Abstract
Angiogenesis is a hallmark of malignant transformation. With improved understanding of angiogenic signaling in both the normal and malignant state, there have been a number of agents developed that target VEGF signaling. These targeted agents can affect downstream VEGF signal transduction via unique mechanisms at different cellular and extracellular locations. The aflibercept, or VEGF-Trap, molecule is the subject of this article. Its molecular structure, pharmacokinetic and pharmacodynamic profile, and preclinical and early clinical data in epithelial ovarian carcinoma is reviewed. For comparison, other anti-angiogenic agents that have been or are currently being studied in epithelial ovarian carcinoma are also summarized. Finally, the anticipated role of aflibercept in the treatment of epithelial ovarian carcinoma is also discussed.
Keywords: aflibercept, antiangiogenesis, epithelial ovarian carcinoma, vascular endothelial growth factor, VEGF-Trap
Epithelial ovarian cancer (EOC) is a malignancy that afflicts one in 70 women. It occurs most frequently at approximately 60 years of age, and is more common in Caucasian women (45%) than in women of other races (Black: 38%; all others: 17%). In the USA, an estimated 22,000 women are diagnosed with EOC annually [1]. In Europe, the incidence is substantially higher, with an estimated 44,000 cases identified per year. Most women who present with EOC are found with advanced disease; approximately 75% of patients are stage III–IV at diagnosis. Surgical cytoreduction and cytotoxic chemotherapy are the cornerstones of primary therapy for advanced disease, and most patients achieve a complete clinical remission in response to primary therapy. Unfortunately, recurrence is both common and lethal; 5-year survival for stage IIIC and IV patients is 29 and 13%, respectively [2].
Like most other solid tumors, EOCs exhibit a large number of molecular abnormalities that are thought to be central to carcinogenesis and metastatic spread. Among these, increased VEGF signal transduction, an important step in tumor-associated angiogenesis, appears to be one of the primary means by which malignant ovarian cells grow and disseminate.
Targeted inhibition of VEGF signaling has been shown to be an effective way to treat many different solid tumors, including EOC [3]. Since the introduction of monoclonal antibodies such as bevacizumab (Avastin™, Genentech, CA, USA), significant emphasis has been placed on the development of other VEGF pathway inhibitors that have the potential to provide improved efficacy in the treatment of malignancy. One of these, the subject of this review, is the drug aflibercept, also known as VEGF-Trap.
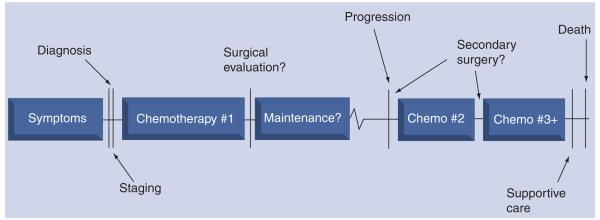
Contemporary adjuvant chemotherapy for EOC in the primary treatment setting is platinum and taxane based. Efforts to improve response rate and progression-free survival by adding cytotoxic agents to platinum and taxane therapy have not been successful [4]. In general, EOC is a relatively chemosensitive disease, as up to 80% of patients achieve a complete clinical remission. Unfortunately, the recurrence rate for EOC in patients who achieve this status is approximately 80% [5]. Treatment in the recurrent setting may include surgery, radiotherapy and chemotherapy, or any combination thereof. In nearly all cases, the approach is considered palliative. As such, there is no clear management algorithm and treatment options are, in large part, individualized. The manner in which they are individualized depends upon a number of factors, such as the location and estimated number of recurrent implants, the patient's previous response to platinum- and taxane-based chemotherapy and functional status. Figure 1 illustrates the typical treatment and survival timeline for patients with EOC. Table 1 lists agents offered by the National Comprehensive Cancer Network (NCCN) as acceptable for use in patients with recurrent EOC [6]. Of note, only one targeted biologic agent is listed, bevacizumab. This agent has become more widely used in the past few years after multiple Phase II studies have demonstrated its activity in EOC [7-9].
Figure 1.
Epithelial ovarian cancer: natural history.
Table 1.
National Comprehensive Cancer Network acceptable drugs in recurrent epithelial ovarian carcinoma.
| Cytotoxic therapy | Hormonal therapy |
Targeted therapy | |
|---|---|---|---|
| Preferred agents | Cisplatin (if platinum-sensitive) | – | – |
| Carboplatin (if platinum-sensitive) | |||
| Gemcitabine | |||
| Carboplatin/paclitaxel (category 1) | |||
| (if platinum-sensitive) | |||
| Gemcitabine/carboplatin | |||
| Liposomal doxorubicin | |||
| Topotecan | |||
| Other potentially active agents |
Altretamine | Anastrozole | Bevacizumab |
| Capecitabine | Letrozole | ||
| Cyclophosphamide | Tamoxifen | ||
| Docetaxel | |||
| Etoposide, oral | |||
| Ifosfamide | |||
| Irinotecan | |||
| Melphalan | |||
| Oxaliplatin | |||
| Paclitaxel | |||
| Vinorelbine | |||
Data taken from [6].
The VEGF family is composed of five glyco-proteins referred to as VEGF-A, VEGF-B, VEGF-C, VEGF-D and PlGF. Expression of the different VEGF ligands are associated, somewhat uniquely, with key events in physiologic vasculo genesis and angiogenesis from embryonic life forward [10]. The most well described of these ligands is VEGF-A (VEGF). In mice, homozygous deletion of the VEGF is embryologically lethal, resulting in defects in vasculogenesis and cardiovascular abnormalities. VEGF-A is also important to a number of postnatal angiogenic processes, including wound healing, ovulation, menstruation, maintenance of blood pressure and pregnancy. PlGF and VEGF-B, in contrast to VEGF-A, do not appear to be essential to organogenesis or early development in mice. PlGF knockout mice, however, show a reduced ability to respond to ischemic damage through angiogenesis and adaptive arteriogenesis, suggesting a role for PlGF in pathologic states in the adult [10]. VEGF is alternatively spliced after translation, forming numerous isoforms of varying lengths (e.g., 121-, 145-, 165- and 206-amino acid proteins). Of these isoforms, VEGF165 is predominant, and is overexpressed in a number of solid tumors. The predominant isoform of PlGF (PlGF-1) has close structural similarity to VEGF-A [11].
VEGF ligands as well as PlGF bind to and activate three closely related transmembrane receptor tyrosine kinases, referred to as VEGF receptor (VEGFR)1 (FLT1), VEGFR2 (KDR) and VEGFR3 (FLT4). The multiple different VEGF ligands have unique binding specificities for each VEGFR tyrosine kinase receptor, contributing to their diversity of function. After ligand binding to VEGF receptors, each tyrosine kinase activates a complex network of unique downstream signaling pathways. VEGFR2 is expressed primarily within the endothelium, and is considered the primary mediator of VEGF-induced angiogenesis [12]. VEGFR1 is expressed in multiple tissues, including vascular endothelium; however, its exact role in signal transduction-mediated angiogenesis is unclear. It has a tenfold higher binding affinity to VEGF than VEGFR2, but VEGFR1 is much less involved with subsequent signal transduction activity than VEGFR2 [12]. VEGFR3 preferentially binds VEGF-C and VEGF-D, and is found primarily with lymphatic endothelial cells. Recent data has demonstrated that VEGFR3 is also located within the vascular endothelium. Targeting VEGFR3 leads to tumor regression in some mouse xenograft studies [13].
Neuropilins are a group of VEGFRs that were described subsequent to initial characterization of the VEGF receptor family. They were originally named based on their role in axonal guidance in the developing nervous system, and were then found expressed on endothelial cells as well as overexpressed on tumor cells. Neuropilins have since been found to be important co-receptors for VEGFR signaling, and also possess intrinsic pro-angiogenic signaling capability. They are promising targets for anti-angiogenic therapy, particularly in combination with cytotoxics and/or other vascular-focused therapy. Development of neuropilin inhibitors is currently underway and awaits clinical investigation [12].
Overview of the market
Despite the promise of anti-angiogenic agents in the treatment of EOC, there are relatively few clinically available drugs available at the current time. There are, however, multiple drugs under study (see below). Each of these agents can be categorized mechanistically. A brief description of each follows (Table 2).
Table 2.
Phase III VEGF-inhibitor trials in epithelial ovarian carcinoma.
| Trial name | Combination | Treatment setting | Anticipated accrual |
|---|---|---|---|
| GOG-218 | Carboplatin, paclitaxel, bevacizumab | Primary | 2000 |
| ICON-7 | Carboplatin, paclitaxel, bevacizumab | Primary | 1444 |
| ICON-6 | Carboplatin, paclitaxel, cediranib‡ | Recurrent | 2000 |
| GOG-213 | Carboplatin, paclitaxel, bevacizumab | Recurrent* | 660 |
| OCEANS | Carboplatin, paclitaxel, bevacizumab | Recurrent* | 440 |
Platinum sensitive.
VEGFR-2 tyrosine kinase inhibitor.
GOG: Gynecologic Oncology Group; ICON: Internationational Collaborative Ovarian Neoplasm.
Monoclonal antibodies targeting the VEGF pathway
VEGF monoclonal antibodies (Mabs) act by antagonizing the VEGF receptors (primarily KDR and FLT1) or their respective ligands. Currently, the most widely used VEGF Mab is bevacizumab, which is a chimeric murine/human antibody targeting the VEGF ligand. It currently has US FDA approval in combination with 5-fluorouracil-based therapy in metastatic colorectal carcinoma, metastatic breast carcinoma in combination with paclitaxel and in nonsquamous, non-small-cell lung cancer patients in combination with carboplatin and paclitaxel [3].
Although not formally approved for EOC, bevacizumab has been widely studied in multiple Phase II trials as both a single agent and in combinations with both cytotoxic and other targeted therapies (Table 3) [7-9]. Several EOC Phase III trials in both the primary and recurrent settings are underway (Table 4).
Table 3.
Bevacizumab in epithelial ovarian carcinoma (Phase II).
| Variable | Cannistra (n = 44) (%) |
Garcia* (n = 70) (%) |
Burger (n = 62) (%) |
|---|---|---|---|
| Previous regimens | |||
| 1 | 100 | 34 | |
| 2 | 52 | ||
| 3 | 48 | ||
| Response rate | |||
| CR | 0 | 0 | 3 |
| PR | 16 | 24 | 18 |
| GI perforations | 11 | 6 | 0 |
| Arterial thrombosis | 7 | 4 | 0 |
| Bevacizumab-related deaths | 7 | 4 | 0 |
| Ref. | [8] | [9] | [7] |
Combined with oral cyclophosphamide.
CR: Complete response; GI: Gastrointestinal; PR: Partial response.
Table 4.
Afibercept-related adverse effects.
| Year | Publication form |
Author | AF doses (mg/kg) |
Combination agent(s) |
G2 events (%) | G3–G4 events (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Phase I | |||||||
| 2008 | Abstract | Rixe | 2–6 | Irinotecan, 5-FU, leucovorin |
Dysphonia (24), epistaxis (18) |
Hypertension (32), proteinuria (11) |
[31] |
| 2008 | Abstract | Freyer | 4, 6 | Docetaxel, cisplatin | Epistaxis (19), proteinuria (17) dysphonia (13) |
Hypertension (13) | [36] |
| 2008 | Absract | Limentani | 2, 3, 4, 5 | Oxaliplatin, 5-FU, leucovorin (FOLFOX-4) |
Not reported | Hypertension (13), proteinuria (13), hemorrhagic events (3), deep vein thrombosis (3) |
[35] |
| 2008 | Abstract | Coleman | 2–6 | Docetaxel 75 mg/kg | Fatigue (33), mucositis (11) |
Hypertension (22), neutropenia (89) |
[37] |
| Phase II | |||||||
| 2007 | Abstract | Tew | 2, 4 | None | Not reported | Hypertension (9), proteinuria (4), encephalopathy (2), renal failure (2) |
[32] |
| 2008 | Unpublished | Coleman | 6 | Docetaxel 75 mg/kg | Study in progress | Study in progress | |
5-FU: 5-fluorouracil; AF: Aflibercept.
Tyrosine kinase inhibitors targeting the VEGF pathway
VEGF tyrosine kinase inhibitors (TKIs) are small molecules that bind specific intracellular domains of VEGFR receptor tyrosine kinases (RTKs), inhibiting phosphorylation and subsequent signal transduction. Most RTK inhibitors are relatively selective, however, not specific for a unique RTK domain. As a result, they function, to varying degrees, as multikinase inhibitors. Commonly, other RTK systems affected are within the EGF and PDGF receptor families. In addition, small-molecule TKIs frequently act on downstream signaling effectors such as Raf, Src and Met [10].
There are no VEGF TKIs currently approved in EOC; however, several have been studied in the Phase II setting and have shown some activity in combination with other agents in heavily pretreated patients [14–23] (Table 5).
Table 5.
Tyrosine kinase inhibitors in epithelial ovarian carcinoma.
| Drug | Generic name | Target | Current trials in ovarian carcinoma |
US FDA approval (indication) |
Ref. |
|---|---|---|---|---|---|
| SU11248 | Sutent | VEGFR, EGFR, PDGFR, c-KIT | Phase II/III | Yes (renal) | [14] |
| ZD6474 | Vandetanib | VEGFR, EGFR | Phase II | Yes | [21] |
| Sorafenib | Sorafenib | RAF-1, VEGFR-2, EGFR-3 PDGFR-β, FLT-3, c-KIT |
Phase I/II/III | Yes (renal) | [16] |
| AZD2171 | Cediranib | VEGFR-1-3, PDGFR, c-KIT | Phase I/II/III | No | [22] |
| GW786034 | Pazopanib | VEGFR1-3, PDGFR-α/β, c-KIT |
Phase II | No | [15] |
| PTK787 | Vatalanib | VEGFR-1-3, PDGFR, c-KIT | Phase I | No | [20] |
| BIBF 1120 | Vargatef | VEGFR, PDGFR, FGFR | Phase I | No | [17] |
| BMS-582664 | Brivanib | VEGFR-2, FGFR | Phase I | No | [18] |
Fusion proteins
Both monoclonal antibodies and TKIs act via direct interaction with binding domains on either soluble VEGF ligands or membrane-bound receptors. Another, unique method to inhibit VEGF-mediated angiogenic signaling involves the use of a soluble fusion protein comprised of truncated VEGFR1 and VEGFR2 binding domains combined with the Fc portion of IgG1. This molecule, via a mechanism similar to that of VEGF Mabs, serves to function as a decoy receptor, binding with high affinity to the VEGF-A ligand and thus preventing VEGFR1 and VEGFR2 binding and subsequent stimulation [24]. Despite the mechanistic similarity between Mabs and fusion proteins, there are clear structural and pharmacokinetic differences between them, the functional significance of which are incompletely understood. The only VEGF fusion protein currently in clinical use is aflibercept.
Introduction to the compound
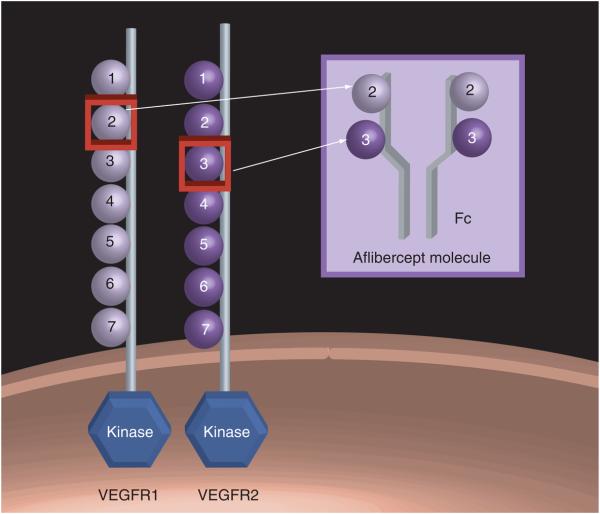
The aflibercept molecule (parental VEGF -Trap) was originally synthesized as a fusion protein combining the constant region (Fc) of IgG1 with the first three domains of VEGFR1 (VEGFδB1). It was found to have impressive, picomolar binding affinity to VEGF ligand and promising anti-tumor activity in transformed cancer cell lines. Unfortunately, it was also found in its parental form to have a significant positive charge, and as a result, to bind nonspecifically to negatively charged extracellular matrix proteins, causing its systemic half-life to be poor. It was then modified to include the Fc region of IgG1 fused with domain two of VEGFR1 and domain three of VEGFR2 (VEGFδR1R2) (Figure 2). This modification served to maintain its high VEGF-A ligand affinity and relative specificity, and to significantly prolong its in vivo half-life, making it clinically useful. It also has strong, picomolar binding affinity for PlGF. All of the aflibercept variants were produced and purified from Chinese hamster ovary cells [24].
Figure 2. Aflibercept/VEGFR1R2 structure.
Domain 2 of VEGFR1 and domain 3 of VEGFR2 complexed with the Fc portion of human IgG1.
Aflibercept has undergone extensive pre-clinical testing to establish it as an effective inhibitor of angiogenesis and tumor growth in animal models. Subcutaneous mouse tumor xenograft studies using cancer cell lines from multiple species (murine melanoma, human rhabdomyosarcoma and rat glioma) were performed. Aflibercept at high (25 mg/kg) and low (2.5 mg/kg) doses was administered subcutaneously twice-weekly. Measurement of tumor volumes at multiple time points from initiation of therapy demonstrated significant regression in tumor in two of the three models. Examination of tumor sections from sacrificed treatment and control mice demonstrated substantial decreases in tumor vascularity in treated mice, particularly those who received high doses [24].
With the knowledge that VEGF plays an important role in the development of malignant ascites, a mouse model of ovarian cancer using VEGF overexpressing SKOV-3 cells was created. Mice were administered aflibercept 25 mg/kg twice-weekly or placebo. Aflibercept treated mice developed minimal to no ascites in comparison to placebo-treated mice. In a similar experiment using OVCAR-3 cells, both inhibition of ascites as well as tumor growth were significantly improved with aflibercept relative to placebo [25].
Initial anti-angiogenic strategies targeting VEGF were tested in preclinical models and in humans who were known to have minimal disease (e.g., early stage or immediately following surgical cytoreduction). It has been postulated that pro-angiogenic growth factors secreted by pericytes found in established or bulky tumors would make them refractory to VEGF antagonism. To test this in the preclinical setting, mice with bulky, orthotopic, SK-NEP-1 (Wilms tumor) xenografts were injected subcutaneously with aflibercept 500 μg or an equivalent placebo volume twice-weekly. Mice were then sacrificed at multiple time points and their tumors were weighed. Significant differences between treated mice and controls were observed at multiple time points, with a 79.3% decrease noted in VEGF treated mice at the longest tested treatment duration (36 days). Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) and platelet endothelial cell adhesion molecule-1 (PECAM-1) staining strongly indicated that apoptosis was the mechanism responsible for the loss of tumor cells [26].
Aflibercept was also evaluated in an orthotopic murine renal cancer cell model. Aflibercept's ability to prevent tumor formation after ortho-topic injection of luciferase-tagged renal cancer cells, as well as its effect on measurable tumor burden, was assessed. In the prevention assay, twice-weekly injections of aflibercept 10 mg/kg versus control were performed starting day 3 or 4 after injection of cancer cells. Luciferase assay at 30 days demonstrated an 87% decrease in tumor growth (p = 0.007), and survival in these mice was increased by 27 days (p < 0.0001). In the intervention assay, aflibercept 10 and 25 mg/kg versus control was administered subcutaneously twice-weekly for up to 30 days. Aflibercept inhibited primary tumor growth by 74 and 78%, respectively by luciferase expression, and this was confirmed by tumor weight ana lysis. Formation of detectable lung metastases was inhibited in 98% of cases (p = 0.004) [27].
Chemistry
As mentioned previously, aflibercept is a soluble fusion protein consisting of the Fc portion of IgG1 combined with the second domain of VEGFR1 and the third domain of VEGFR2. Its molecular weight is 115 kDa, and it is produced from Chinese hamster ovaries [24]. Aflibercept is depicted in Figure 2.
Pharmacokinetics & metabolism
Published data regarding the pharmacodynamic and pharmacokinetic qualities of aflibercept were performed using subcutaneous injection of the drug. Since that time, additional experiments have been performed comparing intra venous dosing with subcutaneous dosing, demonstrating nearly identical bioavailability between the two dosing methods [28].
The pharmacokinetic qualities of aflibercept were obtained by injecting VEGFR1R2 (4 mg/kg) into mice. Cmax and AUC were found to be 16 μg/ml and 36.28 μg × days/ml, respectively. Equilibrium binding assays were also performed, demonstrating a Kd = 1 pM for the predominant VEGF isoform in humans, VEGF165 [24].
Pharmacokinetic studies performed during development of the aflibercept molecule identified that, in comparison with VEGF targeting Mabs, which forms a multimeric immune complex, the Trap molecule forms a 1:1 stable and inert complex with VEGF-A ligand that is measurable and persists systemically for up to 14 days after a single subcutaneous dose. Holash et al. described experiments in murine cancer models where the attainment of an optimal ratio of free VEGF-Trap to complexed VEGF-Trap in measured serum samples correlated with maximal tumor response, allowing the Trap complex to serve as its own biomarker for the achievement of optimal dosing [13].
Pharmacodynamics
Pharmacodynamic performance in vivo was assessed by examining the ability of aflibercept to block dose-dependent hypotension caused by VEGF infusion in mice. A single dose of aflibercept (25 mg/kg) 24 h prior to VEGF administration completely blocked VEGF-induced hypotension. Its in vivo durability was tested by measuring the blockage of hypotension at 1, 3 and 7 days after aflibercept administration (5 mg/kg × 1). It was completely effective at blocking hypotension through day 3, indicating in vivo activity through this duration [24].
One of the potential differences between aflibercept and bevacizumab stems from their different pharmacodynamic properties. As mentioned above, the aflibercept molecule forms a 1:1, relatively low molecular weight complex with the VEGF-A ligand [28]. Bevacizumab, in contrast, is known to form larger molecular weight multimeric immune complexes. Some authors have hypothesized that some of the class effects of VEGF targeted therapies, such as hypertension, proteinuria and arterial thrombosis, are largely a result of immune complex deposition and immunogenicity. For this reason, some authors question whether the smaller molecular weight and presumably less immunogenic aflibercept IC will result in fewer such adverse effects [28]. Interestingly, Meyer et al. recently reported that bevacizumab immune complexes were able to induce platelet aggregation via activation of the FcγRIIa receptor found on human platelets [29].
Clinical efficacy
To date there is only one peer-reviewable published trial regarding aflibercept; however, there have been numerous published abstracts that describe its clinical efficacy in a variety of solid tumors [30]. While it has shown some activity as a single agent, its use in combination with other agents has been more impressive.
A Phase I study of single-agent aflibercept administered intravenously every 2 weeks explored doses ranging between 0.3 and 7.0 mg/kg. Free aflibercept concentrations remained in excess of bound aflibercept concentration during the entire 2-week dosing interval for dose levels at 2.0 mg/ kg and above, suggesting that enough aflibercept was present to bind all endogenous and tumor-produced VEGF [31]. Thus, a 4.0 mg/kg every 2 weeks dose of aflibercept has been utilized in several Phase II studies.
The results of a randomized, double-blind, Phase II study of 215 women with advanced ovarian cancer (predominantly third- and fourth-line therapy) who were treated with single-agent aflibercept at a dose of either 2 mg/kg or 4 mg/kg every 2 weeks were reported in 2007 [32]. Response to treatment was assessed both by the clinical investigators and an independent review committee (IRC). As assessed by the investigators, Response Evaluation In Solid Tumors (RECIST) response rates were 7.3 and 3.8% for the 4 mg/kg and 2 mg/kg dose, respectively. The response rates as assessed by the IRC were 4.6 and 0.9% in the 4 mg/kg and 2 mg/kg arms, respectively. The CA-125 response rates were defined as at least a 50% reduction in CA-125 protein levels, and were approximately 11% in both arms. A total of 18 (13.8%) of the 130 patients evaluable for CA-125 response from the combined groups had either a RECIST (as assessed by the IRC) or CA-125 response. The median progression-free survival was 13.3 and 13.0 weeks with the 4 mg/kg and 2 mg/kg doses, respectively. The median overall survival was 49.3 and 55.4 weeks with the 4 mg/kg and 2 mg/kg doses, respectively. Of the 40 patients in both dose groups who had evaluable ascites at baseline, 77.5% had either a complete disappearance or stabilization of their ascites [32].
Aflibercept is also being evaluated in combination with a variety of chemotherapeutic agents. A Phase I study evaluating aflibercept with docetaxel revealed that it could be safely combined with docetaxel with no increase in docetaxel-related toxicity. The recommended dose was aflibercept 6.0 mg/kg intravenously and docetaxel 75 mg/m2 intravenously every 21 days [33]. There is also a Phase I/II clinical trial in recurrent EOC evaluating aflibercept and docetaxel (in progress). The completed Phase I trial did not identify a maximum tolerated dose, and recommended that the Phase II dose for aflibercept was 6 mg/kg intravenously every 21 days in this combination regimen. There were two out of nine confirmed partial responders in the Phase I; the two-stage designed Phase II has suspended accrual after completing first-stage enrollment and is evaluating for responses in order to determine whether second-stage accrual is warranted [Coleman RC: Update on data analysis regarding VEGF-Trap plus docetaxel in EOC. Pers. Commun.].
Also notable is a Phase I trial performed in patients with advanced ovarian carcinoma and symptomatic ascites. Based on preclinical data describing a dramatic decrease in ascites in a murine model, the goal of this trial was to achieve a repeat paracentesis response rate, defined as a doubling in time between repeat paracentesis procedures compared with a baseline average. Aflibercept 4 mg/m2 was administered every 2 weeks. Eight out of ten evaluable patients achieved a repeat paracentesis response rate. Significant adverse events were notable as well; four out of ten patients experienced a bowel obstruction, one patient experienced a bowel perforation and three out of ten had grade 3/4 nausea and vomiting. Apparent from the abstract is that the majority of enrolled patients were in the terminal phase of their disease, making it less likely that aflibercept was primarily responsible for the adverse events [34].
The current development strategy for aflibercept includes four randomized Phase III trials in combination with standard chemotherapy regimens in patients with prostate, non-small-cell lung, colorectal and pancreatic cancers. These trials are currently underway.
Safety & tolerability
In general, aflibercept has been well-tolerated. A Phase I study in patients with advanced cancer (multiple tumor types) with aflibercept administered intravenously every 2 weeks explored doses ranging between 0.3 and 7.0 mg/kg. Dose-dependent adverse events, particularly hypertension and proteinuria, increased in frequency and severity at doses of 4.0 mg/kg and above [33]. Combination Phase I and II studies have validated these as the primary adverse effects of aflibercept, without significant potentiation when given in combination with docetaxel, irinotecan, 5-fluououracil, cisplatin, gemcitabine and oxaliplatin [31,32,35]. Grade 3/4 hypertension has been described in 9–32% of patients, and proteinuria in 4–13%. Although rare, other well-described anti-angiogenic associated adverse events, such as bowel perforations, bowel obstructions, hemorrhagic events, reversible posterior leukoencephalopathy syndrome and pulmonary emboli, have been described in association with the drug. A summary of adverse effects is illustrated in Table 4 [31,32,35,36]. Overall, it is not clear based on published data whether there is any significant difference in the frequency or severity of the off-target side effects mentioned above when comparing aflibercept and bevacizumab.
Regulatory affairs
Aflibercept has also been identified as VEGF-Trap and AVE0005. (NSC #724770, IND #BB100137). It was developed by Regeneron (NY, USA), and is being developed in collaboration with Sanofi-Aventis Pharmaceuticals (Paris, France). The drug is also distributed by the Clinical Trials Evaluation Program, the Division of Cancer Treatment and Diagnosis and the National Cancer Institute. In addition to the primary tumors mentioned above, ovarian cancer is the subject of a future randomized clinical trial evaluating chemotherapy in combination with aflibercept [Coleman RC: Update on data analysis regarding VEGF-Trap plus docetaxel in EOC. Pers. Commun.].
Executive summary.
■ Epithelial ovarian cancer (EOC) is the predominant form of ovarian cancer, and it is most frequently diagnosed after becoming widely disseminated. Despite its initial chemosensitivity, it is an aggressive disease with few effective treatment options for patients in whom it recurs.
■ There is consensus in the setting of primary treatment regarding cytotoxic chemotherapy agents; however, there is little or no consensus regarding the utility of adjuvant targeted agents in combination with platinum and taxanes in both the setting of primary treatment and recurrence.
■ Clinically available anti-angiogenic agents have shown activity in EOC and can be classified mechanistically as monoclonal antibodies, receptor tyrosine kinase inhibitors, and decoy receptor (Trap) molecules.
■ Aflibercept is a synthetic, soluble decoy receptor with high affinity for the predominant VEGF-ligand. It has impressive anti-tumor activity in murine xenograft models and early clinical trials based on preliminary data. It is well-tolerated and active as monotherapy and in combination therapies in EOC; however, it has shown better clinical activity in combination with cytotoxic agents.
■ The market for VEGF-targeted therapy in EOC is currently dominated by the monoclonal antibody, bevacizumab, which is undergoing multiple Phase III trials in both the primary and recurrent treatment setting. Future competitors, including aflibercept, must compare favorably with regard to clinical efficacy or side effect profile to supplant this agent. Data to support a valid comparison between these two agents as well as other VEGF-targeted therapies has yet to be reported.
Conclusion
Aflibercept has evolved from an experimental molecule with doubtful pharmacokinetic and pharmaco dynamic properties (previous VEGF-Trap variants), to a clinically relevant and clearly active agent in a relatively short period of time. Although peer-reviewable published data are forthcoming, it has to date been a safe and relatively well-tolerated drug, with preliminary reports suggesting anti-tumor activity as a single agent and in combination therapy for EOC patients. Because there is relatively little published prospective data available for aflibercept, it is at this point too early to make helpful comparisons regarding both its safety and efficacy in comparison with bevacizumab or other VEGF-targeted therapies.
Future perspective
Anti-angiogenic therapy has been incorporated into the standard of care (combination therapy) for multiple malignancies, principally in the metastatic and recurrent settings. The only clinically available anti-angiogenic agents are monoclonal antibodies and TKIs, and while both groups contain individual drugs that have demonstrated activity in EOC, there is no clearly superior agent. Other potentially viable strategies for the future include message targeting RNA ribozymes, VEGF antisense and siRNA. Because it has been US FDA labeled and used extensively in multiple cancers, bevacizumab has emerged as the default anti-angiogenic agent by which all future drugs of this nature will be compared. Aflibercept appears to be similar to bevacizumab with regard to safety and tolerability; however, it is not possible at this time to offer an adequate, evidence-based comparison in terms of clinical efficacy. Because of its affinity for PlGF, aflibercept does offer the potential to bind more ligands in the tumor microenvironment, but whether this capacity will translate into better tumor control is yet to be elucidated. It is also unclear whether or not the pharmacodynamic properties of aflibercept will translate into an improved adverse effect profile in comparison with other anti-angiogenic therapies, particularly bevacizumab.
Another important clinical question for aflibercept that remains is whether it would be better suited for EOC patients in the primary or recurrent setting. Bevacizumab, as the first available anti-angiogenic agent, is currently being evaluated in both groups of patients. It is likely that future clinical development will position aflibercept in similar settings, particularly if efficacy is demonstrated in recurrent disease.
Acknowledgments
This work was supported in part by the UTMD Anderson SPORE (P50 CA083639) and the Marcus Foundation.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Choi M, Fuller CD, Thomas CR, Jr, et al. Conditional survival in ovarian cancer: results from the SEER dataset 1988–2001. Gynecol. Oncol. 2008;109:203–209. doi: 10.1016/j.ygyno.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Bevacizumab . US Food and Drug Administration label. MD, USA: 2007. [Google Scholar]
- 4■.Bookman MA. GOG0182-ICON5: 5-arm Phase III randomized trial of paclitaxel (P) and carboplatin (C) vs combinations with gemcitabine (G), PEG-liposomal doxorubicin (D), or topotecan (T) in patients (pts) with advanced-stage epithelial ovarian (EOC) or primary peritoneal (PPC) carcinoma; Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL, USA: 2006. Abstract 5002. This large, prospective study was the first to demonstrate that triplet cytotoxic regimens were not better than the standard of platinum and taxane doublet in advanced epithelial ovarian cancer (EOC) [Google Scholar]
- 5.Markman MLP, Wilczynski S, Monk B, et al. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. J. Clin. Oncol. 2003;21(13):2460–2465. doi: 10.1200/JCO.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Teng NN. NCCN Practice Guidelines in Oncology – v.1.2008: Ovarian Cancer. National Comprehensive Cancer Network. 2008 [Google Scholar]
- 7.Burger RA, Sill MW, Monk BJ, et al. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J. Clin. Oncol. 2007;25:5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 8.Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J. Clin. Oncol. 2007;25:5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 9.Garcia AA, Hirte H, Fleming G, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital Phase II consortia. J. Clin. Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 10.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 11.Ribatti D. The discovery of the placental growth factor and its role in angiogenesis: a historical review. Angiogenesis. 2008;11:215–221. doi: 10.1007/s10456-008-9114-4. [DOI] [PubMed] [Google Scholar]
- 12■.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat. Rev. Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. Excellent review of VEGF signaling and its role in malignancy. [DOI] [PubMed] [Google Scholar]
- 13■.Holash J, Thurston G, Rudge JS, et al. Inhibitors of growth factor receptors, signaling pathways and angiogenesis as therapeutic molecular agents. Cancer Metastasis Rev. 2006;25:243–252. doi: 10.1007/s10555-006-8504-6. Reviews the development of the current VEGF-Trap molecule. [DOI] [PubMed] [Google Scholar]
- 14.Mendel D. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 15.Biagi JJOA, Grimshaw R, Ellard SL, et al. A Phase II study of sunitinib (SU11248) in patients (pts) with recurrent epithelial ovarian, fallopian tube or primary peritoneal carcinoma – NCIC CTG IND 185; Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL, USA: 2008. Abstract 5522. [Google Scholar]
- 16.Friedlander M. Pazopanib (GW 786034) is active in women with advanced epithelial ovarian, fallopian tube and peritoneal cancers: Initial results of a Phase II study; Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL, USA: 2007. Abstract 5561. [Google Scholar]
- 17.Garofalo A. The combination of the tyrosine kinase receptor inhibitor SU6668 with paclitaxel affects ascites formation and tumor spread in ovarian carcinoma xenografts growing orthotopically. Clin. Cancer Res. 2003;9:3476–3485. [PubMed] [Google Scholar]
- 18.Harter HJ, Pfisterer J, Wimberger P, et al. A Phase I dose escalation and pharmacokinetic study of BIBF 1120 in combination with paclitaxel and carboplatin in patients with advanced gynecological malignancies; Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL, USA: 2007. Abstract 3561. [Google Scholar]
- 19.Jonker D. A Phase I study of BMS-582664 (brivanib alaninate), an oral dual inhibitor of VEGFR and FGFR tyrosine kinases, in patients (pts) with advanced/metastatic solid tumors: Safety, pharmacokinetic (PK), and pharmacodynamic (PD) findings; Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL, USA: 2007. Abstract 3559. [Google Scholar]
- 20.Matei SM, DeGeest K, Bristow RE. Phase II trial of sorafenib in persistent or recurrent epithelial ovarian cancer (EOC) or primary peritoneal cancer (PPC): A Gynecologic Oncology Group (GOG) study; Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL, USA: 2008. Abstract 5537. [Google Scholar]
- 21.Schroder W, Campone M, Abadie S, et al. A Phase IB, open label, safety and pharmacokinetic study of escalating doses of PTK787/ZK in combination with paclitaxel and carboplatin in patients with Stage IC to IV epithelial ovarian cancer; Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL, USA: 2006. Abstract 5075. [Google Scholar]
- 22.Wedge S. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002;62:4645–4655. [PubMed] [Google Scholar]
- 23.Wedge S. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 24■■.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc. Natl Acad. Sci. USA. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. Thorough review of the construction and development of the VEGF-Trap molecule, as well as a review of much of the preclinical work performed to justify entry into clinical trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrne AT, Ross L, Holash J, et al. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin. Cancer Res. 2003;9:5721–5728. [PubMed] [Google Scholar]
- 26.Konner J, Dupont J. Use of soluble recombinant decoy receptor vascular endothelial growth factor trap (VEGF-Trap) to inhibit vascular endothelial growth factor activity. Clin. Colorectal. Cancer. 2004;4(Suppl 2):S81–S85. doi: 10.3816/ccc.2004.s.013. [DOI] [PubMed] [Google Scholar]
- 27.Verheul HM, Hammers H, van Erp K, et al. Vascular endothelial growth factor trap blocks tumor growth, metastasis formation, and vascular leakage in an orthotopic murine renal cell cancer model. Clin. Cancer Res. 2007;13:4201–4208. doi: 10.1158/1078-0432.CCR-06-2553. [DOI] [PubMed] [Google Scholar]
- 28■.Rudge JS, Holash J, Hylton D, et al. Inaugural Article: VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proc. Natl Acad. Sci. USA. 2007;104:18363–18370. doi: 10.1073/pnas.0708865104. Describes a series of assays that describe how VEGF-Trap levels can be reliably used to serve as a biomarker for optimal dosing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29■■.Meyer T, Robles-Carrillo L, Robson T, et al. Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J. Thromb. Haemost. 2009;7:171–181. doi: 10.1111/j.1538-7836.2008.03212.x. Recent paper providing a possible mechanism for bevacizumab-related thrombosis. [DOI] [PubMed] [Google Scholar]
- 30.Fricke I, Mirza N, Dupont J, et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin. Cancer Res. 2007;13:4840–4848. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- 31.Rixe VC, Khayat D, Tejpar S, et al. A Phase I dose escalation (DE) and pharmacokinetics (PK) study of intravenous aflibercept (VEGF Trap) plus irinotecan, 5-fluorouracil, and leucovorin (I-LV5FU2) in patients with advanced solid tumors (STs); Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL, USA: 2008. Abstract 3557. [Google Scholar]
- 32■■.Tew CN, Ray-Coquard I, Oza A, del Campo J, Scambia G, Spriggs D. VEGF-Trap for patients with recurrent platinum-resistant epithelial ovarian cancer (EOC): preliminary results of a randomized, multicenter Phase II study; Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL, USA: 2007. Abstract 5508. The largest Phase II evaluation of VEGF-Trap in EOC. [Google Scholar]
- 33.Isambert N. A Phase I dose escalation and pharmacokinetic (PK) study of intravenous aflibercept (VEGF Trap) plus docetaxel (D) in patients (pts) with advanced solid tumors: Preliminary results; Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL, USA: 2008. Abstract 3599. [Google Scholar]
- 34.Colombo N. Aflibercept (VEGF Trap) for advanced epithelial ovarian cancer (EOC) patients (pts) with symptomatic malignant ascites: Preliminary results of a pilot study; Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL, USA: 2008. Abstract 14598. [Google Scholar]
- 35.Limentani JR, Purdham A, Bair A, Tamby L, Chap I, Rosen S. A Phase I dose escalation and pharmacokinetic (PK) study of intravenous (iv) aflibercept (VEGF Trap) plus FOLFOX4 in patients (pts) with advanced solid tumors: Preliminary results; Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL, USA: 2008. Abstract 3556. [Google Scholar]
- 36.Freyer G. A Phase I dose escalation and pharmacokinetic (PK) study of intravenous (iv) aflibercept (VEGF Trap) plus docetaxel (D) and (C) in patients (pts) with advanced solid tumors: preliminary results; Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL, USA: 2008. Abstract 3556. [Google Scholar]
- 37.Coleman RL. Interim efficacy and safety from a Phase I/II study of aflibercept + docetaxel in patients with recurrent epithelial ovarian carcinoma; Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting; Chicago, IL, USA: 2008. Abstract 5549. [Google Scholar]