Abstract
Neurons located in the dorsomedial pontine rapid eye movement (REM) sleep-triggering region send axons to the medial medullary reticular formation (mMRF). This pathway is believed to be important for the generation of REM sleep motor atonia, but other than that they are glutamatergic little is known about neurochemical signatures of these pontine neurons important for REM sleep. We used single-cell reverse transcription and polymerase chain reaction (RT-PCR) to determine whether dorsomedial pontine cells with projections to the mMRF express mRNA for selected membrane receptors that mediate modulatory influences on REM sleep. Fluorescein (FITC)-labeled latex microspheres were microinjected into the mMRF of 26–34 day-old rats under pentobarbital anesthesia. After 5–6 days, rats were sacrificed, pontine slices were obtained and neurons were dissociated from 400–600 μm micropunches extracted from dorsomedial pontine reticular formation. We found that 32 out of 51 FITC-labeled cells tested (63%±7(SE)) contained the orexin type 1 receptor (ORX1r) mRNA, 27 out of 73 (37%±6) contained the adrenergic α2A receptor (α2Ar) RNA, and 6 out of 31 (19%±7) contained both mRNAs. The percentage of cells positive for the ORX1r mRNA was significantly lower (p<0.04) for the dorsomedial pontine cells that were not retrogradely labeled from the mMRF (32%±11), whereas α2Ar mRNA was present in a similar percentage of FITC-labeled and unlabeled neurons. Our data suggest that ORX and adrenergic pathways converge on a subpopulation of cells of the pontine REM sleep-triggering region that have descending projections to the medullary region important for the motor control during REM sleep.
Keywords: adrenergic receptors, hypocretin, REM sleep, single-cell RT-PCR, motor atonia, pons
Introduction
Rapid eye movement (REM) sleep is a stage of sleep characterized by a combination of cortical and hippocampal activation, rapid eye movements, atonia of postural muscles with superimposed muscle twitches, and highly variable respiratory rate, heart rate and blood pressure. Many neurotransmitter receptor agonists and antagonist, including a cholinergic agonist, carbachol [1, 4, 7, 11], GABAA receptor antagonist, bicuculline [3, 21, 24, 26, 38] and the peptide orexin (ORX) [14, 37], can effectively trigger REM sleep-like state when injected into the dorsomedial pontine reticular formation. Other agonists have suppressant effects on REM sleep when injected into the same pontine region, e.g., agonists of α2 adrenergic and type 1A and 2 serotonergic receptors [2, 11, 23, 28, 30; reviewed in ref. 16]. Some cells in the dorsomedial pontine reticular formation are immunoreactive for type 1 and/or type 2 ORX receptors [5, 8, 20] and are excited by ORX in vitro [6], and some cells express α2A adrenergic receptors [29]. Importantly, it appears that stimulation of dorsomedial pontine type 2 ORX receptors promotes REM sleep and its atonia [14, 37], whereas stimulation of either type 1 ORX receptors or α2 adrenergic receptors suppresses REM sleep [2, 22, 30, 35]. Therefore, we investigated the expression and co-expression of type 1 ORX and α2A adrenergic receptor mRNAs in dorsomedial pontine cells that have axonal projections to the medial medullary reticular formation (mMRF), a region also important for the control of REM sleep and its motor atonia.
Among the efferent projection sites from the dorsomedial pontine REM sleep-triggering region [3, 25, 27], the mMRF received particular attention as potentially important for the generation of REM sleep and/or its motor atonia [15, 18, 28, 31, 36]. This very robust pathway is likely to be glutamatergic [17, 19] and targets the medial magnocellular, ventral gigantocellular and medullary raphe nuclei [9, 10, 13, 17, 25, 27]. Lesions of the mMRF region abolish or attenuate REM sleep and its atonia [12, 31]. Since the atonia-controlling pathways can be modulated by both ORX and α2 adrenergic receptors [2, 14, 22, 30, 35, 37], it is of interest to determine whether these effects can be exerted on those cells within the dorsomedial pontine REM sleep-triggering region that have axonal projections to the mMRF. Here, we used single-cell reverse transcription and polymerase chain reaction (RT-PCR) to determine whether cells of the pontine REM sleep-triggering region that have axonal projections to the mMRF express mRNA for adrenergic α2A receptor (α2Ar) and/or type 1 ORX receptor (ORX1r). A preliminary report has been published [34].
Materials and methods
Fifteen Sprague-Dawley rats were housed under a 12h/12h light-dark cycle with food and water available ad libitum. All surgical and animal handling procedures followed the guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Fluorescein (FITC)-labeled latex microspheres (LumaFluor, USA) (100–450 nl; mean volume 248±40 (SE)) were microinjected into the mMRF of 26–34 day-old pentobarbital-anesthetized rats. After 5–6 days, rats were sacrificed under deep isoflurane anesthesia (4%), and 1–2 coronal, 400 μm slices were cut from the level of the caudal inferior colliculus. The medulla was also extracted, fixed in formalin, and then sectioned and mounted to visualize the FITC-labeled microspheres injection site (Fig. 1A–C). Cells from the pontine reticular formation were dissociated as described previously [32, 33]. Briefly, following enzymatic digestion with papain, micropunches (400–600 μm in diameter) were cut from the dorsomedial pontine reticular formation. The pontine slices from which the punches were extracted were fixed in formalin, cut into 20 μm sections and mounted to verify the punch locations (Fig. 1D).
Figure 1.
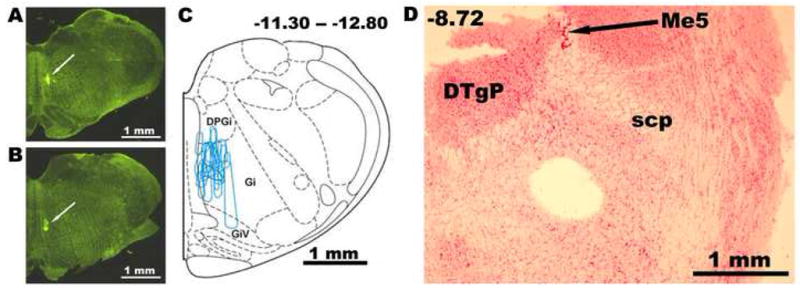
FITC-labeled bead injection sites in the medial medullary reticular formation (A–C) and a typical location of a 600 μm micropunch of tissue taken from a pontine slice from a rat injected 5–6 days earlier with FITC-labeled beads (D). A and B: location of FITC deposit at two antero-posterior (AP) levels in one rat. C: injections sites as those shown in A and B from all animals superimposed on one standard cross-section located in the middle of the rostro-caudal span of the injected area that extended from AP-11.3 to AP-12.8 from bregma. Abbreviations: DPGi-dorsal paragigantocellular region; DTgP-dorsal tegmental region, pericentral; Gi-gigantocellular region; GiV-gigantocellular region, pars ventralis; Me5-mesencephalic trigeminal nucleus; scp- superior cerebellar peduncle.
Cells contained in the micropunch were mechanically dispersed, plated, and those with FITC-labeled beads were identified using an inverted phase contrast microscope equipped with fluorescent light source and FITC filters (IMT-2, Olympus, Japan) (Fig. 2A). Individual cells, labeled and unlabeled, were collected, their intracellular contents treated with DNAse and then subjected to reverse transcription, as described previously [32]. Aliquots of the resulting cDNA (each corresponding to ~1/3 of the total volume from each cell) were used in a two-stage, semi-nested PCR (Fig. 2B–D). The first round of amplification (35–37 cycles) was performed using a conventional thermal cycler (PCR Sprint; Thermo Hybaid, UK) and the second round with a real-time cycler (LightCycler; Roche Diagnostics, USA). Primers were designed using Vector NTI software (Invitrogen, USA). The criteria for primer specificity, reaction quality control and the strategy to optimize PCR conditions were described previously [32]. The primers used in this study included those for the ORX1r (accession: NM_013064) and the adrenergic α2Ar (accession: M62372). The sequences of the ORX1r primers were: 5′-GTGTCGGTGTCAGTGGCAGT-3′ (sense, first and second PCR rounds), 5′-TGAGGGTCGCTTCCAGTTCC-3′ (antisense, first PCR round), and 5′-GAAGAGCCGTGTGCGATTGG-3′ (internal antisense, second PCR round). The primer sets used for the α2Ar have been published previously [32]. The position and size of the melting curve peaks obtained in the second PCR round provided an initial assessment as to whether the expected cDNA was generated (Fig. 2C). Selected PCR products were then separated on ethidium bromide-stained 2% agarose gels to further verify that they were of the expected size (Fig. 2D). To control for false positive results, 8 non-reverse transcribed single-cell samples from two rats were amplified with all primers. To control for mRNA contamination of the medium, one sample of the fluid from above the plated cells was collected at the end of each cell collection session and subjected to the RT-PCR procedures identical to those with single-cell samples. None of those control reactions was positive.
Figure 2.
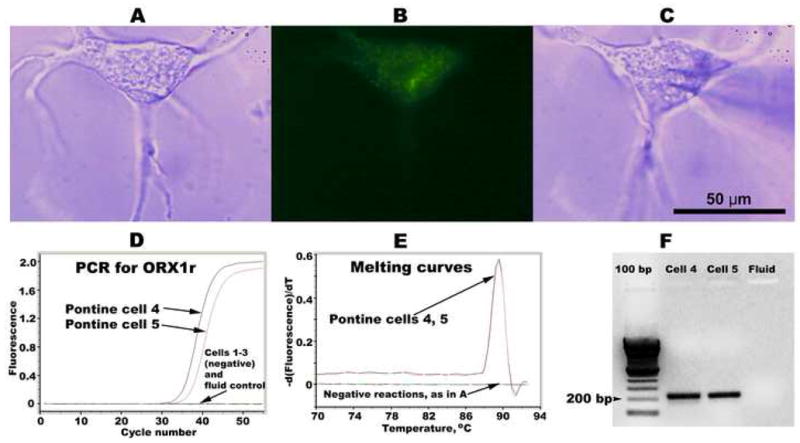
Example of a dissociated and plated dorsomedial pontine cell that was retrogradely labeled from the medial medullary reticular formation (A–C), and results of the second round of real-time cDNA amplification with the material from a set of pontine cells (D–F). A: a dissociated cell, as seen under phase contrast illumination. B: the same cell seen under fluorescent illumination contains FITC-labeled beads transported from the medial medullary reticular formation. C: the same cell with a polished glass pipette placed in position for cell collection. D: real-time PCR curves for a set of five pontine cells and one control sample containing culture medium only; two of the cells in this set were positive for ORX1r cDNA. E: melting curves for the cDNA products from the reactions shown in D. F: gel display of selected PCR products obtained in the reactions shown in D.
The standard errors (SE) for estimation of the proportions of cells positive for distinct mRNAs were determined based on the size of each cell population and the corresponding statistical tests used the assumption of a random bimodal distribution (Analyse-It Software, Leeds, UK). Statistical comparisons of the percentages of cells expressing different mRNAs were conducted using Fisher exact test (Analyse-It Software). Differences were regarded significant at p<0.05.
Results and discussion
Ninety seven FITC-labeled cells were collected from 15 rats, and an additional 32 unlabeled cells were collected from a subset of 9 rats from the same group of 15 animals (7±0.9 (SE) and 4 ±0.9 cells per animal, respectively). Following histological verification, 5 single-cell samples from 2 rats (4 FITC-labeled and 1 unlabeled) were excluded due to improper location of the punch. From the remaining 93 FITC-labeled and 31 unlabeled cells, 31 labeled and 2 unlabeled cells were tested for the presence of both the ORX1r and α2Ar mRNAs. cDNA samples from the remaining 62 labeled cells were tested for the presence of one mRNA species only, 20 for the ORX1r mRNA and 42 for the α2Ar mRNA. Similarly, for the remaining 31 unlabeled cell samples, 17 were tested for the ORX1r mRNA only and 12 for the α2Ar mRNA only. The remaining cDNA from these single-cell samples (~2/3 of the total volume) was used for testing and optimizing various primer sets.
Within the FITC-labeled population, 32 out of 51 cells tested (63%±7) contained the ORX1r mRNA, 27 out of 73 (37%±6) contained α2Ar mRNA (Fig. 3), and 6 out of 31 tested (19%±7) contained both mRNAs. The proportion of cells positive for the ORX1r mRNA was significantly lower (p<0.04) among the dorsomedial pontine cells that were not retrogradely labeled from the mMRF (6 out of 19, or 32%±11). The proportion of FITC-labeled cells positive for the adrenergic α2Ar mRNA did not differ significantly from that for the unlabeled cells (37%±6 vs. 43%±14; Fig. 3). None of the two unlabeled cells tested expressed both mRNAs.
Figure 3.
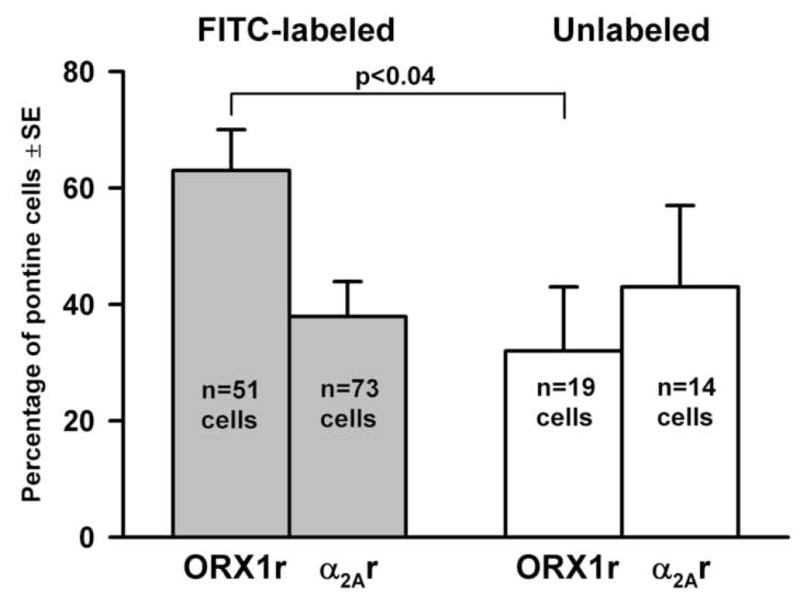
Percentages of dorsomedial pontine reticular cells that contained the orexin type 1 receptor (ORX1r) or adrenergic α2A receptor (α2AAr) mRNA; dorsomedial pontine cells not retrogradely labeled from the medial medullary reticular formation (Unlabeled) were less likely to contain ORX1r mRNA than those that had descending projections to the medial medulla (FITC-labeled).
Since it has been reported that large GABAergic pontine reticular neurons (cross-sectional area >450 μm2, approximately equivalent to mean diameter >24 μm) have higher immunostaining density for type 2 ORX receptor than smaller neurons [5], we also investigated the relationship between the presence of ORX1r or α2Ar mRNA and cell size. During the collection procedure, cells were classified into three groups: small (<20 μm in diameter), medium (20–30 μm), or large (>30 μm). We found a trend for ORX1r mRNA to be more frequently present in small- and medium-size than large cells (75%±13 and 68%±10 vs. 43%±14). The α2Ar mRNA tended to be more often present in medium-size (46%±9) than in either small (33%±14) or large (27%±9) cells.
Our data show that many dorsomedial pontine neurons with axonal projections to the medial medullary reticular formation contain mRNA for the excitatory ORX1r or the inhibitory adrenergic α2Ar, and that both these mRNAs are present in about 20% of such neurons. The dorsomedial pontine neurons with descending projections to the medial medulla were more likely to express the ORX1r mRNA than those that were not retrogradely labeled and tended to be of a small or medium size. Thus, ORX1r may be a useful, albeit not fully reliable when used alone, marker in future neurochemical studies of subpopulations of dorsomedial pontine cells involved in the generation of REM sleep.
Data show that microinjections of ORX can trigger REM sleep atonia when it is injected into the dorsomedial pontine reticular formation [14, 37], whereas clonidine injected in this region suppresses REM sleep [30]. Together with these results, our data suggest that these two opposing effects may postsynaptically converge on those cells of the pontine REM sleep-triggering region that have descending projections to the medial medullary reticular formation. Consistent with this interpretation, cells immunoreactive for both the ORX1r and α2Ar proteins are present in the dorsomedial pontine tegmentum [8, 20, 29], and ORX excites those dorsomedial pontine neurons that are also excited by carbachol [6], a cholinergic agonist widely used to trigger REM sleep-like state [16]. Recent data also show that microinjections of ORX in this region can suppress REM sleep and increase wakefulness [22, 35]. This effect of ORX may be mediated, at least in part, by its action on a different population of dorsomedial pontine cells than those investigated in our study. Such cells may be GABAergic, rather than glutamatergic, and express type 2, rather than type 1, ORX receptors [5]. ORX, by acting on such cells may increases GABA levels in this region [35].
Our finding that dorsomedial pontine neurons with axonal projections to the medial medulla contain the ORX1r and α2Ar mRNAs may also indicate that these receptors function as presynaptic receptors on the medullary and other axon terminals of these dorsomedial pontine neurons. Additional studies at the level of the corresponding receptor proteins are needed to further explore this question.
Acknowledgments
The study was supported by NIH Grant HL-47600.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baghdoyan H, Rodrigo-Angulo M, McCarley R, Hobson J. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 1984;306:39–52. doi: 10.1016/0006-8993(84)90354-8. [DOI] [PubMed] [Google Scholar]
- 2.Bier M, McCarley R. REM-enhancing effects of the adrenergic antagonist idazoxan infused into the medial pontine reticular formation of the freely moving cat. Brain Res. 1994;634:333–338. doi: 10.1016/0006-8993(94)91939-9. [DOI] [PubMed] [Google Scholar]
- 3.Boissard R, Gervasoni D, Schmidt M, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- 4.Bourgin P, Escourrou P, Gaultier C, Adrien J. Induction of rapid eye movement sleep by carbachol infusion into the pontine reticular formation in the rat. Neuroreport. 1995;6:532–536. doi: 10.1097/00001756-199502000-00031. [DOI] [PubMed] [Google Scholar]
- 5.Brischoux F, Mainville L, Jones B. Muscarinic-2 and orexin-2 receptors on GABAergic and other neurons in the rat mesopontine tegmentum and their potential role in sleep-wake state control. J Comp Neurol. 2008;510:607–630. doi: 10.1002/cne.21803. [DOI] [PubMed] [Google Scholar]
- 6.Brown R, Winston S, Basheer R, Thakkar M, McCarley R. Electrophysiological characterization of neurons in the dorsolateral pontine rapid-eye-movement sleep induction zone of the rat: Intrinsic membrane properties and responses to carbachol and orexins. Neuroscience. 2006;143:739–755. doi: 10.1016/j.neuroscience.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenik VB, Kubin L. Differential localization of carbachol- and bicuculline-sensitive pontine sites for eliciting REM sleep-like effects in anesthetized rats. J Sleep Res. 2009;18:99–112. doi: 10.1111/j.1365-2869.2008.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greco M, Shiromani P. Hypocretin receptor protein and mRNA expression in the dorsolateral pons of rats. Brain Res Mol Brain Res. 2001;88:176–182. doi: 10.1016/s0169-328x(01)00039-0. [DOI] [PubMed] [Google Scholar]
- 9.Herbert H, Klepper A, Ostwald J. Afferent and efferent connections of the ventrolateral tegmental area in the rat. Anat Embryol. 1997;196:235–259. doi: 10.1007/s004290050094. [DOI] [PubMed] [Google Scholar]
- 10.Hermann D, Luppi P, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) J Chem Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 11.Hernández-Peón R, Chávez-Ibarra G, Morgane PJ, Timo-Iaria C. Limbic cholinergic pathways involved in sleep and emotional behavior. Exp Neurol. 1963;8:93–111. [Google Scholar]
- 12.Holmes C, Jones B. Importance of cholinergic, GABAergic, serotonergic and other neurons in the medial medullary reticular formation for sleep-wake states studied by cytotoxic lesions in the cat. Neuroscience. 1994;62:1179–1200. doi: 10.1016/0306-4522(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 13.Jones B, Yang T. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- 14.Kiyashchenko L, Mileykovskiy B, Lai Y, Siegel J. Increased and decreased muscle tone with orexin (hypocretin) microinjections in the locus coeruleus and pontine inhibitory area. J Neurophysiol. 2001;85:2008–2016. doi: 10.1152/jn.2001.85.5.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohyama J, Lai Y, Siegel J. Inactivation of the pons blocks medullary-induced muscle tone suppression in the decerebrate cat. Sleep. 1998;21:695–699. doi: 10.1093/sleep/21.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubin L. Carbachol models of REM sleep: recent developments and new directions. Arch Ital Biol. 2001;139:147–168. [PubMed] [Google Scholar]
- 17.Lai Y, Clements J, Wu X, Shalita T, Wu J, Kuo J, Siegel J. Brainstem projections to the ventromedial medulla in cat: retrograde transport horseradish peroxidase and immunohistochemical studies. J Comp Neurol. 1999;408:419–436. doi: 10.1002/(sici)1096-9861(19990607)408:3<419::aid-cne8>3.0.co;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai Y, Siegel J. Medullary regions mediating atonia. J Neurosci. 1988;8:4790–4796. doi: 10.1523/JNEUROSCI.08-12-04790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 20.Marcus J, Aschkenasi C, Lee C, Chemelli R, Saper C, Yanagisawa M, Elmquist J. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 21.Marks G, Sachs O, Birabil C. Blockade of GABA, type A, receptors in the rat pontine reticular formation induces rapid eye movement sleep that is dependent upon the cholinergic system. Neuroscience. 2008;156:1–10. doi: 10.1016/j.neuroscience.2008.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno-Balandrán E, Garzón M, Bódalo C, Reinoso-Suárez F, de Andrés I. Sleep-wakefulness effects after microinjections of hypocretin 1 (orexin A) in cholinoceptive areas of the cat oral pontine tegmentum. Eur J Neurosci. 2008;28:331–341. doi: 10.1111/j.1460-9568.2008.06334.x. [DOI] [PubMed] [Google Scholar]
- 23.Mori S. Contribution of postural muscle tone to full expression of posture and locomotor movements: multi-faceted analyses of its setting brainstem-spinal cord mechanisms in the cat. Jpn J Physiol. 1989;39:785–809. [PubMed] [Google Scholar]
- 24.Pollock MS, Mistlberger RE. Rapid eye movement sleep induction by microinjection of the GABAA antagonist bicuculline into the dorsal subcoeruleus area of the rat. Brain Res. 2003;962:68–77. doi: 10.1016/s0006-8993(02)03956-2. [DOI] [PubMed] [Google Scholar]
- 25.Sakai K, Sastre J, Salvert D, Touret M, Tohyama M, Jouvet M. Tegmentoreticular projections with special reference to the muscular atonia during paradoxical sleep in the cat: an HRP study. Brain Res. 1979;176:233–254. doi: 10.1016/0006-8993(79)90981-8. [DOI] [PubMed] [Google Scholar]
- 26.Sanford LD, Tang X, Xiao J, Ross RJ, Morrison AR. GABAergic regulation of REM sleep in reticularis pontis oralis and caudalis in rats. J Neurophysiol. 2003;90:938–945. doi: 10.1152/jn.00993.2002. [DOI] [PubMed] [Google Scholar]
- 27.Shiromani P, Lai Y, Siegel J. Descending projections from the dorsolateral pontine tegmentum to the paramedian reticular nucleus of the caudal medulla in the cat. Brain Res. 1990;517:224–228. doi: 10.1016/0006-8993(90)91030-k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takakusaki K, Shimoda N, Matsuyama K, Mori S. Discharge properties of medullary reticulospinal neurons during postural changes induced by intrapontine injections of carbachol, atropine and serotonin, and their functional linkages to hindlimb motoneurons in cats. Exp Brain Res. 1994;99:361–374. doi: 10.1007/BF00228973. [DOI] [PubMed] [Google Scholar]
- 29.Talley E, Rosin D, Lee A, Guyenet P, Lynch K. Distribution of α2A-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372:111–134. doi: 10.1002/(SICI)1096-9861(19960812)372:1<111::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Tononi G, Pompeiano M, Cirelli C. Suppression of desynchronized sleep through microinjection of the alpha 2-adrenergic agonist clonidine in the dorsal pontine tegmentum of the cat. Pflügers Arch. 1991;418:512–518. doi: 10.1007/BF00497780. [DOI] [PubMed] [Google Scholar]
- 31.Vanni-Mercier G, Sakai K, Lin J, Jouvet M. Carbachol microinjections in the mediodorsal pontine tegmentum are unable to induce paradoxical sleep after caudal pontine and prebulbar transections in the cat. Neurosci Lett. 1991;130:41–45. doi: 10.1016/0304-3940(91)90222-f. [DOI] [PubMed] [Google Scholar]
- 32.Volgin D, Mackiewicz M, Kubin L. α1B receptors are the main postsynaptic mediators of adrenergic excitation in brainstem motoneurons, a single-cell RT-PCR study. J Chem Neuroanat. 2001;22:157–166. doi: 10.1016/s0891-0618(01)00124-7. [DOI] [PubMed] [Google Scholar]
- 33.Volgin D, Fay R, Kubin L. Postnatal development of serotonin 1B, 2 A and 2C receptors in brainstem motoneurons. Eur J Neurosci. 2003;17:1179–1188. doi: 10.1046/j.1460-9568.2003.02545.x. [DOI] [PubMed] [Google Scholar]
- 34.Volgin D, Malinowska M, Swan J, Kubin L. Dorsomedial pontine neurons with axonal projections to the medullary reticular formation express orexin-1 and adrenergic α2A receptor mRNA. Sleep. 2004;27:A15. doi: 10.1016/j.neulet.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson C, Soto-Calderon H, Lydic R, Baghdoyan H. Pontine reticular formation (PnO) administration of hypocretin-1 increases PnO GABA levels and wakefulness. Sleep. 2008;31:453–464. doi: 10.1093/sleep/31.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webster H, Friedman L, Jones B. Modification of paradoxical sleep following transections of the reticular formation at the pontomedullary junction. Sleep. 1986;9:1–23. doi: 10.1093/sleep/9.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Xi M, Fung S, Yamuy J, Morales F, Chase M. Induction of active (REM) sleep and motor inhibition by hypocretin in the nucleus pontis oralis of the cat. J Neurophysiol. 2002;87:2880–2888. doi: 10.1152/jn.2002.87.6.2880. [DOI] [PubMed] [Google Scholar]
- 38.Xi MC, Morales FR, Chase MH. The motor inhibitory system operating during active sleep is tonically suppressed by GABAergic mechanisms during other states. J Neurophysiol. 2001;86:1908–1915. doi: 10.1152/jn.2001.86.4.1908. [DOI] [PubMed] [Google Scholar]


