Abstract
At this time, the pathophysiology of macrophage involvement and their role in stroke progression is poorly understood. Recently, T2- and T2*-weighted MRI following intravenous administration of iron-oxide particles have been used in understanding the inflammatory cascade. Previous studies report that image enhancement following stroke is from iron-laden macrophages, however, they do not account for potential blood-brain barrier disruption and non-specific contrast enhancement. In this study, spontaneously-hypertensive (SHR) rats were preloaded with Feridex seven days prior to stroke, permitting labeling of bone-marrow-derived macrophages. 3D-GRE imaging revealed average signal decreases of 13–23% in preloaded animals, concentrated on the lesion periphery and reaching a maximum days 2–4 post stroke. Immunohistochemistry revealed ED-2+, PB+, MHC-II+, and TNF-α+ perivascular macrophages (PVM), meningeal macrophages (MM), and choroid plexus macrophages (CPM). ED-1+ and IBA+ tissue macrophages and/or activated microglia were located throughout the lesion, but were PB−. These findings indicate the following: 1) Feridex preloading permits tracking of CNS-resident macrophages (PVM, MM, CPM) and 2) CNS-resident macrophages likely play an integral role in the inflammatory cascade via antigen presentation and expression of proinflammatory cytokines. Further refinement of this method should permit non-invasive monitoring of inflammation and potential evaluation of anti-inflammatory therapies in pre-clinical models of stroke.
Keywords: stroke, MRI, feridex, inflammation, macrophages
Introduction
Ischemia-reperfusion (I-R) triggers the production of inflammatory cytokines, resulting in increased expression of cellular adhesion molecules, microglia activation, and leukocyte infiltration (Wang et al 2007). While microglia play an integral role in the hyperacute time-frame (minutes), macrophages are recruited heavily in the subacute to chronic phase of stroke (days) (Flaris et al 1993; Hallenbeck et al 2005; Kochanek and Hallenbeck 1992). The importance of macrophage activity to overall stroke progression, from the hyperacute phase through the chronic phase, has largely been underappreciated. Recently, it has been suggested that they may play a larger role in the initiation and continuation of the inflammatory cascade (Becher et al 2006; Greter et al 2005).
Macrophages of the brain may be divided into two major subtypes: tissue macrophages, and central nervous system (CNS) resident macrophages (Lassmann et al 1993; McMenamin 1999). Tissue macrophages are derived from activated microglia that have become phagocytic. CNS-resident macrophages include perivascular macrophages (PVM), meningeal macrophages (MM), and macrophages within the choroid plexus (CPM). Given their strategic location within the CNS, these macrophages (PVM, MM, CPM) may be considered the ‘gatekeepers’ of the brain. It has been speculated that PVM and MM may serve as antigen presenting cells (APCs), expressing major histocompatibility complex (MHC) class II and mediating the immune response via interaction with T-cells (Angelov et al 1998; Becher et al 2006; Hickey and Kimura 1988; Polfliet et al 2002). They have also been reported to express pro-inflammatory cytokines, suggesting direct involvement in the progression of disease (Bauer et al 1996; Becher et al 2006; Lehrmann et al 1998). Given this duality, understanding the role(s) of CNS-resident macrophages in stroke is vital.
The development of iron-oxide contrast agents, such as superparamagnetic iron-oxides (SPIOs, size 60–150 nm) and ultrasmall paramagnetic iron-oxides (USPIOs, size 10–40 nm) prompted interest in tracking inflammation via macrophage activity (Thorek et al 2006; Wang et al 2001; Weissleder et al 1990). SPIOs and USPIOs may be internalized by macrophages via receptor-mediated endocytosis (SR-A Types I/II) or phagocytosis (Raynal et al 2004), making them visible on T2-weighted imaging (T2WI) and gradient-echo imaging (GRE). Previous work in experimental (Rausch et al 2001; Saleh et al 2004b; Wiart et al 2007) and clinical (Manninger et al 2005; Saleh et al 2004a) stroke has shown promise in the ability to monitor iron-laden macrophages, however, these studies do not account for potential blood-brain barrier (BBB) disruption and pooling of contrast on the lesion periphery prior to macrophage uptake. Because iron oxide administration has generally involved systemic injection shortly after insult, the question arises as to whether non-specific contrast enhancement is a problem.
In this study, we preloaded spontaneously-hypertensive (SHR) rats with the SPIO Feridex 7 days prior to tMCAO (Fig. 1). We hypothesize that this will permit iron-labeling of bone-marrow derived macrophages and imaging of their activity following tMCAO. We also hypothesize that this particular population of macrophages, termed CNS-resident macrophages, are essential to the initiation and maintenance of the inflammatory response via antigen presentation and cytokine expression. While this is an accepted concept in experimental and clinical multiple sclerosis (MS), it is new to stroke and has yet to be investigated thoroughly. The results presented herein provide a basis for potential investigation of anti-inflammatory therapies in preclinical models of stroke.
Figure 1. Hypothesized Method of Feridex Accumulation with 7-day Preloading.
Feridex has a blood half-life of 5–6 hours, the majority of which is cleared by the reticuloendothelial system (RES). The remainder accumulates in the bone marrow previous to ischemic insult. Following ischemia-reperfusion (I–R), these iron-laden macrophages are recruited to the stroke region. Using non-invasive MRI, their involvement in the acute and subacute/chronic phase of the inflammatory cascade may be monitored.
Methods
Animal Preparation
The present study was approved by the institutional animal care and use committee (IACUC) of the National Institute of Neurological Disorders and Stroke, National Institutes of Health (IACUC protocol #1225-05). Sixteen spontaneously-hypertensive rats (SHR/NCrl: male, 3–4 months, 350±20g, Charles River Laboratories, White Plains, NY) were anesthetized with isoflurane (5% induction, 2.5% maintenance) delivered in a 2:2:1 mixture of breathing quality air, nitrogen, and oxygen. Twelve animals were subjected to 30 minutes of right middle cerebral artery occlusion followed by reperfusion (tMCAO) using the intraluminal suture model (Koizumi et al 1986; Longa et al 1989). The remaining four animals had sham surgery (no suture insertion). Rats were monitored for two hours following recovery, and then returned to their cages.
Feridex Administration
Animals were divided into four groups (n = 4 per group). Group 1 (Preloaded tMCAO): animals were injected with 0.5ml I.V. Feridex (16 mg/kg of 11.2 mg/mL iron) 7 days prior to tMCAO. Group 2 (Postloaded tMCAO): animals were injected with Feridex 5 minutes after tMCAO. Group 3 (Control tMCAO): animals had tMCAO without Feridex administration. Group 4 (Preloaded SHAM): animals were injected with Feridex 7 days prior to sham surgery.
MRI
Imaging was performed using a Bruker Biospin 7.0T/30cm imaging spectrometer equipped with ± 45G/cm self-shielded gradients. Diffusion-weighted echo-planar imaging (DW-EPI), T2-weighted imaging (T2WI), and 3D gradient-echo imaging (GRE) were performed on days 1, 2, 3, 4, and 7. Twelve 1.5-mm-thick slices were acquired using a field of view (FOV) = 25.6×25.6 mm2. DWI: matrix size = 96×96; TR/TE = 6200/46 msec; Δ = 13 msec; δ = 4 msec; b-values = 0, 250, 500, 750, 1000 s/mm2 (acquired for all three principle directions, x, y, z); NEX = 4. T2WI: matrix size = 128×128; TR = 2000 msec; TE = 15, 30, 45, 60, 75, 90, 105, 120 msec; NEX = 1. 3D-GRE: matrix size = 256×256×256; TR/TE = 30/8 msec; flip angle = 30°, NEX = 1. These parameters yielded an in-plane resolution of 267µm for DWI and 200µm for T2WI. 3D-GRE scans had an isotropic resolution of 100µm. Total scan time was approximately 53 minutes per animal.
Inclusion/Exclusion Criteria
Animals (n = 3) were excluded for lesion volumes less than 50 mm3, considered to be an incomplete middle cerebral artery occlusion (Gerriets et al 2004). All remaining animals (n = 13) were included in the study, yielding a success rate of 13/16 (81%).
Immunohistochemistry
After final MRI on day 7, rats were sacrificed by means of transcardial perfusion fixation following an overdose of isoflurane anesthesia. Brains were extracted and placed in 4% paraformaldehyde for 1–2 days and then transferred to 20% sucrose overnight. They were then snap frozen in isopentane and stored at −73° C. Serial 16-µm-thick sections were taken beginning at the olfactory bulb (+5.0 from bregma) and extending to the start of the cerebellum (-13.0 from bregma). For every set of 10 slides, the cryostat was advanced 500µm.
ED-1, ED-2, and IBA (ionized calcium binding adaptor molecule) staining were performed for the identification of macrophages and microglia [ED-1: non-specific macrophage marker (monocytes, macrophages, activated/phagocytic microglia), ED-2: specific to well-differentiated macrophages, IBA: specific to activated microglia]. Sections were incubated for 10 min. in methanol containing 0.6% hydrogen peroxide, permeabilized with 0.3% Tween for 1 hr., and blocked with 5% NDS for 1 hr. Three primary antibodies were used on consecutive slides: ED-1 (1:200, Abcam, CD68), ED-2 (1:100, AbD Serotec, CD163), or IBA (1:150, Wako, Iba1). Negative control sections were prepared with mouse IgG (1:1500) for ED-1, ED-2 and rabbit IgG (1:1500) for IBA. Slides were incubated in a humidity chamber at 4°C overnight. Biotinylated secondary antibodies (donkey anti-mouse or anti-rabbit, 1:1500, Jackson Labs) were applied to all sections and incubated for 1 hr. followed by Avidin-biotin-peroxidase complex (ABC-Elite Kit, Vector Laboratories) for 1 hr. Binding between antibody and antigen was visualized with diaminobenzidine (DAB Kit, Sigma) for 2–5 minutes. All slides were counterstained with nuclear fast red for nuclear identification.
Prussian blue (PB) staining was performed for identification of iron-labeled cells [PB: specific to ferric iron (Fe3+)]. Rehydrated sections were immersed in a stock solution of 10% potassium ferrocyanide for 5 minutes, followed by a working solution composed of 3% concentrated hydrochloric acid and 7% potassium ferrocyanide for 30 minutes. All slides were counterstained with nuclear fast red for nuclear identification.
ED-1, ED-2, and PB double-staining was performed in order to verify which population(s) of macrophages contain iron. Single sections were initially run through the standard IHC protocol, with staining of contiguous sections for ED-1 or ED-2. Following the DAB step, PB staining was performed per protocol, followed by counterstaining with nuclear fast red for nuclear identification.
ED-2, MHC-II, TNF-α (tumor necrosis factor alpha), and IL-1 (interleukin-1) expression were employed for a rough assessment of antigen presentation and cytokine expression within the well-differentiated macrophage population. Double-Immunofluorescence for ED-2:TNF-α, MHC-II:TNF-α, ED-2:IL-1, and MHC-II:IL-1 was performed as follows: incubation with anti-rat TNF-α (1:100, YC-032, Yanaihara Institute) and anti-rat IL-1 (1:100, AF-501-NA, R&D Systems) were followed by incubation with FITC conjugated secondary antibodies (1:200, Jackson Labs), anti-CD163 (ED-2) (1:100, AbD Serotec, CD163) and anti-MHC-II (1:250, Abcam, OX6) were followed by incubation with Rhodamine conjugated secondary antibodies (1:200, Jackson Labs), All slides were mounted using Prolong Antifade (Invitrogen).
Image Analysis: MRI
Image reconstruction was performed using Paravision’s Image Processing and Display Software (XTip). Image analysis and parameter map production (ADC, T2) were performed using MIPAV software (BIRSS, NIH) and routines written in IDL (Research Systems Inc, Boulder, CO). Lesion volumes were manually drawn by two readers using T2 parameter maps. ADC and T2 values were determined for volumes of interest in both healthy and ischemic tissue. In order to correct for edema, the ipsilateral (right) and contralateral (left) hemispheres were compared [edema = (ipsi-contra)/contra]. Inter-reader variability was determined and the mean volumes (uncorrected, corrected) of the two readers were used for statistical analysis.
A separate reader blinded to lesion volume determined Feridex-positive and Feridex-negative regions on 3D-GRE images. Feridex-positive volumes were manually drawn, with the equivalent contralateral region serving as a Feridex-negative control. Percent signal reduction was calculated [% SI decrease = (SIFeridex-positive-SIFeridex-negative)/SIFeridex-negative) × 100].
Image Analysis: Immunohistochemistry
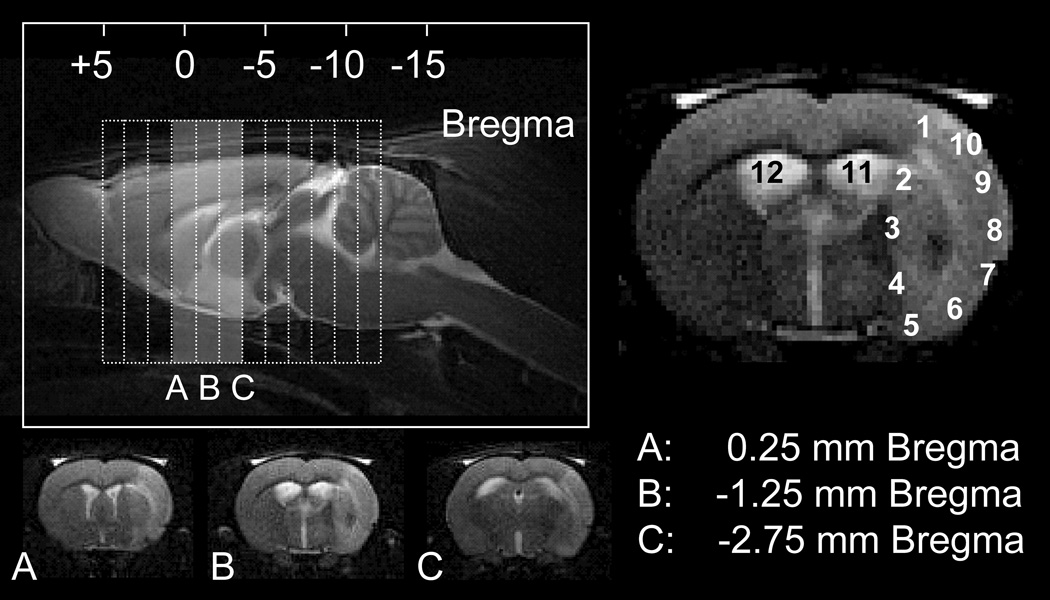
For ED-1, ED-2, IBA, and PB staining, photos were taken using an Axioplan light microscope (Carl Zeiss Microimaging GmbH, Berlin, Germany) at 20× magnification in 10 locations along the entire lesion periphery (interior, exterior). Additional photos were taken in the lateral ventricles (left, right) for assessment of the choroid plexus. These 12 images were acquired for the central portion of the lesion, corresponding to the central three imaging slices (Fig. 2). Cell counts were performed using ImageJ software (BIRSS, NIH) by a single reader blinded to all animal identifiers.
Figure 2. Schematic of imaging slice selection and corresponding immunohistochemistry.
Top left: sagittal T2-weighted image with slice package (dotted lines) for T2-weighted and diffusion-weighted imaging. Bottom left (A–C): T2-weighted images corresponding to the central three imaging slices (shaded area). For ED-1, ED-2, IBA, and PB staining, 20× photos were taken at three locations with respect to bregma (+0.25,−1.25,−2.75). For each location, twelve regions were investigated (numbered 1–12), ten along the lesion periphery and two within the lateral ventricles.
For double-staining and double-immunofluorescence, photos were taken at 40× magnification in multiple locations along the lesion periphery with particular attention to perivascular regions and the meninges along the exterior edge. For double-immunofluorescence, additional photos were taken in the lateral ventricles (left, right) for assessment of the choroid plexus.
Statistical Analysis
Data are presented as mean (SD). Normality was tested using the Shapiro-Wilk and Kolmogorov-Smirnov tests. For the temporal evolution of lesion volume, edema, ADC, and T2 values, one-way ANOVA with post-hoc Tukey tests were performed. For between-group comparisons (i.e., lesion versus healthy tissue), two-way between groups ANOVA and Kruskal-Wallis tests were employed. For Feridex-positive volumes, the comparison of % SI decrease was performed using the two-way between groups ANOVA and one-way ANOVA with post-hoc Tukey tests. For immunohistochemistry, the comparison of cell counts per staining method and region were performed using Kruskal-Wallis tests with post-hoc testing using Mann-Whitney. P-values < 0.05 were considered statistically significant.
Results
Lesion Progression Post Ischemia-Reperfusion
Inter-reader variability was 20 mm2 (20) [r = 0.961, 96.2% within ±2 SD]. There was a significant increase in lesion volume from day 1 [150 mm3 (50)] to day 2 [220 mm3 (40), p < 0.01], which persisted out to day 4 [210 mm3 (50), p = 0.967]. Concomitant with this volume increase was a significant increase in vasogenic edema (10% (7), p = 0.025), increased T2 [90 ms (5), p < 0.01], and a persistent reduction in ADC [0.62 × 10−5 cm2s−1 (0.06), p < 0.01] when compared to healthy tissue. By day 7, this edema had subsided [2% (2), p = 0.334], and lesion volume had returned to approximately day 1 values [140 mm3 (40), p = 0.987]. ADC values remained significantly reduced until day 2, with renormalization by day 4 [0.75 × 10−5 cm2s−1 (0.07), p = 0.951]. T2 values remained elevated through day 7 [75 ms (4), p < 0.01], indicative of permanent damage.
Feridex Visualization of the Inflammatory Response
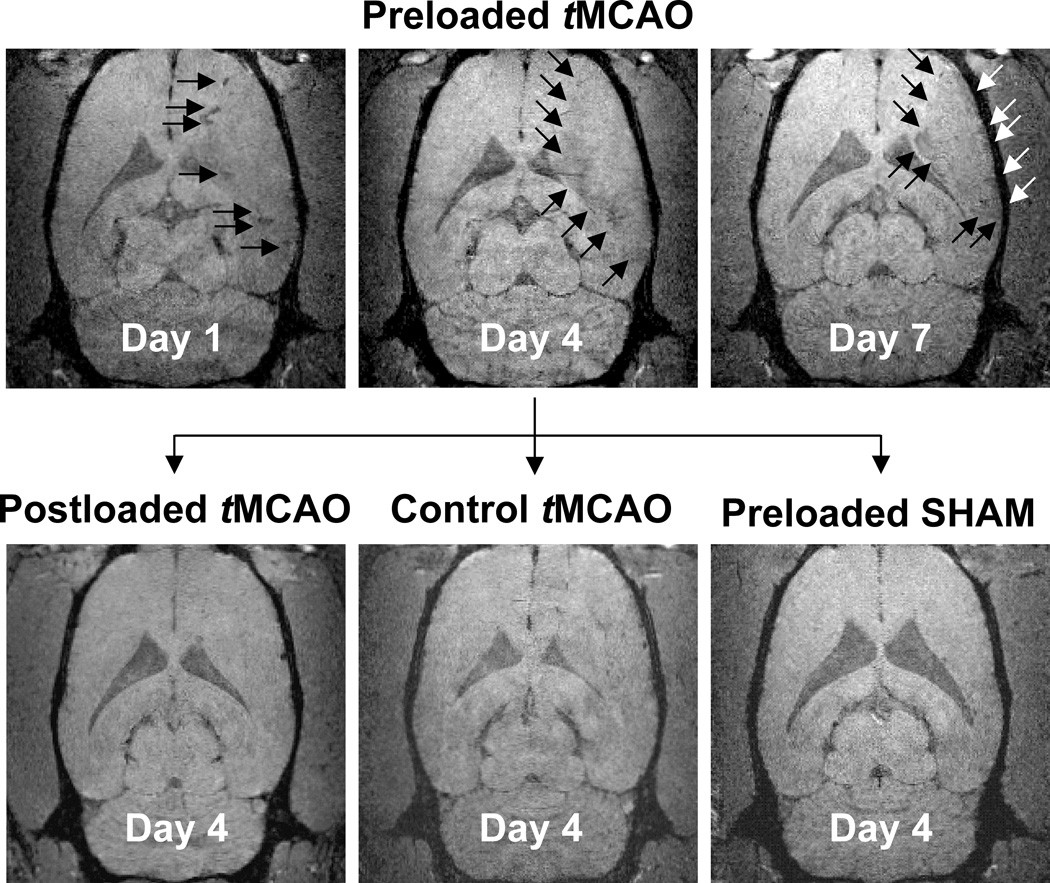
Figure 3 shows 3D-GRE images for Group 1: Preloaded tMCAO on days 1, 4, and 7 (top row) and for Groups 2–4 (Postloaded tMCAO, Control tMCAO, Preloaded SHAM) on day 4. Within the Preloaded tMCAO group, vasodilation and blood vessel darkening was evident on day 1 GRE images (Figure 3, top left, arrows). Total lesion hypointensity was significantly greater in magnitude than the Preloaded SHAM group for day 1 (p < 0.01), day 4 (p < 0.05), and day 7 (p < 0.05), with a trend toward significance for day 3 (p = 0.107). Marked hypointensity developed along the lesion periphery by day 2 [-21%], reaching a maximum by day 4 [-22% (8)], and remaining present through day 7 [-23% (3)]. The GRE signal decrease along the lesion periphery (arrows) was significantly greater in magnitude than the Preloaded SHAM group for all days post I-R injury (p < 0.01 for days 1, 2, 4, and 7; p < 0.05 for day 3). There appeared to be no major GRE signal changes in the Postloaded tMCAO group (Fig.3, bottom left). There was a slight GRE signal decrease in the Control tMCAO group [∼3–10%] (Fig.3, bottom middle). 3D–GREs for the Preloaded SHAM (Fig. 3, bottom right) group were considered negative. Table 1 provides a detailed, quantitative summary of 3D-GRE signal evolutions for individual groups post tMCAO. For the Preloaded tMCAO group, time-averaged signal changes for total lesion [-15% (4)] and the lesion periphery [-22% (2)] were significantly different than Postloaded tMCAO, Control tMCAO, and Preloaded SHAM groups (p < 0.05). Signal changes within the infarct core [-11% (3%)] were significantly different than the Postloaded tMCAO and Preloaded SHAM groups (p < 0.05), but similar to the Control tMCAO group [-7% (3), p = 0.09]. ED-1 Correlates with IBA and ED-2 Correlates with PB
Figure 3. Feridex Visualization of the Inflammatory Response.
Day 1, 4, and 7 coronal GRE images for Group 1: 7-day Preloaded tMCAO (top row). Vasodilation and blood vessel darkening was evident on day 1 (top left). Marked hypointensity developed along the lesion periphery, reaching a maximum by day 4 (top middle), and remaining present through day 7 (top right). Day 4 coronal GRE images for Group 2: Postloaded tMCAO (bottom left), Group 3: Control tMCAO (bottom middle), and Group 4: Preloaded SHAM (bottom right). The GRE signal decrease along the lesion periphery (top middle) was significantly greater in magnitude than the 7d-preloaded SHAM group (bottom right). The GRE signal decrease within the lesion core was not statistically different than the 7d-preloaded SHAM group (p ≥ 0.183).
Table 1.
3D-GRE Signal Changes Following 30 Minutes Transient Middle Cerebral Artery Occlusion (tMCAO)
|
Time (Days) |
30 minutes tMCAO | SHAM | ||||
|---|---|---|---|---|---|---|
| Group 1: 7d Preloaded |
Group 2: Postloaded |
Group 3: Control |
Group 4: 7d Preloaded |
|||
| Total | Core | Periphery | ||||
| 1 | −13 (3)** | −15 (20) | −13 (4)** | −3 (3) | −4 | 4 (4) |
| 2 | −20 | −10 (10) | −21 | 2 (2) | −10 | 2 (1) |
| 3 | −20 (10) | −10 (20) | −20 (8)* | 8 (5) | −10 | 2 (5) |
| 4 | −9 (7)* | −8 (2) | −22 (8)** | −2 (5) | −3 | −3 (3) |
| 7 | −15 (2)* | −10 (9) | −23 (3)** | −10 (10) | −7 | −2 (5) |
| Average | −15 (4)# | −11 (3)* | −22 (2)** # † | 0 (1) | −7 (3) | 1 (3) |
Significance with p < 0.01, versus SHAM
Significance with p < 0.05, versus SHAM
Significance with p < 0.05, versus Postloaded tMCAO, Control tMCAO
Significance with p < 0.05, Periphery versus Core
Figure 4 shows the correlations between ED-1, IBA, ED-2, and PB immunohistochemical staining within the Preloaded tMCAO group. ED-1 and IBA staining were well-correlated on a region-by-region basis (r = 0.521, p < 0.01), with no significant difference in cell counts (p = 0.33, averaged across R1-R12). ED-2 and PB staining were well-correlated visually, however, while the two had the same magnitude of cell counts, ED-2 counts [20 (20)] were significantly higher than PB counts [10 (20)] by an approximate factor of two (p < 0.01).
Figure 4. Correlation Between ED-1/IBA and ED-2/PB Staining.
ED-1+ staining visually correlated with IBA+ staining and there were no significant difference in cell counts. ED-2+ staining (arrows, inset, brown) visually correlated with PB+ staining (arrows, inset, blue). However, while the two had the same magnitude of cell counts, ED-2+ was significantly higher than PB+ by an approximate factor of two (p < 0.01). Colocalized images, 20×, scale bar = 50 µm.
On the inner edge of the peri-infarct area (R1-R5), ED-2+ and PB+ staining was observed primarily in perivascular locations and as a limited quantity in the parenchyma. On the outer edge of the peri-infarct area (R6-R10), ED-2+ and PB+ staining were observed primarily within the meninges. Within the infarct core, ED-2+ and PB+ staining were extremely limited, if not completely absent. ED-1+ and IBA+ staining was present throughout the infarct. Similar to ED-2+ and PB+ staining, ED-1+ and IBA+ staining was greater in the peri-infarct area versus that of the core.
Table 2 provides a detailed, quantitative summary of cell counts for ED-1, IBA, ED-2, and PB immunohistochemical staining for individual groups on day 7 post tMCAO. In Postloaded tMCAO and Control tMCAO groups, although IBA and ED-1 cell counts were of the same magnitude, IBA cell counts were significantly higher than ED-1 cell counts (p < 0.01). ED-2 counts and PB counts were significantly lower than ED-1 counts and IBA counts across all groups (p < 0.01). ED-2 counts were similar for all tMCAO animals, but differences were present for PB. The Preloaded tMCAO group had significantly higher PB counts than all other groups (p < 0.01). PB cell counts for the Postloaded tMCAO and Control tMCAO groups were similar to the Preloaded SHAM group (p = 0.782, 0.347), with means close to zero. Within the Preloaded SHAM group, there were no differences between ED-1, ED-2, and PB counts (p ≥ 0.319) and IBA counts were extremely low.
Table 2.
Cell Counts on a Group-by-Group Basis (per 20× field)
| Staining Method |
30 Minutes tMCAO | SHAM | ||
|---|---|---|---|---|
| Group 1: 7d Preloaded |
Group 2: Postloaded |
Group 3: Control |
Group 4: 7d Preloaded |
|
| ED-1 | 460 (290) [0–1127] | 320 (250) [0–945] | 230 (160) [0–544] | 1 (1) [0–10] |
| IBA | 520 (290) [0–1294] | 590 (290) [37–1101] | 460 (230) [11–906] | 30 (20) [0–216] |
| ED-2 | 20 (20) [0–92]* | 8 (1) [0–76]* | 10 (20) [0–67]* | 1 (4) [0–45] |
| PB | 10 (20) [0–85]*#† | 2 (9) [0–85] *# | 1 (2) [0–11] *# | 1 (2) [0–10] |
versus IBA and ED-1 (within group), significance with p < 0.01
versus ED-2 (within group), significance with p < 0.01
versus all groups for PB staining, significance with p < 0.01
Verification of ED-2:PB Double-staining
Figure 5 shows double-staining for ED-2:PB (top, middle) and ED1:PB (bottom) for CNS-resident macrophages located in the meninges. ED-2+ and PB+ macrophages were identified (top, middle row), validating the presence of iron in well-differentiated macrophages. While some of the ED-2+/PB+ macrophages stained brown with an intense blue color (middle right), the majority stained brown and light blue (top). ED-1+ macrophages were PB-negative (bottom row), confirming the absence of iron in monocytes/macrophages, and activated microglia (bottom right).
Figure 5. Confirmation of ED-2:PB Double-staining.
ED-2+ and PB+ macrophages were identified (top, middle row), validating the presence of iron in well-differentiated macrophages. ED-1+ and PB− macrophages were identified (bottom row), confirming the absence of iron in monocytes/macrophages, and activated microglia. Brown = ED-2+ (top, middle) or ED-1+ (bottom), Blue = PB+, Background = nuclear fast red counterstain. Colocalized images, 40×, scale bar = 25 µm.
CNS-Resident Macrophages Express MHC-II and TNF-alpha
Figure 6 shows the colocalization of ED-2+ and PB+ stained macrophages and the colocalization of ED-2+ macrophages expressing MHC-II complex and TNF-α. ED-2+ macrophages were isolated to three distinct locations, the meninges, choroids plexus, and perivascular regions. These macrophages stained positive for MHC-II complex and TNF-α. An additional small number of ED-2−, PB− and MHC-II+ cells were observed scattered throughout the lesion area (not shown). It is likely that these correspond to ED-1+ and IBA+ activated microglia. Overall IL-1 staining was weak, with high background fluorescence and non-specific staining. For these reasons, IL-1 results were excluded from this study.
Figure 6. CNS-Resident Macrophages express MHC-II complex and TNF-α.
Immunohistochemistry (Set A, contiguous sections): ED-2+ and PB+ macrophages (arrows) were identified within the meninges, choroid plexus, and perivascular locations [colocalized images, 20×, scale bar = 25 µm]. Brown = ED-2+, Blue = PB+, Background = nuclear fast red counterstain. Immunofluorescence (Set B, contiguous sections): These ED-2+ and PB+ macrophages, located at the interface between the blood-brain barrier and the extravascular space, were found to express both MHC-II complex and TNF-α [colocalized images, 40×, scale bar = 25µm]. Blue = DAPI for nuclei. Red = Rhodamine for ED-2/MHC-II. Green = FITC for TNF-α.
Discussion
In this study, we monitored CNS-resident macrophages using Feridex-preloading and non-invasive MRI following tMCAO. In addition, we verified that these CNS-resident macrophages likely play an integral role in the inflammatory response using immunohistochemistry and immunofluorescence techniques.
We observed an approximate 20% signal decrease along the lesion periphery between days 2–7 post I-R injury, corresponding to ED-2+, PB+, MHC-II+, and TNF-α+ macrophages. These findings, based on Feridex preloading, are in contrast to previous work employing administration of iron oxides post stroke (Farr et al 2008; Rausch et al 2002; Schroeter et al 2006). This difference may largely be attributed to the time-frame of macrophage labeling. According to Raynal and colleagues (Raynal et al 2004), a 3-day incubation resulted in a 1.1–3.0% uptake of Fe-injected dose. Without adequate time for macrophage labeling, injection of iron oxide post I-R injury merely serves as a negative contrast method for perfusion status and assessment of BBB permeability, not as a marker of macrophage activity. The choice of 7-day preloading was based on Feridex blood-pool kinetics and liver/spleen clearance rates (Wang et al 2001) and previous testing in traumatic brain injury (Foley et al 2006), ensuring the MRI signal is from macrophages of systemic origin. Given the difficulty in isolating bone-marrow derived macrophages, we do not know the precise labeling efficiency in our study. However, we do know that the signal decrease observed on 3D-GRE images is consistent with the 24% (Vande Berg et al 1999) and 27.8% (Hundt et al 2000) signal decreases in MRI studies directly labeling the bone marrow. As further support, the pattern of signal evolution from the subacute to chronic timeframe post tMCAO mirrors the direct quantification of GFP-labeled bone-marrow derived macrophages in radiation-induced chimeras post-stroke (Tanaka et al 2003).
There was a small decrease in GRE signal over time (∼3–10%) in the Control tMCAO group. This decrease could potentially be from endogenous iron deposition during the process of tissue necrosis, but it is more likely that this signal change is from the formation of edema and the concomitant change in T1 related to increased water content. In this animal model, edema reached a maximum at 2–3 days post-stroke, with some resolution, but not full resolution, by day 7. To utilize Feridex-preloading or other USPIO preloading to monitor the inflammatory cascade, a correction would need to be applied to account for the presence of vasogenic edema. An alternative approach would be to perform a separate set of experiments with appropriate controls (i.e., non-preloaded).
We found quantitative and qualitative correlations between ED-1 and IBA staining (i.e., activated microglia) as well as ED-2 and PB staining (i.e., iron-labeled, well-differentiated macrophages), validating the signal changes observed on 3D-GRE images. Additional double-staining for ED-2:PB and ED-1:PB verified both the presence of iron within ED-2+ macrophages and the absence of iron in ED-1+ macrophages. These findings are in contrast to previous work in permanent ischemia, where there exists an inherent disconnect between the findings on MRI and those on histology/immunohistochemistry (Rausch et al 2001; Saleh et al 2004b). We propose that this discordance is a result two factors. First, iron oxide administration several hours after permanent ischemia is potentially troublesome. Depending on their size, iron oxides can remain in the blood pool for anywhere from several minutes to several hours. If BBB disruption is present, they may extravasate, travel along the Virchow-Robin spaces, and diffuse into the brain (Thorek et al 2006). This increases the potential for non-specific accumulation of contrast when smaller iron oxides, such as combidex and mion particles, are being employed. Second, ED-1 is a non-specific marker of macrophage activity (Beelen et al 1987). ED-1 has been shown to be coincident with OX-42 staining, typically employed for identification of activated microglia (Lassmann et al 1993). It is, therefore, not surprising the number of ED-1+ cells far exceeded the number of PB+ cells in these previous studies.
To circumvent these problems, we employed methods known to accurately differentiate between various populations of macrophages and brain microglia (Beelen et al 1987; Ito et al 1998; Lassmann et al 1993). OX-42 staining is more widely used in the identification of microglia, however, we chose to employ IBA, a recently identified marker specific to a 17kDa calcium binding protein expressed only on activated microglia (Ito et al 1998). ED-1 is a marker that recognizes the rat homologue of human CD68, a 110kDa cell-surface glycoprotein readily expressed on the membrane surface of monocytes, macrophages, dendritic cells, and microglia. ED-2 is marker that recognizes the rat homologue of human CD163, a 175kDa cell-surface glycoprotein expressed only by resident or well-differentiated macrophages, not monocytes or microglia. PB staining, most commonly known as Perl’s method, targets the release of ferric iron (Fe3+) from binding proteins and the production of an insoluble blue compound. Therefore, if a macrophage contained ferric iron, it will be labeled blue.
To reiterate, we found a significant correlation between IBA+ and ED-1+ cells. IBA+ counts were either equivalent to or slightly higher than ED-1+ counts, indicating that the majority of ED-1+ cells were in fact activated microglia. The number of ED-2+ cells in our study were significantly higher than the number of PB+ cells, however, the quantitative results were of the same magnitude and were significantly different than both IBA+ and ED-1+ cell counts. This difference between ED-2+ and PB+ counts is easily explained by reported turnover of macrophages every 1–2 weeks (Whitelaw 1966). We preloaded animals with Feridex 7 days prior to I-R injury, and cell counts were not performed until 7 days post I-R injury. This means that upon insult, macrophages will begin to turnover, leading to two populations of macrophages—one that is Feridex-labeled and a newly recruited non-labeled population. We do not know the temporal dynamics of Feridex-labeled versus non-labeled macrophages, and admittedly, further study is warranted to determine the effect of macrophage turnover on MRI signal variability. Investigation into the kinetics of iron uptake by the bone marrow, spleen, lymph organs, and the CNS post Feridex administration, but prior to insult, as well as at serial time points following insult would be required. Regardless of this shortcoming, the correlations between ED-1+ and IBA+ macrophages, ED-2+ and PB+ macrophages, and the observed 3D-GRE signal changes confirm our primary hypothesis—Feridex-preloading permits labeling of well-differentiated macrophages and monitoring of their activity post tMCAO.
We discovered that these Feridex-labeled macrophages (ED-2+, PB+) were confined to three distinct locations, including perivascular regions (PVM), meninges (MM), and choroid plexus (CPM). These ED-2+ and PB+ macrophages were also MHC-II+ and TNF-α+. Unfortunately, we were unable to draw any conclusions about IL-1. Because we employed a polyclonal antibody, IL-1 staining results had extremely high background, making true IL-1 expression difficult to differentiate from non-specific IL-1 staining. Despite our lack of conclusive evidence on IL-1 expression, the strategic location of these ED-2+, PB+ macrophages and their dual expression of MHC-II+ and TNF-α+ leads us to believe that CNS resident macrophages play an active role in the continuation of the inflammatory response following tMCAO. The best way to confirm their role in reperfusion injury would be to perform a selective depletion prior to stroke induction. These experiments are extremely difficult even in healthy animals, so it would be more challenging to perform them in hypertensive animals with stroke. Additionally, it would be important to investigate these CNS-resident macrophages, their expression of MHC-II+, and their interaction with T-cells. MHC-II+ expression is not synonymous with, but is required for T-cell activation. While this only permits us to state that CNS-resident macrophages expressing MHC-II may mediate T-cell activation and the immune response following tMCAO, prior work in EAE supports this contention (Becher et al 2006; Greter et al 2005; McMahon et al 2005).
Extensive investigation of this specific population of macrophages has been underway since the 1990s in experimental allergic encephalitis (EAE) and clinical MS. These macrophages actively express MHC-II antigen following administration of interferon-gamma and TNF in bone-marrow chimeras (Lassmann et al 1991). They participate in the pathogenesis of EAE through production and secretion of products such as proteases (MMPs) and inflammatory cytokines (IL-1, IL-6, TNF-α), and may interact with leukocytes through ICAM-1 expression (Bauer et al 1996). More recently, and probably most importantly, it was demonstrated that their selective depletion results in complete suppression of disease (Polfliet et al 2002). Given that this population of macrophages forms a first line of defense at the interface between the blood and the brain, their manipulation could provide attenuation of reperfusion-injury in both the acute and subacute to chronic phases of stroke.
In conclusion, we have shown that preloading with Feridex 7 days prior to tMCAO permits selective monitoring of CNS-resident macrophages using 3D-GRE imaging, a unique population of cells that seem to be playing an active rather than passive role in inflammation and disease progression. Feridex preloading, therefore, provides an attractive avenue for the non-invasive assessment of anti-inflammatory therapies in preclinical models of stroke and EAE.
Acknowledgements
This research was supported by the Division of Intramural Research of the National Institute of Neurological Disorders and Stroke, National Institutes of Health. The authors would like to thank Christina A. Rabinak, B.S. and Narendra C. Panapitiya, B.S. for preliminary work with respect to MRI analysis and immunohistochemistry.
Footnotes
Disclosure/Conflict of Interest:
None.
Part of this work was presented at the International Stroke Conference, San Francisco, California, February 2007.
References
- Angelov DN, Walther M, Streppel M, Guntinas-Lichius O, van Dam AM, Stennert E, Neiss WF. ED2-positive perivascular phagocytes produce interleukin-1beta during delayed neuronal loss in the facial nucleus of the rat. J Neurosci Res. 1998;54:820–827. doi: 10.1002/(SICI)1097-4547(19981215)54:6<820::AID-JNR10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Bauer J, Ruuls SR, Huitinga I, Dijkstra CD. The role of macrophage subpopulations in autoimmune disease of the central nervous system. Histochem J. 1996;28:83–97. doi: 10.1007/BF02331413. [DOI] [PubMed] [Google Scholar]
- Becher B, Bechmann I, Greter M. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J Mol Med. 2006;84:532–543. doi: 10.1007/s00109-006-0065-1. [DOI] [PubMed] [Google Scholar]
- Beelen R, Eestermans I, Döpp E, Dijkstra C. Monoclonal antibodies ED1, ED2 and ED3 against rat macrophages: expression of recognized antigens in different stages of differentiation. Transpl Proc XIX. 1987;3:3166–3170. [Google Scholar]
- Farr T, Hoehn M, Seehafer J. Imaging macrophage infiltration of ischemic tissue is not possible following transient middle cerebral artery occlusion. ISMRM Proceedings. 2008 [Google Scholar]
- Flaris NA, Densmore TL, Molleston MC, Hickey WF. Characterization of microglia and macrophages in the central nervous system of rats: definition of the differential expression of molecules using standard and novel monoclonal antibodies in normal CNS and in four models of parenchymal reaction. Glia. 1993;7:34–40. doi: 10.1002/glia.440070108. [DOI] [PubMed] [Google Scholar]
- Foley LM, Hitchens TK, Kochanek PM, Melick JA, Ho C. Visualization of phagocytic cells in a mouse brain after traumatic brain injury with micron-sized iron-oxide particles: a preliminary report. ISMRM Proceedings. 2006 [Google Scholar]
- Gerriets T, Stolz E, Walberer M, Muller C, Rottger C, Kluge A, Kaps M, Fisher M, Bachmann G. Complications and pitfalls in rat stroke models for middle cerebral artery occlusion: a comparison between the suture and the macrosphere model using magnetic resonance angiography. Stroke. 2004;35:2372–2377. doi: 10.1161/01.STR.0000142134.37512.a7. [DOI] [PubMed] [Google Scholar]
- Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nature medicine. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM, Hansson GK, Becker KJ. Immunology of ischemic vascular disease: plaque to attack. Trends Immunol. 2005;26:550–556. doi: 10.1016/j.it.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hundt W, Petsch R, Helmberger T, Reiser M. Effect of superparamagnetic iron oxide on bone marrow. Eur Radiol. 2000;10:1495–1500. doi: 10.1007/s003300000350. [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Hallenbeck JM. Polymorphonuclear leukocytes and monocytes/macrophages in the pathogenesis of cerebral ischemia and stroke. Stroke. 1992;23:1367–1379. doi: 10.1161/01.str.23.9.1367. [DOI] [PubMed] [Google Scholar]
- Koizumi J, Yoshida Y, Nakazawa TGO. Experimental studies of ischemic brain edema. I. A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- Lassmann H, Zimprich F, Vass K, Hickey WF. Microglial cells are a component of the perivascular glia limitans. J Neurosci Res. 1991;28:236–243. doi: 10.1002/jnr.490280211. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Schmied M, Vass K, Hickey WF. Bone marrow derived elements and resident microglia in brain inflammation. Glia. 1993;7:19–24. doi: 10.1002/glia.440070106. [DOI] [PubMed] [Google Scholar]
- Lehrmann E, Kiefer R, Christensen T, Toyka KV, Zimmer J, Diemer NH, Hartung HP, Finsen B. Microglia and macrophages are major sources of locally produced transforming growth factor-beta1 after transient middle cerebral artery occlusion in rats. Glia. 1998;24:437–448. doi: 10.1002/(sici)1098-1136(199812)24:4<437::aid-glia9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Manninger SP, Muldoon LL, Nesbit G, Murillo T, Jacobs PM, Neuwelt EA. An exploratory study of ferumoxtran-10 nanoparticles as a blood-brain barrier imaging agent targeting phagocytic cells in CNS inflammatory lesions. AJNR Am J Neuroradiol. 2005;26:2290–2300. [PMC free article] [PubMed] [Google Scholar]
- McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nature medicine. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–562. [PubMed] [Google Scholar]
- Polfliet MM, van de Veerdonk F, Dopp EA, van Kesteren-Hendrikx EM, van Rooijen N, Dijkstra CD, van den Berg TK. The role of perivascular and meningeal macrophages in experimental allergic encephalomyelitis. J Neuroimmunol. 2002;122:1–8. doi: 10.1016/s0165-5728(01)00445-3. [DOI] [PubMed] [Google Scholar]
- Rausch M, Sauter A, Frohlich J, Neubacher U, Radu EW, Rudin M. Dynamic patterns of USPIO enhancement can be observed in macrophages after ischemic brain damage. Magn Reson Med. 2001;46:1018–1022. doi: 10.1002/mrm.1290. [DOI] [PubMed] [Google Scholar]
- Rausch M, Baumann D, Neubacher U, Rudin M. In-vivo visualization of phagocytotic cells in rat brains after transient ischemia by USPIO. NMR Biomed. 2002;15:278–283. doi: 10.1002/nbm.770. [DOI] [PubMed] [Google Scholar]
- Raynal I, Prigent P, Peyramaure S, Najid A, Rebuzzi C, Corot C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest Radiol. 2004;39:56–63. doi: 10.1097/01.rli.0000101027.57021.28. [DOI] [PubMed] [Google Scholar]
- Saleh A, Schroeter M, Jonkmanns C, Hartung HP, Modder U, Jander S. In vivo MRI of brain inflammation in human ischaemic stroke. Brain. 2004a;127:1670–1677. doi: 10.1093/brain/awh191. [DOI] [PubMed] [Google Scholar]
- Saleh A, Wiedermann D, Schroeter M, Jonkmanns C, Jander S, Hoehn M. Central nervous system inflammatory response after cerebral infarction as detected by magnetic resonance imaging. NMR Biomed. 2004b;17:163–169. doi: 10.1002/nbm.881. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Oros-Peusquens AM, Irkens M, Celik A, Saleh A, Shah NJ, Jander S. Spatiotemporal pattern of USPIO enhancement in experimental stroke lesions depends on the type of ischemic injury: a 9.4T MRI study. ISMRM Proceedings. 2006 [Google Scholar]
- Tanaka R, Komine-Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, Shimada T, Mizuno Y, Urabe T. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003;117:531–539. doi: 10.1016/s0306-4522(02)00954-5. [DOI] [PubMed] [Google Scholar]
- Thorek DL, Chen AK, Czupryna J, Tsourkas A. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann Biomed Eng. 2006;34:23–38. doi: 10.1007/s10439-005-9002-7. [DOI] [PubMed] [Google Scholar]
- Van de Berg BC, Lecouvet FE, Kanku JP, Jamart J, Van Beers BE, Maldague B, Malghem J. Ferumoxides-enhanced quantitative magnetic resonance imaging of the normal and abnormal bone marrow: preliminary assessment. J Magn Reson Imaging. 1999;9:322–328. doi: 10.1002/(sici)1522-2586(199902)9:2<322::aid-jmri26>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11:2319–2331. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- Weissleder R, Elizondo G, Wittenberg J, Rabito CA, Bengele HH, Josephson L. Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology. 1990;175:489–493. doi: 10.1148/radiology.175.2.2326474. [DOI] [PubMed] [Google Scholar]
- Whitelaw DM. The intravascular lifespan of monocytes. Blood. 1966;28:455–464. [PubMed] [Google Scholar]
- Wiart M, Davoust N, Pialat JB, Desestret V, Moucharrafie S, Cho TH, Mutin M, Langlois JB, Beuf O, Honnorat J, Nighoghossian N, Berthezene Y. MRI monitoring of neuroinflammation in mouse focal ischemia. Stroke. 2007;38:131–137. doi: 10.1161/01.STR.0000252159.05702.00. [DOI] [PubMed] [Google Scholar]