Abstract
The objectives of our present experiments were to determine whether the BKCa channel agonist NS1619 is able to induce immediate preconditioning in cultured rat cortical neurons and to elucidate the role of BKCa channels in the initiation of immediate preconditioning. NS1619 depolarized mitochondria and increased reactive oxygen species (ROS) generation, but neither of these effects was inhibited by BKCa channel antagonists. NS1619 also activated the extracellular signal-regulated kinase signaling pathways. One-hour treatment with NS1619 induced immediate protection against glutamate excitotoxicity (viability 24 h after glutamate exposure: control, 58.45±0.95%; NS1619 50 μM, 78.99±0.90%*; NS1619 100 μM, 86.89±1.20%*; NS1619 150 μM, 93.23±1.23%*; mean±SEM; *p<0.05 vs. control; n=16–32). Eliminating ROS during the preconditioning phase effectively blocked the development of cytoprotection. In contrast, the BKCa channel blockers iberiotoxin and paxilline, the phosphoinositide 3-kinase inhibitor wortmannin, the protein kinase C blocker chelerythrine, and the mitogen activated protein kinase antagonist PD98059 were unable to antagonize the immediate neuroprotective effect. Finally, preconditioning with NS1619 reduced the calcium load and ROS surge upon glutamate exposure and increased superoxide dismutase activity. Our results indicate that NS1619 is an effective inducer of immediate neuronal preconditioning, but the neuroprotective effect is independent of the activation of BKCa channels.
Keywords: neuroprotection, mitochondria, BKCa channel, reactive oxygen species, glutamate
INTRODUCTION
Preconditioning (PC) is a naturally occurring phenomenon in which a sublethal or nontoxic stimulus renders cells, tissues, and organs tolerant to a subsequent, otherwise lethal insult. This endogenous protective mechanism was first recognized more than 60 years ago by Noble (1943), and later by Dahl and Balfour (1964). The term ‘preconditioning’ was first used for tolerance induction in the same year (Janoff, 1964). Despite these early reports, research in this field evolved only from the second half of the 1980s (Kitagawa et al., 1990; Murry et al., 1986; Schurr et al., 1986). Subsequent studies demonstrated an early or classical phase occurring virtually immediately after the triggering stimulus and lasting 2–3 h, and a late phase or second window of protection appearing 24 h later and lasting 1–3 days (Kuzuya et al., 1993; Marber et al., 1993).
Mitochondrial potassium channels are generally accepted to be major components of PC-induced cytoprotection. Since the first demonstration of ATP-sensitive potassium channels in the inner membrane of mitochondria (Inoue et al., 1991), numerous studies, including our own, have demonstrated neuroprotection using selective channel openers (Busija et al., 2004; Domoki et al., 1999; Gaspar et al., 2008b; Kis et al., 2003; Kis et al., 2004; Mayanagi et al., 2007). Recently, another type of potassium channel, the large conductance calcium activated potassium (BKCa) channel was reported to be present in the mitochondria (mitoBKCa) of a glioma cell line (Siemen et al., 1999), heart muscle (Xu et al., 2002), brain (Douglas et al., 2006), and skeletal muscle cells (Skalska et al., 2008). Studies have also demonstrated that cardiac PC with the BKCa channel opener 1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H- benzimidazol-2-one (NS1619) could be inhibited by BKCa channel antagonists (Cao et al., 2005; Sato et al., 2005; Shintani et al., 2004; Wang et al., 2004; Xu et al., 2002). In contrast to the heart studies, our recent publication provides convincing evidence that delayed neuronal PC with NS1619 is independent of BKCa channels (Gaspar et al., 2008a). In that study we found that NS1619 induced depolarization and reactive oxygen species (ROS) generation in isolated mitochondria and subsequent activation of the phosphoinositide-3-kinase (PI3K) – Akt signal transduction pathway which ultimately led to delayed neuroprotection against various toxic insults. Of the numerous effects, however, only cell membrane hyperpolarization was effectively counteracted by BKCa channel inhibitors. We also looked for the constituents of the BKCa channel in mitochondrial preparations, but were unable to demonstrate the presence of the pore forming a subunit. Nevertheless, no immediate neuronal PC studies are currently available using BKCa channel activators; thus, the function of BKCa channels in immediate neuronal PC is yet to be determined.
The purpose of our present study was to examine whether NS1619 induces immediate PC in rat cortical neuronal cultures. We also investigated the role of BKCa channels and several signaling molecules in the development of immediate cytoprotection. Finally, we tested the effect of immediate PC with NS1619 on ROS generation and cytosolic free calcium levels in neurons.
RESULTS
Preliminary experiments showed that treatment with NS1619 for 30 min, or less, did not induce immediate PC (viability 24 h after exposure to 200 μM glutamate for 1 h: untreated control, 53.75±1.62%; NS1619 150 μM for 15 min, 55.09±2.37%; NS1619 150 μM for 30 min, 58.67±2.27%; percent of untreated control unexposed to glutamate; mean±SEM; n=8 in each group). However, treatment with NS1619 for 60 minutes induced immediate PC.
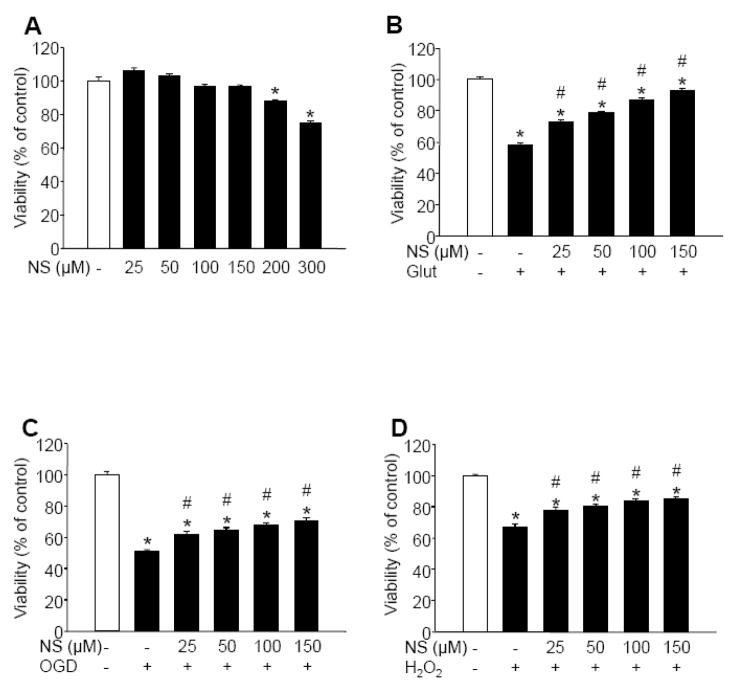
Treatment of quiescent cells with concentrations of NS1619 up to 150 μM for 1 hour had no detectable effect on neuronal survival whereas higher doses resulted in decreased viability (Figure 1A). Therefore, a 1-hour treatment with 25–150 μM NS1619 was used to elicit immediate PC in subsequent experiments. Within this range, the compound induced a dose-dependent protection against glutamate excitotoxicity (Figure 1B) and, to a lesser extent, against combined oxygen and glucose deprivation (OGD) (Figure 1C) and exogenous hydrogen peroxide (Figure 1D). Because the best protection was observed against glutamate, this experimental paradigm was chosen for further experiments investigating the mechanism of immediate PC. To determine the duration of the protection, neuronal cultures were treated with 150 μM NS1619 for 1 h, then the compound was washed out and the cells were incubated in the regular culture medium for 0, 1, or 2 h before exposure to glutamate (200 μM; 60 min). These experiments showed that the window of immediate PC is very narrow and provides protection only for about 2 h (viability 24 h after exposure to glutamate: untreated, 64.09±1.27%; NS1619 150 μM + 0 h incubation before glutamate exposure, 94.66±1.05%*; NS1619 150 μM + 1 h incubation, 73.18±1.66%*; NS1619 150 μM + 2 h incubation, 63.42±1.42%; mean±SEM; *p<0.05 vs. untreated; n=12–24).
Figure 1. Immediate preconditioning with NS1619 protects neuronal cultures against various toxic insults.
Neuronal cultures were treated with increasing doses of NS1619 (25–300 μM) for 60 min then the compound was thoroughly washed out. Panel A shows the direct effect of NS1619 on cell viability 24 h after treatment. To test the protective effect of NS1619, other cultures were exposed to 200 μM glutamate (Glut) for 60 min (Panel B), combined oxygen and glucose deprivation (OGD) for 180 min (Panel C), or 50 μM exogenous hydrogen peroxide (H2O2) in the presence of glucose oxidase (1 U/L) for 60 min (Panel D), immediately after washing out NS1619. In all experiments, viability was measured by assaying lactate dehydrogenase released from living cells 24 hours after the insults and was expressed as a percent of the viability of control cultures which were not treated with NS1619 and were not exposed to any of the toxic insults (white bars). *Significant difference (p<0.05) compared to untreated control neurons which were not exposed to any of the insults. #Significant difference (p<0.05) compared to untreated cultures which were exposed to the corresponding toxic insult. Data are expressed as mean ± SEM; cells from at least two individual cultures (n = 16–32).
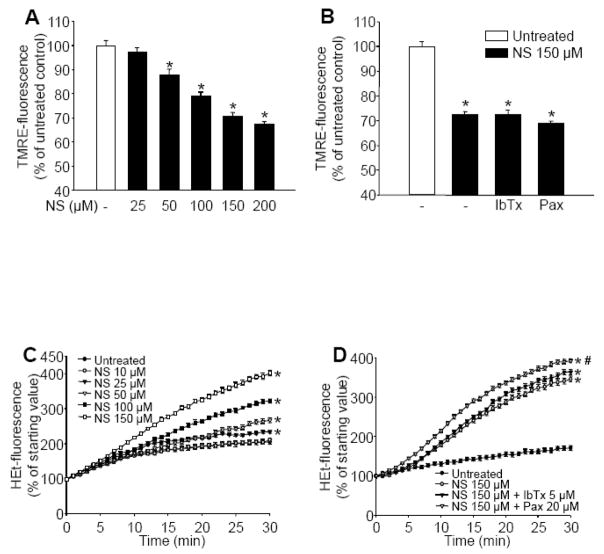
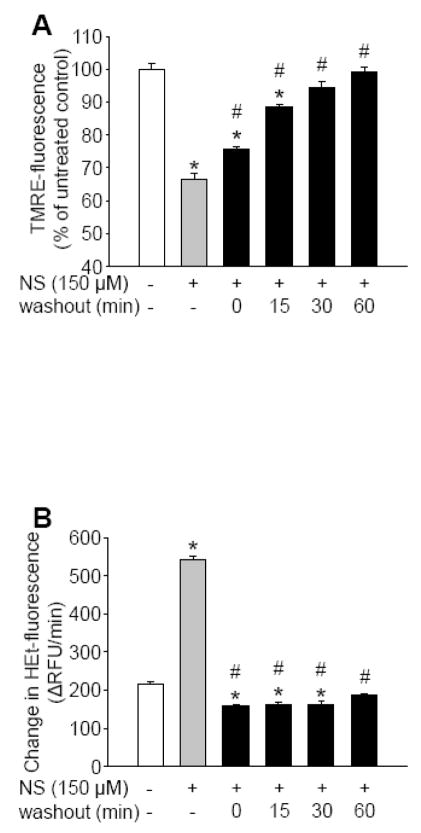
Application of NS1619 resulted in a dose-dependent depolarization of in situ mitochondria in cultured cortical neurons (Figure 2A). The BKCa channel inhibitors, iberiotoxin (IbTx; 5 μM) and paxilline (Pax; 20 μM) could not block this effect (Figure 2B). Control neurons showed a moderate increase in hydroethidine (HEt) fluorescence over time which resulted from the basal ROS formation by the cells (Figure 2C). NS1619 induced a dose-dependent elevation of ROS production (Figure 2C) which, similar to the effect on the mitochondrial membrane potential, was unresponsive to BKCa channel antagonists (Figure 2D). To determine whether ROS generation induced by NS1619 was responsible for mitochondrial depolarization, we measured TMRE fluorescence in the presence of either catalase or the combination of the superoxide dismutase (SOD) mimetic M40401 and catalase and found that the antioxidants did not influence the depolarizing effect of NS1619 (TMRE fluorescence: NS1619 150 μM, 69.51±1.98%; NS1619 150 μM + catalase 100 U/ml, 66.58±1.84%; NS1619 150 μM + catalase 100 U/ml + M40401 50 μM, 68.46±1.90%; percent of untreated control; mean±SEM, n=14 in each group). Removal of NS1619 quickly reversed effects on mitochondrial membrane potential and ROS generation. Washing out the compound resulted in a rapid repolarization, reaching a steady state slightly below baseline within 30 minutes (Figure 3A). Stimulated levels of ROS generation fell to and even a little bit below baseline immediately after washout (Figure 3B).
Figure 2. NS1619 induces mitochondrial depolarization and reactive oxygen species (ROS) generation in intact neurons, independently of large conductance calcium activated potassium channels.
To assess mitochondrial membrane potential, cultured neurons in black-walled 96-well plates were treated with NS1619 (NS; 25–200 μM) and loaded with tetramethylrhodamine ethyl ester (TMRE) for 20 min (Panel A). Another set of neurons was pretreated with iberiotoxin (IbTx; 5 μM) or paxilline (Pax; 20 μM) for 5 min before loading with TMRE and treatment with NS (150 μM) (Panel B). After 20 min, the cells were washed, and TMRE fluorescence was measured using a fluorescent microplate reader. *Significant difference (p<0.05) compared to untreated control cultures (n = 16–24). ROS production was monitored using the fluorescent dye hydroethidine (HEt). Cultured neurons were treated with NS1619 (10–150 μM) and loaded with HEt at the same time, in the same buffer, 1 min before ROS determination (Panel C). HEt-fluorescence was recorded every minute for 30 minutes using a fluorescent microplate reader. NS1619 at 10 μM (○) did not induce any changes in HEt fluorescence; therefore, this curve overlies untreated control (●). *Significant difference (p<0.05) compared to the fluorescent intensity of untreated control neurons (n = 16 in each group). To evaluate the effect of K+ channel inhibitors on NS1619 induced ROS generation, neurons were pre-treated with IbTx (5 μM), or Pax (20 μM) for 5 min and then were treated with NS1619 (150 μM) and loaded with HEt (5μM) 1 min before ROS determination (Panel D). HEt fluorescent intensity was measured every minute for 30 minutes with a fluorescent microplate reader. *Significant difference (p<0.05) compared to the fluorescent intensity of untreated control neurons. #Significant difference (p<0.05) compared to the fluorescent intensity of neurons treated with NS1619 (150 μM). Data are expressed as mean ± SEM (n = 24 in each group).
Figure 3. Washing out NS1619 quickly restores mitochondrial membrane potential and reactive oxygen species (ROS) generation.
Mitochondrial membrane potential was monitored with tetramethylrhodamine ethyl ester (TMRE). TMRE-fluorescence was determined before (open bar; baseline), during (grey bar), and 0, 15, 30, and 60 minutes after (black bars) washout following 1 hour of NS1619 treatment (150 μM) using a fluorescent microplate reader (Panel A). *Significant difference (p<0.05) compared to the fluorescent intensity of untreated control neurons. #Significant difference (p<0.05) compared to the fluorescent intensity of neurons during treatment with 150 μM NS1619 (grey bar). Data are expressed as mean ± SEM (n = 16 in each group). ROS generation before (open bar; baseline), during (grey bar), 0, 15, 30, and 60 minutes after (black bars) washout following 1 hour of NS1619 treatment (150 μM) was determined with the superoxide sensitive fluorescent dye, hydroethidine (HEt) in a fluorescent microplate reader (Panel B). HEt-fluorescence was detected every minute for 30 minutes then changes in fluorescent intensity per minute were calculated. *Significant difference (p<0.05) compared to the fluorescent intensity of untreated control neurons. #Significant difference (p<0.05) compared to the fluorescent intensity of neurons during treatment with 150 μM NS1619 (grey bar). Data are expressed as mean ± SEM (n = 16 in each group).
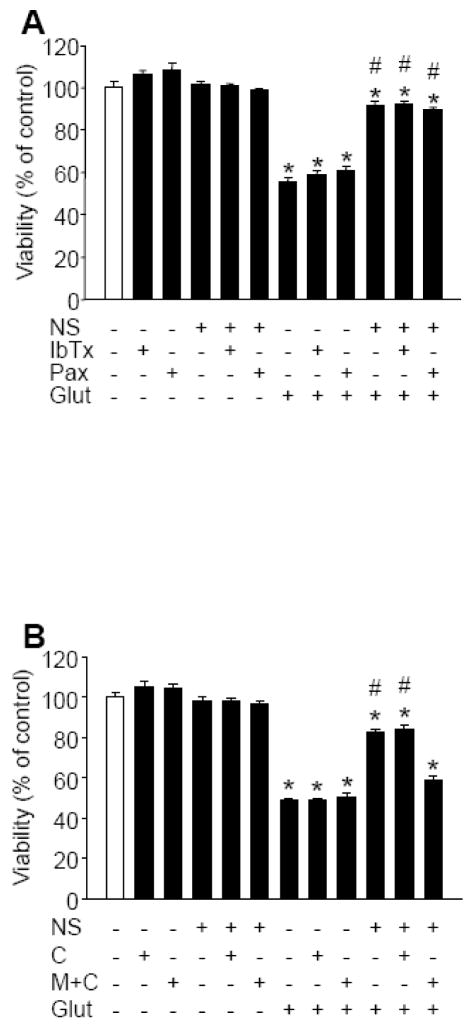
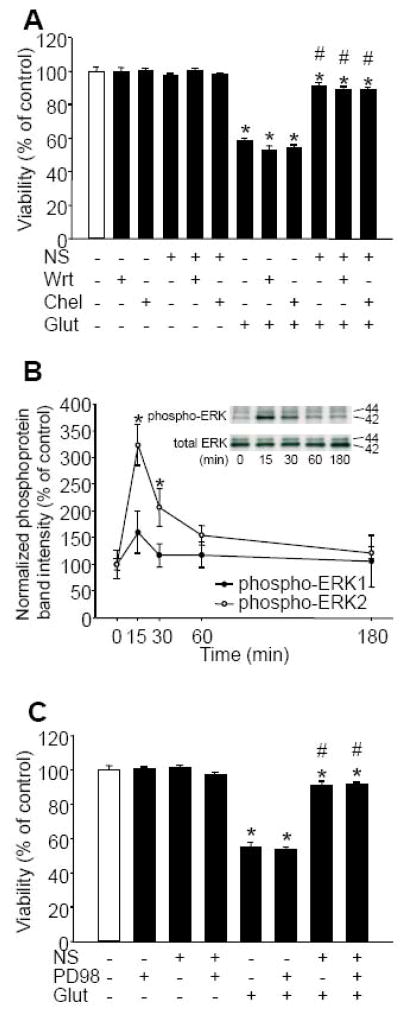
To determine the role of BKCa channels in the induction of immediate PC, neuronal cultures were co-treated with NS1619 (150 μM), and with either IbTx (5 μM) or Pax (20 μM) for 60 min and then were exposed to glutamate. The selected concentrations of BKCa antagonists showed no toxicity. Additionally, neither of them could antagonize the protective effect of NS1619 against glutamate (Figure 4A). We also tested whether ROS contributed to the immediate PC effect of NS1619. The cells were treated with NS1619, with or without the combination of M40401 (50 μM) and catalase (100 U/ml) or catalase (100 U/ml) alone for 60 min, then the compounds were thoroughly washed and the cultures were exposed to glutamate (200 μM, 60 min). The ROS scavengers, alone or with NS1619, did not affect cell viability. However, whereas catalase alone did not inhibit the protection the combination of M40401 and catalase significantly reduced the neuroprotective effect of NS1619 against glutamate (Figure 4B).
Figure 4. Reactive oxygen species (ROS) but not large conductance calcium activated potassium (BKCa) channel activation is required for the immediate preconditioning effect of NS1619.
Cultured cortical neurons were treated with NS1619 (150 μM) with or without the BKCa channel inhibitors, iberiotoxin (IbTx; 5μM) or paxilline (Pax; 20 μM) to block BKCa activity (Panel A). In a second experiment, catalase (100 U/ml) alone (C) or the superoxide dismutase mimetic M40401 (50 μM) and 100 U/ml catalase (M+C) were applied together with NS1619 (150 μM) to eliminate ROS generated during the initiating phase of preconditioning (Panel B). After 60 min the compounds were washed out and the cultures were exposed to 200 μM glutamate for 60 min. Cell viability was measured 24 h later and was expressed as a percent of control cultures which were not treated with any of the compounds and were not exposed to glutamate. *Significant difference (p<0.05) compared to untreated control cultures which were not exposed to glutamate. #Significant difference (p<0.05) compared to untreated cultures which were exposed to glutamate. Data are expressed as mean ± SEM; cells from at least two individual cultures (n = 8–32).
To elucidate the function of the PI3K and protein kinase C (PKC) signaling pathways in immediate PC, we tested whether the PI3K inhibitor wortmannin (Wrt) and the PKC blocker chelerythrine (Chel) would antagonize NS1619-induced tolerance against glutamate exposure. The doses of Wrt and Chel were selected so that they did not significantly affect the viability of control neurons unexposed to glutamate. The presence of antagonists, however, did not change the neuroprotection elicited by NS1619 against glutamate excitotoxicity (Figure 5A). We also tested whether the compound would also affect mitogen-activated protein kinases (MAPK) and found a rapid phosphorylation of extracellular signal-regulated kinase 2 (ERK-2) which reached its maximum within 15 minutes. A similar tendency was observed with ERK-1; however, the changes were not significant (Figure 5B). We then treated neuronal cultures with the MAPK kinase inhibitor PD98059 (PD; 50 μM) along with NS1619 for 1 h before exposure to glutamate. The dose of PD was chosen so that it did not influence the viability of control neurons. In addition, blocking MAPKs did not inhibit immediate PC with NS1619 (Figure 5C).
Figure 5. The activation of the phosphoinositide 3-kinase (PI3K), protein kinase C (PKC), and extracellular signal-regulated kinase (ERK) pathways does not contribute to the immediate preconditioning effect of NS1619.
Cultured cortical neurons were treated with NS1619 (150 μM) and with the PI3K inhibitor wortmannin (Wrt; 300 nM), or the PKC inhibitor chelerythrine (Chel; 5 μM) for 60 min (Panel A). Then the compounds were washed out and the cells were exposed to glutamate (200 μM) for 60 min. Cell viability was measured 1 day later and was expressed as a percent of control cultures which were not treated with any of the compounds and were not exposed to glutamate.*Significant difference (p<0.05) compared to untreated control cultures which were not exposed to glutamate. #Significant difference (p<0.05) compared to untreated cultures which were exposed to glutamate. Data are expressed as mean ± SEM; cells from at least two individual cultures (n = 8–16). Neuronal cultures in 35 mm dishes were treated with NS1619 (100 μM) for 15 min, 30 min, 1 h, and 3 h after which proteins were extracted and subjected to western blot analysis for total and phosphorylated ERKs (Panel B). The levels of phosphorylated forms were normalized to the total amount of the same protein. Inset shows representative blots. *Significant difference (p<0.05) compared to the normalized protein level of untreated control. Data are expressed as mean ± SEM (n = 4 in each group). Cultured cortical neurons were treated with NS1619 (150 μM) with or without the mitogen activated protein kinase inhibitor PD98059 (PD98; 50 μM) for 60 min (Panel C). Subsequently, the cultures were exposed to glutamate (200 μM) for 60 min. Cell viability was measured 24 h later and was expressed as a percent of control cultures which were not treated with any of the compounds and were not exposed to glutamate. *Significant difference (p<0.05) compared to untreated control cultures which were not exposed to glutamate. #Significant difference (p<0.05) compared to untreated cultures which were exposed to glutamate. Data are expressed as mean ± SEM; cells from at least two individual cultures (n = 12–16).
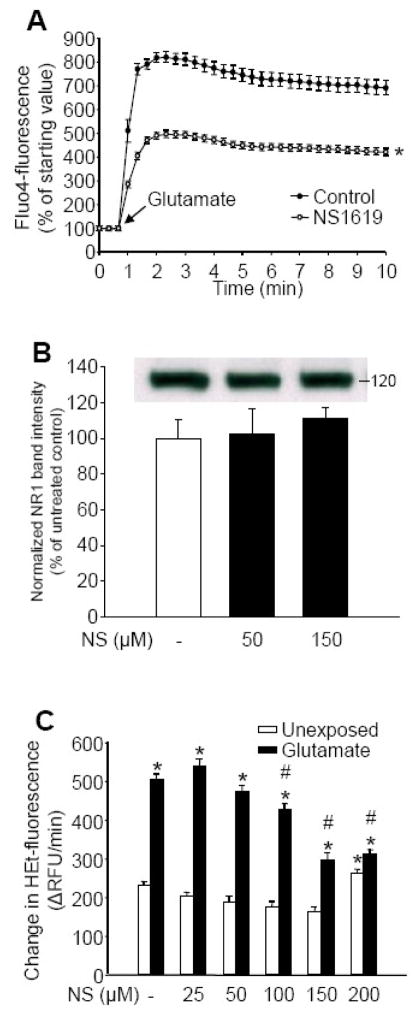
In the last set of experiments we attempted to identify the effectors of immediate PC by NS1619. We first incubated neurons with NS1619 to induce PC then measured the changes of intracellular free calcium ([Ca2+]i) and ROS levels in response to exposure to glutamate using the fluorescent dyes, Fluo-4 and HEt, respectively. The Fluo-4 fluorescent signal of quiescent control and NS1619-treated neurons did not show any difference and remained stable over time. When exposed to glutamate (200 μM), the peak [Ca2+]i value of the cultures pretreated with NS1619 was significantly lower than that of the control cultures, whereas the rate of calcium elimination did not seem to be affected by NS1619 treatment (Figure 6A). Since reduced calcium response to glutamate may be a consequence of NMDA receptor down-regulation, we performed western blot analysis of the NR1 subunit of the NMDA receptor using the membrane fraction isolated from neurons after 60 minutes treatment with NS1619. However, NS1619 did not change the expression of the examined subunit (Figure 6B).
Figure 6. Immediate preconditioning with NS1619 results in reduced Ca2+ load and decreased ROS availability upon exposure to glutamate without altering the expression of the NR1 subunit of the NMDA receptor.
Intracellular free calcium levels in cultured neurons were measured by a confocal microscope using Fluo-4 AM during exposure to 200 μM glutamate (panel A). To induce preconditioning, neuronal cultures were treated with NS1619 (150 μM) for 60 min prior to measurement. Fluorescent intensity of the untreated control at the starting point was regarded as 100%. *Significant difference (p<0.05) compared to untreated control. Data are expressed as mean ± SEM (n = 50–112). Neuronal cultures in 35 mm dishes were treated with NS1619 (50, 150 μM) for 1 hour then protein was extracted and subjected to western blot analysis for the NMDA receptor subunit NR1 (Panel B). Bands were normalized to β-actin and were expressed as percent of untreated control. Inset demonstrates a representative blot. Data are shown as mean ± SEM (n=4 in each group). Cultured neurons in poly-D-lysine coated black-walled 96-well plates were treated with NS1619 (25–200 μM) 1 h before loading with ROS sensitive fluorescent dye hydroethidine (HEt) (Panel C). Neurons were exposed to glutamate (200 μM) then HEt fluorescent intensity was measured for 30 min with a fluorescent microplate reader and was expressed as a change in relative fluorescent units/minute (ΔRFU/min). *Significant difference (p<0.05) compared to the fluorescent intensity of untreated control neurons unexposed to glutamate. #Significant difference (p<0.05) compared to untreated neurons that were exposed to glutamate. Data are expressed as mean ± SEM; cells from at least two individual cultures (n = 16–32).
Challenging quiescent neurons with glutamate (200 μM) resulted in an increase in free radical production detected as an increased rate of conversion of HEt to the fluorescent end product, ethidium (Figure 6C). Immediate PC with NS1619 caused a mild dose-dependent decrease in non-stimulated ROS levels. Additionally, a dose-dependent reduction in glutamate induced ROS generation was observed in NS1619-preconditioned cultures. Incubation with 200 μM NS1619 resulted in a higher basal ROS level and no further decrease in glutamate stimulated ROS generation (Figure 6C). Beside reduced calcium influx upon glutamate exposure, another cause of reduced ROS response might be an increased elimination of radicals by antioxidant systems. Therefore, we examined superoxide dismutase activity and expression. Incubation of neurons with NS1619 for 1 hour resulted in a mild but significant and dose-dependent increase in total SOD activity (untreated control, 100.00±2.07%; NS1619 50 μM, 108.66±5.62%; NS1619 100 μM, 117.49±6.37%; NS1619 150 μM, 124.66±5.24%*; *p<0.05 vs. untreated control; mean±SEM; n=10 in each group), whereas western blot analysis did not detect any increase in protein levels (data not shown).
Cellular ATP content is a sensitive indicator of stress induced by glutamate exposure. Pretreatment with 150μM NS1619 did not influence resting ATP levels, but NS1619-treated neurons contained significantly higher ATP levels after a 60-minute exposure to 200 μM glutamate as compared with control cells (ATP levels in 10−18 M/cell measured immediately after challenge: untreated control unexposed to glutamate, 2893±23; NS1619 150 μM unexposed to glutamate, 2951±21; untreated cells exposed to glutamate, 1706±14*; NS1619 150 μM exposed to glutamate, 2146±25*#; *p<0.05 vs. untreated control unexposed to glutamate; #p<0.05 vs. untreated cells exposed to glutamate; mean±SEM, n=16 in each group).
DISCUSSION
This is the first study demonstrating immediate neuronal PC with NS1619. Here we show that 1) 1 h incubation with NS1619 induces immediate neuronal PC that is protective against various neurotoxic insults; 2) the immediate effects of NS1619 are transient mitochondrial depolarization and increased ROS generation in neurons; 3) elimination of ROS during the PC phase significantly reduces the neuroprotective effect of NS1619; 4) antagonizing BKCa channels, and blocking the PI3K, PKC, and ERK signal transduction pathways do not counteract immediate PC; and finally, 5) consequences of immediate NS1619 PC include decreased Ca2+ influx through glutamate receptors, increased SOD activity, a consequent reduced ROS response during glutamate stimulation, and better preservation of ATP.
The first report on the neurovascular protective effect of a BKCa channel opener was published by our laboratory a decade ago in which we showed that pretreatment with NS1619 preserved cerebrovascular response to NMDA after combined hypoxia and ischemia (Veltkamp et al., 1998). However, in that study we did not identify the exact mechanism of the neuroprotection; nevertheless, our results suggested that the protection was independent of the vasodilator effect of the potassium channel openers. Parallel with the recent reports on BKCa channels in the inner mitochondrial membrane of several cell types (Douglas et al., 2006; Siemen et al., 1999; Skalska et al., 2008; Xu et al., 2002), a surge of studies appeared reporting cardiac PC using BKCa channel activators, most frequently NS1619 (Cao et al., 2005; Sato et al., 2005; Stowe et al., 2006; Wang et al., 2004; Xu et al., 2002). Although claimed to be selective by many authors, NS1619 is well known to have numerous BKCa channel-independent effects (Debska et al., 2003; Edwards et al., 1994; Holland et al., 1996; Kicinska and Szewczyk, 2004; Korper et al., 2003; Park et al., 2007; Yamamura et al., 2001) which might, at least partially, be responsible for the protective effect. Additionally, in our previous study we could not confirm the presence of the a subunit of the BKCa channel whereas we could detect the modulatory β4 subunit in our mitochondrial preparations isolated from the rat cortex using commercially available antibodies (Gaspar et al., 2008a). Since then we have tested other antibodies directed against the pore forming subunit (from Sigma and BD) but were unable to demonstrate its presence in mitochondria (unpublished data). These results are seemingly in contrast with the report of Douglas et al. (2006) who demonstrated the presence of the a subunit of the channel in brain mitochondria. However, the pore forming subunit was only present in cerebellar neurons, including Purkinje cells and granular cells, and only in a small fraction of mitochondria which questions its role as an inducer of neuronal PC and supports our view.
A one-hour treatment with NS1619 induced a dose-dependent immediate protection against several toxic challenges. Shorter treatment periods were insufficient to evoke significant tolerance providing evidence for the time-dependence of the development of immediate PC. Whereas in our previous study the best delayed protection was seen against hydrogen peroxide (Gaspar et al., 2008a), here we found that the immediate PC effect of NS1619 provided the highest level of protection against glutamate excitotoxicity. Previously we reported similar neuroprotection against glutamate after immediate PC using the mitochondrial ATP-sensitive potassium channel opener BMS-191095 (Kis et al., 2004). Our present results also confirm that the protective effect of immediate PC lasts only for a short period.
Compared to heart studies in which 10–30 μM NS1619 was usually used to induce cardiac PC (Sato et al., 2005; Wang et al., 2004; Xu et al., 2002), in our current and previous experiments, optimal neuroprotection by immediate and delayed neuronal PC was achieved using 100–150 μM NS1619. The need for higher concentrations to induce neuronal PC might be explained by the fact that our experiments were performed in protein containing media and buffers which may reduce the availability of the compound. This hypothesis is supported by the fact that at least 100 μM NS1619 was needed to induce significant membrane hyperpolarization in a protein containing transport medium (Gaspar et al., 2008a) as compared with 10–20 μM in a protein-free buffer (Gaspar et al., 2007; Mayanagi et al., 2007). This effect of NS1619 could be antagonized by BKCa channel inhibitors, providing pharmacological evidence for the presence of plasma membrane BKCa channels in our neuronal preparations (Gaspar et al., 2008a). Similar to our recent experiments using NS1619, in previous studies with diazoxide, a higher concentration of the compound was needed to induce PC in neurons (Kis et al., 2003) than that reported in the heart (Garlid et al., 1997).
In our previous study we examined the effects of NS1619 on isolated mitochondria and found that it caused depolarization and ROS generation that were not antagonized by BKCa channel blockers (Gaspar et al., 2008a). However, the isolation procedure may influence the integrity of mitochondria and experiments on isolated mitochondria do not always correspond to findings in intact cells. Therefore, in the present study, we examined the direct effects of NS1619 on mitochondrial function in intact neurons. Verifying our previous results with isolated mitochondria, NS1619 induced mitochondrial depolarization and elevated ROS production in cultured cells. These findings are in partial agreement with those of Stowe et al. (2006) who demonstrated that NS1619-induced cardioprotection could be abolished with a ROS scavenger, confirming increased ROS generation after NS1619 application. These authors also reported that the protection was similarly antagonized by Pax. In our experiments, however, neither mitochondrial depolarization nor enhanced ROS formation could be blocked with BKCa channel antagonists even using the lipophilic Pax as well as the polypeptide IbTx. To determine whether ROS generation is upstream to mitochondrial depolarization, we performed TMRE measurements in the presence of antioxidants. Catalase and M40401, however, did not influence the depolarizing effect of NS1619 suggesting that both mitochondrial ROS generation and depolarization may be the consequences of the inhibitory effect of NS1619 on the respiratory chain (Debska et al., 2003; Kicinska and Szewczyk, 2004). Mitochondria quickly repolarized after washing out NS1619, but reached a slightly lower-than-baseline resting potential after about 30 minutes. Washout also caused a rapid fall in ROS generation, resulting in an about 25% lower than baseline level of free radical production. This reduced rate of ROS generation was dose-dependent (Figure 6C) and returned to control levels 1 hour after washout (Figure 3B). These experiments demonstrated that the mitochondrial effects of NS1619 were only transient and reversible by washout.
To identify the underlying mechanisms of tolerance induction, we applied BKCa channel antagonists along with NS1619 before glutamate exposure. Consistent with our results on mitochondrial depolarization and ROS generation, we found that the BKCa blockers were ineffective in terms of inhibition of protection. Consequently, our results provide strong evidence that BKCa channel activation (mitochondrial and/or plasmalemmal) does not induce tolerance in neurons and does not play any role in the immediate neuronal PC effect of NS1619. Since PI3K activation was shown to be crucial in delayed PC by NS1619 (Gaspar et al., 2008a), we also tested whether the pro-survival signal transduction pathways known to be activated by the compound play any particular role in the immediate neuroprotective effect. Thus, we co-applied NS1619 with the PI3K blocker Wrt, the PKC inhibitor Chel, and the MAPK kinase inhibitor PD. However, none of these compounds blocked immediate PC, suggesting that the activation of these pathways is not essential in immediate tolerance induction.
Several studies, including those from our laboratory, have provided evidence for the role of ROS in the initiation of PC (Gaspar et al., 2007; Kis et al., 2003). ROS can act as second messengers and activate ROS-sensitive kinases (Otani, 2004). We and others have shown that NS1619 induces mitochondrial ROS generation that is required for the initiation of PC (Gaspar et al., 2008a; Heinen et al., 2007; Stowe et al., 2006). In the present study, we were able to block the immediate protective effect of NS1619 using ROS scavengers during the initiation of PC. The fact that catalase alone did not but the combination of a SOD mimetic and catalase did antagonize the protection suggests a major role for superoxide in the initiation of immediate PC. In contrast, Kulawiak et al. (2008) recently demonstrated that the direct effect of BKCa channel activators in rat brain mitochondria is a potassium-dependent decrease in ROS formation. However, the dose of NS1619 applied in that study (3 μM) was well below that used in PC studies. Thus, the relevance of putative mitochondrial BKCa channels in the initiation of PC was not clarified. Finally, NS1619 is known for its ability to inhibit the mitochondrial electron transport chain at Complex I (Debska et al., 2003; Kicinska and Szewczyk, 2004). The latter effect explains the finding of increased ROS production and the subsequent chain of events leading to cytoprotection.
To identify the final effectors of NS1619-induced immediate PC, we examined the Ca2+ and ROS response upon glutamate stimulation. Glutamate exposure results in massive calcium influx in cortical neurons with a consequent mitochondrial calcium accumulation, increased consumption and decreased generation of ATP, and increased production of ROS (Nicholls, 2004). We found that incubation with NS1619 significantly reduced Ca2+ influx as compared with untreated controls whereas the elimination phase of the curves seemed to be unaffected. Because NS1619 was thoroughly washed out in all experiments, a direct effect of the compound on glutamate receptors is not likely. Additionally, to our knowledge, such an effect has not been described. Earlier we demonstrated that glutamate-induced neuronal cell death is almost exclusively dependent on NMDA receptor activation (Gaspar et al., 2008a). Therefore, we tested whether NS1619-treatment would down-regulate NMDA receptors but found that the compound did not affect the expression of the NR1 subunit. NMDA receptors are known to be sensitive to the oxidizing effect of free radicals (Aizenman et al., 1990). Thus, ROS produced in response to NS1619 may represent the link between the direct effect of the compound on mitochondria (i.e. inhibition of the electron transport chain) and the subsequent indirect effect on glutamate receptors. The same authors reported similar alterations in NMDA receptor properties after chemical preconditioning with KCN (Aizenman et al., 2000). This hypothesis of oxidative modulation is supported by the evidence that the elimination of ROS during NS1619 treatment abolished the protection. It has to be noted that, while PI3K, PKC, and ERKs are not part of the cascade, the involvement of a supposedly ROS-sensitive kinase other than the ones tested in this study in the signaling cannot be excluded.
Decreased ROS availability in response to potentially lethal stimuli is a major component of PC induced cytoprotection. Similar to our previous results using different compounds and PC protocols (Gaspar et al., 2007; Kis et al., 2003; Kis et al., 2004), our present study showed that immediate PC with NS1619 resulted in a dose-dependent decrease in ROS levels upon exposure to glutamate. In neurons, the ROS response upon NMDA receptor stimulation is dependent on Ca2+ influx (Kahlert et al., 2005) which provides a direct link between reduced Ca2+ influx and the decreased ROS availability observed in the present study and explains the reduced vulnerability of our neuronal cultures to glutamate after immediate PC by NS1619. In addition to decreased production, we also found that immediate PC by NS1619 mildly increased SOD activity in the neurons. Others have also shown a superoxide-induced rapid up-regulation of SOD in acute cardiac PC by the anesthetic isoflurane (Chen et al., 2008).
During NMDA receptor activation, mitochondria accumulate calcium and, consequently, use more ATP to expel protons. Excessive glutamate receptor stimulation ultimately causes mitochondrial calcium overload resulting in the rupture of the mitochondrial inner membrane and a failure to generate ATP (for a review see Nicholls, 2004). Therefore, a reduced calcium response in NS1619-treated neurons is expected to result in better maintenance of mitochondrial integrity and function. Indeed, we found that whereas short-term treatment with the compound did not influence cellular ATP content, NS1619-treated neurons could better preserve ATP during a glutamate challenge. Besides being the consequence of reduced calcium response to glutamate exposure, higher ATP levels may also allow a quicker restoration of normal cellular function after glutamate excitotoxicity.
In summary, our present study is the first demonstrating immediate neuronal PC using NS1619. Similar to our previous study reporting delayed PC, the immediate PC effect is initiated by ROS generation and is independent of BKCa channel activation. While delayed PC by NS1619 required PI3K activation with a subsequent inhibition of apoptosis and protection against hydrogen peroxide toxicity, immediate PC perhaps results from direct or indirect modulation of NMDA receptors by ROS and up-regulation of SOD activity followed by decreased Ca2+ influx and a reduction in oxidative stress upon glutamate exposure.
MATERIALS AND METHODS
Materials
Cell culture plastics were purchased from Becton-Dickinson (San Jose, CA, USA). Dulbecco’s modified Eagle medium (DMEM), Neurobasal medium, B27 Supplement, 2-mercaptoethanol, and horse serum were obtained from Gibco BRL (Grand Island, NY, USA). Percoll was purchased from Amersham Biosciences (Uppsala, Sweden), dispase I from Roche (Mannheim, Germany), and M40401 from Metaphore Pharmaceuticals (St. Louis, MO, USA). NS1619, Pax, Wrt, and PD were purchased from Sigma (St. Louis, MO, USA), and IbTx from California Peptide Research (Napa, CA, USA). CellTiter 96 AQueous One Solution Assay and CellTiter-Glo Luminescent Assay were procured from Promega (Madison, WI, USA), and the SOD Assay Kit from Fluka (Buchs, Switzerland). HEt, Fluo-4 AM, Pluronic F-127, and tetramethylrhodamine ethyl ester (TMRE) were obtained from Molecular Probes (Eugene, OR, USA). The Bio-Rad DC Protein Assay was procured from Bio-Rad (Hercules, CA, USA), and the “Cytotoxicity Detection Kit (LDH)” from Roche Diagnostics (Indianapolis, IN, USA). All other chemicals were from Sigma. Antibodies were obtained from the following sources: anti-glial fibrillary acidic protein antibody from Chemicon (Temecula, CA, USA), anti-microtubule associated protein-2 and anti-NMDA-receptor subunit 1 (NR1) antibodies from Becton-Dickinson, anti-MAPK antibody from Sigma, anti-active MAPK from Promega, and anti-rabbit IgG and anti-mouse IgG from Jackson Immuno-Research (West Grove, PA, USA).
Primary rat cortical neuronal culture
Timed pregnant Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN, USA) and were maintained and used in compliance with the principles set forth by the Animal Care and Use Committee of Wake Forest University Health Sciences. Primary rat cortical neurons were isolated from E18 Sprague-Dawley fetuses, as described elsewhere (Gaspar et al., 2006). Briefly, the cells were plated at a density of 2×105 cells/cm2 onto poly-D-lysine coated glass coverslips for confocal microscopic analysis and 106 cells/cm2 were placed onto poly-D-lysine coated plates or dishes for the other experiments in plating medium consisting of 60% DMEM, 20% Ham’s F-12 Nutrient Mixture, 20% horse serum, and L-glutamine (0.5 mM). After cell attachment, the plating medium was replaced with regular cell culture medium [feeding medium (FM)] consisting of Neurobasal medium supplemented with B27 (2%), L-glutamine (0.5 mM), 2-mercaptoethanol (55 μM), and KCl (25 mM). Positive immunostaining for microtubule-associated protein-2 and negative immunostaining for glial fibrillary acidic protein verified that the cultures were composed of more than 98% of neurons on day 7 in vitro. Experiments were carried out on 7–9-day old cultures, during which period neurons expressed NMDA, αamino-3-hydroxy-5-methylisoxazole-4-propionate, and kainate receptors and were vulnerable to glucose deprivation (Mattson et al., 1991; Mattson et al., 1993). For medium changes, fresh FM was used in all experiments.
Treatment with NS1619
To induce immediate PC, 9-day old neuronal cultures were treated with increasing doses of NS1619 (10, 25, 50, 100, 150, and 200 μM) for 60 min in FM. In other experiments, the cells were treated for 60 min with NS1619 (150 μM) and with the BKCa channel antagonists IbTx (5 μM) or Pax (20 μM), the PI3K inhibitor Wrt (300 nM), the MAPK inhibitor PD (50 μM), the PKC blocker Chel (5 μM), or with catalase (100 U/ml) and M40401 (50 μM). After each treatment, the plates were washed twice with phosphate buffered saline (PBS) and then the cells were kept in FM.
Combined oxygen and glucose deprivation
Neurons in 96-well plates were exposed to OGD for 180 min at 37 °C using a protocol described previously (Goldberg and Choi, 1993; Kis et al., 2003). Briefly, after the treatment with NS1619, the cells were rinsed, the medium was replaced with glucose-free Earle’s balanced salt solution (EBSS) and the cultures were placed in a ShelLab Bactron Anaerobic Chamber (Sheldon Manufacturing Inc., Cornelius, OR, USA) filled with a humidified anaerobic gas mixture (5% CO2, 5% H2 and 90% N2) at 37 °C. Oxygen level was kept below 0.1% in the chamber. Control cell cultures were incubated in glucose-containing (5.5 mM) EBSS in a regular 5% CO2 cell culture incubator for 180 min. OGD was terminated by reoxygenation and replacing the glucose-free EBSS with FM. Thereafter the cultures were maintained in the regular 5% CO2 incubator within normoxic conditions.
Glutamate excitotoxicity
After 60-min treatment with NS1619, cell cultures in 96-well plates were washed and then exposed to glutamate (200 μM) for 60 min in FM at 37°C in the 5% CO2 incubator. Afterward the cells were rinsed and returned to the 5% CO2 incubator in FM.
Exogenous hydrogen peroxide toxicity
Neuronal cultures in 96-well plates were rinsed and the cells were incubated in Neurobasal medium containing H2O2 (50 μM) and glucose oxidase (1 U/L) at 37°C in the 5% CO2 incubator for 1 h. The continuous generation of H2O2 by glucose oxidase was shown to compensate for the consumption of the initial dose of H2O2 by neurons (Antunes and Cadenas, 2001; Vauzour et al., 2007). After the challenge, the cultures were washed, FM was restored, and the cells were returned to the 5% CO2 incubator.
Quantification of neuronal survival
The viability of neuronal cultures was determined 24 h after the neurotoxic insults. The plates were washed twice with PBS which was then replaced with Neurobasal medium containing 1% TritonX to release lactate dehydrogenase (LDH) from living cells. LDH levels were then determined using a commercially available LDH detection kit according to the manufacturer’s instructions. Absorbance at 492 nm was measured with a microplate reader (FLUOstar OPTIMA, BMG Labtech GmbH, Offenburg, Germany). Comparisons were always made in the same manner, between sister cultures exposed to the neurotoxic stimulus on the same day, and cell viability was expressed as a percentage of the corresponding control culture (untreated, and not exposed to the lethal insult) as follows: % viability = (absorbanceTREATED − absorbanceBACKGROUND) × 100/(absorbanceCONTROL − absorbanceBACKGROUND).
Fluorescent measurements
The changes of mitochondrial membrane potential were determined using TMRE. Neurons in FM were loaded with TMRE (0.5 μM) and treated with NS1619 (25 – 200 μM). A second set of neurons was pretreated with IbTx (5 μM) or Pax (20 μM) for 5 min before loading and NS1619 application (150 μM). After 20 min of incubation in the dark at 37°C that allowed the redistribution of the dye in proportion to the membrane potential of mitochondria, the drugs and the dye were washed out and TMRE-fluorescence was measured in each well using the same microplate reader that was used for viability measurements (λex = 510 nm, λem = 590 nm). Measurements were performed in PBS containing 1 mg/ml glucose at 37 °C. Data were expressed as a percentage of the intensity of the untreated control culture.
ROS generation was assessed in black-walled 96-well plates with HEt using the same microplate reader used in the TMRE measurements. Neurons in PBS containing 1 mg/ml glucose were loaded with HEt (5 μM) and were treated with NS1619 (10 – 150 μM) at the same time, in the same buffer. In another experiment, neurons were pretreated with either IbTx (5 μM) or Pax (20 μM) for 5 min then were loaded with HEt (5 μM) and treated with NS1619 (150 μM). ROS determination was started 1 min after NS1619 application. HEt-fluorescence (λex = 510 nm, λem = 590 nm) in each well was measured at 37 °C every minute for 30 min. Data were expressed as percent of the starting intensity of the untreated control or as a change in relative fluorescent intensity/minute (ΔRFU/min).
Changes of intracellular free calcium ([Ca2+]i) were monitored using the Ca-indicator dye Fluo-4 AM in glucose-containing PBS (1 mg/ml). Neuronal cultures were loaded with 2 μM Fluo-4 AM and 1 μM Pluronic F-127 in PBS in the dark for 60 min at room temperature (22 °C). In a second group, cells were treated with NS1619 (150 μM) or vehicle during loading with Fluo-4 AM. After loading, neurons were rinsed three times with PBS and confocal images of cellular Fluo-4 AM fluorescence (λex = 488 nm, λem = 520 nm) were acquired using a laser scanning microscope (LSM 510; Zeiss, Jena, Germany) with a 63× water immersion objective (Zeiss). Glutamate (200 μM) was added to the medium after 1 minute. Fluorescent images of randomly selected fields were recorded every 20 s for 10 min and the average pixel intensity of individual cell bodies was determined using the software supplied by the manufacturer (LSM 510, Zeiss). Data were expressed as a percentage of the starting intensity of the untreated control culture.
Western blotting for extracellular signal-regulated kinases and for the NR1 subunit of the NMDA receptor complex
Cultured cells were washed twice in ice-cold PBS and were harvested by scraping in ice-cold Nonidet P40 lysis buffer supplemented with a protease inhibitor cocktail and a phosphatase inhibitor cocktail (both from Sigma). Equal amounts of protein for each sample were separated by 4–20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto a polyvinylidine difluoride sheet (Polyscreen PVDF; Perkin Elmer Life Sciences, Boston, MA, USA). Membranes were incubated in a blocking buffer (Tris-buffered saline, 0.05% Tween 20, and 1% bovine serum albumin) for 1 h at room temperature after which the blots were incubated with polyclonal anti-MAPK (1:10 000), polyclonal anti-active MAPK (1:5000), and monoclonal anti-NR1 (1:1000) antibodies overnight at 4 °C. The membranes were then washed three times in Tris-buffered saline with 0.05% Tween 20 and incubated for 1 h in the blocking buffer with anti-rabbit IgG (1:100 000) or anti-mouse IgG (1:100 000) conjugated to horseradish peroxidase. The final reaction products were visualized using enhanced chemiluminescence (SuperSignal West Pico; Pierce, Rockford, IL, USA) and recorded on X-ray film. Films were digitalized using a FOTO/Analyst® Investigator-PC Electronic Documentation System with the provided Image v5.00 software (FOTODYNE Inc., Hartland, WI, USA). Densitometry of digitalized blots was performed with the ImageJ 1.30v software (http://rsb.info.nih.gov/ij; National Institutes of Health, USA).
SOD activity
SOD activity was measured using the tetrazolium-based SOD Assay Kit as directed by the manufacturer. Briefly, neurons on 96-well plates were incubated with different doses of NS1619 (50, 100, and 150 μM) for 1 hour. Then the plates were rinsed with PBS, and enzyme activity was measured with the same microplate reader that was used for other measurements (λabs = 460 nm). An SOD activity standard curve was generated and then data were expressed as a percentage of the activity of the nontreated control culture.
ATP Assay
The ATP level of neurons was measured with the glowtype CellTiter-Glo Luminescent Assay, as directed by the manufacturer. Cortical neurons cultured in opaque-walled 96-well plates were equilibrated to room temperature (21 °C) for 30 mins. CellTiter-Glo was added to each well and the plates were incubated at room temperature for 10 mins to stabilize the luminescent signal, which was then measured with the same microplate reader that was used for other measurements. An ATP standard curve was generated for each measurement to calculate the ATP contents of cells.
Statistical analysis
Statistical analyses were performed with SigmaStat (SPSS, Chicago, IL, USA). Data are presented as means ± SEM. Differences between groups were assessed by one way ANOVA or two way repeated measures ANOVA, where appropriate, followed by Tukey comparison tests. A value of p<0.05 was considered to be statistically significant.
Acknowledgments
The authors gratefully thank Nancy Busija, M.A., for editing the manuscript. This work was supported by the National Institutes of Health Grants (HL-030260, HL-065380, and HL-077731), and by the Hungarian Science Research Fund (OTKA K63401, K68976, and IN69967).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenman E, Hartnett KA, Reynolds IJ. Oxygen free radicals regulate NMDA receptor function via a redox modulatory site. Neuron. 1990;5:841–6. doi: 10.1016/0896-6273(90)90343-e. [DOI] [PubMed] [Google Scholar]
- Aizenman E, Sinor JD, Brimecombe JC, Herin GA. Alterations of N-methyl-D-aspartate receptor properties after chemical ischemia. J Pharmacol Exp Ther. 2000;295:572–7. [PubMed] [Google Scholar]
- Antunes F, Cadenas E. Cellular titration of apoptosis with steady state concentrations of H(2)O(2): submicromolar levels of H(2)O(2) induce apoptosis through Fenton chemistry independent of the cellular thiol state. Free Radic Biol Med. 2001;30:1008–18. doi: 10.1016/s0891-5849(01)00493-2. [DOI] [PubMed] [Google Scholar]
- Busija DW, Lacza Z, Rajapakse N, Shimizu K, Kis B, Bari F, Domoki F, Horiguchi T. Targeting mitochondrial ATP-sensitive potassium channels--a novel approach to neuroprotection. Brain Res Brain Res Rev. 2004;46:282–94. doi: 10.1016/j.brainresrev.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Cao CM, Xia Q, Gao Q, Chen M, Wong TM. Calcium-activated potassium channel triggers cardioprotection of ischemic preconditioning. J Pharmacol Exp Ther. 2005;312:644–50. doi: 10.1124/jpet.104.074476. [DOI] [PubMed] [Google Scholar]
- Chen CH, Liu K, Chan JY. Anesthetic preconditioning confers acute cardioprotection via up-regulation of manganese superoxide dismutase and preservation of mitochondrial respiratory enzyme activity. Shock. 2008;29:300–8. doi: 10.1097/SHK.0b013e3181454295. [DOI] [PubMed] [Google Scholar]
- Dahl NA, Balfour WM. Prolonged Anoxic Survival Due to Anoxia Pre-Exposure: Brain ATP, Lactate, and Pyruvate. Am J Physiol. 1964;207:452–6. doi: 10.1152/ajplegacy.1964.207.2.452. [DOI] [PubMed] [Google Scholar]
- Debska G, Kicinska A, Dobrucki J, Dworakowska B, Nurowska E, Skalska J, Dolowy K, Szewczyk A. Large-conductance K+ channel openers NS1619 and NS004 as inhibitors of mitochondrial function in glioma cells. Biochem Pharmacol. 2003;65:1827–34. doi: 10.1016/s0006-2952(03)00180-1. [DOI] [PubMed] [Google Scholar]
- Domoki F, Perciaccante JV, Veltkamp R, Bari F, Busija DW. Mitochondrial potassium channel opener diazoxide preserves neuronal-vascular function after cerebral ischemia in newborn pigs. Stroke. 1999;30:2713 –8. doi: 10.1161/01.str.30.12.2713. discussion 2718–9. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Lai JC, Bian S, Cummins L, Moczydlowski E, Haddad GG. The calcium-sensitive large-conductance potassium channel (BK/MAXI K) is present in the inner mitochondrial membrane of rat brain. Neuroscience. 2006;139:1249–61. doi: 10.1016/j.neuroscience.2006.01.061. [DOI] [PubMed] [Google Scholar]
- Edwards G, Niederste-Hollenberg A, Schneider J, Noack T, Weston AH. Ion channel modulation by NS 1619, the putative BKCa channel opener, in vascular smooth muscle. Br J Pharmacol. 1994;113:1538–47. doi: 10.1111/j.1476-5381.1994.tb17171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D’Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–82. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- Gaspar T, Kis B, Snipes JA, Lenzser G, Mayanagi K, Bari F, Busija DW. Transient glucose and amino acid deprivation induces delayed preconditioning in cultured rat cortical neurons. J Neurochem. 2006;98:555–65. doi: 10.1111/j.1471-4159.2006.03899.x. [DOI] [PubMed] [Google Scholar]
- Gaspar T, Kis B, Snipes JA, Lenzser G, Mayanagi K, Bari F, Busija DW. Neuronal preconditioning with the antianginal drug, bepridil. J Neurochem. 2007;102:595–608. doi: 10.1111/j.1471-4159.2007.04501.x. [DOI] [PubMed] [Google Scholar]
- Gaspar T, Katakam P, Snipes JA, Kis B, Domoki F, Bari F, Busija DW. Delayed neuronal preconditioning by NS1619 is independent of calcium activated potassium channels. J Neurochem. 2008a;105:1115–28. doi: 10.1111/j.1471-4159.2007.05210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar T, Snipes JA, Busija AR, Kis B, Domoki F, Bari F, Busija DW. ROS-independent preconditioning in neurons via activation of mitoK(ATP) channels by BMS-191095. J Cereb Blood Flow Metab. 2008b;28:1090–103. doi: 10.1038/sj.jcbfm.9600611. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci. 1993;13:3510–24. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen A, Camara AK, Aldakkak M, Rhodes SS, Riess ML, Stowe DF. Mitochondrial Ca2+-induced K+ influx increases respiration and enhances ROS production while maintaining membrane potential. Am J Physiol Cell Physiol. 2007;292:C148–56. doi: 10.1152/ajpcell.00215.2006. [DOI] [PubMed] [Google Scholar]
- Holland M, Langton PD, Standen NB, Boyle JP. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br J Pharmacol. 1996;117:119–29. doi: 10.1111/j.1476-5381.1996.tb15163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–7. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- Janoff A. Alterations in Lysosomes (Intracellular Enzymes) During Shock; Effects of Preconditioning (Tolerance) and Protective Drugs. Int Anesthesiol Clin. 1964;2:251–69. doi: 10.1097/00004311-196402000-00008. [DOI] [PubMed] [Google Scholar]
- Kahlert S, Zundorf G, Reiser G. Glutamate-mediated influx of extracellular Ca2+ is coupled with reactive oxygen species generation in cultured hippocampal neurons but not in astrocytes. J Neurosci Res. 2005;79:262–71. doi: 10.1002/jnr.20322. [DOI] [PubMed] [Google Scholar]
- Kicinska A, Szewczyk A. Large-Conductance Potassium Cation Channel Opener NS1619 Inhibits Cardiac Mitochondria Respiratory Chain. Toxicol Mech Methods. 2004;14:59–61. doi: 10.1080/15376520490257482. [DOI] [PubMed] [Google Scholar]
- Kis B, Rajapakse NC, Snipes JA, Nagy K, Horiguchi T, Busija DW. Diazoxide induces delayed pre-conditioning in cultured rat cortical neurons. J Neurochem. 2003;87:969–80. doi: 10.1046/j.1471-4159.2003.02072.x. [DOI] [PubMed] [Google Scholar]
- Kis B, Nagy K, Snipes JA, Rajapakse NC, Horiguchi T, Grover GJ, Busija DW. The mitochondrial K(ATP) channel opener BMS-191095 induces neuronal preconditioning. Neuroreport. 2004;15:345–9. doi: 10.1097/00001756-200402090-00027. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K, Kamada T. ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res. 1990;528:21–4. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- Korper S, Nolte F, Rojewski MT, Thiel E, Schrezenmeier H. The K+ channel openers diazoxide and NS1619 induce depolarization of mitochondria and have differential effects on cell Ca2+ in CD34+ cell line KG-1a. Exp Hematol. 2003;31:815–23. doi: 10.1016/s0301-472x(03)00199-1. [DOI] [PubMed] [Google Scholar]
- Kulawiak B, Kudin AP, Szewczyk A, Kunz WS. BK channel openers inhibit ROS production of isolated rat brain mitochondria. Exp Neurol. 2008;212:543–7. doi: 10.1016/j.expneurol.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, Hori M, Kamada T, Tada M. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993;72:1293–9. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–72. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Wang H, Michaelis EK. Developmental expression, compartmentalization, and possible role in excitotoxicity of a putative NMDA receptor protein in cultured hippocampal neurons. Brain Res. 1991;565:94–108. doi: 10.1016/0006-8993(91)91740-r. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Zhang Y, Bose S. Growth factors prevent mitochondrial dysfunction, loss of calcium homeostasis, and cell injury, but not ATP depletion in hippocampal neurons deprived of glucose. Exp Neurol. 1993;121:1–13. doi: 10.1006/exnr.1993.1066. [DOI] [PubMed] [Google Scholar]
- Mayanagi K, Gaspar T, Katakam PV, Kis B, Busija DW. The mitochondrial K(ATP) channel opener BMS-191095 reduces neuronal damage after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2007;27:348–55. doi: 10.1038/sj.jcbfm.9600345. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr Mol Med. 2004;4:149–77. doi: 10.2174/1566524043479239. [DOI] [PubMed] [Google Scholar]
- Noble RL. The development of resistance by rats and guinea pigs to amounts of trauma usually fatal. Am J Physiol. 1943;138:346–351. [Google Scholar]
- Otani H. Reactive oxygen species as mediators of signal transduction in ischemic preconditioning. Antioxid Redox Signal. 2004;6:449–69. doi: 10.1089/152308604322899521. [DOI] [PubMed] [Google Scholar]
- Park WS, Kang SH, Son YK, Kim N, Ko JH, Kim HK, Ko EA, Kim CD, Han J. The mitochondrial Ca2+-activated K+ channel activator, NS 1619 inhibits L-type Ca2+ channels in rat ventricular myocytes. Biochem Biophys Res Commun. 2007;362:31–6. doi: 10.1016/j.bbrc.2007.07.057. [DOI] [PubMed] [Google Scholar]
- Sato T, Saito T, Saegusa N, Nakaya H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation. 2005;111:198–203. doi: 10.1161/01.CIR.0000151099.15706.B1. [DOI] [PubMed] [Google Scholar]
- Schurr A, Reid KH, Tseng MT, West C, Rigor BM. Adaptation of adult brain tissue to anoxia and hypoxia in vitro. Brain Res. 1986;374:244–8. doi: 10.1016/0006-8993(86)90418-x. [DOI] [PubMed] [Google Scholar]
- Shintani Y, Node K, Asanuma H, Sanada S, Takashima S, Asano Y, Liao Y, Fujita M, Hirata A, Shinozaki Y, Fukushima T, Nagamachi Y, Okuda H, Kim J, Tomoike H, Hori M, Kitakaze M. Opening of Ca2+-activated K+ channels is involved in ischemic preconditioning in canine hearts. J Mol Cell Cardiol. 2004;37:1213–8. doi: 10.1016/j.yjmcc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Siemen D, Loupatatzis C, Borecky J, Gulbins E, Lang F. Ca2+-activated K channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line. Biochem Biophys Res Commun. 1999;257:549–54. doi: 10.1006/bbrc.1999.0496. [DOI] [PubMed] [Google Scholar]
- Skalska J, Piwonska M, Wyroba E, Surmacz L, Wieczorek R, Koszela-Piotrowska I, Zielinska J, Bednarczyk P, Dolowy K, Wilczynski GM, Szewczyk A, Kunz WS. A novel potassium channel in skeletal muscle mitochondria. Biochim Biophys Acta. 2008;1777:651–9. doi: 10.1016/j.bbabio.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Stowe DF, Aldakkak M, Camara AK, Riess ML, Heinen A, Varadarajan SG, Jiang MT. Cardiac mitochondrial preconditioning by Big Ca2+-sensitive K+ channel opening requires superoxide radical generation. Am J Physiol Heart Circ Physiol. 2006;290:H434–40. doi: 10.1152/ajpheart.00763.2005. [DOI] [PubMed] [Google Scholar]
- Vauzour D, Vafeiadou K, Rice-Evans C, Williams RJ, Spencer JP. Activation of pro-survival Akt and ERK1/2 signalling pathways underlie the anti-apoptotic effects of flavanones in cortical neurons. J Neurochem. 2007;103:1355–67. doi: 10.1111/j.1471-4159.2007.04841.x. [DOI] [PubMed] [Google Scholar]
- Veltkamp R, Domoki F, Bari F, Busija DW. Potassium channel activators protect the N-methyl-D-aspartate-induced cerebral vascular dilation after combined hypoxia and ischemia in piglets. Stroke. 1998;29:837–42. doi: 10.1161/01.str.29.4.837. discussion 842–3. [DOI] [PubMed] [Google Scholar]
- Wang X, Yin C, Xi L, Kukreja RC. Opening of Ca2+-activated K+ channels triggers early and delayed preconditioning against I/R injury independent of NOS in mice. Am J Physiol Heart Circ Physiol. 2004;287:H2070–7. doi: 10.1152/ajpheart.00431.2004. [DOI] [PubMed] [Google Scholar]
- Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O’Rourke B. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–33. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- Yamamura H, Ohi Y, Muraki K, Watanabe M, Imaizumi Y. BK channel activation by NS-1619 is partially mediated by intracellular Ca2+ release in smooth muscle cells of porcine coronary artery. Br J Pharmacol. 2001;132:828–34. doi: 10.1038/sj.bjp.0703885. [DOI] [PMC free article] [PubMed] [Google Scholar]