Abstract
Nitric oxide is well established as a major signaling molecule. Evidence is accumulating that carbon monoxide and hydrogen sulfide also are physiologic mediators in the cardiovascular, immune, and nervous systems. This Review focuses on mechanisms whereby they signal by binding to metal centers in metalloproteins, such as in guanylyl cyclase, or modifying sulfhydryl groups in protein targets.
Introduction
Signaling molecules come in all sizes and chemical dispositions, ranging from relatively large proteins, lipids, and peptides through biogenic amines and amino acids, to gaseous molecules. Variations in the properties of signaling molecules that depend upon their chemical nature are best exemplified among neurotransmitters. Amine, amino acid, and peptide neurotransmitters share a number of features. All are stored in synaptic vesicles, so that with each nerve impulse only a small proportion of the stores is released, leaving a large safety net of reserve pools. Release involves exocytosis, in which the vesicle fuses with the plasma membrane to expel its contents. These neurotransmitters bind to receptor proteins on the external surface of adjacent membranes. Inactivation occurs by reuptake of neurotransmitters into the releasing nerve terminal or adjacent glia, by enzymatic degradation, or by simple diffusion away from the synapse. Nitric oxide (NO) was the first identified gasotransmitter, a term that refers to a gaseous messenger molecule involved in any signaling process. More recent studies have established carbon monoxide (CO) and hydrogen sulfide (H2S) as physiologically relevant gasotransmitters.
The gasotransmitters have overturned conventional concepts of intercellular communication. For instance, a gaseous substance is not readily stored in vesicular structures and so must be resynthesized as needed. This implies that, rather then supply depending on regulation of exocytosis, the biosynthetic enzymes must be subject to extraordinarily nuanced regulatory mechanisms. Instead of binding to plasma membrane receptors, gasotransmitters diffuse into adjacent cells to interact with their targets. Two of the gasotransmitters, NO and H2S, display notable chemical reactivity. Thus, were they to diffuse randomly throughout cells, they would likely be intercepted and inactivated by substances such as glutathione, which is present in abundant concentrations. Instead, NO typically reaches its targets through the binding of its biosynthetic enzyme, one or another form of NO synthase (NOS), to the protein target. Alternatively, a scaffolding protein such as CAPON (carboxy-terminal PDZ ligand of neuronal NO synthase protein) delivers NOS to the target. Similar regulatory mechanisms may also exist to direct H2S to specific targets, though none have been reported.
Perhaps the most remarkably unique feature of gasotransmitters relates to the molecular mechanisms whereby they signal to their targets. Classical messenger molecules act through amplifying signal cascades. For instance, hormones and neurotransmitters that act through G protein (heterotrimeric guanine nucleotide–binding protein)–coupled receptors (GPCRs) elicit alterations in properties of the G proteins, which in turn affect enzymes that generate cyclic nucleotides or inositol 1,4,5-trisphosphate (IP3). The cyclic nucleotides influence various protein kinases, whereas IP3 releases intracellular calcium, which in turn affects diverse intracellular proteins. Peptides and proteins that act through tyrosine kinase receptors elicit a distinct but similarly lengthy sequence of molecular events. By contrast, gasotransmitters chemically modify intracellular proteins, thus affecting cellular metabolism in a more immediate fashion. These signaling processes make up the bulk of this review.
Gasotransmitter Properties
Nitric oxide
NO was first identified as the mediator of two processes, blood-vessel relaxation (1) and macrophage activation (2–4). The classic work of Robert Furchgott established that substances such as acetylcholine do not relax blood vessels by acting directly on the smooth muscle but instead activate the endothelium to produce a diffusible substance, endothelial-derived relaxing factor (EDRF), which was subsequently indentified as NO (Table 1 and Fig. 1) (5–7). Independently, other investigators sought the molecule(s) that enables activated macrophages to kill tumor cells and bacteria and identified NO as a culprit. Rapid enhancement of our understanding of NO function came with the isolation and molecular cloning of neuronal NO synthase (nNOS, also known as NOS1), enabling the subsequent cloning of the macrophage form, inducible NO synthase (iNOS, also known as NOS2) and endothelial NO synthase (eNOS, also known as NOS3) (8). These enzymes generate NO from a guanidino nitrogen of arginine, with citrulline formed as a by-product. The NOS isoforms are heavily regulated. Their oxidative functions are mediated by FAD (flavin adenine dinucleotide), FMN (flavin mononucleotide), and tetrahydrobiopterin, and they are phosphorylated by a wide range of serine kinases. Swift activation of eNOS and nNOS reflects calcium-calmodulin stimulation, a rapid but evanescent process. eNOS is regulated by its translocation between intracellular caveolar structures and the plasma membrane (9). For continuous stimulation in blood vessels, viscous drag and shear stress activate the phosphoinositide 3-kinase (PI 3-kinase)–Akt phosphorylation pathway to elicit long-term activation of eNOS (10). This is exemplified in penile erection, which involves vasodilation of the corpora cavernosae with erection being initiated by calcium-calmodulin activation of nNOS followed by Akt stimulation of eNOS (11). By contrast, enhancement of iNOS function predominantly involves marked augmentation of new protein synthesis, hence the term “inducible” NOS. Within 1 to 2 hours of endotoxin stimulation of macrophages, very large amounts of iNOS are synthesized to produce the substantial quantities of NO needed to attack bacteria or tumor cells. These are just a few examples of the myriad facets of NO biology.
Table 1.
Signaling pathways for NO, H2S, and CO in the vasculature. ACh, acetylcholine; SMC, smooth muscle cell.
| Vasculature | NO | H2S | CO |
|---|---|---|---|
| Synthetic enzymes | eNOS | CSE | HO2 |
| Endothelial localization | Yes | Yes | Yes |
| Activation signal | ACh | ACh | ACh |
| Ca2+-CaM regulation | Yes | Yes | Yes |
| Downstream target(s) | sGC-cGMP* | K+ channel (KATP)? | sGC-cGMP |
| Target location | SMC | SMC | SMC |
| Chemical modification (Cys) | S-nitrosylation? | S-sulfhydration | ? |
| Vasorelaxation | Yes | Yes | Yes |
| KO phenotype | Hypertension | Hypertension | ? |
| Concentration (μM) | <0.2 | 40−150 | ? |
NO has various other targets in vascular smooth muscle in addition to sGC.
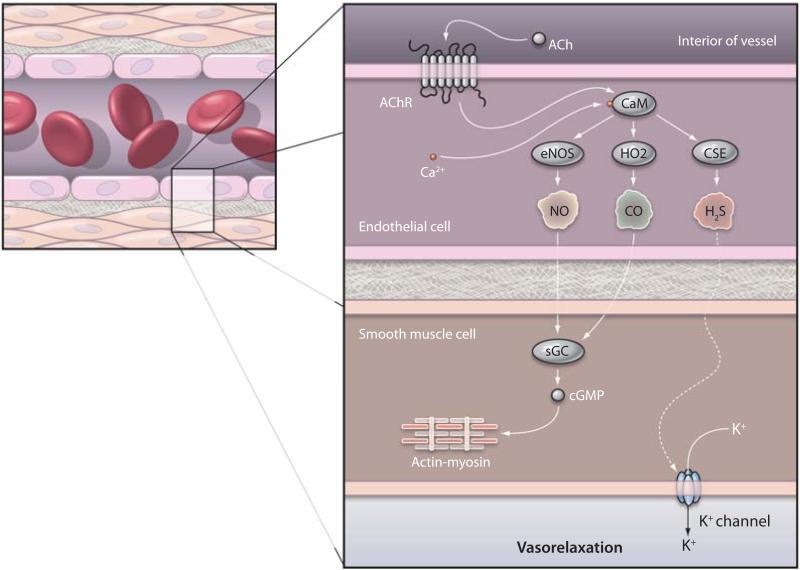
Fig. 1.
Physiologic vasorelaxation by NO, CO, and H2S. Stimulation of muscarinic acetylcholine receptors (AChRs) on endothelial cells activates Ca2+-calmodulin (CaM), which in turn binds to and stimulates endothelial NO synthase (eNOS), cystathionine γ-lyase (CSE), and HO2 to produce NO, H2S, and CO, respectively. NO and CO diffuse into the adjacent smooth muscle cells and activate soluble guanylyl cyclase (sGC) to produce cyclic GMP (cGMP), ultimately affecting the actin-myosin cross-bridge. H2S also diffuses into smooth muscle cells, where it likely activates K+ channels. These actions of all three gases lead to vasorelaxation.
The carrier protein CAPON delivers nNOS to its targets. The C terminus of CAPON interacts with the PDZ domain of nNOS, competing with the binding of nNOS to PSD95 (postsynaptic density protein–95) (12). Glutamate, acting through its N-methyl-D-aspartate (NMDA) subtype receptor, signals to nNOS via PSD95 (Table 2) (13). Thus, CAPON may regulate nNOS by influencing its ability to associate with PSD95-NMDA receptor complexes. CAPON also binds to Dexras1, a brain-enriched member of the Ras family of small guanosine triphosphatases, thereby linking Dexras1 to nNOS. The generated NO serves as a guanine nucleotide exchange factor for Dexras1 (14). Dexras1, in turn, via the benzodiazepine receptor–associated protein (PAP7), is linked to divalent metal transporter 1 (DMT1), which mediates iron accumulation in cells. NMDA receptor activation, via nNOS, leads to S-nitrosylation and activation of Dexras1, which physiologically induces iron uptake through DMT1. This iron accumulation may contribute to NMDA neurotoxicity, because selective iron chelation prevents NMDA toxicity (14).
Table 2.
How NO, H2S, and CO signal in the nervous system. EC, endothelial cell; GI, gastrointestinal; KO, knockout; mGluR, metabotropic glutamate receptor.
| Nervous system | NO | H2S | CO |
|---|---|---|---|
| Synthetic enzymes | nNOS | CBS | HO2 |
| Cellular localization | Neuron, EC, etc. | Neuron (and glia?) | Neuron |
| Activation signal | NMDA receptors | ? | mGluR |
| Ca2+ regulation | Ca2+-CaM | ? | Ca2+-CaM |
| Downstream target(s) | sGC-cGMP, etc. | K+ channel, etc. | sGC-cGMP |
| Chemical modification (Cys) | S-nitrosylation | S-sulfhydration | ? |
| KO phenotype | Resists ischemia aggression | Developmental deficits | Decreased GI motility |
| Concentration (μM) | <0.1 | 50−160 | 2* |
See (73).
Carbon monoxide
Whereas NO was isolated as the chemical mediator of an important biological function, CO was known to be formed physiologically long before much attention had been devoted to its function (15). Heme oxygenase 1 (HO1) is an inducible enzyme responsible for degrading heme that exits aging red blood cells. HO1 cleaves the heme ring to the straight-chain biliverdin, thereby releasing CO. Biliverdin is rapidly reduced to bilirubin by biliverdin reductase, which occurs in high densities in cells so that biliverdin rarely accumulates. Bilirubin was long thought to be only a toxic end product, which in high concentrations gives rise to jaundice in newborn infants and which in excess can cause kernicteric damage to the brain. Bilirubin is now appreciated as a physiologic antioxidant cytoprotectant comparable in importance to glutathione (16). The lipophilic bilirubin protects cell membranes against lipid peroxidation, whereas the water-soluble glutathione protects soluble proteins (17). Low tissue concentrations of bilirubin can protect against much higher concentrations of reactive oxygen species through a cycle in which the antioxidant actions of bilirubin are accompanied by its oxidation to biliverdin, upon which it is immediately reduced back to bilirubin by biliverdin reductase (16).
The anti-stress and anti-inflammatory role of HO1 may involve CO as well as bilirubin. Exogenous CO inhibits the formation of pro-inflammatory cytokines and increases the production of anti-inflammatory cytokines (18). CO also prevents arteriosclerotic lesions by inhibiting smooth muscle cell proliferation (19). Following vascular injury, activation of HO1 directly reduces vasoconstriction and inhibits cell proliferation (19). CO stabilizes hypoxia-inducible factor 1α (HIF1α), leading to cytoprotection (20).
HO1 is induced following virtually all forms of cell stress and is even designated a heat shock protein. This induction presumably affords cytoprotection through bilirubin and CO. By contrast, iNOS, via its product NO, mediates inflammatory responses of macrophages. Like heme oxygenase, NOS generates two products, NO and citrulline. Citrulline is a component of the urea cycle but, in contrast to bilirubin, is not thought to act itself as a messenger molecule.
In analogy to distinctions between the inducible iNOS and the constitutive nNOS and eNOS, a constitutive heme oxygenase 2 (HO2) exists. Moreover, like eNOS and nNOS, HO2 is activated by calcium-calmodulin (21). Just as phosphorylation of NOS isoforms can augment their activity, HO2 is phosphorylated and activated by casein kinase 2 (CK2) (22).
CO and NO have several functional parallels. HO2, like eNOS, is localized to the endothelial layer of blood vessels (Table 1 and Fig. 1) (23). CO relaxes blood vessels and may be an EDRF, although the evidence for this is much weaker than for NO and H2S (see below). Both HO2 and nNOS are localized to discrete populations of neurons in the brain with some overlap (Table 2) (24). In the myenteric plexus of the intestines, HO2 and nNOS are colocalized to many neurons and both mediate nonadrenergic, noncholinergic neurotransmission (25–27).
Hydrogen sulfide
Effects of H2S on numerous organs were well characterized before unequivocal evidence for its physiologic enzymatic formation in mammals was obtained. Like NO, H2S is chemically reactive and for years was regarded as a noxious toxin. However, H2S gas or H2S donors had long been known to relax blood vessels and to relieve hypoxic pulmonary hypertension and ischemia-reperfusion injury in the heart, to reduce leukocyte adherence in the blood vessels, to be anti-inflammatory, and to reduce blood vessel restenosis (28). H2S gas has been evaluated for possible clinical actions, and sulfide-releasing nonsteroidal anti-inflammatory drugs (NSAIDs) as well as other H2S-related agents have been developed by pharmaceutical companies. H2S induces a suspended animation–like state in mice, and the resulting reduced metabolic demands are potentially useful in improving outcomes after surgery or stroke (29). In Caenorhabditis elegans, H2S exposure renders the worms tolerant to heat stress and increases their life span (30). The reduced cardiovascular risk associated with ingestion of garlic appears to be related to the generation of H2S in red blood cells from garlic-derived organic polysulfides (31).
Very recently, physiologic biosynthetic pathways for H2S have been established. It has long been known that cystathionine γ-lyase (cystathionase; CSE) and cystathionine β-synthase (CBS) can generate H2S from cysteine. Abe and Kimura (32, 33) showed that CBS inhibitors decrease production of H2S in the brain, whereas the CBS activator S-adenosylmethionine augments H2S formation. However, definitive evidence that H2S is generated in intact organisms by these enzymes was lacking. Recent studies show that targeted deletion of CSE leads to a profound decrease in H2S generation in the aorta, heart, and serum, although not in the brain (34). CBS appears to be the primary H2S-synthesizing enzyme in the brain, as deletion of CBS leads to loss of H2S formation by brain tissue (Table 2) (35). Like HO2, nNOS, and eNOS, CSE activity is calcium-calmodulin dependent (34). A role for calcium-calmodulin in CBS activity has not been established. Neither CSE nor CBS are dramatically inducible in the manner of iNOS and HO1.
Like NO, H2S is a robust EDRF (Table 1 and Fig. 1) (34). Like eNOS and HO2, CSE protein is highly localized to the endothelial layer of blood vessels. Moreover, cholinergic relaxation of blood vessels is profoundly reduced in CSE knockout mice. The CSE knockouts manifest increased blood pressure comparable to that of eNOS knockouts. The extent to which NO, H2S, and CO contribute to overall EDRF activity in different vascular beds remains to be determined.
Signaling Targets
Nitric oxide
NO signals in multiple ways. One of its first elucidated functions, mediating macrophage-elicited tumoricidal and bactericidal actions, involves reactions of NO with superoxide to generate peroxynitrite. This reactive species in turn decomposes to the highly toxic hydroxyl free radical which, along with other related reactive oxygen species, damages multiple intracellular organelles.
Relaxation of blood vessels was known to involve cyclic guanosine monophosphate (cGMP) long before the identification of NO as an EDRF. cGMP activates cGMP-dependent protein kinase to phosphorylate myosin and other proteins that regulate muscle contractility. NO activates the cGMP-forming enzyme guanylyl cyclase by binding with nanomolar affinity to iron in the heme at the active site of the enzyme (Table 1 and Fig. 1). NO influences numerous mitochondrial heme proteins through a similar mechanism. For instance, NO may directly modulate cellular energetics by interfering with the O2 binding of cytochrome c oxidase, altering its ability to catalyze the oxidation of cytochrome c and the reduction of O2 to water (36).
NO also binds to the iron-sulfur cluster of cytosolic aconitase, also known as iron regulatory protein 1 (IRP1) (37). This enzyme interconverts between an active cytosolic enzyme and an RNA-binding protein. In iron-depleted cells, cytosolic aconitase loses its enzymatic activity and functions as IRP1, binding to specific RNA stem-loop structures called iron-responsive elements, whereas in iron-replete cells the converse takes place, with the enzymatic activity of aconitase returning. By binding to the mRNA of ferritin and of the transferrin receptor, IRP1 controls the iron homeostasis of cells. Glutamate-NMDA neurotransmission, via NO, stimulates the IRE-binding function of IRP1 and diminishes its cytosolic aconitase activity.
The chemical reactivity of NO is particularly well suited for interacting with sulfhydryl groups of cysteine. Elegant studies of Stamler and associates (38) established that NO can S-nitrosylate numerous proteins (Table 3). Because S-nitrosylation is extremely labile, demonstrating that proteins are physiologically nitrosylated under basal conditions was challenging. The development of the biotin-switch method for identifying endogenously nitrosylated proteins established definitively that a number of prominent proteins in the brain are basally nitrosylated, with this modification vanishing in mice with targeted deletion of nNOS (39). In the initial study, proteins shown to be basally nitrosylated in wild-type but not nNOS knockout mice included glyceraldehyde-3-phosphate dehydrogenase (GAPDH), glycogen phosphorylase, creatine kinase, retinoblastoma gene product (Rb), heat-shock protein-72 (HSP72), sodium/potassium adenosine triphosphatase (ATPase), the NMDA receptor, β-tubulin, actin, and neurofilament heavy protein. Thus, in these first experiments, the range of endogenously nitrosylated proteins was considerable and included metabolic enzymes, ion channels, neurotransmitter receptors, a sodium-pumping enzyme, and structural proteins. Identifying nitrosylation by the biotin-switch or related techniques, and demonstrating loss of signal in NOS-depleted tissues, are now regarded as essential criteria in establishing the physiologic relevance of a protein's nitrosylation. Numerous proteins have now been shown to be physiologically nitrosylated. Here, we focus upon some well-characterized examples that illustrate certain principles.
Table 3.
Selected physiologic targets of NO, H2S, and CO.
| Selected protein targets | NO | H2S | CO | References |
|---|---|---|---|---|
| Dexras1 | ↑ Activity | (14) | ||
| HIF1α | ↑ Stability/Activity | ↑ Stability/Activity | (20, 61) | |
| Arginase | ↑ Activity | (40) | ||
| Serine racemase | ↓ Activity | (42) | ||
| NSF | ↑ Activity | (43, 44) | ||
| ↓ Exocytosis of Weibel-Palade bodies | ||||
| ↑ Surface AMPA receptor | ||||
| GRK2 | ↓ Activity | (45) | ||
| β-Arrestin 2 | ↑ β-Adrenergic receptor endocytosis | (46) | ||
| RyR1 | ↑ Temperature sensitivity | (47, 48) | ||
| TRP channels | ↑ Activity | (49) | ||
| MAP1B | ↑ Binding to microtubule | (50) | ||
| ↑ Neurite retraction | ||||
| PDI | ↓ Activity | (51) | ||
| Parkin | ↓ Activity | (52) | ||
| COX2 | ↑ Activity | (53, 58) | ||
| GAPDH | ↓ Activity | ↑ Activity | (54, 55, 72) | |
| Nuclear translocation | ||||
| Ras | ↑ Activity | (56) | ||
| MMP-9 | ↑ Activity | (57) | ||
| CREB | ↑ DNA binding | (62) | ||
| HDAC2 | ↑ Chromatin remodeling | (63) | ||
| E75 | ↓ Activity | (68) | ||
| NPAS2 | ↑ Activity | (69) | ||
| KATP | ↑ Activity | (71) |
A number of enzymes and other proteins related to neurotransmission are nitrosylated. The generation of NO by NOS from arginine can be influenced by consumption of arginine by arginase. Nitrosylation of arginase increases its affinity for arginine about sixfold (40). Nitrosylation of arginase is markedly increased in blood vessels of aging rats; this leads to increased arginase activity with less arginine available for eNOS, accounting for the impaired NO bioavailability and endothelial dysfunction characteristic of aging.
NO directly influences the biosynthesis of D-serine, which is a co-agonist with glutamate at NMDA receptors (41). Serine racemase, which converts L-serine to D-serine, is physiologically nitrosylated, which inhibits its enzymatic activity (42). NO is formed in response to NMDA transmission and may diffuse in a retrograde manner to cells generating D-serine as a form of feedback inhibition.
The exocytotic release of chemical mediators and surface expression of neurotransmitter receptors may be regulated by nitrosylation of NSF (N-ethylmaleimide–sensitive factor), an ATPase that modulates vesicular transport. Nitrosylation of NSF influences exocytosis of granules from endothelial cells (43). Glutamate, the major excitatory neurotransmitter in brain, mediates excitation primarily through α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)–type receptors. Recycling of AMPA receptors by endocytosis followed by surface expression is a principal mechanism for the synaptic plasticity that underlies learning and memory. NSF facilitates the recycling of AMPA receptors into synaptic membranes (44). S-nitrosylation of NSF enhances its binding to AMPA receptors, which leads to their increased surface expression. In this way, NO is a major regulator of synaptic plasticity.
Nitrosylation also regulates critical proteins that influence GPCRs. GPCR kinases (GRKs) attenuate G-protein signaling and target GPCRs for internalization. Nitrosylation of the GRKs inhibits their activity, leading to diminished phosphorylation and increased surface expression of the receptors (45). The scaffolding protein β-arrestin binds activated GPCRs to inhibit their coupling to G proteins. Nitrosylation of β-arrestin promotes its binding to clathrin and β-adaptin, which accelerates the internalization of GPCRs bound to β-arrestin (46). Thus, nitrosylation of GRK and β-arrestin exert opposite effects upon surface expression of GPCRs.
Various ion channels are also nitrosylated, a modification that sometimes has major clinical relevance. The ryanodine receptor (RyR), a calcium channel that occurs ubiquitously but is most prominent in cardiac and skeletal muscle, is S-nitrosylated under basal conditions (47, 48). nNOS knockout mice show impaired RyR S-nitrosylation, which alters calcium dynamics. Diastolic calcium concentrations are increased in the hearts of the knockouts, presumably reflecting diastolic calcium leakage. This leads to defects in cardiac electrical stability, predisposing toward arrhythmias.
Mutations in the RyR of skeletal muscle occur in patients who are sensitive to environmental heat stroke as well as in patients who develop malignant hyperthermia, a related condition in which elevated body temperature leads to uncontrolled muscle contractions that can be triggered by halogenated anesthetics such as halothane and isofluorane (47). Exercise-induced sudden death often occurs in young healthy individuals with family histories of malignant hyperthermia. Hamilton and associates (47) have shown that the enhanced calcium leak with RyR mutations that in humans are associated with malignant hyperthermia leads to increased oxidative and nitrosative stress, which causes RyR nitrosylation. This further augments the calcium leak and, in a feed-forward system, increases susceptibility to heat-induced sudden death.
Transient receptor potential (TRP) proteins are cation channels that respond to numerous stimuli. Most TRP channels appear to be physiologically nitrosylated (49). The nitrosylated cysteines are uniformly associated with the N-terminal side of the pore region of the channel. Nitrosylation of TRPC5 following activation of the G protein–coupled ATP receptor regulates channel function.
Numerous cytoskeletal proteins are regulated by nitrosylation. The microtubule-associated protein-1B (MAP1B) binds actin as well as microtubules, is necessary for neuronal migration, and has been implicated in neurological disorders such as fragile X syndrome. It is nitrosylated under basal conditions (50). nNOS binds MAP1B, thereby “delivering” NO to its target (50). Nitrosylation of MAP1B increases microtubule binding and causes neurite retraction. Activation of nNOS leads to axon retraction, which is lost in MAP1B-deleted tissues, implying that nitrosylation of this protein is responsible for NO-induced axon retraction.
Protein disulf ide isomerase (PDI) regulates the maturation and transport of unfolded secretory proteins by catalyzing thioldisulfide exchange. In the brains of Parkinson's or Alzheimer's disease patients, PDI is nitrosylated, which inhibits its catalytic activity and leads to the accumulation of polyubiquitinated proteins (51). PDI physiologically attenuates neuronal cell death caused by stressors; nitrosylation impairs this response, which may contribute to the pathophysiology of neurodegenerative diseases.
An analogous situation occurs with parkin, an E3 ubiquitin ligase whose ubiquitination of proteins helps dopamine neurons survive. Nitrosylation of parkin in a mouse model of Parkinson's disease may participate in pathophysiology, because nitrosylation inhibits parkin's E3 ligase activity and protective function (52).
Nitrosylation plays a direct role in a prominent signaling pathway underlying apoptotic cell death. Cell stressors lead to induction of iNOS in multiple tissues (2), whereas neurotoxic insults activate nNOS in the brain (53). The resultant NO nitrosylates the glycolytic enzyme GAPDH terminating its catalytic activity but conferring upon it the ability to bind to Siah1, an E3 ubiquitin ligase that possesses a nuclear localization signal (54). Siah1 “escorts” GAPDH to the nucleus. In the nucleus GAPDH is acetylated by the acetyltransferase p300/CREB binding protein (CBP), which in turn stimulates the acetylation and catalytic activity of p300/CBP. Downstream targets of p300/CBP, such as p53, are then activated and mediate apoptosis (55).
In a contrasting model, nitrosylation participates in the maintenance of tumors. The protein kinase Akt phosphorylates many targets involved in enhancing cell and tumor growth. Selective blockade of its phosphorylation of eNOS inhibits tumor initiation and maintenance (56). NO generated by eNOS nitrosylates and activates endogenous wild-type Ras proteins that are required for tumorigenesis.
Inflammatory mediators are also sometimes involved in cell death as exemplified by matrix metalloproteinases (MMPs). MMPs have been implicated in the pathophysiology of neurodegenerative diseases, because MMP inhibitors or genetic deletion of MMP-9 reduce vascular stroke damage. During cerebral ischemia MMP-9 colocalizes with nNOS, leading to nitrosylation of MMP-9, which activates its catalytic activity and is associated with neuronal apoptosis (57).
General inflammatory responses may also involve nitrosylation. Prostaglandin formation by cyclo-oxygenase-2 (COX2) and NO formed by iNOS are two predominant small-molecule mediators of inflammation. They appear to be functionally linked by nitrosylation (58). Thus, iNOS and COX2 physiologically bind to each other; NO generated by iNOS nitrosylates and activates COX2. Selectively disrupting iNOS-COX2 binding prevents NO-mediated activation of COX2. This synergy between these two major inflammatory systems may afford a means for developing new therapeutic agents. The site on iNOS that is most critical for binding COX2 is in close proximity to the catalytic site. Conceivably, NOS-inhibitor drugs may be modified to interfere with iNOS-COX2 binding so that a single molecule could inhibit the generation of both NO and prostaglandins. In the heart, iNOS and COX2 may be regulated somewhat differently, as Bolli and associates (59, 60) have shown that both of these are cardioprotective. In the brain, neurotoxicity associated with stroke and neurodegenerative diseases is elicited by activation of NMDA-type glutamate receptors. NMDA signals, at least in part, by activation of nNOS through calcium traversing NMDA receptor channels (24). Through its PDZ domain, nNOS binds COX2, with the generated NO nitrosylating and activating COX2. Selective disruption of nNOS-COX2 binding prevents NMDA neurotoxicity.
Nuclear signaling systems are also regulated by nitrosylation. The transcription factor HIF1α is suppressed at normal oxygen tension. Nitrosylation of normoxemic HIF1α in the oxygen-dependent degradation (ODD) domain that causes the protein's degradation stabilizes HIF1α (61). Selective inhibition of nitrosylation attenuates HIF1α activation.
The transcription factor CREB (cyclic AMP response element–binding protein) is also regulated by nitrosylation. Treating neurons with brain-derived neurotrophic factor (BDNF) elicits binding of CREB to DNA coincident with its phosphorylation at serine-133 (62). However, CREB-DNA binding is independent of this phosphorylation (62). Instead, BDNF initiates an NO-dependent signaling pathway leading to nitrosylation of nuclear proteins that then associate with CREB target genes. One of the nuclear proteins nitrosylated in response to the BDNF-nNOS signaling pathway in neurons is histone deacetylase-2 (HDAC2) (63). Nitrosylation of HDAC2 does not influence its deacetylase activity. Rather, it elicits release of HDAC2 from chromatin, which facilitates augmented acetylation of histones that surround neurotrophin-dependent gene promoters, leading to enhanced transcription.
Nitrosylation is evanescent. Because the nitrosothiol bond is labile, denitrosylation might be spontaneous. However, there is evidence for specific catalytic denitrosylation by thioredoxin (64). Several caspases, enzymes that mediate apoptosis, are inhibited by nitrosylation. Moreover, stimulation of the Fas death receptor leads to caspase denitrosylation. Stamler and associates (64) purified a caspase-denitrosylating activity, based on the reactivation of caspase following denitrosylation, and isolated thioredoxin. Depletion of thioredoxin by RNA interference or pharmacologic inhibition led to increased abundance of nitrosylated caspase-3 as well as caspase-9, protein tyrosine phosphatase1B, and GAPDH. Thus, thioredoxin may be a physiologic denitrosylating enzyme for at least some nitrosylated proteins. Specific denitrosylating enzyme activity also affects peptides that are nitrosylated, as glutathione is physiologically nitrosylated and then denitrosylated by the glutathione-dependent GSNO reductase (65).
Carbon monoxide
Like NO, CO signals in multiple ways. Both molecules stimulate guanylyl cyclase to form cGMP (Table 3). They both act by binding to iron at the active site of heme. Such binding to heme in hemoglobin and mitochondrial proteins underlies the lethality of inhaled CO. NO is substantially more potent than CO in activating soluble guanylyl cyclase in vitro (66, 67). However, in intact organisms, CO physiologically activates cGMP. Thus, in mice with genetic deletion of the CO biosynthetic enzyme HO2, intestinal cGMP is depleted to the same extent as in mice with nNOS knockout (27).
Nuclear transcription factors may also be regulated by CO binding to heme. The ligand-binding pocket of the Drosophila nuclear receptor E75 contains a heme prosthetic group. Oxidation-reduction of E75 regulates its binding to its heterodimer partner DHR3 (Drosophila hormone receptor 3). Binding of CO, NO, or both to the heme of E75 modulates the E75-DHR3 interaction and subsequent transcription (68).
Neuronal PAS (Per, Arnt, Sim) domain protein 2 (NPAS2) is a mammalian transcription factor that binds DNA as a dimeric partner with BMAL1 to regulate circadian rhythms. The PAS domains of NPAS2 bind heme as a prosthetic group with the heme status controlling DNA binding. CO in low concentrations inhibits the DNA binding activity of holo-NPAS2, leading to the formation of inactive BMAL1 homodimers instead of active NPAS-BMAL1 heterodimers (69). Thus, CO-heme binding may influence the transcriptional activity that underlies circadian rhythms.
Hydrogen sulfide
How does H2S signal? Like NO and CO, H2S relaxes blood vessels (70). Because H2S is chemically reactive, in principle it might bind to the heme in guanylyl cyclase to augment cGMP abundance and thereby relax smooth muscle. However, evidence indicates that dilation of blood vessels by H2S involves activation of ATP-sensitive potassium channels (71). How does H2S influence these channels? How does it affect other target proteins?
Recent evidence indicates that H2S signals by sulfhydrating target proteins, a process analogous to nitrosylation (Table 3) (72). Sulfhydration, the formation of SSH in targeted cysteines, is detected by a modification of the biotin-switch assay for nitrosylation (39). In the conventional assay, ascorbate reduction removes NO, permitting binding by a biotin derivative, whereas for sulfhydrated cysteines such reduction is not required (72). This modified biotin-switch assay reveals substantial degrees of sulfhydration for a wide range of proteins, conceivably most of the proteins in mammalian liver. Whereas nitrosylation of proteins more often inhibits than augments their activity, the reverse appears to be the case with sulfhydration. Thus, sulfhydration of GAPDH at cysteine-150, critical for catalytic activity, stimulates the enzyme about 700%. This regulation is physiologically relevant, because in CSE knockout mice, overall catalytic activity of GAPDH in the liver is reduced by about 30% even though GAPDH protein abundance is unaltered. Similarly, actin-dependent polymerization is enhanced by sulfhydration.
Sulfhydration is substantially more prevalent than nitrosylation. About 10 to 25% of endogenous GAPDH, β-tubulin, and actin are sulfhydrated under basal conditions, 5 to 10 times as much as the basal levels of protein nitrosylation (39, 72). It appears likely that sulfhydration mediates the diverse physiologic actions of H2S. Because sulfhydration affects such a large proportion of mammalian proteins and alters their biological activity, it may represent a previously unappreciated, major posttranslational modification.
Conclusions
The gasotransmitters have revolutionized our thinking about messenger molecules in multiple ways. Their mode of biosynthesis, storage, and release differs markedly from those of other hormones, neurotransmitters, and growth factors. Recent studies indicate that their modes of signaling also differentiate them from other chemical messengers. The ability of NO and H2S to directly modify a wide range of protein targets amplifies strikingly the possibilities for information transmission in biology.
Footnotes
Citation: A. K. Mustafa, M. M. Gadalla, S. H. Snyder, Signaling by gasotransmitters. Sci. Signal. 2, re2 (2009).
References and Notes
- 1.Murad F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N. Engl. J. Med. 2006;355:2003–2011. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- 2.Bogdan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 3.Hibbs JB, Jr., Vavrin Z, Taintor RR. L-Arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J. Immunol. 1987;138:550–565. [PubMed] [Google Scholar]
- 4.Stuehr DJ, Gross SS, Sakuma I, Levi R, Nathan CF. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J. Exp. Med. 1989;169:1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 6.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br. J. Pharmacol. 2006;147:S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bredt DS. Nitric oxide signaling specificity—the heart of the problem. J. Cell Sci. 2003;116:9–15. doi: 10.1242/jcs.00183. [DOI] [PubMed] [Google Scholar]
- 9.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu. Rev. Pharmacol. Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 10.Sessa WC. eNOS at a glance. J. Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 11.Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, Snyder SH, Burnett AL. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4061–4066. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaffrey SR, Snowman AM, Eliasson MJ, Cohen NA, Snyder SH. CAPON: A protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron. 1998;20:115–124. doi: 10.1016/s0896-6273(00)80439-0. [DOI] [PubMed] [Google Scholar]
- 13.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 14.Cheah JH, Kim SF, Hester LD, Clancy KW, Patterson SE, III, Papadopoulos V, Snyder SH. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron. 2006;51:431–440. doi: 10.1016/j.neuron.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maines MD, Gibbs PE. 30 some years of heme oxygenase: From a “molecular wrecking ball” to a “mesmerizing” trigger of cellular events. Biochem. Biophys. Res. Commun. 2005;338:568–577. doi: 10.1016/j.bbrc.2005.08.121. [DOI] [PubMed] [Google Scholar]
- 16.Sedlak TW, Snyder SH. Messenger molecules and cell death: Therapeutic implications. JAMA. 2006;295:81–89. doi: 10.1001/jama.295.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Sedlak TW, Saleh M, Higginson D, Paul B, Juluri K, Snyder SH. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5171–5176. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 19.Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Smith RN, Csizmadia E, Tyagi S, Akamatsu Y, Flavell RJ, Billiar TR, Tzeng E, Bach FH, Choi AM, Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat. Med. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 20.Chin BY, Jiang G, Wegiel B, Wang HJ, Macdonald T, Zhang XC, Gallo D, Cszimadia E, Bach FH, Lee PJ, Otterbein LE. Hypoxia-inducible factor 1α stabilization by carbon monoxide results in cytoprotective preconditioning. Proc. Natl. Acad. Sci. U.S.A. 2007;104:5109–5114. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boehning D, Sedaghat L, Sedlak TW, Snyder SH. Heme oxygenase-2 is activated by calcium-calmodulin. J. Biol. Chem. 2004;279:30927–30930. doi: 10.1074/jbc.C400222200. [DOI] [PubMed] [Google Scholar]
- 22.Boehning D, Moon C, Sharma S, Hurt KJ, Hester LD, Ronnett GV, Shugar D, Snyder SH. Carbon monoxide neurotransmission activated by CK2 phosphorylation of heme oxygenase-2. Neuron. 2003;40:129–137. doi: 10.1016/s0896-6273(03)00596-8. [DOI] [PubMed] [Google Scholar]
- 23.Zakhary R, Gaine SP, Dinerman JL, Ruat M, Flavahan NA, Snyder SH. Heme oxygenase 2: Endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc. Natl. Acad. Sci. U.S.A. 1996;93:795–798. doi: 10.1073/pnas.93.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boehning D, Snyder SH. Novel neural modulators. Annu. Rev. Neurosci. 2003;26:105–131. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- 25.Battish R, Cao GY, Lynn RB, Chakder S, Rattan S. Heme oxygenase-2 distribution in anorectum: Colocalization with neuronal nitric oxide synthase. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G148–G155. doi: 10.1152/ajpgi.2000.278.1.G148. [DOI] [PubMed] [Google Scholar]
- 26.Ny L, Alm P, Larsson B, Andersson KE. Morphological relations between haem oxygenases, NO synthase and VIP in the canine and feline gastrointestinal tracts. J. Auton. Nerv. Syst. 1997;65:49–56. doi: 10.1016/s0165-1838(97)00034-9. [DOI] [PubMed] [Google Scholar]
- 27.Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14848–14853. doi: 10.1073/pnas.94.26.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szabó C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 29.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 30.Miller DL, Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20618–20622. doi: 10.1073/pnas.0710191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. U.S.A. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura H, Nagai Y, Umemura K, Kimura Y. Physiological roles of hydrogen sulfide: Synaptic modulation, neuroprotection, and smooth muscle relaxation. Antioxid. Redox Signal. 2005;7:795–803. doi: 10.1089/ars.2005.7.795. [DOI] [PubMed] [Google Scholar]
- 34.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gadalla MM, Snyder SH. unpublished observation.
- 36.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 37.Jaffrey SR, Cohen NA, Rouault TA, Klausner RD, Snyder SH. The iron-responsive element binding protein: A target for synaptic actions of nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12994–12998. doi: 10.1073/pnas.91.26.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: Purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 39.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 40.Santhanam L, Lim HK, Lim HK, Miriel V, Brown T, Patel M, Balanson S, Ryoo S, Anderson M, Irani K, Khanday F, Di Costanzo L, Nyhan D, Hare JM, Christianson DW, Rivers R, Shoukas A, Berkowitz DE. Inducible NO synthase dependent S-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ. Res. 2007;101:692–702. doi: 10.1161/CIRCRESAHA.107.157727. [DOI] [PubMed] [Google Scholar]
- 41.Wolosker H, Dumin E, Balan L, Foltyn VN. D-amino acids in the brain: D-serine in neurotrans-mission and neurodegeneration. FEBS J. 2008;275:3514–3526. doi: 10.1111/j.1742-4658.2008.06515.x. [DOI] [PubMed] [Google Scholar]
- 42.Mustafa AK, Kumar M, Selvakumar B, Ho GP, Ehmsen JT, Barrow RK, Amzel LM, Snyder SH. Nitric oxide S-nitrosylates serine race-mase, mediating feedback inhibition of D-serine formation. Proc. Natl. Acad. Sci. U.S.A. 2007;104:2950–2955. doi: 10.1073/pnas.0611620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O'Rourke B, Lowenstein JM, Pevsner J, Wagner DD, Lowenstein CJ. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Y, Man HY, Sekine-Aizawa Y, Han Y, Juluri K, Luo H, Cheah J, Lowenstein C, Huganir RL, Snyder SH. S-Nitrosylation of N-ethylmaleimide sensitive factor mediates surface expression of AMPA receptors. Neuron. 2005;46:533–540. doi: 10.1016/j.neuron.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 45.Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA, Koch WJ, Daaka Y, Lefkowitz RJ, Stamler JS. Regulation of β-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 46.Ozawa K, Whalen EJ, Nelson CD, Mu Y, Hess DT, Lefkowitz RJ, Stamler JS. S-Nitrosylation of γ-arrestin regulates γ-adrenergic receptor trafficking. Mol. Cell. 2008;31:395–405. doi: 10.1016/j.molcel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durham WJ, Aracena-Parks P, Long C, Rossi AE, Goonasekera SA, Boncompagni S, Galvan DL, Gilman CP, Baker MR, Shirokova N, Protasi F, Dirksen R, Hamilton SL. RyR1 S-Nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133:53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-Nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20612–20617. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-Nitrosylation. Nat. Chem. Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- 50.Stroissnigg H, Trancíková A, Descovich L, Fuhrmann J, Kutschera W, Kostan J, Meixner A, Nothias F, Propst F. S-Nitrosylation of micro-tubule-associated protein 1B mediates nitric-oxide-induced axon retraction. Nat. Cell Biol. 2007;9:1035–1045. doi: 10.1038/ncb1625. [DOI] [PubMed] [Google Scholar]
- 51.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-Nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 52.Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-Nitrosylation of parkin regulates ubiquiti-nation and compromises parkin's protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 53.Tian J, Kim SF, Hester L, Snyder SH. S-Nitrosylation/activation of COX-2 mediates NMDA neurotoxicity. Proc. Natl. Acad. Sci. U.S.A. 2008;105:10537–10540. doi: 10.1073/pnas.0804852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-Nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 55.Sen N, Hara MR, Kornberg MD, Cascio MB, Bae BI, Shahani N, Thomas B, Dawson TM, Dawson VL, Snyder SH, Sawa A. Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nat. Cell Biol. 2008;10:866–873. doi: 10.1038/ncb1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature. 2008;452:646–649. doi: 10.1038/nature06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-Nitrosylation of matrix metalloproteinases: Signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 58.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–1970. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- 59.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J. Mol. Cell. Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 60.Bolli R, Shinmura K, Tang XL, Kodani E, Xuan YT, Guo Y, Dawn B. Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/ reperfusion injury and mediates the late phase of preconditioning. Cardiovasc. Res. 2002;55:506–519. doi: 10.1016/s0008-6363(02)00414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, Li CY. Regulation of HIF-1α stability through S-nitrosylation. Mol. Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riccio A, Alvania RS, Lonze BE, Ramanan N, Kim T, Huang Y, Dawson TM, Snyder SH, Ginty DD. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Mol. Cell. 2006;21:283–294. doi: 10.1016/j.molcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodeling in neurons. Nature. 2008;455:411–415. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- 64.Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 66.Lee YC, Martin E, Murad F. Human recombinant soluble guanylyl cyclase: Expression, purification, and regulation. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10763–10768. doi: 10.1073/pnas.190333697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stone JR, Marletta MA. Soluble guanylate cyclase from bovine lung: Activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 68.Reinking J, Lam MM, Pardee K, Sampson HM, Liu S, Yang P, Williams S, White W, Lajoie G, Edwards A, Krause HM. The Drosophila nuclear receptor E75 contains heme and is gas responsive. Cell. 2005;122:195–207. doi: 10.1016/j.cell.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Dioum EM, Rutter J, Tuckerman JR, Gonzalez G, Gilles-Gonzalez MA, McKnight SL. NPAS2: A gas-responsive transcription factor. Science. 2002;298:2385–2387. doi: 10.1126/science.1078456. [DOI] [PubMed] [Google Scholar]
- 70.Wang R. Two's company, three's a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 71.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mustafa AK, Gadalla MM, Sen N, Mu W, Gazi SK, Kim S, Barrow RK, Yang G, Wang R, Snyder SH. unpublished observations.
- 73.Vreman HJ, Wong RJ, Kadotani T, Stevenson DK. Determination of carbon monoxide (CO) in rodent tissue: Effect of heme administration and environmental CO exposure. Anal. Biochem. 2005;341:280–289. doi: 10.1016/j.ab.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 74.This study has been supported by a NIH National Research Service Award (1 F30 MH074191-01A2) to A.K.M., a Medical Scientist Training Program Award (T32 GM007309) to M.M.G., and U.S. Public Health Service Grant (MH18501) and Research Scientist Award (DAOOO74) to S.H.S.