Abstract
Background
Liver transplantation is the best treatment option for endstage liver disease. The Human T-Cell Lymphotrophic Virus (HTLV) has been associated with leukemia/lymphoma and progressive neurological disease. There has, however, been an increased utilization of HTLV (+) grafts with little data available to support or discourage their use.
Methods
We performed uni- and multivariate analyses related to graft and patient survival for recipients of HTLV (+) donors and compared them to recipients of HTLV (−) donors utilizing the UNOS database. Complete analysis of recipient and donor clinical and demographic factors was performed.
Results
There were 81 adult recipients of HTLV (+) donors and 29,747 HTLV (−) donor recipients. HTLV (+) donors were more likely to be older, female, and black, with a higher average donor risk index and creatinine, and were more likely to be shared nationally. Recipients of HTLV (+) organs were at slightly elevated risk of graft failure (HR = 1.39, 95% CI 0.91–2.11) and death (HR=1.20, CI 0.71–2.02) relative to HTLV(−) donor recipients (p=0.12 and 0.5, respectively). The risk decreased after multivariate analysis - graft survival (HR=1.20, CI 0.79–1.83) and patient survival (HR=1.06, CI 0.63–1.79).
Conclusion
Our analysis reveals no statistically significant difference in graft or patient survival between recipients of HTLV (+) and (−) donors. Serious limitations of these data are that serologic testing for HTLV has a high false positive rate and that there was a short follow up period. Until these issues are addressed, extreme caution should be exercised when utilizing these organs.
Keywords: Human T-cell Lymphotrophic Virus, Extended Criteria Donors, Liver Transplantation, UNOS data
INTRODUCTION
Liver transplantation is the best treatment option for endstage liver disease. There are currently almost 100,000 recipients on the national organ transplant waiting list with more than 17,000 recipients awaiting liver transplant. Unfortunately, the availability of standard criteria donor organs has fallen far short of the need. According to UNOS, in 2005, nearly 2000 people died while awaiting a liver transplant. This discrepancy has led to an expansion of the criteria by which many transplant centers determine acceptability of potential donor organs.
While there are ample data to suggest that the MELD-based allocation system has had a significant impact on wait list mortality (1), the system, by design, fails to take into consideration the toll that ascites and encephalopathy have on the quality of life of potential liver transplant recipients (2). Furthermore, the system only partially addresses the urgent organ need of recipients with hepatocellular carcinoma. These issues affect the willingness of transplant professionals to accept, as well as potential recipients to receive, organs from donors that might present an increased risk of post-transplant morbidity and mortality. In contrast to the known survival benefit of liver transplantation, for some (those with MELD scores <15), earlier access to transplantation with extended criteria donors might provide them with the ability to improve their quality of life, albeit with increased risk.
The Human T-Cell Lymphotrophic Virus (HTLV) has been associated with leukemia/lymphoma (3) and progressive neurological disease (HTLV-associated myelopathy (HAM)/tropical spastic paraparesis) (4). Few reports exist in the literature regarding the use of organs from HTLV positive donors (5), and fewer still report viral transmission (HTLV seroconversion)(6) or development of disease (7, 8). In this article, we attempt to fill this gap in the literature by evaluating the graft and patient survival of liver transplant recipients of HTLV (+) donors and comparing them to HTLV (−) liver transplant recipients, using data from the United Network for Organ Sharing (UNOS) database.
MATERIALS AND METHODS
Study Design
The UNOS database was retrospectively queried for the recipients of donors who were HTLV (+) with recipients of HTLV (−) grafts serving as the comparison group. Complete analysis of recipient and donor clinical and demographic factors was performed. Since the primary outcomes of interest were recipient and graft survival, we selected a cohort study design rather than a case control design.
Data
Data was obtained on all recipients receiving liver transplants prior to 08/28/07 from the UNOS Standard Transplant Analysis and Research Files (STAR). The full data set included information on 84,054 liver transplants. The UNOS data included information on both HTLV1 and HTLV2 status up until June of 2004, but only recorded overall HTLV (+/−) status after that point. Only 18 transplants were identified as HTLV1 positive, and 17 of these also tested positive for HTLV2. Since there was considerable overlap between HTLV1 (+) and HTLV2 (+) transplants, all transplants were simply identified as being either HTLV (+) or (−). These data were screened by excluding donors with an unknown HTLV status (40,957 out of 84,054 donors). In addition, one donor with a positive HIV antibody serum result was removed. Since HTLV(+) organs are nearly always transplanted into adult patients, recipients under the age of 18 (3,349 out of 33,096 remaining recipients) were also excluded. The final data set contained information on 29,747 transplants where the donor HTLV status was known to be negative (both HTLV1 and HTLV2 negative, 29,666 transplants) or positive (either HTLV1 or HTLV2 positive, 81 transplants). Only deceased donors were included in the analysis.
Outcomes and Covariates
The primary outcomes evaluated were recipient and graft survival. Donor covariates examined included age, ethnicity, gender, history of diabetes and hypertension, hepatitis C status, organ share type (local, regional, national), donor risk index (DRI), creatinine, bilirubin, AST, and ALT levels, and organ cold ischemic time. Recipient covariates examined included age, ethnicity, gender, functional status at transplant, previous malignancy, MELD score, creatinine, bilirubin, and INR levels. A functional status of ‘good’ was defined as having at least 80% functionality or the ability to perform daily activities without any assistance, a status of ‘average’ was defined as having between 40% and 70% functionality or the ability to perform daily activities with some assistance, and a status of ‘poor’ was defined as having 30% or less functionality or the inability to perform daily activities without total assistance. Recipients coded as ‘unknown’ or ‘not applicable’ were given ‘unknown’ functional status, all others were reported as missing. DRI and MELD scores were calculated using standard formulas (9, 10. Organ cold times were restricted to between 2 hours and 18 hours; all times outside of that range were removed as these times were outside what would be expected for deceased donor grafts and might be erroneous.
Statistical methods
Differences in recipient and donor covariates between the recipients of HTLV (+) and HTLV (−) grafts were assessed using either Student’s t-test (continuous covariates) or the chi-square test/Fisher’s exact test (categorical covariates). Univariate differences in recipient and graft survival based on donor HTLV(+)/(−) status were evaluated using Kaplan-Meier curves and the log-rank test (11). Survival proportions at one, three, and five years were also calculated and compared. A power analysis was performed to assess the power of our sample to detect clinically relevant differences in patient and graft survival. Covariates were evaluated for their influence on recipient and graft survival by fitting Cox proportional hazards (PH) regression models, and calculating hazard ratios (HRs) along with 95% confidence intervals (CI) (12). Continuous covariates were dichotomized by picking the cut point with the largest log-rank statistic, and statistical significance was determined using the method in (13). Dichotomization of continuous covariates improves interpretation from a clinical standpoint, and also puts HRs on a scale that is more directly comparable with categorical covariates. To assess the confounding affects of covariates on the association between donor HTLV status and recipient and graft survival, multivariable Cox PH models were fitted using covariates that were significant (p<0.05) from univariate analysis. Only covariates which had a significant impact on the coefficient for donor HTLV status were added to the model. HRs and 95% CIs were reported for all variables included in the final model. Statistical analyses were conducted using SAS version 9.1 and R version 2.7. Power analysis was carried out using PASS/NCSS.
RESULTS
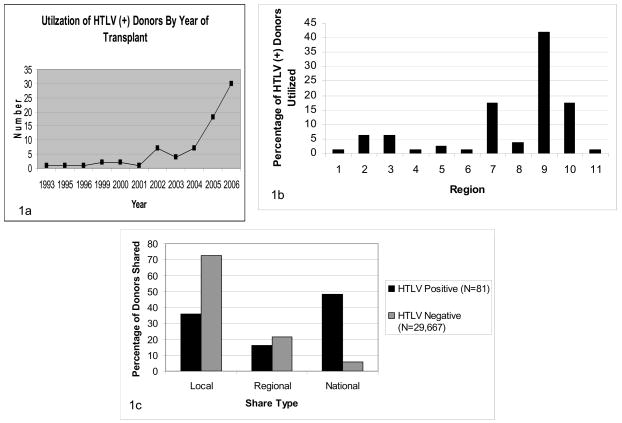
The UNOS STAR data contained 82 HTLV (+) donors (either HTLV1 (+) or HTLV2 (+)), with 81 of those transplanted into recipients at least 18 years old (the other recipient was 15 years old). The number of donors known to be HTLV (−) (both HTLV1 (−) and HTLV2 (−)) was 33,097, while the remainder (40,957) had unknown HTLV status. The number of donor HTLV (+) recipients by year is shown in Figure 1a, and show a clear rise within the past three years. Considering only the adult transplant recipients with known HTLV status (29,748 patients), Figure 1b shows the distribution of HTLV (+)/(−) donor transplants by region. The percentage of HTLV (+) donor transplants occurring in regions 7, 9, and 10 is significantly higher compared to other regions (p<0.001). The average length of follow-up for a recipient of an HTLV (−) graft was 3.83 years (median 3.69 years) with a standard deviation (sd) of 2.76, while the average length of follow-up for an HTLV (+) graft was 1.21 years (median 0.62 years) with a sd of 1.49 (p-value for difference <0.001). The maximum follow-up for HTLV (−) recipients was 13 years, while for HTLV (+) recipients it was 7.7 years (minimum of zero in both cases). The earliest and latest HTLV (−) transplants in our data set were 02/14/1994 and 06/29/2004, while the earliest and latest HTLV (+) transplants were 06/24/1993 and 04/07/2007. Twenty-seven (33%) recipients of HTLV (+) donors were followed for more than 1 year, fifteen (19%) for more than 2 years and nine (11%) for more than 3 years.
Figure 1.
a. Utilization of HTLV (+) Donors by Year of Transplant, b. Utilization of HTLV (+) Donors by Region, c. Distribution of sharing type between HTLV (+) vs (−) Donors
Summary demographics for HTLV (+) and HTLV (−) grafts and their recipients are shown in Table 1a for categorical covariates and Table 1b for continuous covariates. Statistically significant differences were observed in many of the donor characteristics, specifically gender, ethnicity, history of diabetes and hypertension, age, hepatitis C status, DRI, creatinine, and AST. HTLV (+) donors were more likely to be female and black compared to HTLV (−) donors, and the average age was nearly seven years higher (45.7 versus 38.8 years). HTLV (+) donors also had a higher percentage with a history of diabetes and hypertension, and were more likely to be hepatitis C positive. The average DRI and creatinine were both higher for HTLV (+) donors. Interestingly, the average AST was lower for HTLV (+) donors, but the variability in AST and ALT scores was quite high and included many outliers, making it difficult to draw any conclusions related to the role of enzyme elevation and outcomes. HTLV (+) organs were more likely to be distributed nationally compared to HTLV (−) organs (48.15% versus 5.74%) (Figure 1c). The cold ischemic time, however, was not significantly different for HTLV (+) (8.04 hours) and HTLV (−) (7.50 hours) organs (note that cold times below 2 hours and above 18 hours were excluded, see Materials and Methods).
Table 1.
a.Summary demographics for HTLV+/− donors and recipients, continuous covariates. b. Summary demographics for HTLV+/− donors and recipients, categorical covariates.
| Table 1a | HTLV Positive (N=81) | HTLV Negative (N=29,666) | ||||
|---|---|---|---|---|---|---|
| Covariate | Level | Frequency | Percent | Frequency | Percent | P-value |
| Gender - Recipient | F | 28 | 34.6 | 10556 | 35.6 | 0.941 |
| M | 53 | 65.4 | 19110 | 64.4 | ||
| Gender - Donor | F | 51 | 63.0 | 12033 | 40.6 | <0.001 |
| M | 30 | 37.0 | 17633 | 59.4 | ||
| Ethnicity - Recipient | White | 61 | 75.3 | 22472 | 75.8 | 0.74 |
| Black | 5 | 6.2 | 2318 | 7.8 | ||
| Hispanic | 12 | 14.8 | 3428 | 11.6 | ||
| Other | 3 | 3.7 | 1448 | 4.9 | ||
| Ethnicity - Donor | White | 41 | 50.6 | 22101 | 74.5 | <0.001 |
| Black | 30 | 37.0 | 3479 | 11.7 | ||
| Hispanic | 9 | 11.1 | 3168 | 10.7 | ||
| Other | 1 | 1.2 | 918 | 3.1 | ||
| History Diabetes - Donor | Yes | 10 | 12.4 | 1622 | 5.5 | <0.001 |
| No | 70 | 86.4 | 27907 | 94.0 | ||
| Unknown | 1 | 1.2 | 137 | 0.5 | ||
| History Hypertension - Donor | Yes | 35 | 43.2 | 7114 | 24.0 | <0.001 |
| No | 45 | 55.6 | 22251 | 75.0 | ||
| Unknown | 1 | 1.2 | 301 | 1.0 | ||
| Previous Malignancy - Recipient | Yes | 15 | 18.5 | 1539 | 5.2 | <0.001 |
| No | 60 | 74.1 | 25808 | 87.0 | ||
| Unknown | 6 | 7.4 | 2319 | 7.8 | ||
| HIV Serostatus - Recipient | Negative | 54 | 66.7 | 10216 | 34.4 | <0.001 |
| Positive | 2 | 2.5 | 34 | 0.1 | ||
| Unknown | 14 | 17.3 | 12227 | 41.2 | ||
| Missing | 11 | 13.6 | 7189 | 24.2 | ||
| Share Type | Local | 29 | 35.8 | 21564 | 72.7 | <0.001 |
| Regional | 13 | 16.1 | 6400 | 21.6 | ||
| National | 39 | 48.2 | 1702 | 5.7 | ||
| Hep C - Donor | Negative | 70 | 86.4 | 28988 | 97.7 | <0.001 |
| Positive | 10 | 12.4 | 590 | 2.0 | ||
| Unknown | 1 | 1.2 | 88 | 0.3 | ||
| Age - Recipient | (0,39] | 3 | 3.7 | 3606 | 12.2 | 0.002 |
| (39,49] | 19 | 23.5 | 9247 | 31.2 | ||
| (49,59] | 32 | 39.5 | 11012 | 37.1 | ||
| (59,69] | 23 | 28.4 | 5344 | 18.0 | ||
| >69 | 4 | 4.9 | 457 | 1.5 | ||
| Age – Donor | (0,39] | 26 | 32.1 | 15099 | 50.9 | 0.008 |
| (39,49] | 16 | 19.8 | 5582 | 18.8 | ||
| (49,59] | 24 | 29.6 | 4895 | 16.5 | ||
| (59,69] | 10 | 12.4 | 2811 | 9.5 | ||
| >69 | 5 | 6.2 | 1257 | 4.2 | ||
| Missing | 0 | 0.0 | 22 | 0.1 | ||
| Functional Status, Transplant | Well | 34 | 42.0 | 11383 | 38.4 | <0.001 |
| Ave | 24 | 29.6 | 5699 | 19.2 | ||
| Poor | 10 | 12.4 | 535 | 1.8 | ||
| Unknown | 12 | 14.8 | 11709 | 39.5 | ||
| Missing | 1 | 1.2 | 340 | 1.2 | ||
| Table 1b | HTLV Positive (N=81) | HTLV Negative (N=29,667) | |||||
|---|---|---|---|---|---|---|---|
| Covariate | Number | Missing | Mean | Std Dev | Mean | Std Dev | P-value |
| Age - Recipient | 29747 | 0 | 55.54 | 9.38 | 50.6 | 10.35 | <0.001 |
| Age – Donor | 29725 | 22 | 45.65 | 15.79 | 38.83 | 17.58 | <0.001 |
| DRI | 25510 | 4237 | 1.75 | 0.47 | 1.39 | 0.40 | <0.001 |
| MELD | 16069 | 13678 | 18.33 | 8.47 | 20.2 | 9.25 | 0.06 |
| Creatinine - Recipient | 29287 | 460 | 1.31 | 0.85 | 1.44 | 1.26 | 0.196 |
| Creatinine - Donor | 29601 | 146 | 2.09 | 2.72 | 1.34 | 1.73 | 0.015 |
| Bilirubin - Recipient | 29233 | 514 | 5.79 | 8.88 | 7.47 | 10.37 | 0.096 |
| Bilirubin - Donor | 29105 | 642 | 0.9 | 0.73 | 1.05 | 1.95 | 0.074 |
| NR - Recipient | 16112 | 13636 | 1.58 | 0.62 | 1.89 | 2.05 | <0.001 |
| Cold Ischemic Time (Hours) | 24979 | 4768 | 7.50 | 2.98 | 8.04 | 2.42 | 0.076 |
| SGOT/AST - Donor | 29242 | 505 | 49.65 | 47.83 | 74.43 | 166.57 | <0.001 |
| SGPT/ALT - Donor | 28914 | 833 | 43.06 | 58.03 | 55.5 | 151.22 | 0.064 |
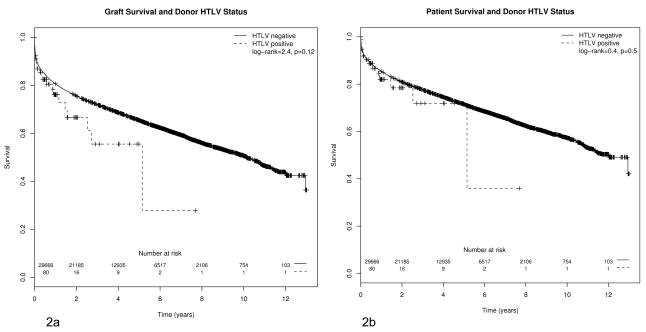
Though gender and ethnicity were not different among HTLV (+)/(−) graft recipients, statistically significant differences were observed for previous malignancy and age. HTLV (+) graft recipients had a larger percentage of previously reported malignancies, and a higher average age (55.5 years versus 50.6 years). The Kaplan-Meier (KM) curves for HTLV (+)/(−) graft recipients are shown in Figure 2a for graft survival and in Figure 2b for patient survival. The log-rank test for differences between HTLV (+)/(−) was non-significant in both cases (p=0.12, graft survival, p=0.5, recipient survival). Survival at one, three, and five years was also non-significant in either case.. Using the observed five year patient and graft survival rates in the HTLV(−) graft group, the power of our sample to detect a HR of 1.5 between HTLV(+/−) status was 0.56 for patient survival and 0.68 for graft survival (α =0.05). This HR corresponded to a difference in five year patient survival of around 15% (0.715 vs 0.605 for HTLV(−) and (+) grafts, respectively) and in five year graft survival of around 19% (0.654 vs 0.529 for HTLV(−) and (+) grafts, respectively). Thus, our study sample provided modest power for detecting differences in survival of clinical importance. To achieve 80% power for the same effect size and assuming only 0.4% of transplanted grafts are HTLV(+), a sample size of 155 HTLV(+) grafts would be required for patient survival and 112 HTLV(+) grafts would be required for graft survival (out of a total transplanted patient population with known HTLV graft status of 38,881 and 28,010, respectively).
Figure 2.
a. Kaplan-Meier curves for HTLV+/− recipients, graft survival. b. Kaplan-Meier curves for HTLV+/− recipients, overall patient survival
Table 2a gives univariate HRs for overall recipient survival for HTLV status and other clinically relevant recipient and donor characteristics. All donor covariates except HTLV status and those involving laboratory tests (creatinine, bilirubin, AST, and ALT) were significant. Note that all continuous covariates (creatinine, bilirubin, AST, ALT, DRI, and cold ischemic time) were dichotomized, and p-values were adjusted according to the method in (13) (CIs were calculated using an unadjusted standard error). After adjustment, none of these dichotomized covariates were significant except DRI. All recipient covariates were significant except gender and ethnicity. Again, continuous covariates (MELD, creatinine, and bilirubin) were dichotomized, and adjusted p-values were significant in each case. Univariate HRs and 95% CIs for graft survival are given in Table 2b. The graft survival significance of the covariates was nearly the same as for overall recipient survival.
Table 2.
| Table 2. a. Univariate hazard ratios for patient survival. | ||||
|---|---|---|---|---|
| DONOR VARIABLES | Levels | Hazard Ratio | 95% CI | P-value |
| HTLV | Pos vs Neg | 1.195 | (0.708, 2.02) | 0.5 |
| Gender | Male vs Female | 0.933 | (0.893, 0.975) | 0.0019 |
| Ethnicity | Black vs White | 1.056 | (0.986, 1.13) | 0.12 |
| Hispanic vs White | 1.092 | (1.018, 1.171) | 0.014 | |
| Other vs White | 1.277 | (1.135, 1.437) | < 0.001 | |
| Age | 40–49 vs <40 | 1.189 | (1.121, 1.262) | < 0.001 |
| 50–59 vs <40 | 1.341 | (1.263, 1.424) | < 0.001 | |
| 60–69 vs <40 | 1.587 | (1.478, 1.705) | < 0.001 | |
| 70+ vs <40 | 1.659 | (1.499, 1.835) | < 0.001 | |
| Creatinine | > 1.5 | 1.115 | (1.054, 1.18) | > 0.3 |
| Bilirubin | > 0.6 | 0.928 | (0.888, 0.97) | > 0.3 |
| AST | > 40 | 0.9 | (0.861, 0.94) | > 0.3 |
| ALT | > 45 | 0.893 | (0.85, 0.938) | > 0.3 |
| DRI | > 1.3 | 1.39 | (1.33, 1.46) | < 0.001 |
| Cold Ischemia Time | > 10 hours | 1.17 | (1.11, 1.233) | > 0.3 |
| History of Diabetes | Yes vs No | 1.28 | (1.17, 1.40) | < 0.001 |
| Unknown vs No | 1.08 | (0.80, 1.45) | 0.63 | |
| History of Hypertension | Yes vs No | 1.24 | (1.18, 1.30) | < 0.001 |
| Unknown vs No | 1.21 | (0.988, 1.47) | 0.065 | |
| Hepatitis C | Pos vs Negative | 1.20 | (1.04, 1.39) | 0.014 |
| Unknown vs Negative | 1.11 | (0.78, 1.59) | 0.56 | |
| Share Type | Regional vs Local | 1.164 | (1.105, 1.226) | < 0.001 |
| National vs Local | 1.485 | (1.364, 1.616) | < 0.001 | |
| PATIENT VARIABLES | ||||
| Gender | Male vs Female | 0.981 | (0.937, 1.026) | 0.39 |
| Ethnicity | Black vs White | 1.298 | (1.204, 1.398) | < 0.001 |
| Hispanic vs White | 0.957 | (0.891, 1.027) | 0.22 | |
| Other vs White | 0.931 | (0.838, 1.036) | 0.19 | |
| Age | 40–49 vs <40 | 1.154 | (1.066, 1.25) | < 0.001 |
| 50–59 vs <40 | 1.254 | (1.16, 1.355) | < 0.001 | |
| 60–69 vs <40 | 1.581 | (1.455, 1.717) | < 0.001 | |
| 70+ vs <40 | 2.009 | (1.71, 2.361) | < 0.001 | |
| MELD | > 25 | 1.488 | (1.39, 1.593) | < 0.001 |
| Creatine | > 1.8 | 1.737 | (1.653, 1.825) | < 0.001 |
| Bilirubin | > 6 | 1.264 | (1.206, 1.324) | < 0.001 |
| HIV Serostatus | Pos vs Negative | 1.39 | (0.77, 2.51) | 0.28 |
| Unknown/Missing vs Negative | 1.04 | (0.99, 1.09) | 0.09 | |
| Previous Malignancy | Yes vs No | 1.36 | (1.25, 1.49) | < 0.001 |
| Unknown vs No | 1.09 | (1.01, 1.18) | 0.032 | |
| Functional Status at Transplant | Average vs Good | 1.133 | (1.062, 1.208) | < 0.001 |
| Poor vs Good | 1.542 | (1.334, 1.784) | < 0.001 | |
| Unknown vs Good | 1.592 | (1.515, 1.674) | < 0.001 | |
| Table 2. b. Univariate hazard ratios for graft survival. | ||||
|---|---|---|---|---|
| DONOR VARIABLES | Levels | Hazard Ratio | 95% CI | P-value |
| HTLV | Pos vs Neg | 1.389 | (0.914, 2.112) | 0.12 |
| Gender | Male vs Female | 0.888 | (0.854, 0.923) | < 0.001 |
| Ethnicity | Black vs White | 1.128 | (1.064, 1.197) | < 0.001 |
| Hispanic vs White | 1.075 | (1.01, 1.145) | 0.023 | |
| Other vs White | 1.239 | (1.115, 1.378) | < 0.001 | |
| Age | 40–49 vs <40 | 1.209 | (1.147, 1.275) | < 0.001 |
| 50–59 vs <40 | 1.401 | (1.329, 1.477) | < 0.001 | |
| 60–69 vs <40 | 1.651 | (1.551, 1.758) | < 0.001 | |
| 70+ vs <40 | 1.839 | (1.688, 2.003) | < 0.001 | |
| Creatine | > 1.5 | 1.102 | (1.049, 1.159) | > 0.3 |
| Bilirubin | > 0.6 | 0.904 | (0.87, 0.941) | > 0.3 |
| AST | > 40 | 0.9 | (0.866, 0.936) | > 0.3 |
| ALT | > 45 | 0.911 | (0.873, 0.952) | > 0.3 |
| DRI | > 1.3 | 1.48 | (1.42, 1.55) | < 0.001 |
| Cold Ischemia Time | > 10 hours | 1.198 | (1.144, 1.255) | 0.107 |
| History of Diabetes | Yes vs No | 1.33 | (1.23, 1.44) | < 0.001 |
| Unknown vs No | 1.03 | (0.97, 0.785) | 0.83 | |
| History of Hypertension | Yes vs No | 1.29 | (1.23, 1.35) | < 0.001 |
| Unknown vs No | 1.16 | (0.963, 1.39) | 0.12 | |
| Hepatitis C | Pos vs Neg | 1.19 | (1.04, 1.35) | 0.009 |
| Unknown vs Neg | 1.09 | (0.786, 1.50) | 0.62 | |
| Share Type | Regional vs Local | 1.187 | (1.134, 1.243) | < 0.001 |
| National vs Local | 1.529 | (1.42, 1.647) | < 0.001 | |
| PATIENT VARIABLES | ||||
| Gender | Male vs Female | 0.997 | (0.957, 1.037) | 0.86 |
| Ethnicity | Black vs White | 1.311 | (1.228, 1.401) | < 0.001 |
| Hispanic vs White | 0.975 | (0.916, 1.038) | 0.43 | |
| Other vs White | 0.915 | (0.833, 1.006) | 0.067 | |
| Age | 40–49 vs <40 | 1.023 | (0.958, 1.092) | 0.5 |
| 50–59 vs <40 | 1.006 | (0.943, 1.073) | 0.86 | |
| 60–69 vs <40 | 1.18 | (1.1, 1.266) | < 0.001 | |
| 70+ vs <40 | 1.442 | (1.242, 1.675) | < 0.001 | |
| MELD | > 25 | 1.356 | (1.275, 1.441) | < 0.001 |
| Creatine | > 1.8 | 1.549 | (1.481, 1.62) | < 0.001 |
| Bilirubin | > 6 | 1.242 | (1.192, 1.295) | < 0.001 |
| HIV Serostatus | Pos vs Neg | 1.44 | (0.87, 2.4) | 0.15 |
| Unknown/Miss vs Neg | 1.03 | (0.985, 1.07) | 0.21 | |
| Previous Malignancy | Yes vs No | 1.25 | (1.15, 1.36) | < 0.001 |
| Unknown vs No | 1.10 | (1.03, 1.18) | 0.007 | |
| Functional Status at Transplant | Average vs Good | 1.108 | (1.047, 1.172) | < 0.001 |
| Poor vs Good | 1.443 | (1.265, 1.644) | < 0.001 | |
| Unknown vs Good | 1.497 | (1.432, 1.564) | < 0.001 | |
We performed a subset analysis of the 27 recipients of HTLV (+) donor organs with more one year of follow-up. In this set of patients, there were three deaths at 1.46, 2.53, and 5.15 years, with corresponding number at risk of 23, 12, and 2 and Kaplan-Meier survival estimates of 0.96, 0.88, and 0.44. The estimated median survival time (minimum time t such that S(t) < 0.5) was 5.15 years. There were six graft failures at 1.16, 1.46, 1.56, 2.53, 2.72, and 5.15 years, with corresponding number at risk of 24, 23, 22, 12, 11, and 2, and survival estimates of 0.96, 0.92, 0.88, 0.80, 0.73, and 0.37. Again, the estimated median survival time was 5.15 years. The average recipient age in this cohort was 55.4 (range 39–72), and average donor age was 42.1 (range 12–72). The average DRI was 1.57 (range 0.99–2.26) and average MELD score was 16.42 (range 7–40).
All variables that were significant in the univariate survival analysis and were associated with HTLV(+/−) status, except MELD score, were examined for potential confounding with HTLV status in a multivariable Cox model. MELD score was excluded due to the extremely large number of missing values needed to calculate the MELD score. Since this was mainly due to missing INR values, recipient creatinine and bilirubin were used directly instead. Since the primary interest is in the effect the covariate has on the estimate associated with HTLV status, we examined the difference in the estimated HR associated with HTLV status once the covariate was included in the model. Table 3a displays the results for patient survival. The majority of covariates decreased the estimated HR associated with HTLV status once they were included in the model, indicating that HTLV(+) graft recipients were affiliated with risk factors which put them at greater risk of failure. To fit a multivariable model and obtain adjusted risk estimates, we selected those covariates which changed the HR for HTLV status by greater than +/− 5%, which included patient functional status at transplant, share type, and donor and patient age. The HR for HTLV status in this multivariable model was reduced to 1.06, though the 95% confidence interval was rather wide (0.63 to 1.79). Inclusion of patient bilirubin and creatine levels, which increased the estimate for HTLV status individually, did not alter the estimate if they were included in the multivariable model. Inclusion of cold ischemia time in the multivariable model increased the HTLV status HR to 1.10, but there were a substantial number of missing values (5,454) and only 63 HTLV(+) patients remained in the analysis.
Table 3.
| Table 3. a. Influence of covariates on hazard ratio (HR) estimate for patient survival associated with HTLV (+/−) status, based on multivariable Cox regression models. Changes in HR for HTLV status are given relative to a baseline Cox model which includes only HTLV status. | ||
|---|---|---|
| Models with Donor Variables | HR for HTLV (+/−) | Change in HR associated with HTLV (+/−) (%) |
| HTLV | 1.195 | Ref |
| HTLV + Cold Ischemia Time | 1.255 | 0.060 (4.7) |
| HTLV + Ethnic Category | 1.184 | −0.011 (−0.9) |
| HTLV + DRI | 1.180 | −0.016 (−1.3) |
| HTLV + Hepatitis C | 1.174 | −0.021 (−1.8) |
| HTLV + Gender | 1.173 | −0.022 (−1.9) |
| HTLV + History of Diabetes | 1.168 | −0.028 (−2.4) |
| HTLV + History of Hypertension | 1.144 | −0.051 (−4.5) |
| HTLV + Age | 1.127 | −0.068 (−6.0) |
| Models with Patient Variables | ||
| HTLV + Functional Status at Transplant | 1.293 | 0.098 (7.6) |
| HTLV + Bilirubin | 1.240 | 0.045 (3.6) |
| HTLV + Creatine | 1.232 | 0.036 (3.0) |
| HTLV + Previous Malignancy | 1.140 | −0.056 (−4.9) |
| HTLV + Age | 1.126 | −0.069 (−6.1) |
| HTLV + Share Type | 1.018 | −0.177 (−17.4) |
| Final Multivariate Model | ||
| HTLV + Functional Status at Transplant + Share Type + Age of Donor +Age of Patient | 1.06 (95% CI 0.63 to 1.79) | −0.14 (−11.5) |
| Table 3. b. Influence of covariates on hazard ratio (HR) estimate for graft survival associated with HTLV (+/−) status, based on multivariable Cox regression models. Changes in HR for HTLV status are given relative to a baseline Cox model which includes only HTLV status. | ||
|---|---|---|
| Models with Donor Variables | HR for HTLV (+/−) | Change in HR associated with HTLV (+/−) (%) |
| HTLV | 1.389 | Ref |
| HTLV + Hepatitis C | 1.367 | −0.023 (−1.7) |
| HTLV + Ethnic Category | 1.356 | −0.034 (−2.5) |
| HTLV + History of Diabetes | 1.352 | −0.038 (−2.8) |
| HTLV + Gender | 1.346 | −0.043 (−3.2) |
| HTLV + Cold Ischemia Time | 1.332 | −0.058 (−4.3) |
| HTLV + History of Hypertension | 1.319 | −0.070 (−5.3) |
| HTLV + Age | 1.300 | −0.090 (−6.9) |
| HTLV + DRI | 1.127 | −0.263 (−23.3) |
| Models with Patient Variables | ||
| HTLV + Functional Status at Transplant | 1.487 | 0.098 (6.6) |
| HTLV + Bilirubin | 1.437 | 0.048 (3.3) |
| HTLV + Creatine | 1.420 | 0.030 (2.1) |
| HTLV + Previous Malignancy | 1.346 | −0.043 (−3.2) |
| HTLV + Age | 1.340 | −0.050 (−3.7) |
| HTLV + Share Type | 1.167 | −0.222 (−19.0) |
| Final Multivariate Model | ||
| HTLV + Functional Status at Transplant + Share Type + Age of Donor + Donor History of Hypertension + Donor History of Diabetes | 1.204 (95% CI 0.79 to 1.83) | −0.184 (−13.2) |
Results for analysis of potential confounding effects of variables associated with HTLV status and graft survival are shown in Table 3b. Again, the majority of the covariates decreased the risk estimate associated with HTLV status once included in the multivariable model. Covariates which decreased the HR for HTLV status by more than +/− 5% were included in a multivariable model, which included patient functional status at transplant, donor history of hypertension and diabetes, donor age, donor risk index, and share type. This multivariable model gave a HR for HTLV(+) vs (−) status of 1.11 (95% CI 0.68 to 1.81). However, including donor risk index again resulted in many missing values (4,554) and only 65 HTLV(+) patients remaining, so this variable was dropped from the multivariable model. The resulting model gave a HR estimate for HTLV (+) vs (−) status of 1.20 (95% CI 0.79 to 1.83).
DISCUSSION
HTLV types I and II are RNA viruses related to the human immunodeficiency virus (HIV). The type I subtype has been associated with leukemia/lymphoma (3) and progressive neurological disease (HTLV-associated myelopathy (HAM)/tropical spastic paraparesis) (4). HAM results in a slowly progressive spastic paraparesis and bladder dysfunction (14). No clear relationship has been found between HTLV type II and disease (15, 16).
Prevalence of the virus varies among different populations. It is common in the Caribbean, Middle East, Japan, and Africa (17). In the United States, the prevalence of positive serologies among voluntary blood donors is 0.035–0.046% (18) but it is as high as 4% in certain areas where immigrants from endemic areas have congregated (19, 20). Viral transmission can occur through contact with blood, blood products and bodily fluids such as breast milk, sexual contact, and the sharing of needles (5). The incidence of HTLV infection in organ donors is reported to be 0.027% (5).
While the diseases associated with HTLV-I can be quite devastating, most recipients are asymptomatic, with a lifetime incidence of disease in known HTLV-I positive populations between 2 and 5% (21). Moreover, the incidence of HAM in HTLV-1 carriers is less than 2% (22). In addition, in the non-immunosuppressed population, the disease does not occur until many years after infection, and when it does, progression is over many years (23).
Transplant recipients with positive pre-transplant HTLV serology have not definitively developed more rapid HTLV-related disease, but the development of HAM has been reported. Tanabe reported no disease development in 16 renal transplant recipients after 8 years of follow-up (24). Kawano reported on post-transplant recipients who developed HTLV-related disease but the origin of the disease was from recipient HTLV-infected cells (25). Recently, an HTLV (+) recipient received an HTLV (+) living donor liver allograft from his sister with the development of HAM 20 months following transplantation (26). The recipient is alive 8 months later (28 months post transplant) but with a gait disturbance and bladder dysfunction. The authors noted that 5 other HTLV-I carriers were transplanted in the past at their institution and none have developed symptomatic HTLV-related disease.
There are, however, documented reports in the literature of solid organ donor-related transmission of HTLV-related disease. Three recipients (1 liver, 2 kidney recipients) from a single donor in Spain rapidly (within 2 years) developed HAM (8). Transmission was clearly linked to the donor. Another investigator reported HTLV transmission through a kidney transplant but no donor serology was performed (27). In a review of the literature, fewer than 5 recipients from 2 donors have developed disease related to transmission of HTLV from solid organ transplantation.
Kauffman in a letter to the editor of Transplantation in 2003 responded to the Spanish report by stating that only in the setting of “extreme medical emergency” in non-renal organs is the utilization of HTLV organs advised (28). The last report utilizing UNOS data addressing the use of HTLV (+) donors was in 2002 and focused on donors in the database until the year 2000 (5). The 2002 report included 9 liver transplant recipients with no HTLV-related disease discovered post-transplant with a mean follow up of 435 days. Since this time there has been a dramatic increase in the utilization of HTLV (+) donors (Fig. 1a) and these organs are predominantly being used in regions such as New York where there is a relatively long waiting time and higher MELD requirement for transplantation (Fig 1b). Urgent need has expanded the willingness of patients to receive and their doctors to offer, these “risky” life-saving grafts.
While we observed no statistically significant differences in patient and graft survival when comparing recipients of HTLV(+) and HTLV(−) grafts, this was based on a relatively small number of HTLV(+) graft recipients which provided only modest power to detect clinically relevant differences in survival. Kaplan-Meier curves and Cox regression models for HTLV(+/−) status showed HTLV(+) patients were at slightly elevated risk of failure compared to HTLV(−) patients, although this slight elevation did not reach statistical significance. Our inspection of potential confounding variables associated with HTLV (+/−) status and survival revealed that HTLV(+) graft recipients were affiliated with a number of risk factors which put them at greater risk of failure. After adjustment for these risk factors in a multivariable Cox model, the HR associated with HTLV(+) vs (−) status was reduced from 1.20 to 1.06 for patient survival and from 1.39 to 1.15 for graft survival. However, the confidence intervals around these risk estimates were quite wide, ranging from 0.62 to 1.78 for patient survival and 0.75 to 1.77 for graft survival. Hence, though there is no clear evidence suggesting diminished patient and graft survival for HTLV(+) graft recipients, such an association cannot be entirely ruled out based on the current study.
It is impossible to glean from UNOS data that no HTLV-related disease developed after utilization of an HTLV (+) donor because of the lack of required follow-up data entry fields relating to HTLV disease in the data set and the relatively short follow-up period in the HTLV (+) donor cohort (mean 1.2 years). Only 33% of the patients have been followed for more than 1 year with 19% and 11% followed for more than 2 and 3 years, respectively. As mentioned above, the progression of disease in most patients with HTLV infection is slow and it is possible that disease may develop in this population with prolonged follow-up. This problem is compounded by the large number of patients who had missing data in the registry. Clearly, longer follow up time and further study is imperative prior to drawing any conclusions on the safety of utilizing these grafts.
The most important and serious limitation of these data is the fact that serologic testing for HTLV has a very significant incidence of false positive test results. How can one make an educated decision about the risks of potential viral disease transmission if patients included in the literature are not actually at risk at all because of false positive testing? Shames reported a 100% incidence of false positive testing in their University of Wisconsin series (5). More recently, Renz’s group from Columbia has presented 2 abstracts documenting their NY experience which demonstrated a false positive rate of 62% in their series of 25 recipients (30, 31). They did however note that 2 of the 25 patients seroconverted from HTLV (−) serology pre-transplant to HTLV (+) serology post transplant. No patient has developed HTLV-related disease. One of their patients died from an encephalitis/neurological event 3 months post OLT but this was not clearly attributed to any HTLV-related disease and is inconsistent with HTLV infection.
We recommend that there be mandatory Western blot confirmation of all HTLV (+) donors so as to definitively determine if the donor was truly HTLV (+). The determination of the true HTLV (+) status is critical both for the management of individual recipients and to the transplant community as a whole. The testing would most likely need to occur in a retrospective fashion as the time constraints inherent in Western blotting most often precludes prospective testing. If a false positive test result has occurred and the organs already transplanted, no further testing or follow up of HTLV status would be required in the recipient. However, if it is determined that the donor was truly positive for HTLV, close follow up with serial serology and measurement of proviral loads would be warranted. In addition, determination of the true incidence of false positive testing for HTLV is critical in this era of organ shortage so that no potentially life saving organs are unnecessarily discarded.
Clearly, recipients of HTLV (+) grafts need to be followed very closely for the development of HTLV-associated illnesses. Serial determinations of HTLV serology as well as measurement of proviral loads will potentially permit early recognition of patients at risk for development of disease, as it has been demonstrated that symptomatic patients with known HTLV infection in the non-transplant setting manifest high proviral loads (32).
An informal survey of organ procurement organizations that we conducted and presented at the 2008 American Transplant Congress (29) indicated that many potentially life saving organs were not able to be placed because of a lack of knowledge by OPO staff of transplant centers willing to use these grafts (data not shown). Recent updates to DonorNet will assist with this problem in that centers will be asked to indicate within DonorNet their willingness to accept an HTLV (+) organ.
The utilization of HTLV (+) donors is increasing as a result of the well-known growing disparity between organ availability and patient need. Patients with severe recurrent encephalopathy, intractable ascites or hepatocellular tumors outside of Milan criteria receive no special consideration in the MELD-based allocation system. Many of these patients might be very willing to accept an increased risk in terms of HTLV-related disease transmission in order to undergo liver transplantation. While there may indeed be a risk of disease transmission from HTLV (+) donors (true magnitude unknown), the risk may be warranted in selected recipients who agree to accept these risks in order to more rapidly obtain a life-saving and life-enhancing organ. These patients must, however, be clearly informed of the potential risks of major life-threatening disease from their acceptance of these organs.
Conclusion
While extreme caution must be exercised in the utilization of these grafts and there are clearly serious limitations in this registry analysis of the HTLV status of donors, this is currently all we have to assist us in making the decision of whether or not to accept an HTLV (+) graft. Mandatory donor confirmatory testing with serial serological testing and measurement of proviral loads in the recipient combined with HTLV specific disease fields in the mandatory UNOS reporting from transplant centers, would enable us to eventually make a well informed decision regarding the use of these organs.
Acknowledgments
Funding sources: GNB is supported in part by NIH grant P30ES014443. This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Jr, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2006;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 2.Saab S, Ibrahim A, Surti B, Durazo F, Han S, Yersiz H, Farmer D, Ghobrial R, Goldstein L, Tong M, Busutil R. Pretransplant variables associated with quality of life in liver transplant recipients. Liver International. 2008;28(8):1087–1094. doi: 10.1111/j.1478-3231.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- 3.Popovic M, Reitz MS, Kalyanaraman VS, Gallo RC. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sezary T-cell leukemia. Nature. 1981;294:268–271. doi: 10.1038/294268a0. [DOI] [PubMed] [Google Scholar]
- 4.Osame M, Usuku K, Izumo S, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;I:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 5.Shames BD, D’Alessandro AM, Sollinger HW. Human T-Cell Lymphotrophic Virus Infection in Organ Donors: A Need to Reassess Policy? Am J Trnas. 2002;2:658–663. doi: 10.1034/j.1600-6143.2002.20712.x. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Perez MP. Human T-cell leukemia virus type I infection in various recipients of transplants from the same donor. Transplantation. 2003 Apr 15;75(7):1006–11. doi: 10.1097/01.TP.0000058470.15921.CA. [DOI] [PubMed] [Google Scholar]
- 7.Zarranz JJ, Rouco I, Gomez-Esteban JC. Human T lymphotropic virus type I (HTLV-1) associated myelopathy acquired through a liver transplant. J Neurol Neurosurg Psychiatry. 2001 December;71:818. doi: 10.1136/jnnp.71.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toro C, Rodes B, Poveda E, Soriano V. Rapid Development of Subacute Myelopathy in Three Organ Transplant Recipeints after Transmission of Human T-Cell lymphotropic virus Type I from a Single Donor. Transplantation. 2003;75(1):102–104. doi: 10.1097/00007890-200301150-00019. [DOI] [PubMed] [Google Scholar]
- 9.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 10.Wiesner RH, Feuer G, Green PL, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 11.Klein JP, Moeschberger ML. Survival Analysis. 2. Springer; 2003. [Google Scholar]
- 12.Cox DR. Regression Models and Life Tables (with Discussion) Journal of the Royal Statistical Society B. 1972;34:187–220. [Google Scholar]
- 13.Contal C, O’Quigley J. An Application of Change Point Methods in Studying the Effect of Age on Survival in Breast Cancer. Computational Statistics and Data Analysis. 1999;30:253–270. [Google Scholar]
- 14.Araujo AQ, Silva MT. The HTLV-1 neurological complex. Lancet Neurol. 2006 Dec;5(12):1068–76. doi: 10.1016/S1474-4422(06)70628-7. [DOI] [PubMed] [Google Scholar]
- 15.Feurer G, Green PL. Comparative biology of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2. Oncogene. 2005;24:5996–6004. doi: 10.1038/sj.onc.1208971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araujo A, Hall WW. Human T-lymphotropic virus type II and neurological disease. Ann Neurol. 2004;56:10–19. doi: 10.1002/ana.20126. [DOI] [PubMed] [Google Scholar]
- 17.Holsberg P, Hafler DA. Pathogenesis of diseases induced by human lymphotrophic virus type I infection. N Engl J Med. 1993;328:1173–1182. doi: 10.1056/NEJM199304223281608. [DOI] [PubMed] [Google Scholar]
- 18.Glynn SA, Kleinman SH, Schreiber GB, et al. Trends in the incidence and prevalence of major transfusion-transmissible viral infections in US blood donors, 1991–96. JAMA. 2000;284:229–235. doi: 10.1001/jama.284.2.229. [DOI] [PubMed] [Google Scholar]
- 19.Levine PH, Dosik H, Joseph EM, et al. A study of adult T-cell leukemia/lymphoma incidence in central Brooklyn. Int J Cancer. 1999;80:662–666. doi: 10.1002/(sici)1097-0215(19990301)80:5<662::aid-ijc5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Harrington WJ, Ucar A, Gill P, et al. Clinical spectrum of HTLV-I in south Florida. J Acquir Immune Defic Syndr. 1995;8:466–473. doi: 10.1097/00042560-199504120-00006. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe T. HTLV-1 associated diseases. Int J Hematol. 1997 doi: 10.1016/s0925-5710(97)00077-7. [DOI] [PubMed] [Google Scholar]
- 22.Maloney EM, Cleghorn FR, Morgan OSC, et al. Incidence of HTLV-I associated myelopathy/tropical spastic paraparesis (HAM/TSP) in Jamaica and Trinidad. J Acquir Immune Defic Syndro. 1997;17:167–170. doi: 10.1097/00042560-199802010-00011. [DOI] [PubMed] [Google Scholar]
- 23.Olindo S, Cabre P, Lezin A, Merle H, Saint-Vil M, Signate A, Bonnan M, Chalon A, Magnani L, Cesaire R, Smadja D. Natural history of human T-lymphotropic virus 1-associated myelopathy: a 14-year follow-up study. doi: 10.1001/archneur.63.11.1560. [DOI] [PubMed] [Google Scholar]
- 24.Tanabe K, Kitani R, Takahashi K, et al. Long-term results in human T-cell leukemia virus type 1-positive renal transplant recipients. Tansplant Proc. 1998;30 (7):3168. doi: 10.1016/s0041-1345(98)00980-4. [DOI] [PubMed] [Google Scholar]
- 25.Kawano N, Shimoda K, Ishikawa F, Taketomi A, Yoshizumi T, Shimoda S, Yoshida S, Uozumi K, Suzuki S, Maehara Y, Harada M. Adult T-cell leukemia development from a human T-cell leukemia virus type I carrier after a living-donor liver transplantation. Transplantation. 2006 Sep 27;82(6):840–3. doi: 10.1097/01.tp.0000235186.30113.c7. [DOI] [PubMed] [Google Scholar]
- 26.Soyama A, Eguchi S, Takatsuki M, Ichikawa T, Moriuchi M, Moriuchi H, Nakamura T, Tajima Y, Kanematsu T. Human T-cell leukemia virus type I-associated myelopathy following living-donor liver transplantation. Liver Transpl. 2008 May;14(5):647–50. doi: 10.1002/lt.21414. [DOI] [PubMed] [Google Scholar]
- 27.Nakatsuji Y, Sugai F, Watanabe S, Kaido M, Koguchi K, Abe K, Sakoda S. HTLV-I associated myelopathy manifested after renal transplantation. J Neurol Sci. 2000;177:154–156. doi: 10.1016/s0022-510x(00)00332-4. [DOI] [PubMed] [Google Scholar]
- 28.Kauffman H, Taranto S. Human T-Cell Lymphotrophic Virus Type-1 and Organ Donors. Transplantation. 2003;76:745–746. doi: 10.1097/01.TP.0000071847.36786.C1. [DOI] [PubMed] [Google Scholar]
- 29.Nagubandi R, Brock GN, Ravindra KV, Eng M, Buell JF, Marvin MR. Optimal Utilization of HTLV I/II Positive Organs A Nationwide Survey. Am J Trans. 2008;8(S2):231. [Google Scholar]
- 30.Alkofer B, Kin C, Lo I, Guarrera J, Samstein B, Bellemare S, Jan D, Kinkhabwala M, Schilsky M, Sigal S, Dove L, Gaglio P, Brown R, Emond JC, Renz JF. Utilization of HTLV Infected Liver Allografts for Liver Transplantation. Abstract ASTS Winter Symposium, Solving the Organ Shortage Crisis: 32Implications of Expanding the Donor Pool; Jan 12–14, 2007. [Google Scholar]
- 31.Lo I, Alkofer B, Kin C, Guarrera J, Samstein B, Jan D, Schilsky M, Sigal S, Dove L, Gaglio P, Brown R, Emond J, Renz J. Utilization of HTLV Serology Positive Allografts for Liver Tranpslantation. Abstract - ASTS Winter Symposium, Solving the Organ Shortage Crisis: 32Implications of Expanding the Donor Pool; Jan 12–14, 2007. [Google Scholar]
- 32.Silva MT, Harab RC, Leite AC, Schor D, Araujo A, Andrada-Serpa MJ. Human T lymphotropic virus type 1 (HTLV-1) proviral load in asymptomatic carriers, HTLV-1-associated myelopathy/tropical spastic paraparesis, and other neurological abnormalities associated with HTLV-1 infection. Clin Infect Dis. 2007 Mar 1;44(5):689–92. doi: 10.1086/510679. [DOI] [PubMed] [Google Scholar]