Abstract
Objective To examine the association between biological stress regulation and somatic complaints in young girls prior to the onset of clear psychopathology such as somatization disorder. Methods Salivary cortisol, heart rate variability (HRV), and negative mood were assessed in 48 12-year-old girls in response to the Trier Social Stress Test for Children (TSST-C). Parent and child report on the Children's Somatization Inventory was used to identify girls with high and low somatic complaints. Results Girls with high levels of somatic complaints had significantly higher initial levels of cortisol, which decreased over time, and showed a trend for a more limited HRV in response to the TSST-C than girls with low levels of somatic complaints. Conclusions High levels of cortisol and possibly low HRV among girls with somatic complaints may interfere with flexibility in responding to typical psychosocial stressors, which may increase vulnerability to the onset of somatic illnesses in females.
Keywords: girls, preadolescence, somatic complaints, stress reactivity.
Introduction
Understanding the interface between physical and psychological functioning is highly relevant for women's mental health. In childhood, girls are more likely than boys to report a range of somatic symptoms (Abu-Arafeh & Russell, 1996; Kristjansdottir, 1997) and this gender difference increases with age from childhood into adolescence (Garber, Walker, & Zeman, 1991). Girls also show more consistency in symptom reporting over time than boys (Walker, Garber, & Greene, 1991) and are more likely to seek support and services for physical symptoms compared with boys (Lewis & Lewis, 1989; Tsao & Zeltzer, 2003). Thus, girls seem to be more at risk for developing impaired functioning as a result of somatic problems.
Little is known about the underlying causes of somatic complaints. However, it is reasonable to posit that regulation of physiological arousal systems in response to physical and psychological stress may be altered in girls who are at risk for developing somatization disorders. Individual differences in stress response systems, such as the hypothalamic–pituitary–adrenal (HPA) axis, appear early in life (Gunnar, Mangelsdorf, Larson, & Hertsgaard, 1989) and among adults, it is known that chronic stimulation of this axis is associated with both physical complaints (Rohleder, Wolf, & Kirschbaum, 2003) and psychiatric disorders (Sapolsky, 2000). Preliminary research suggests that individual differences in HPA stress reactivity are also involved in the etiology and development of childhood emotional and behavioral problems (Granger, Weisz, & Kauneckis, 1994; van Goozen et al., 1998). Cardiovascular reactivity has also been identified as an individual characteristic that emerges early in childhood and is associated with emotional disorders. Low variability in heart rate, indicative of reduced parasympathetic cardiac control, has been concurrently and prospectively associated with symptoms and disorders in children (Mezzacappa et al., 1997; Monk et al., 2001). Despite growing interest in the links between physiologic functioning and the development of childhood psychopathology (primarily anxiety disorders, and often with male samples), the association between physiological arousal and somatic complaints has rarely been explored in children. In one notable exception, Dorn et al. (2003) reported a trend for greater physiological responses to stress, assessed via cortisol levels, heart rate, and blood pressure, among 14 children with recurrent abdominal pain compared with healthy controls. The small cell sizes in this study, however, did not allow specific conclusions to be drawn about girls.
Finally, individual factors such as obesity, race, and menarche may be related to psychophysiological stress reactivity and to somatic complaints. For example, studies of adults have shown greater and more prolonged cardiovascular reactivity to stress among individuals with high body mass index (BMI) and among individuals of African-American race (Barnes et al., 2000; Davis, Twamley, Hamilton, & Swan, 1999). Somatic complaints have also been shown to be more common among non-white research participants (Campo, Jansen-McWilliams, Comer, & Kelleher, 1999). Furthermore, early menarche is related to higher levels of menstrual pain (Klein & Litt, 1981), and menstrual symptoms in turn, are associated with a heightened preoccupation with bodily sensations and somatic complaints (Sigmon, Dorhofer, Rohan, & Boulard, 2000), and are exacerbated by stress (Lewis, Wasserman, Denney, & Gerrard, 1983).
The current study examined psychophysiological characteristics of girls that are associated with somatic symptoms in the absence of known physical disease. Thus, comparisons were made between cortisol levels, heart rate variability (HRV), and negative mood before, during and after a social stressor among young adolescent girls with high, versus low levels of somatic complaints in a non-clinical sample. Our goal was to explore the feasibility of identifying a profile of psychophysiological responses to stress prior to the onset of, but among girls who appear to be at risk for, somatization disorder. For this reason, we focused on the early adolescent period before increases in somatic complaints and onset of serious psychopathology typically occur (Borge, Nordhagen, Moe, Botten, & Bakketeig, 1994; Hyams, Burke, Davis, Rzepski, & Andrulonis, 1996; Offord, Boyle, Fleming, Blum, & Grant, 1989). The first aim was to determine whether there were significant group differences in prestressor levels of arousal, which would suggest more stable psychophysiological differences. We also tested whether obesity, race, and menarche were associated with prestressor psychophysiology. The second aim was to determine whether patterns of stress response differed by group. More specifically, it was hypothesized that girls with high levels of somatic complaints would show higher resting cortisol levels, lower resting HRV, and more negative mood than girls with low levels of somatic complaints. In addition, it was anticipated that the high somatic group would show greater stress-induced increases in cortisol and negative mood, and decreases in HRV than the low comparison group.
Methods
Participants
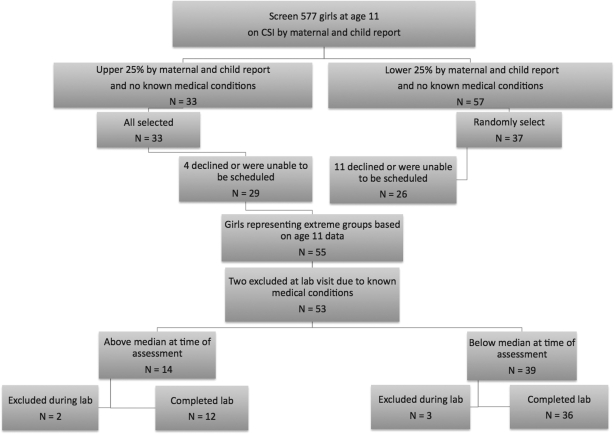
Research participants were drawn from a sample of 577 girls enrolled in an ongoing longitudinal study, the Pittsburgh Girls Study (see Hipwell et al., 2002 for further details). In order to increase the probability of detecting significant differences using a small sample, an extreme group sampling approach was adopted in the current study. To this end, the sample was enriched by selecting girls who scored in the upper or lower quartiles by both parent and child report on the Child Somatization Inventory (CSI; Walker et al., 1991) at age 11, in the absence of a known medical condition. Additional selection criteria included residence in Allegheny County due to the need for families to come to the research laboratory for assessments, and residence with the biological mother to allow for the collection of family history health data for the purpose of another study.
Thirty-three (5.7%) of the 577 girls scored in the upper quartile on the CSI according to both informants and had no known medical condition. Fifty-seven girls (9.9%) with no known medical conditions scored in the lower quartile, and from this group 37 girls were randomly selected for participation (Figure 1). Of the 70 families invited to participate, 55 (78.6%) consented and attended the laboratory visits, and 15 (21.4%) either declined or could not be scheduled within the study's timeframe. At the time of the laboratory assessment (girls aged 12), two of the 55 girls reported known medical conditions (i.e., arthritis and diabetes) and were excluded. Although the participants were selected to represent extreme groups based on complaints in the previous year, examination of the concurrent association with stress reactivity required that somatic complaints be re-assessed at age 12 to identify a clinically relevant group of girls with stable complaints. Therefore, the girls in the high somatic group were defined as those scoring above the median on both maternal (median = 3) and child (median = 7) reports of somatic complaints at the time of the laboratory visit. Thus, if the informant reports differed, the girl was assigned to the low somatic group. All 14 12-year-old girls who met the criteria for high levels of somatic complaints had scores in the upper quartile at the time of screening for eligibility. Thus, there were no girls who “switched” from having low levels of somatic complaints at screening to having high levels at the time of the laboratory assessment.
Figure 1.
Sampling of participants.
As per the Institutional Review Board's request, girls’ blood pressure (BP) was used to determine safety of administration and tolerability of the stressor, and laboratory assessments were discontinued if resting BP exceeded 132/90 mmHg, or if BP rose above 150/100 mmHg during the stressor. One girl was discontinued due to above-normal resting BP, and four girls were discontinued due to BP increasing above the threshold during the stress paradigm: two of the five were from the high somatic group and the other three were from the low somatic group.
Procedures
Informed consent to participate in the study was obtained from a legal guardian with assent obtained from the child. The study was approved by the Institutional Review Board of the University of Pittsburgh. All laboratory sessions took place between 3 pm and 7 pm to control for diurnal variations in cortisol. On the day of testing, participants were asked to avoid any nonprescription medications or exercise (above that required in the usual daily schedule) and to abstain from eating or drinking (except water) for 2 hr prior to the laboratory assessment.
Following the consent procedures, both informants completed a questionnaire about the child's somatic complaints. Next, height and weight were recorded, and an occluding cuff was placed on the nondominant arm for automated measurement of BP. A three-lead electrocardiogram (ECG) was attached to the shoulders and xyphoid process for continual measurement of HRV. Following instrumentation, participants were asked to rest and remain seated in a comfortable armchair in the testing chamber for a 20 min baseline habituation period. Participants were then administered a modified version of the Trier Social Stress Test for Children (TSST-C; Buske-Kirschbaum et al., 1997, 2003). In the current study, the speech task consisted of three min to prepare, and then three min to deliver a speech about a recent experience when the girl felt she was treated unfairly. The arithmetic task required serial subtraction of the number 7 from 758 as fast and as accurately as possible for three min. Both tasks were performed in front of an unfamiliar adult who gave minimal facial feedback. Following the tasks, participants were asked to rest quietly while viewing a neutral film of marine life for a 45-min recovery period. Seven samples of salivary cortisol and four ratings of mood were obtained before, during and after the TSST-C.
Measures
Somatic Complaints
The short form of the Child Somatization Inventory (CSI; Walker et al., 1991; Walker & Garber, 2003) was used to measure the severity of girls’ somatic complaints. This inventory comprises 18 somatic symptoms based on DSM-IV criteria for somatization disorder with modifications made for use in children, and has a response format ranging from 0 (not at all) to 4 (a whole lot). Good internal consistency, test–retest reliability, and concurrent validity with measures of functional impairment and depression have been reported for the CSI-18 with both clinical and well-patient samples of children ages 7–17 years (Walker & Garber, 2003). In the validation samples, the mean parent-reported score for well patients was 5.2 (SD = 5.9), and the mean score of healthy school children was 12.9 (SD = 10.5). In the current study, the most frequently endorsed items were headaches, nausea, and upset stomach. Girls in the low somatic group had mean scores of 4.8 (SD = 4.6) by self-report, and 2.4 (SD = 3.0) by parent report. In contrast girls in the high somatic group had mean scores of 15.0 (SD = 6.8) by self-report, and 9.0 (SD = 5.5) by parent report. The internal consistency of the children's and mothers’ reports on the CSI for the current sample was high (Cronbach's α = .86 and .83, respectively).
Heart Rate Variability
HRV was measured from a continuous time series of inter-beat intervals measured using CardioPro software (Thought Technology Ltd., Montreal, Canada). Prior to calculating estimates of HRV, the digitalized ECG signals were examined and artifactual detections of R-wave occurrences corrected. HRV analysis was conducted using HRV Analysis Software (Biosignal Analysis and Medical Imaging Group, University of Kuopio, Finland). The square root of the mean of successive differences (RMSSD) in inter-beat intervals was determined as a time-domain estimate of HRV. This measure has been shown to provide a reliable estimate of cardiac vagal activity (Task Force, 1996).
To examine changes in HRV across the protocol, mean RMSSD was generated for nine separate periods: the first and second 10 min during prestressor, 3 min of speech preparation, 3 min of speech delivery, 3 min of mental arithmetic, and minutes 0–2.5, 2.6–5.0, 5.1–10.0, 10.1–20.0 of the recovery period. The HRV data were log10 transformed to better approximate normality. Inter-rater reliability for the editing of artifacts was established using 40 × 5 min epochs (10 per participant for four randomly selected participants). Coefficients of agreement across the primary HRV outcome measures ranged from 0.93 to 1.0.
Salivary Cortisol
Saliva samples were collected seven times: following consent, 15 min and immediately before the task onset, immediately following the task, and at 10, 20, and 45 min into the recovery period. Participants were asked to gently chew on a sterile cotton dental roll until it was saturated with saliva. Samples were frozen at −20°C until assayed. Cortisol assays were conducted in one batch at the end of the study with reagents from the same lot to minimize variability. Samples were assayed in duplicate using the Salimetrics HS Salivary Cortisol EIA Kit for unbound cortisol. The test has an average intra-assay coefficient of variation of 3.5% and an inter-assay coefficient of 5.1%. In the current analyses, the average of the duplicate tests was used. For four girls an insufficient volume of saliva was collected to analyze one of the seven samples in duplicate, so the single test value was substituted. In addition, insufficient saliva was gathered at two time points for one girl, so her data were excluded from the cortisol analyses. Cortisol data were also log10 transformed to better approximate normality. Although there are no established norms for salivary cortisol levels in children, studies with normal children (often control groups) suggest that values typically range from 1.00 to 20 nmol/l (.04 µg/dl to .72 µg/dl), depending on the time of day (Groschl, Rauh, & Dorr, 2003). As with adults, cortisol values peak in the morning and drop to low and stable values in the late afternoon.
Negative Mood
Girls’ concurrent mood was assessed using the seven negative affect states (tired, sad, sleepy, mad, tense, unhappy, and angry) from the Profile of Mood States (POMS; Usala & Hertzog 1989; Turner Cobb & Steptoe, 1998). This mood adjective checklist is scored on a 5-point likert scale from 0 (not at all) to 4 (a whole lot). Ratings were summed to generate a negative mood score (α = .65). Negative mood was assessed four times: at the beginning and end of baseline, immediately after the stressor, and at the end of the recovery period.
Obesity, Race, And Menarche
BMI was calculated and obesity was defined using Centers for Disease Control and Prevention (CDC, 2000) criteria. Mothers reported their daughter's race as African American or European American. Girls who reported that they had had three periods in the past 3 months were classified as having reached menarche.
Analytic Plan
To address the first aim, prestressor levels of cortisol, HRV, and negative mood among girls in the high (n = 12) and low (n = 36) somatic groups were compared using ANOVA. We also tested whether race, menarche, and obesity were associated with prestressor psychophysiology. For the second aim, repeated measures analyses of variance were conducted to test the interaction effect of time and somatic complaints on cortisol, HRV, and negative mood responses to the stressor. Analyses were run using SPSS software. Given the small sample, effect sizes (the square root of partial eta squared, indicated by eta, η) and exact p-levels were reported.
Results
At the time of the laboratory assessment, the girls’ mean age was 12.9 years (SD = 0.28) and 35 girls (72.9%) had reached menarche. The mean BMI was 23.7 (SD = 5.53) with 54% of scores falling within the healthy range, 18% at risk for overweight, and 28% in the overweight range (CDC, 2000). Sixty percent of the sample was African American. The proportion of menarche, obesity, and African-American and European-American race did not differ significantly between the two groups (Table I).
Table I.
Descriptive Data by Somatic Group
| High somatic (n = 12) |
Low somatic (n = 36) |
|||
|---|---|---|---|---|
| Mean (SD) | N (%) | Mean (SD) | N (%) | |
| African-American race | 9 (75.0) | 19 (52.8) | ||
| Menarche | 9 (75.0) | 26 (72.2) | ||
| Obesity | 6 (50.0) | 16 (44.4) | ||
| CSI score (child report) | 15.00 (6.79) | 4.83 (4.59) | ||
| CSI score (parent report) | 9.0 (5.54) | 2.42 (3.03) | ||
| Baseline cortisol raw (µg/dl) | 0.16 (0.09) | 0.11 (0.06) | ||
| Baseline cortisol log10 (µg/dl) | −0.85 (0.24) | −1.02 (0.21) | ||
| Baseline HRV (RMSSD) | 58.45 (30.26) | 71.02 (37.08) | ||
| Baseline negative mood | 2.33 (0.90) | 3.63 (0.53) | ||
Prestressor Psychophysiological Arousal
The results of ANOVA revealed higher cortisol levels upon arrival at the laboratory among girls in the high somatic group (mean raw cortisol = .16, SD = 0.09) compared with the low group (mean raw cortisol = .11, SD = 0.06) (F[1,46] = 4.99, p = .03, η = .31). In contrast, prestressor HRV did not differ between girls in the high (mean = 58.45, SD = 30.26) compared with the low somatic group (mean = 71.02, SD = 37.08) (F[1,46] =1.12, p = .29, η = .15). Similarly, the groups did not differ on ratings of prestressor negative mood: high somatic group mean = 2.33 (SD = 0.90), low somatic group mean = 3.63 (SD = 0.53) (F[1,46] = 1.53, p = .22, η = .18). Furthermore, race, obesity, and menarche were unrelated to group membership, prestressor cortisol levels, HRV, or negative mood.
Effect of Time and Group on Physiological Arousal
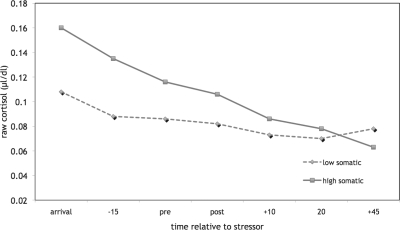
There was an interaction effect of time-by-group on cortisol levels (F[1,46] = 10.30, p = .002, η = .43). As shown in Fig. 2, both groups demonstrated a decrease in cortisol from arrival to 20 min poststressor, at which point girls in the low somatic group appeared to show a slight rise. In contrast, girls in the high somatic group continued to show a steady decline in cortisol level from 20 min poststressor. The interaction effect on cortisol response to the stressor was not only driven by the group differences in pre-stressor cortisol levels. A test of the poststressor response (the last four data points) also revealed a significant interaction effect of time-by-group (F[1,46] = 6.99, p = .011, η = .36). Moreover, the function of the cortisol levels poststressor within each group differed: cortisol levels followed a significant linear function among girls in the high somatic group (F[1,11] = 29.06, p < .001, η = .85) but a significant quadratic function among girls in the low somatic group (F[1,35] = 4.49, p = .041, η = .34).
Figure 2.
Cortisol in response to the TSST-C in girls with low vs. high somatic complaints.
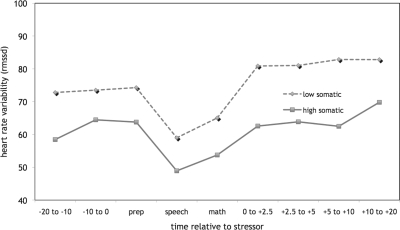
Results showed a significant quadratic effect of time on HRV (F[1,43] = 23.73, p < .001, η = .59) and a trend for a significant interaction effect of time-by-group on HRV (F[1,43 = 3.12, p < .08, η = .26). Depicted in Fig. 3, HRV decreased from pre-stressor to the onset of the stressor, followed by a return to baseline on termination of the stressor for all girls. To explore the interaction trend, we examined the within-group patterns of HRV prestressor to recovery. Within the low somatic complaints group, girls achieved a level of HRV that was significantly higher than prestressor levels (71.1 vs. 78.8; F[1,33] = 4.22, p < .05, η = .34) within 2.5 min poststressor. However, within the high somatic complaints group poststressor HRV did not reach levels that were significantly higher than those prestressor until 10–20 min after the stressor ended (69.8 vs. 58.4; F[1,11] = 4.89, p < .05, η = .55). During the majority of the recovery period, girls high on somatic complaints had levels of HRV that were 15–20% lower than the levels of girls with low somatic complaints.
Figure 3.
Heart rate variability in response to the TSST-C in girls with low vs. high somatic complaints.
Finally, an effect of time on negative mood was revealed, but no interaction effect of time-by-group. Thus, for both groups, there was a significant cubic effect of time, with negative mood increasing at the onset of the stressor, decreasing, and then increasing again at the end of the recovery period (F[1,45] = 6.14, p = .017, η = .35).
Discussion
In the current study, we examined cortisol levels, HRV, and negative mood before, during, and after a social stressor in two groups of 12- year-old girls: those with low and high levels of somatic complaints. The results revealed some support for our hypotheses of group differences in psychophysiological arousal both prior to, and in response to the experimental stressor. Thus, girls high on somatic complaints had significantly higher cortisol levels upon arrival at the laboratory. Cortisol response to the laboratory stressor also differed significantly by group, and medium effect sizes (Cohen, 1988) were revealed for both of these group comparisons. Among the girls with low somatic complaints, the pattern of change in salivary cortisol showed a quadratic function, with a steady decline from arrival at the laboratory followed by a slight upturn at 20 to 45 min poststressor, which is around the time it takes for HPA activation to be detected in salivary cortisol (Dickerson & Kemeny, 2004). In contrast, girls with high levels of somatic complaints showed a steady decline in cortisol levels across the entire study period. The results also showed that the group differences in cortisol during the recovery period were not only due to baseline differences. Nevertheless, it is recognized that prestressor levels are likely to affect the capacity to respond and recover. Although the values observed across both groups prestressor generally fell within the normal range, if stable, a .05 µg/dl group difference in the late afternoon could be clinically meaningful and is suggestive of a higher level of HPA activation in the high somatic group.
We also observed a significant effect of the stressful task on HRV. Although group differences in HRV did not reach statistical significance, the trend was consistent with the pattern observed in cortisol response. Thus, girls with low levels of somatic complaints appeared to demonstrate more tightly modulated HRV in response to the social stressor, whereas girls with high levels had a longer latency to recover optimal HRV. It is possible that these laboratory observations reflect a more stable pattern of hyperarousal (high cortisol and low HRV) that characterizes girls with somatic complaints. Such a heightened state may impact the ability to respond to mild social stressors, resulting in a deficit in maintaining homeostasis. Based on a sample of children with and without anxiety disorders exposed to a CO2 inhalation stressor, Monk et al. (2001) reported data that support such a possibility. In addition, Gerra and colleagues (Gerra et al., 2000) reported that high levels of stress hormones at baseline among anxious boys resulted in a lack of responsiveness to laboratory stress paradigms. Although the social stressor in the current study appeared to elicit negative mood for all girls, no group differences in affective reactivity were revealed. It is possible however, that this lack of effect reflected the global nature of the negative mood construct, and that the groups differed on levels of anxiety, which often co-occurs with somatic disorders (Walker, Garber, & Greene, 1993). Investigating whether somatic complaints, over and above the effects of anxiety, are associated with stable differences in stress reactivity, and understanding the degree to which cortisol levels and/or HRV stability and reactivity account for the development of somatic problems among young girls are clearly important avenues for future research.
Modulation of the biological systems in response to stress is critical to long-term physical and mental health. Chronic activation of the HPA axis has a detrimental impact on multiple systems in the body, leading to increased susceptibility to physical disease (McEwen, 2000). Neuroendocrine dysregulation may also contribute to the development and maintenance of somatic symptoms. Cortisol response to challenge has been shown to enhance the perception of sensory stimuli (Fehm-Wolfsdorf et al., 1993), lower the threshold at which symptoms are perceived (Reif, Shaw, & Fichter, 1998) and increase attention to interoceptive signals (Pennebaker, 1982) among adults. Thus, chronic overactivation of the HPA axis may contribute to stable individual differences in the experience of physical symptoms. Blunted parasympathetic modulation to a stressor has been shown to be associated with ratings of pain in response to the Cold Pressor Task (Appelhans & Luecken, 2008). Associations noted in cross-sectional studies, however, also leave open the possibility that chronic somatic complaints result in system dysregulation. Therefore, longitudinal studies are necessary to further explore these pathways and examine whether somatic complaints precede the development of altered functioning of the HPA axis and cardiovascular system or whether dysregulation of these systems leads to altered physical sensations and perceptions of pain or discomfort.
Several limitations should be considered in the interpretation of our results. First, the findings were based on a small sample, limiting the power to detect significant differences. This is particularly true for examining the effects of potentially important contextual factors such as race, obesity and menarcheal status. Nevertheless, where significant group differences were revealed, the medium effect sizes suggested that the differences detected were meaningful. Second, the cross-sectional design of the study precludes conclusions from being drawn about the direction of effects. Thus, our results could indicate that high levels of somatic complaints result in changes to the HPA axis, or that inherent individual differences in HPA axis functioning result in a greater likelihood of experiencing somatic complaints, or indeed, that there are reciprocal, escalating influences. Further work is clearly needed to extend the current findings via longitudinal studies that also begin prior to the onset of serious psychopathology, in order to identify precursor states and to resolve these questions of causality. Third, to protect the safety of the participants, five girls were excluded due to high initial BP or high BP reactivity. Although the absence of data from these girls likely reduced levels of stress reactivity for the sample as a whole, the excluded girls were evenly distributed between the high and low somatic complaints groups, thus limiting concerns about attrition-related bias. A further limitation of the current study is the assessment of physiological response to a single stressor; multiple assessments over time using different types of stimuli to elicit a stress response might provide more reliable measures of individual differences that are less influenced by situational factors (Musante et al., 1994). Moreover, a true baseline of typical circadian rhythm is necessary to determine whether more stable individual differences are associated with somatic complaints.
Future work with a larger sample would also enable the inclusion of covariates such as depressive symptoms, anxiety, disruptive behaviors, functional impairment, and stressful life events. For example, both state and trait anxiety, as well as depression, have been shown to influence pain perception as well as sympathetic nervous system and HPA functioning (Friedman & Thayer, 1998). Engaging in disruptive behaviors such as conduct disorder is associated with lower levels of cortisol in girls (Pajer, Gardner, Rubin, Perel, & Neal, 2001). Thus, generating a more comprehensive psychological profile would provide an opportunity to differentiate the potential influences on group differences like those observed in the current study.
The requirement that girls in the high somatic group be identified as such by both parent and child report was instituted with the goal of generating a sample that was clinically relevant in terms of level of symptom manifestation. The sampling strategy may, however, have resulted in an atypical sample: one that is characterized by high levels of agreement with parents, or by somatic complaints that are readily recognized and acknowledged by others. Close to 60% of the girls who reported high levels of somatic complaints had a parent who confirmed this. Among the 40% who did not have parent confirmation, there were some girls with scores just below the parent median. The results may have been affected by excluding these sub-threshold girls from the high somatic group. On the other hand, the somatic scores for the two groups were within the range of those found for girls with and without clinically significant somatic symptoms (Walker & Garber, 2003), indicating that this approach likely captured girls with the most significant and/or chronic somatic complaints, who were not seeking health services for such problems. It is also possible however that the girls who reported more transient somatic complaints (i.e., those girls who were in the high group at screening but in the low group at the time of the laboratory visit) represent an important group that is distinct from both stable-high and -low groups in terms of clinical presentation and subsequent functioning. Further investigation of psychophysiological indices associated with stability versus change in somatic symptom reporting is clearly warranted. Finally, although the internal consistency of both parent and child reports in the current study was high, parental reports generated lower scores in both the high and low somatic groups and the range of values overlapped within 1 SD suggesting that there was less distinction between the two groups by parental report. Future research is clearly needed to test of the validity of separate and combined informants’ reports of somatic symptoms.
Despite these limitations, our findings contribute to, and extend the literature linking stress reactivity to psychopathology. By demonstrating associations between psychophysiological stress response and somatic complaints in a nonclinical sample of young adolescent females, the results from the present study raise the possibility that individual differences in stress reactivity may be a risk factor for the development of somatization and/or pain disorders. Determining the interface between severity of somatic complaints and individual differences in physiological arousal over time will be important for understanding the mechanisms underlying pain and somatic disorders.
Funding
National Institutes of Health (MH30915, MH07179, MH66167).
Conflicts of interest: None declared.
Acknowledgments
Special thanks to Dr Kupfer for providing the infrastructure for this study. The authors thank Dara Babinski, Susan Gillo, Amy Grottenthaler, Amanda Hinze, Gloria Konwick, Maria Wrozcek, and Jackie Fury for assisting with data collection, to Amanda Hinze and Kate Buddie for editing HRV data, and to Kristen Kasza for her statistical advice. Special thanks go to the families of the Pittsburgh Girls Study who participated in the present study.
References
- Abu-Arafeh I, Russell G. Recurrent limb pain in school. Archives of Diseases in Childhood. 1996;74:336–339. doi: 10.1136/adc.74.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhans B, Luecken L. Heart rate variability and pain: Associations of two interrelated homeostatic processes. Biological Psychology. 2008;77:174–182. doi: 10.1016/j.biopsycho.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Barnes V, Treiber F, Musante L, Turner J, Davis H, Strong W. Ethnicity and socioeconomic status: Impact on cardiovascular activity at rest and during stress in youth with a family history of hypertension. Ethnicity and Disease. 2000;10:4–16. [PubMed] [Google Scholar]
- Borge A, Nordhagen R, Moe B, Botten G, Bakketeig L. Prevalence and persistence of stomach ache and headache among children. Follow-up of a cohort of Norwegian children from 4 to 10 years of age. Acta Paediatrica. 1994;83:433–437. doi: 10.1111/j.1651-2227.1994.tb18137.x. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, von Auer K, Krieger S, Weis S, Rauh W, Hellhammer D. Blunted cortisol responses to psychosocial stress in asthmatic children: A general feature of atopic disease? Psychosomatic Medicine. 2003;65:806–810. doi: 10.1097/01.psy.0000095916.25975.4f. [DOI] [PubMed] [Google Scholar]
- Campo J, Jansen-McWilliams L, Comer D, Kelleher K. Somatization in pediatric primary care: Association with psychopathology, functional impairment, and use of services. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:1093–1101. doi: 10.1097/00004583-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Clinical Growth Charts, Girls 2–20 years BMI for agse. National Center for Health Statistics and Center for Chronic Disease Prevention and Health Promotion. 2000. Retrieved May 14, 2007, from http://www.cdc.gov/growthcharts.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Lawrence Erlbaum Association; 1988. [Google Scholar]
- Davis M, Twamley E, Hamilton N, Swan P. Body fat distribution and hemodynamic stress responses in premenopausal obese women: A preliminary study. Health Psychology. 1999;18:625–33. doi: 10.1037//0278-6133.18.6.625. [DOI] [PubMed] [Google Scholar]
- Dickerson S, Kemeny M. Acute stressors and cortisol response: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dorn L, Campo J, Thato S, Dahl R, Lewin D, Chandra R, et al. Psychological comorbidity and stress reactivity in children and adolescents with recurrent abdominal pain and anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:66–75. doi: 10.1097/00004583-200301000-00012. [DOI] [PubMed] [Google Scholar]
- Fehm-Wolfsdorf G, Soherr U, Arndt R, Kern W, Fehm H, Nagel D. Auditory reflex thresholds elevated by stress-induced cortisol secretion. Psychoneuroendocrinology. 1993;18:579–589. doi: 10.1016/0306-4530(93)90035-j. [DOI] [PubMed] [Google Scholar]
- Friedman B, Thayer J. Anxiety and autonomic flexibility: a cardiovascular approach. Biological Psychiatry. 1998;49:303–323. doi: 10.1016/s0301-0511(98)00051-9. [DOI] [PubMed] [Google Scholar]
- Garber J, Walker L, Zeman J. Somatization symptoms in a community sample of children and adolescents: Further validation of the Children's Somatization Inventory. Psychological Assessment. 1991;3:588–595. [Google Scholar]
- Gerra G, Zaimovic A, Zambelli U, Timpano M, Reali N, Bernasconi S, et al. Neuroendocrine responses to psychological stress in adolescents with anxiety disorder. Neuropsychobiology. 2000;42:82–92. doi: 10.1159/000026677. [DOI] [PubMed] [Google Scholar]
- Granger D, Weisz J, Kauneckis D. Neuroendocrine reactivity, internalizing behavior problems and control-related cognitions in clinic-referred children and adolescents. Journal of Abnormal Psychology. 1994;103:267–276. doi: 10.1037//0021-843x.103.2.267. [DOI] [PubMed] [Google Scholar]
- Groschl M, Rauh M, Dorr H. Circadian rhythm of salivary cortisol, 17α-hydroxy-progesterone, and progesterone in healthy children. Clinical Chemistry. 2003;49:1688–1691. doi: 10.1373/49.10.1688. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Mangelsdorf S, Larson M, Hertsgaard L. Attachment, temperament, and adrenocortical activity in infancy: A study of psychoendocrine regulation. Developmental Psychology. 1989;3:355–363. [Google Scholar]
- Hipwell AE, Loeber R, Stouthamer-Loeber M, Keenan K, White HR, Kroneman L. Characteristics of girls with early onset disruptive and delinquent behaviour. Criminal Behaviour and Mental Health. 2002;12:99–118. doi: 10.1002/cbm.489. [DOI] [PubMed] [Google Scholar]
- Hyams J, Burke G, Davis P, Rzepski B, Andrulonis P. Abdominal pain and irritable bowel syndrome in adolescents: A community-based study. Journal of Pediatrics. 1996;129:220–226. doi: 10.1016/s0022-3476(96)70246-9. [DOI] [PubMed] [Google Scholar]
- Klein J, Litt I. Epidemiology of adolescent dysmenorrhea. Pediatrics. 1981;68:661–664. [PubMed] [Google Scholar]
- Kristjansdottir G. Prevalence of pain combinations and overall pain: A study of headache, stomach pain and back pain among schoolchildren. Scandinavian Journal of Social Medicine. 1997;25:58–63. doi: 10.1177/140349489702500112. [DOI] [PubMed] [Google Scholar]
- Lewis C, Lewis M. Educational outcomes and illness behaviors in participants in a child-initiated care system: A 12-year follow-up study. Pediatrics. 1989;84:845–850. [PubMed] [Google Scholar]
- Lewis R, Wasserman E, Denney N, Gerrard M. The etiology and treatment of primary dysmenorrhea: A review. Clinical Psychology Review. 1983;3:371–389. [Google Scholar]
- McEwen B. The neurobiology of stress: From serendipity to clinical relevance. Brain Research. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- Mezzacappa E, Trembaly R, Kindlon D, Saul J, Arsenault L, Pihl R, et al. Anxiety, antisocial behavior, and heart rate regulation in adolescent males. Journal of Child Psychology and Psychiatry. 1997;38:457–469. doi: 10.1111/j.1469-7610.1997.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Monk C, Kovelenko P, Ellman L, Sloan R, Bagiella E, Gorman J, et al. Enhanced stress reactivity in paediatric anxiety disorders: Implications for future cardiovascular health. International Journal of Neuropsychopharmacology. 2001;4:199–206. doi: 10.1017/S146114570100236X. [DOI] [PubMed] [Google Scholar]
- Musante L, Raunikar R, Treiber F, Davis H, Dysart J, Levy M, et al. Consistency of children's hemodynamic responses to laboratory stressors. International Journal of Psychophysiology. 1994;17:65–71. doi: 10.1016/0167-8760(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Offord D, Boyle M, Fleming J, Blum H, Grant N. Summary of selected results. The Canadian Journal of Psychiatry. 1989;34:483–491. doi: 10.1177/070674378903400602. [DOI] [PubMed] [Google Scholar]
- Pajer K, Gardner W, Rubin R, Perel J, Neal S. Decreased cortisol levels in adolescent girls with conduct disorder. Archives of General Psychiatry. 2001;58:297–302. doi: 10.1001/archpsyc.58.3.297. [DOI] [PubMed] [Google Scholar]
- Pennebaker J. The psychology of physical symptoms. New York: Springer; 1982. [Google Scholar]
- Reif W, Shaw R, Fichter M. Elevated levels of psychophysiological arousal and cortisol in patients with somatization syndrome. Psychosomatic Medicine. 1998;60:198–203. doi: 10.1097/00006842-199803000-00016. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Wolf J, Kirschbaum C. Glucocorticoid sensitivity in humans-interindividual differences and acute stress effects. Stress. 2003;6:207–222. doi: 10.1080/1025389031000153658. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sigmon S, Dorhofer D, Rohan K, Boulard N. The impact of anxiety sensitivity, bodily expectations, and cultural beliefs on menstrual symptom reporting: a test of the menstrual reactivity hypothesis. Journal of Anxiety Disorders. 2000;14:615–33. doi: 10.1016/s0887-6185(00)00054-2. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and North American Society of Pacing Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. European Heart Journal. 1996;17:354–381. [PubMed] [Google Scholar]
- Tsao J, Zeltzer L. Sex differences in pain-related symptoms and self-initiated school nurse visits among pre-adolescents. Journal of Pain and Symptom Management. 2003;25:472–480. doi: 10.1016/s0885-3924(03)00076-9. [DOI] [PubMed] [Google Scholar]
- Turner Cobb J, Steptoe A. Psychosocial influences on upper respiratory infectious illness in children. Journal of Psychosomatic Research. 1998;45:319–330. doi: 10.1016/s0022-3999(97)00311-5. [DOI] [PubMed] [Google Scholar]
- Usala P, Hertzog C. Measurement of affective states in adults: Evaluation of an adjective rating scale instrument. Research on Aging. 1989;11:403–426. doi: 10.1177/0164027589114001. [DOI] [PubMed] [Google Scholar]
- van Goozen S, Matthys W, Cohen-Kettenis P, Gispen-de Wied C, Wiegant V, van Engelend H. Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biological Psychiatry. 1998;43:531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- Walker L, Garber J. Manual for the Children's Somatization Inventory. 2003. Unpublished manual. [Google Scholar]
- Walker L, Garber J, Greene J. Somatization symptoms in pediatric abdominal pain patients: relation to chronicity of abdominal pain and parent somatization. Journal of Abnormal Child Psychology. 1991;19:379–394. doi: 10.1007/BF00919084. [DOI] [PubMed] [Google Scholar]
- Walker L, Garber J, Greene J. Psychosocial correlates of recurrent childhood pain: A comparison of pediatric patients with recurrent abdominal pain, organic illness, and psychiatric disorders. Journal of Abnormal Psychology. 1993;102:248–258. doi: 10.1037//0021-843x.102.2.248. [DOI] [PubMed] [Google Scholar]