knockout mice and particularly implicates AC5 in the NAc in mediating this phenotype. We also provide direct evidence that the lowered anxiety state in AC5−/− mice reflects altered function of several neurotransmitter systems, including D1 dopamine receptors, prodynorphin, and CCK. Together, these findings strengthen the importance of the cAMP pathway in the NAc in the regulation of affective behavior, and specifically demonstrate the usefulness of AC5 knockout mice in dissecting the neurobiological mechanisms underlying anxiety.
Supplementary Material
Effect of diazepam and type 5 adenyl cyclase knockout on the anxiety state.
Effect of haloperidol on locomotion and open-field behavior of AC5−/− mice.
Full list of identified genes that were up- or down-regulated in the nucleus accumbens of AC5−/− mice greater than approximately 1.2-fold in their expression levels.
Acknowledgments
This research was supported by a grant (M103KV010009 03K2201 00910) from Brain Research Center, The 21st Century Frontier Research Program of the Ministry of Education, Science and Technology, Korea.
Abbreviations used
- AC 5
type 5 adenyl cyclase
- BNI
binaltorphimine
- CCK
cholecystokinin
- DHX
dihydrexidine
- EPM
elevated plus maze
- NAc
nucleus accumbens
- VTA
ventral tegmental area
Footnotes
Competing interest statement
The authors declare that they have no competing financial interests.
Additional supporting information may be found in the online version of this article.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Balerio GN, Aso E, Maldonado R. Involvement of the opioid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology (Berlin) 2005;181:260–269. doi: 10.1007/s00213-005-2238-y. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci USA. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Wallace DL, Bolanños CA, Graham DL, Perrotti LI, Neve RL, Chambliss H, Yin JC, Nestler EJ. Regulation of anxiety and initiation of sexual behavior by CREB in the nucleus accumbens. Proc Natl Acad Sci USA. 2005;102:8357–8362. doi: 10.1073/pnas.0500587102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate, a practical and powerful approach to multiple testing. J Royal Soc Ser. 1995;57:289–300. [Google Scholar]
- Biró E, Penke B, Telegdy G. Role of different neurotransmitter systems in the cholecystokinin octapeptide-induced anxio-genic response in rats. Neuropeptides. 1997;31:281–285. doi: 10.1016/s0143-4179(97)90060-3. [DOI] [PubMed] [Google Scholar]
- Clifford JJ, Usiello A, Vallone D, Kinsella A, Borrelli E, Waddington JL. Topographical evaluation of behavioural phenotype in a line of mice with targeted gene deletion of the D2 dopamine receptor. Neuropharmacology. 2000;39:382–390. doi: 10.1016/s0028-3908(99)00150-1. [DOI] [PubMed] [Google Scholar]
- Corbett R, Fielding S, Cornfeldt M, Dunn RW. GABA-mimetic agents display anxiolytic-like effects in the social interaction and elevated plus maze procedures. Psychopharmacology. 1991;104:312–316. doi: 10.1007/BF02246029. [DOI] [PubMed] [Google Scholar]
- Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM. Exploration of mice in a black and white box, validation as a model of anxiety. Pharmacol Biochem Behav. 1989;32:777–785. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Flugge G. Animal models of anxiety disorders. In: Charnery D, Nestler EJ, editors. Neurobiology of Mental Illness. 2. Oxford University Press; Oxford: 2004. pp. 546–557. [Google Scholar]
- Glatt CE, Snyder SH. Cloning and expression of an adenylyl cyclase localized to the corpus striatum. Nature. 1993;361:536–538. doi: 10.1038/361536a0. [DOI] [PubMed] [Google Scholar]
- Ha HY, Kim JB, Choi IH, et al. Morphogenetic lung defects of JSAP1-deficient embryos proceeds via the disruptions of the normal expressions of cytoskeletal and chaperone proteins. Proteomics. 2008;8:1071–1080. doi: 10.1002/pmic.200700815. [DOI] [PubMed] [Google Scholar]
- Haber S. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan HI, Sadock BJ. Clinical Psychiatry. 10. Lippincott Williams & Wilkins; Philadelphia: 2007. Synopsis of Psychiatry: Behavioral Sciences. [Google Scholar]
- Kelly MA, Rubinsteinl M, Phillips TJ. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci. 1998;18:3470–3479. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Han PL. Optimization of chronic stress paradigms using anxiety- and depression-like behavioral parameters. J Neurosci Res. 2006;83:497–507. doi: 10.1002/jnr.20754. [DOI] [PubMed] [Google Scholar]
- Kim KS, Lee KW, Lee KW, Im JY, Yoo JY, Kim SW, Lee JK, Nestler EJ, Han PL. Adenylyl cyclase type 5 (AC5) is an essential mediator of morphine action. Proc Natl Acad Sci USA. 2006;103:3908–3913. doi: 10.1073/pnas.0508812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Hamon M. 5-HT1 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:1–10. doi: 10.2174/1568007043482570. [DOI] [PubMed] [Google Scholar]
- Lee KW, Hong JH, Choi IY, et al. Impaired D2 dopamine receptor function in mice lacking type 5 adenylyl cyclase. J Neurosci. 2002;22:7931–7940. doi: 10.1523/JNEUROSCI.22-18-07931.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Im JY, Song JS, et al. Progressive neuronal loss and behavioral impairments of transgenic C57BL/6 inbred mice expressing the carboxy terminus of amyloid precursor protein. Neurobiol Dis. 2006;22:10–24. doi: 10.1016/j.nbd.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Martis B, Malizia A, Rauch SL. Functional neuroanatomy of anxiety disorders. In: D’haenen HAH, Boer JA, Willner P, editors. Biological Psychiatry. John Wiley & Sons Press; Manhattan: 2002. pp. 989–1002. [Google Scholar]
- Matsuoka I, Suzuki Y, Defer N, Nakanishi H, Hanoune J. Differential expression of type I, II, and V adenyl cyclase gene in the postnatal developing rat brain. J Neurochem. 1997;68:498–506. doi: 10.1046/j.1471-4159.1997.68020498.x. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Xu M, Tonegawa S, Graybiel AM. Cellular responses to psychomotor stimulant and neuroleptic drugs are abnormal in mice lacking the D1 dopamine receptor. Proc Natl Acad Sci USA. 1996;93:14928–14933. doi: 10.1073/pnas.93.25.14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Rohrer DK, Kobilka BK. G Protein-coupled receptors: functional and mechanistic insights through altered gene expression. Physiol Rev. 1998;78:35–52. doi: 10.1152/physrev.1998.78.1.35. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Suzuki T, Misawa M, Nagase H. Involvement of the opioid system in the anxiolytic effect of diazepam in mice. Eur J Pharmacol. 1996;307:7–14. doi: 10.1016/0014-2999(96)00219-1. [DOI] [PubMed] [Google Scholar]
- Wang H, Wong PT, Spiess J, Zhu YZ. Cholecystokinin-2 (CCK2) receptor-mediated anxiety-like behaviors in rats. Neurosci Biobehav Rev. 2005;29:1361–1373. doi: 10.1016/j.neubiorev.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Zanoveli JM, Netto CF, Guimarães FS, Zangrossi H., Jr Systemic and intra-dorsal periaqueductal gray injections of cholecystokinin sulfated octapeptide (CCK-8s) induce a panic-like response in rats submitted to the elevated T-maze. Peptides. 2004;25:1935–1941. doi: 10.1016/j.peptides.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang L, Lou DW, Nakabeppu Y, Zhang J, Xu M. The dopamine D1 receptor is a critical mediator for cocaine-induced gene expression. J Neurochem. 2002;82:1453–1464. doi: 10.1046/j.1471-4159.2002.01089.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of diazepam and type 5 adenyl cyclase knockout on the anxiety state.
Effect of haloperidol on locomotion and open-field behavior of AC5−/− mice.
Full list of identified genes that were up- or down-regulated in the nucleus accumbens of AC5−/− mice greater than approximately 1.2-fold in their expression levels.