Abstract
The skin is the ultimate example of the function of innate immunity, it alerts the host of danger by many systems including sensing pathogen-associated molecule patterns (PAMPs) through Toll-like receptors and other pattern recognition receptors (PRRs), yet normally provides defense without inflammation. The skin responds rapidly to invading microbes by producing antimicrobial peptides or other antimicrobial intermediates before cytokine release results in inflammation. To achieve maximal immune responses for clearing invading microbes, the activation of select PRRs in skin then initiates and shapes adaptive immune responses through the activation of dendritic cells and recruitment of T cell subsets. Importantly, cross-talk between TLRs can influence this system in several ways including augmenting or suppressing the immune response. As a consequence of their pivotal role, TLR responses need to be tightly controlled by associated negative regulators or negative feedback loops to prevent detrimental effects from TLRs overactivation. This review focuses on describing the involvement of TLRs in the development of skin infectious and inflammatory diseases, and highlights the potential application of TLR agonists or antagonists in these skin diseases.
Keywords: Toll-like receptors, TLR signaling, inflammatory cytokines, antimicrobial peptides, cross talk, negative regulators, skin infectious and inflammatory diseases
1. Introduction
The innate immune system is evolutionally conserved and is the first line of the host for protecting it from invading microbial pathogens[1, 2]. Previously, innate immunity was considered only as a series of nonspecific responses that mediate cell killing through phagocytotic cells such as neutrophils and macrophages. However, the discovery of Toll receptor in Drosophila broke this traditional concept. The Toll receptor in Drosophila was found to play an important role in the induction of antifungal peptide expression against fungal infection, providing the first evidence that Drosophila expresses a specific receptor responsible for sensing fungi infection[3, 4]. Subsequently, a human homolog of Toll(hToll) was identified, and showed that this protein had an ability to induce production of inflammatory cytokines and expression of costimulatory molecules[5]. Remarkably, a loss-of-function mutation of mouse homolog of hToll was identified in lipopolysaccharide(LPS)-hyporesponsive mice[6, 7], which resulted in the development of Gram-negative sepsis but otherwise leaving most other immune functions intact. All these findings, and many others in other pattern recognition systems, suggest PRRs are a key component of innate immunity. Here we will focus on describing the roles of toll-like receptors in infectious and inflammatory diseases of the skin.
2. Toll-like receptor signaling
To date, 13 members of Toll-like receptors have been identified in humans and mice (table 1). TLRs1-9 are conserved between these two species, whereas tlr10 is functional only in humans. In mouse the C-terminal half of tlr10 gene is substituted by a non-related sequence. Therefore, mouse TLR10 is non-functional[8]. In contrast, mouse TLR11 is functional and can be activated by uropathogenic bacteria, whereas a stop codon is present in the human TLR11 gene, resulting in lack of its translation[9]. Furthermore, TLRs 12–13 have only been found in mice.
Table 1.
Toll-like receptor ligands and adaptor molecules
| TLR family | Exogenous ligands | Endogenous ligands | Adaptors proteins |
|---|---|---|---|
| TLR1 | Tri-acyl lipopeptide from bacteria and mycobacteria | N.D. | MyD88/TIRAP |
| TLR2 | Lipoprotein/lipopeptides(a variety of pathogens), lipoteichoic acid (Gram-positive bacteria), PGN(Gram-positive bacteria), lipoarabinomannan (mycobacteria), atypical LPS (Leptospira interrogans and Porphyromonas gingivalis), phenol-soluble modulin (Staphylococcus epidermidis), glycoinositolphospholipids (Trypanosoma Cruzi), glycolipids (Treponema maltophilum), porins(Neisseria), zymosan(fungi) | HsP70 | MyD88/TIRAP |
| TLR3 | Double-strand RNA (virus), poly(I:C) (synthetic) | mRNA | TRIF |
| TLR4 | LPS from Gram-negative bacteria, mannan from Candida albicans, viral envelope proteins from RSV and MMTV, GIPLs from Trypanosoma, HSP from Chlamydia pneumoniae, and taxol from plant | HsP60, fibrinogen, fibronectin, oligosaccharides of HA | MyD88, TIRAP, TRIF, TRAM |
| TLR5 | Bacterial flagellin | N.D. | MyD88 |
| TLR5 | Di-acyl lipopeptides (mycoplasma), zymosan (fungi), soluble tuberculosis factor | N.D. | MyD88/TIRAP |
| TLR7 | GU rich single-stranded RNA (virus), imiquimod, resiquimod (R848), synthetic polyU RNA | N.D. | MyD88 |
| TLR8 | GU rich single-stranded RNA (virus), Resquimod (R848) | N.D. | MyD88 |
| TLR9 | Bacterial and viral CpG DNA, hemozoin from Plasmodium | Chromatin immune complexes | MyD88 |
| TLR10 | N.D. | N.D. | N.D. |
| mTLR11 | Profilin-like protein from Toxoplasma gondii, unknown ligand(s) from uropathogenic bacteria | N.D. | MyD88 |
| mTLR12 | N.D. | N.D. | N.D. |
| mTLR13 | N.D. | N.D. | N.D. |
HSP:heat-shock protein; PGN: peptidoglycan; LPS: lipopolysaccharide; HA: hyaluronic acid; ODNs:ligodeoxynucleotides; N.D.: not determined. m: murine.
The TLRs are expressed in a variety of cell types and in two general cellular locations, plasma membrane(TLR1,2,4–6), or intracellular compartments such as the endoplasmic reticulum (ER) and endosomes (TLR3, 7–9)[10–12]. TLRs recognize and respond to a variety of molecules, such as lipids(TLR1,2,4,6), proteins(TLR5) and nucleic acids(TLR3, 7–9). Accumulating evidence suggests that TLRs are able to recognize both exogenous ligands such as those produced by microbes, and endogenous ligands including damage-associated molecular patterns and extracellular matrix molecules such as hyaluronan[13,14], heat-shock proteins(Hsps), and fibronectin (Table1).
The general structure of Toll-like receptors are that they are transmembrane proteins with a series of leucine-rich repeats in the N-terminal extracellular domain and a cytoplasmic portion greatly similar in structure to that of IL-1 receptor, hence referred to as the Toll-IL-1 receptor homology domain[15]. Based on the similarity in the cytoplasmic Toll/IL-1R (TIR) domain, TLRs are related to IL-1 receptors(IL-1Rs). With IL-1R signaling, TLRs have been shown to activate both NF-kappaB and MAP knase pathways via MyD88(myeloid differential factor 88), a common adaptor molecule recruited towards a Toll-IL-1 receptor (TIR)-domain of TLRs. Furthermore, four additional adaptor proteins involved in MyD88 independent pathways have been identified[6, 12]. They are the TIR domain containing adaptor protein(TIRAP, also called Mal) for TLR2/4-MyD88 dependent pathway[16, 17]; TIR domain containing adaptor inducing interferon-beta (TRIF, also called TICAM-1) and TRIF-related adaptor molecule (TRAM) for TLR3- and TLR4-MyD88 independent pathways[18–23]; and SAM and ARM-containing protein (SARM)[24]. As a result of TLR stimulation by cognate ligands, the proinflammatory response genes including cytokines such as TNFα, IL-6 and IL-12, and co-stimulatory molecules are induced via the activation of NF-κB and MAP kinases, while type-1 IFNs and their inducible genes are induced via interferon regulatory factors(IRF3) and/or 7[7, 25–27]. Therefore, stimulation of individual TLRs leads to the robust but specific activation of innate immune responses.
2.1 The MyD88 dependent pathway
Myeloid differentiation factor 88(MyD88) is a universal adaptor for almost all TLRs identified with the exception of TLR3, since poly(I:C)/TLR3-induced NF-kappaB is normal in the absence of MyD88[28](Fig. 1). The association of TLRs and MyD88 recruits members of the IRAK family, including IRAK1 and IRAK4. Upon TLR activation by cognate ligands IRAKs subsequently dissociate from MyD88 and the phosphorylated IRAK-1 interacts with tumor necrosis factor receptor-associated factor 6(TRAF6)[29–31].
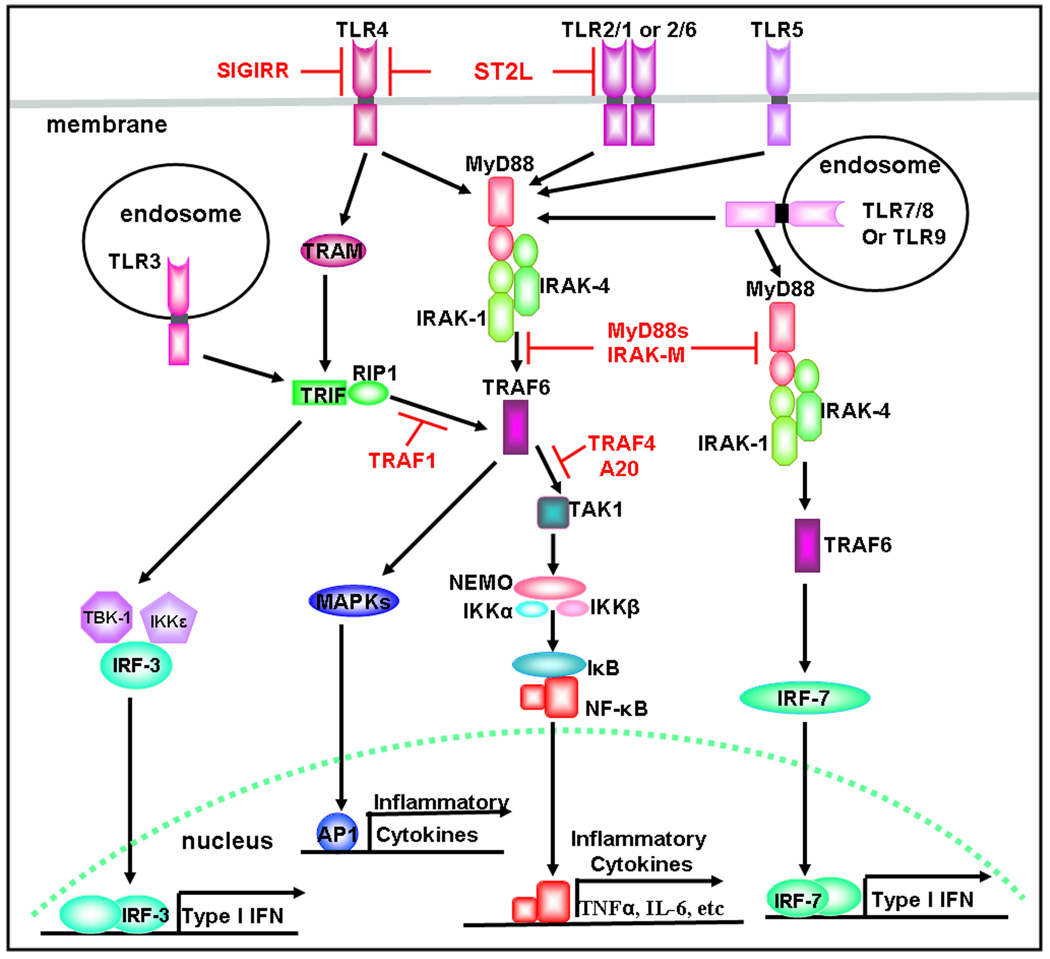
Fig 1. TLR signaling.
Upon the stimulation by cognate ligands, all TLRs, except for TLR3, recruit MyD88, IRAKs and TRAF6 to activate TLR signaling. Subsequently, TLR signaling diverges at TRAF6 to two different pathways to produce inflammatory cytokines. However, TLR3 utilizes TRIF for the activation of the TRIF-dependent pathway to produce type I interferons and inflammatory cytokines.
TRAF6 is the signaling molecule following IRAK. The activation of TRAF6 in turn activates TAK1. TAK1, in combination with TAB1, TAB2 and TAB3, activates two downstream pathways involving the IKK complex and MAPK family. Phosphorylation of TAK1 and TAB2 occurs initiating the dissociation of TRAF6/TAK1/TAB1/TAB2 from the membrane to the cytosol, and IRAK-1 is degraded[32, 33]. TAK1 is subsequently active, resulting in phosphorylation and degradation of IκB, which leads to the translocation of transcription factor NF-kappaB and, consequently, controlling the expression of different inflammatory cytokine genes[34–38]. TRAF6 has been shown to have a role in all TLR pathways to NF-kappaB tested to date, functioning as a central signaling molecule that can dock with multiple effectors and thus lead to NF-κB and MAP kinase activation by different routes. Moreover, phosphorylation of MAPK activates the transcription factor AP-1 in responses to multiple TLRs activation.
2.2 The MyD88 independent/TRIF dependent pathway
TLR3 is unique in that it signals exclusively through the third adapter to be discovered, TRIF(Fig 1)[19]. In addition to TLR3, TLR4 signaling independent of MyD88 has also been shown to require both TRIF and the fourth adapter, TRAM. TRIF binds to TLR3 and recruits TRAF6 directly through a TRAF6-binding motif in its N-terminal domain, which leads to TAK1 activation and subsequent NF-κB activation in an IRAK1 and IRAK4 independent manner[39–41]. TRIF can also activate NF-κB through an alternative pathway. The C-terminal of TRIF possesses a RIP homotypic interaction motif (RHIM), and it associates with receptor interacting protein 1 (RIP1) through homophilic interaction of RHIM domains[42]. A dominant negative form of RIP1 inhibits TRIF-mediated NF-kappaB activation. Thus, TRAF6 and RIP1 are involved in TRIF-dependent activation of NF-kappaB. However, although TRAF6 is involved in the TLR3 induced TRIF-mediated NF-kappaB activation, TLR4-mediated NF-kappaB activation was still inducible in MyD88/TRAF6 double knockout mice[26], indicating the presence of TRAF6-independent NF-kappaB activation in the TRIF-dependent pathway. There is also one study where it was shown that TLR3-mediated activation of NF-kappaB was not affected in TRAF6 deficient macrophages[43]. In TRIF- and TRAM-deficient mice, normal inflammatory cytokine production induced by TLR2, TLR7 and TLR9 ligands was observed, and TLR4-mediated phosphorylation of IRAK was induced normally, indicating that activation of the MyD88-depedent pathway was unaffected. However, TLR4-mediated inflammatory cytokine production, which is believed to be mainly induced by the MyD88-dependent pathway, was defective in TRIF- and TRAM-knockout mice[44]. Therefore, TRIF must be involved in TLR4-mediated induction of inflammatory cytokines although the mechanisms remain to be elucidated.
TRIF, but not MyD88, activate the type I interferon promoters[19]. It has been shown that the noncanonical IKKs, IKKε and TANK-binding kinase-1(TBK1), mediate activation of IRF3 through interaction with TRIF and TRAM, thereby inducing the IFNβ promoter[23, 40, 45].
3. Toll-like receptors in skin
Skin is an ideal example of the innate immune system at work, providing physical barriers and other cellular rapid innate immune responses. All TLRs are expressed in the variety of cells that reside in the skin, but the expression and function of TLRs differs greatly between individual cell types. Relevant cells in the skin include keratinocytes and Langerhans cells in the epidermis, resident and trafficking bone-marrow-derived cells in the dermis such as macrophages, dendritic cells (DCs), T and B cells, mast cells, endothelial cells of the skin microvasculature, and skin stromal cells such as fibroblasts and adipocytes[46–49]. As a consequence of its location, human skin is exposed to a myriad of microorganisms. The cutaneous innate immune system selectively alerts the host of the presence of microbial pathogens by sensing PAMPs or endogenous signals of injury through TLRs and other pattern recognition receptors (PRRs) and responds rapidly by producing cytokines, antimicrobial peptides or antimicrobial intermediates (e.g. radical oxygen species and nitric oxide)[50]. When the innate immune system is unable to combat a microbial infection, as is frequently the case, the adaptive immune system then must play a role as a second line of defense. TLR activation also contributes to this process by initiating and shaping the adaptive immune response through the activation of dendritic cell maturation and influencing T and B cell function. Thus, maximal immunity is achieved to clear pathogens when TLRs coordinate the rapid innate response with the slower adaptive response (Fig 2). However, overactivated responses could be dangerous to the host as exemplified by sepsis or autoimmune diseases. Therefore, the responses need to be tightly controlled by associated negative regulators, negative feedback loop and/or by anti-inflammatory factors such as TGF-beta, interleukin(IL)-10 and steroid hormones.
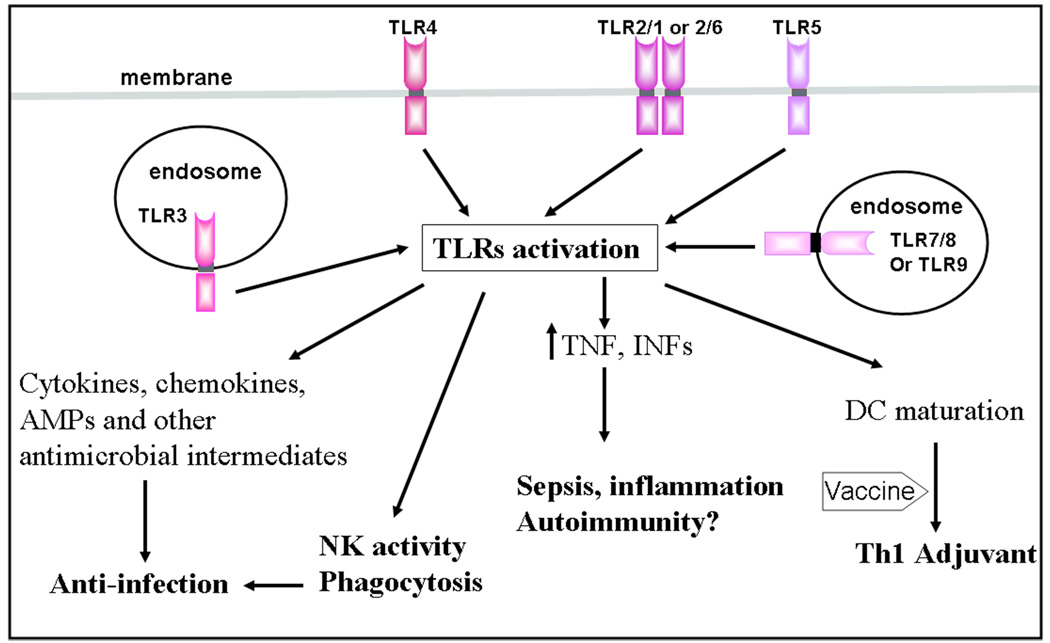
Fig 2. The immunomodulatory effects of TLR activation.
The activation of TLRs is double-edge sword. In addition to promoting NK activity and phagocytosis, appropriated TLR stimulation also boosts the host to produce inflammatory cytokines, chemokines, antimicrobial peptides and other intermediates against infectious diseases in skin. Moreover, the activation of TLRs can initiate and shape an adaptive immune response to co-administered vaccine as vaccine adjuvant. However, excessive TLR stimulation may lead to some inflammatory diseases, sepsis or autoimmune diseases in the skin.
3.1 Toll-like receptors and inflammatory cytokines and chemokines
The production of inflammatory cytokines and chemokines is a direct outcome of the activation of TLR signaling. Upon stimulation by microbial pathogens, TLRs induce the production of interferons and inflammatory cytokines, including IL-6 and TNFalpha. These cytokines then activate surrounding cells to produce chemokines or adhesion molecules, thereby recruiting various inflammatory cells into the infected sites to clear invading pathogens. The appropriate production of these cytokines and chemokines is required for the functioning of both innate and adaptive immune responses. They control the direction, amplitude, and duration of immune responses and the (re)modeling of tissues, be they developmentally programmed, constitutive, or unscheduled. Unscheduled remodeling is that which accompanies inflammation, infection, wounding, and repair. However, overactivation or dysregulation of TLRs signaling also causes severe disease, such as sepsis, atherosclerosis, and autoimmune diseases.
In skin the activation of TLRs regulates gene expression profiles including the production of cytokines such as TNFalpha, IL-1, IL-6 and IL-12, chemokines such as IL-8 and MIP2, and upregulation of co-stimulatory molecules such as CD40, CD80, and CD86. Our unpublished data and data from Lebre et al. [47]demonstrated that activation of TLR3 results in the production of TNFalpha, IL-8, type I interferons (IFNs), the monocyte and basophil chemokine CCL2, and the macrophage inflammatory protein 3 (CCL20) in human kerationcytes. Moreover, activation of TLRs 3 and 5 results in an increased production of CCL27, which promotes memory T-cell recruitment specifically to the skin[47]. In monocytes/macrophages the activation of TLRs induces the production of proinflammatory cytokines, such as TNFalpha and IL-6, chemokines, and increases the phagocytic ability of macrophages[51]. TLR3 stimulation of langerhans cell-like DCs but not monocyte-derived DCs increased the production of type I INF, suggesting that LCs can initiate direct antiviral activity after TLR3 activation[52]. Furthermore, MHC molecules and co-stimulatory molecules such as CD80 and CD86 on the cell surface were upregulated during the process of myeloin DC maturation by TLRs. Therefore, activated myeloid DCs migrate from the skin to draining lymph nodes where they can express antigen to T cells and elicit cell-mediated immune responses[53]. Activation of myeloid DCs also induced the production of IL-12, which promotes Th1-type immune responses[53]. Taken together, all these data suggest that inflammatory cytokines and chemokines induced by TLRs play a critical role in eliciting distinct host defense mechanisms against invading pathogens in the skin.
3.2 Toll-like receptors and antimicrobial peptides (AMPs)
The endogenous antimicrobial peptides are effector molecules of the innate host defense system and are secreted by epithelial and other cell types[54, 55]. AMPs have a broad antimicrobial spectrum and inactivate microorganisms by direct interaction with biomembranes or other organelles. Besides their direct antimicrobial function, it has been suggested that AMPs play multiple roles as mediators of inflammation with impact on epithelial and inflammatory cells influencing diverse processes such as cytokine release, cell proliferation, angiogenesis, wound healing, chemotaxis, immune induction, and protease antiprotease balance[56, 57].
Human skin is exposed to millions of microbial organisms and these microorganisms produce various kinds of TLR ligands. However, the skin is an effective barrier against invading microorganisms both with and without inflammation. This protective function is partly mediated by the presence of AMPs as well as inflammatory cytokines and chemokines after TLRs have been activated in the skin. Several AMPs have been detected in skin keratinocytes, such as beta-defensins, cathelicidin and psoriasin[58–61]. Expression of murine beta-defensin was upregulated by bacterial lipopeptides in wild-type keratinocytes, while it was attenuated in TLR2-deficient keratinocytes in vitro. The grafted tail skin from TLR2-deficient mice resulted in erosion when it has been inoculated with Staphylococcus aureus. These studies strongly suggest that the TLR2-dependent pathway in keratinocytes is essential for antimicrobial activity[62]. Flagellin from Escherichia coli triggers Toll-like receptor 5 in human keratinocytes and strongly induced the expression of S100A7c(psoriasin)[61]. Moreover, recent work in Modlin’s group and our group showed hormonally active vitamin D(3)-1,25-dihydroxyvitamin D(3) (1,25D3)-acts as a signaling molecule in cutaneous immunity by increasing pattern recognition through Toll-like receptor-2 (TLR2), increasing the expression and function of antimicrobial peptide, cathelicidin and killing of intracellular Mycobacterium tuberculosis[63]. In addition, our group further found that the epigenetic control of gene transcription by histone acetylation is important for 1,25D3-regulated antimicrobial and TLR function of keratinocytes against S.aureus[64].
Besides the ability to kill microbes, AMPs can mediate both proinflammatory and anti-inflammatory effects in skin to link innate and adaptive immune responses. AMPs stimulate chemokine and cytokine secretion from a variety of cell types and can act through receptor-dependent mechanisms[59, 65, 66]. In monocytes and dendritic cells hBD3 can induce expression of the costimulatory molecules CD80, CD86, and CD40 by interaction with TLRs 1 and 2, resulting in signaling that requires MyD88 and leads to IL-1 receptor-associated kinase-1 phosphorylation[67]. Our study of allergic contact dermatitis showed cathelicidins inhibited TLR4 but not TLR2 mediated induction of dendritic cell maturation and cytokine release, and this inhibition was associated with an alteration of cell membrane function and structure. Further analysis in vivo showed inhibition of sensitization by exogenous cathelicidin was dependent on the presence of functional TLR4. These observations provide evidence that cathelicidin antimicrobial peptides mediate an anti-inflammatory response through TLR4[68].
3.3 Toll-like receptors link innate and adaptive immunities
TLR activation not only produces AMPs and proinflammatory cytokines, but also bridges the link between innate and adaptive immunity. This link comes in part through dendritic cell (DC) maturation. DCs couple TLR-mediated innate immune recognition to the initiation of T cell and B cell activation. Accumulating evidences show DC maturation, which involves up-regulation of co-stimulatory molecules[69], is controlled by TLRs of the innate immune system[70]. In normal skin, DCs are comprised of epidermal Langerhans cells (LC) and dermal DC (DDC). These cells express none and/or very low levels of major histocomopatibility (MHC) and co-stimulatory molecules, and are incapable of inducing T cell priming. In case of an infection, microbial presence is detected by TLRs expressed on DCs. Subsequently, DCs degrade antigens into MHC class I and II binding peptides, upregulate co-stimulatory molecule expression, and translocate from the site of injury/infection to regional lymph nodes where they interact with naïve T cells[71]. In addition to controlling expression of co-stimulatory molecules, TLRs are also responsible for induction of cytokine and chemokine production by DCs[5, 72], including IL-12, which subsequently promotes Th1 cell-mediated adaptive immune response. Thus, in responses to microbial infection, TLRs not only produce early inflammatory and antimicrobial responses of the innate immune response but also initiate and subsequent adaptive immune responses[48].
3.4 Toll-like receptor cross-talk
Individual TLR agonists are unique in their response profile in spite of the use of apparently common activation pathways [73, 74]. These TLR agonists can cross-talk to each other to augment or suppress their responses either directly through ligand interactions or via secondary adaptor molecules. A great number of in vitro and in vivo studies have demonstrated that TLR stimulation induces different but overlapping Th1–Th2 cytokine–chemokine profiles, suggesting a complex interplay of stimulatory signals between TLRs[75, 76]. The induction of type-1 IFN-gamma production is common to both activated endosomal TLRs (TLR3, 7, 7/8, 8, 9)[77–79] as well as cell surface TLRs (e.g. TLR4)[73, 79, 80]. In addition, it is known that the host target cells express discrete but multiple TLRs and an invading pathogen can have multiple TLR agonists[73, 81, 82]. As a result, a pathogen can activate multiple TLRs [25]and that in turn allows the immune system to determine the profile of the invading pathogen and to launch an appropriate immune response [83]. Furthermore, the TLR cross-talk also can establish a cytokine-chemokine network and set point of one pathogen that may differ from another which in turn may be detrimental to the survival of one another and may serve as beneficial to the host [84].
TLR cross talk may also provide a mechanism by which cells that are non- or low-responsive to a particular TLR may switch to a “responsive mode” due to concomitant activation with another TLR agonist or cytokine[85], and hence drive responses toward preferred T cell polarization. Although this upregulation of TLRs by TLRs often results in augmenting immune responses by engaging more TLRs, the initial stimulating dose and the timing of stimulation by the second TLR agonist can influence the immune response[10, 86]. Thus, studies on TLR cross talk may provide a better understanding of the mechanisms by which innate and adaptive immunity interact, and provide clues towards development of more successful vaccination strategies and immunotherapies. In human and mouse DCs, TLR3 and TLR4 potently acted in synergy with TLR7, TLR8 and TLR9 in the induction of a selected set of genes. Synergic TLR stimulation increased production of interleukins 12 and 23 and increased the Delta-4/Jagged-1 ratio, leading to DCs with enhanced and sustained T helper type 1-polarizing capacity [86]. Furthermore, TLR activation of innate immunity prevents the induction of transplantation tolerance and shortens skin allograft survival in mice treated with costimulation blockade. It has been reported that administration of the TLR agonists LPS (TLR4) and/or polyinosinic:polycytidylic acid (TLR3) to mice treated with costimulation blockade prevents alloreactive CD8(+) T cell deletion, primes alloreactive CTLs, and shortens allograft survival.
Although TLRs cross talk can boost the signaling and thus the immune response, strong first stimulation of TLR may also induce TLR-signaling inhibitors such as MyD88s(a splice variant of MyD88), or third TLR signaling may involve in inducing negative regulators, such as IRAKM and TRAF1, resulting in attenuating or inhibiting TLR signaling until the overactivation of TLR signaling goes back to normal.
3.5 Negative regulators in TLR overactivation
TLRs are critical for the initiation of inflammatory and immune responses by detecting conserved microbial ligands [72]. These ligands trigger TLRs for the induction of inflammatory cytokines[87], which is required for effectively clearing pathogens, and plays an instructive role in the development of the adaptive immune response, in particular the Th1 response[88]. As discussed previously, TLR cross-talk can either boost or suppress these responses. However, TLR activation is a double-edged sword. On one hand its activation is essential for provoking the innate response and enhancing adaptive immunity against pathogens [1, 70] and on the other hand TLRs are also involved in the pathogenesis of autoimmune, chronic inflammatory and infectious diseases[89]. Overactivation of TLRs causes persistence of producing proinflammatory cytokines, such as TNFα, IL-6. IL-6 has been implicated in autoimmune diseases, such as pristine-induced lupus, collagen-induced arthritis and experimental autoimmune encephalomyelitis (EAE)[90–93]. The fact that IL-6-deficient animals are resistant to several autoimmune diseases suggests that this may be, at least in part, due to its ability to help effector T cells to overcome suppression mediated by Tr cells. In chronic infections, persistence of pathogens and their products can lead to enhanced production of several proinflammatory cytokines, including IL-6, leading to a situation conducive to activation of self-reactive T cells. Therefore, the inflammatory response must be tightly regulated and indeed, multiple regulatory mechanisms control the extent and duration of TLR-induced inflammation[94]. These include the inhibition of TLR signaling by inducible negative regulators, production of anti-inflammatory cytokines and alterations of the TLR signaling complex [94, 95].
To limit TLR-induced inflammation several negative regulators of TLR signaling are involved via sequestration of signaling molecules, blockade of their recruitment, degradation of target proteins or inhibition of transcription (Fig. 3)[96, 97]. This negative regulation is achieved at multiple levels. During acute bacterial infection, soluble TLRs (such as MyD88s) are produced and provide the first line of regulation by functioning as decoy receptors that prevent a direct interaction between TLRs and their microbial ligands. Once TLR and ligand interact, TLR signaling can be further controlled by intracellular regulators, which can inhibit TLR signaling pathway[95]. Several intracellular proteins have been identified that negatively regulate the function of TLR signaling. In the IRAK family, the overexpression of IRAK-M results in prevention of dissociation of IRAK4 and IRAK1 from MyD88[92]. And IRAK-M deficient mice produce more inflammatory cytokines in response to different TLR ligands than wild type mice do. Another regulator family, TRAF, have shown dual functions in TLR signaling. Whereas TRAF3 and TRAF6 can positively regulate TLR signaling, TRAF1 and TRAF4 are implicated negatively regulating TLR signaling. TRAF1 interacts with TRIF and overexpression of TRIF results in the activation of caspase 8 to cleave TRAF1 to release N-terminal TRAF1[98]. The N-terminal TRAF1 can suppress TRIF-dependent activation of NF-kappaB and IRF3, suggesting that TRAF1 is responsible for turning off the TRIF-dependent pathways. TRAF4 also suppresses MyD88 dependent NF-κB activation. It has been implicated that TRAF4 serves to antagonize TRAF6 function by preventing recruitment of TRAF6 to the adapter complex. Indeed, several independent negative regulators have been suggested to involve in negatively regulating TRAF6. In response to LPS, β-arrestins interact with TRAF6 to prevent its oligomerization, resulting in inhibition of TRAF6 polyubiquitination and subsequent activation of NF-κB and MAPK[99]. A20, an inducible de-ubiquitination enzyme, removes ubiquitin moieties from TRAF6 to terminate TLR signaling. Therefore, A20-deficient mice had increased NF-kappaB activation in response to several TLR ligands, including TLR2,4 and 9[100] Furthermore, some negative regulators are also involved in negative feedback of TLR signaling. LPS triggers TLR4 signaling to continuously produce inflammatory cytokines. IRAK-M is inducible by LPS to negatively regulate this signaling to control the excessive production of cytokines [101]. Overall, TLR signaling is tightly controlled by negative regulators to terminate immune and inflammatory responses, prevent excessive inflammation, and balance the beneficial or detrimental roles of TLRs in host defense.
Fig 3. Negative regulators for TLR signaling.
TLR signaling is negatively regulated by several negative regulators (Shown in Red). Both MyD88s and IRAK-M suppress the downstream signaling of MyD88. TRAF1 turns off TRIF signaling. TRAF4 and A20 bind and block TRAF6. SIGIRR expresses in epithelial cells and antagonize TLR4 signaling. ST2L inhibits both TLR2 and TLR4 signaling.
4. Toll-like receptors and skin infectious and inflammatory diseases
Upon microbial infection, the innate immune system alerts the host of the presence of microbial pathogens, activates TLR signaling to produce cytokines and antimicrobial intermediates, further orchestrates the adaptive immune response, and thus achieves the maximal immunity to clear pathogens. TLRs thereby play a critical role in infectious and inflammatory diseases. Indeed, the engagement of TLRs appears to be intricately associated with certain skin diseases. However, although the activation of TLRs is necessary for host action against various pathogens, in some cases, microbes also take advantage of the TLR-induced innate responses to develop infections. Therefore, the better understanding of TLRs and their detailed signaling cascades will expand more successful strategies for the prevention and treatment of various skin infectious and inflammatory diseases. The following are examples of some skin infectious and inflammatory diseases relevant to TLRs.
4.1 S.aureus-induced infections
Staphylococcus aureus (S.aureus) is a common type of bacteria that live on the skin and mucous membranes (eg. in nose) of humans. About 20% of the population is persistently colonized and 50% are intermittent carriers. When crossing the skin barrier, S. aureus causes skin abscesses from which the bacteria can disseminate and cause bacteremia, sepsis, endocarditis and keratitis[102]. S. aureus infections also often happen to people with some skin diseases, such as atopic dermatitis.
Staphylococci have several TLR inducers. Bacterial lipoproteins and lipoteichoic acid (LTA) serve as TLR2/6 or TLR2/2 agonists[103–105]. And peptidoglycan is sensed by NOD2[106, 107]. Studies showed that TLR adapter molecule MyD88 is required in response to all S.aureus infection models in mice. In skin abscesses and systemic infection, MyD88 plays a pivotal role in bacterial clearance and limitation of the disease[108, 109]. Furthermore, cytokine production and neutrophil recruitment were proven to be MyD88-dependent in cutaneous and corneal infections. In cutaneous infection, IL-1R and MyD88-deficient mice show similar phenotypes, as reflected by increased skin lesions, reduced cytokine levels and impaired neutrophil recruitment. Moreover, polymorphisms in the TLR2 gene have been demonstrated to fail to clear S.aureus[110]. Through a mutation screen of the TLR2 gene in 110 healthy subjects, arginine-to-glutamine substitution at residue 753 of Toll-like receptor 2 (TLR2 Arg753Gln) was associated with a diminished responsiveness to various gram-positive bacterial LPS. In addition, this mutation was noted in 2 of 22 (9%) of patients with gram-positive septic shock, both of whom were found to have systemic staphylococcemia. However, whether Toll-like receptor 2 abnormalities are relevant to the susceptibility of skin colonization with S.aureus remains unknown[111].
4.2 Vaccinia virus
Vaccinia virus (VV), a member of poxviruses family, has been used extensively as a vaccine vehicle in the clinical application for infectious diseases and cancer. Fetal vaccinia, manifested by skin lesions and organ involvement, often results in fetal or neonatal death. Poxviruses employ many strategies to evade and neutralize the host immune response. VV encodes proteins that antagonize important components of host antiviral defense. A46R and A52R, two VV proteins, that share amino acid sequence similarity with the Toll/IL-1 receptor (TIR) domain, have a key role in innate immunity and inflammation. When expressed in mammalian cells, the protein products of both ORFs were shown to interfere specifically with IL-1 signal transduction. A46R partially inhibited IL-1-mediated activation of the transcription factor NF-κB, and A52R potently blocked both IL-1- and TLR4-mediated NF-κB activation[15]. In addition to IL-1- and TLR4-mediated NF-κB activation, VV protein A52R also blocks the activation of NF-κB by TLR3, a recently identified receptor for viral RNA. A52R associates with both interleukin 1 receptor-associated kinase 2 (IRAK2) and tumor necrosis factor receptor-associated factor 6 (TRAF6), two key proteins important in TLR signal transduction. Furthermore, A52R could disrupt signaling complexes containing these proteins[16]. Therefore, A46R and A52R are likely to represent a mechanism used by vaccinia virus of suppressing TIR domain-dependent intracellular signaling. A recent study showed that VV elicited innate immune response also through TLR2 and MyD88, leading to the production of proinflammatory cytokines[35], suggesting that VV can target multiple TLRs pathways to work together in achieving efficient activation of host defense.
4.3 Acne vulgaris
Acne vulgaris is a common cutaneous disorder of the pilosebaceous follicle characterized by inflammatory papules, pustules, nodules and noninflammatory comedones, affecting more than 45 million people in the United States alone. The pathogenesis of acne is multifactorial, involving abnormal hyperkeratinization, increased sebum production, hormones, cutaneous microbes, and immunological mechanisms. Many of the immunological processes that contribute to the formation of acne lesions take place at the very site of disease, the skin[112].
It’s well known that Propionibacterium acne (P. acne) is a key bacterium that causes acne vulgaris. P.acne is a gram-positive bacterium and petidoglycan is one of major components from cell wall. Petidoglycan is an exogenous ligand for TLR2, and TLR2 forms homodimers and acts as one of the signal-transducing coreceptors for CD14. On the basis of these findings and the paradigm of Toll-like receptor engagement as a critical step in the elaboration of proinflammatory cytokine, Kim and collaborators investigated the role of TLR2 in acne[113]. TLR2 was abundantly expressed on perifollicular and peribulbar macrophages, and the concentrations of the TLR2-bearing cells increased with the evolution of acne lesions. Inflammatory cytokine production (IL-6,−8 and −12) in response to P. acnes was critically dependent on TLR2. In vivo TLR2 and TLR4 expression is increased in the epidermis of acne lesions. In vitro, an increase in TLR2 and TLR4 expression by human keratinocytes occurred in the first hours of incubation with bacterial fractions as well as an increase of the expression and secretion by the keratinocytes of MMP-9, which plays a role in inflammation[114]. A recent study indicated that retinoids, used for treatment of acnes, can down-regulate TLR2 signaling in monocytes and, therefore, dampen the inflammatory reaction of monocytes exposed to P.acnes in vitro, potentially explaining the anti-inflammatory action of retinoids in acnes[111].
4.4 Leprosy
Leprosy is a chronic disease caused by infection with Mycobacterium leprae (M.leprae). Usually the organisms are found in the subepidermal zone, inside the nerves, sweat glands, sweat ducts, arrector pili muscle, macrophages and around the hair follicle. Sometimes, in patients with a high bacteriological index (BI≥4+) the bacilli may be seen throughout the dermis, even scattered in dermal collagen[115].
M. leprae is a gram-positive bacterium. And pathogen associated molecule patterns (PAMPs) from Mycobacteria are capable of altering the expression levels of TLR genes such as TLR2 and TLR4[116]. Stronger levels of TLR2 and TLR1 expression have been found in human leprosy lesions from patients with the self-healing tuberculoid form of leprosy when compared to lesions from patients with largely disseminated lepromatous form of leprosy[52]. It has been suggested that these differences in TLR expression may be due to the differences in cytokines found at the site of disease. Type 2 cytokines predominate in lepromatous lesions and are able to reduce TLR2 expression levels. However, type 1 cytokines predominate in tuberculoid lesions and are able induce TLR1 expression. The expression of TLRs in skin together with the ability of TLR2/TLR1 heterodimers to recognize M. leprae will allow cells of the skin to modify inflammatory responses and modulate subsequent adaptive immune responses. Furthermore, polymorphisms in TLRs and their signaling molecules have revealed the importance of TLRs in human defense against disease. The mutation of TLR2, R677W, was found to associated in Asian people with lepromatous leprosy[111].
4.5 Atopic dermatitis
Atopic dermatitis (AD) is a widespread inflammatory skin condition marked by flares and remissions. Patients with AD, compared to patients with other skin inflammatory diseases, exhibit defects in innate and acquired immune responses, resulting in a heightened susceptibility to bacterial, fungal and viral infections, most notably colonization by S.aureus[117]. What caused the S.aureus tropism for AD skin? One logical hypothesis is a defect in the production of antimicrobial peptides in AD patients. A recent study showed the skin from AD patients, compared with normal skin and psoriasis skin lesion, had much decreased production of 2 endogenous antimicrobial peptides, LL-37 and beta-defensin 2[118]. Activation of TLR2 signaling has been shown to induce beta-defensin 2 [119]. Another hypothesis is that Staphylococcal exacerbation of AD patients may be due to abnormalities/dysfunction in the TLR2 signaling. Polymorphisms in the TLR2 gene have been shown to lead to a decreased ability to clear S.aureus [110]. Although the precise role of TLR2 in atopic dermatitis is not well understood, the dysfunction of TLR2 as a potential mechanism in the pathogenesis of atopic dermatitis is rational theory[111].
4.6 Psoriasis
Psoriasis is a chronic inflammatory skin disease and considered to be an immune-mediated, organ-specific (skin, or skin and joints) inflammatory disease, in which intralesional T lymphocytes trigger primed basal stem keratinocytes(KCs) to proliferate and perpetuate the disease process. A study that compared TLR expression and HSP expression in normal and psoriatic skin showed heat shock proteins (HSPs) are naturally occurring ligands that can stimulate APCs by way of TLR4[120], which may be relevant to the pathogenesis of psoriasis because HSPs are overexpressed by KCs in psoriatic lesions. HSPs trigger TLR4 on APCs such as Langerhans cells, leading to maturation, and TNFalpha and IL-12 secretion, thus, contributing to psoriasis immunopathology. Moreover, an immunohistochemical analysis of normal and psoriatic skin demonstrated that TLR1 and TLR2 expression was increased in psoriatic KCs in the suprabasal layer, whereas TLR5 was down-regulated by basal KCs in psoriasis compared with normal human skin[46]. Another recent interesting observation is that the cathelicidin antimicrobial peptide LL-37 can serve to present self-DNA to activate INF-g release from plasmacytoid dendritic cells[121]. That this event may perpetuate inflammation in psoriatic patients remains to be demonstrated.
Toll-like receptors (TLRs) are crucial players in the innate immune response to microbial invaders. The lipophilic yeast Malassezia furfur has been implicated in the triggering of scalp lesions in psoriasis. Human keratinocytes infected with M. furfur had an up-regulation of TLR2, MyD88, HBD-2, HBD-3 and IL-8 mRNA compared to the untreated cells. The same results were obtained when psoriatic skin biopsies were analyzed. The M. furfur-induced increase in HBD-2 and IL-8 gene expression is inhibited by anti-TLR2 neutralizing antibodies, suggesting that TLR2 is involved in the M. furfur-induced expression of these molecules [122].
5. Therapeutic applications of TLRs
As TLRs are critical in both innate immune responses and adaptive immune systems, regulation of TLR expression at sites of skin diseases such as in acne, psoriasis and leprosy may be important in the pathophysiology of these diseases. Normally, modulation of TLR expression and activation protects the host from the severity of disease. TLR overactivation, however, also promotes excessive inflammation and apoptosis contributing to the pathology of disorders such as sepsis. Additionally, the recognition of S.aureus or P. acnes through TLR2 to induce inflammatory cytokine production or the inhibition of TLR3 by vaccinia to produce inflammatory cytokines may also involve in the pathogenesis of the diseases. TLRs play a vital role in skin infectious and inflammatory diseases, thereby making them potential therapeutic targets. Therefore, the ability of TLR to combat disease could be used in dermatological clinic through the development of drugs that act as TLR agonists or antagonists.
TLR ligands are potent inducers of innate immunity. Vaccine adjuvants are perhaps the most extensively explored applications for TLR ligands. Accumulating evidence suggests that many TLR agonists are effective adjuvants acting as signals for initiating efficient adaptive immune responses. At present, two improved adult hepatitis B virus vaccines and a papillomavirus vaccine that use TLR4 agonists as the adjuvant have been approved[123]. Also, TLR3 ligand, poly(I:C), has already proven to be beneficial as a mucosal adjuvant for influenza virus vaccine in a murine infection model[124], and there is also considerable research and activity exploring the adjuvant activities of ligands for most of TLRs. All these studies suggest that TLR agonists have potential to enhance the therapeutic vaccination for treatment of infectious diseases.
Furthermore, TLR stimulation by the respective ligands protects hosts against subsequent lethal infection from bacteria and viruses. It was initially found that TLR2 and 4 differentially confer protective immunity against Gram-positive and –negative bacteria due to their specific recognition of lipoproteins and LPS. TLR3, 7 and 9 have been shown to be critical for viruses, such as herpes virus recognition. Of interest, pre-treatment of animals with TLR agonists confers protection against lethal infections of a variety of infectious microbes without any antigen exposure[125]. These protective innate immune responses elicited by TLR ligands are being developed as anti-infection agents. The imidazoquimolones, imiquimod and R-848, are the most successful TLR agonists used for the treatment for skin viral infections. Application of 1% imiquimod cream on the skin of hairless mice demonstrated increased transcription of INFgamma in the treated skin, indicating INFgamma may act locally as an antiviral agent[126]. And imiquimod was also approved by the Food and Drug Administration (FDA) to treat anogenital HPV infections. One randomized, controlled trial examined skin biopsies of HPV patients treated with 5% imiquimod cream. Examination of biopsies demonstrated that wart clearance revealed elevated INFalpha, INFbeta, INFgamma, and TNFα mRNA as well as decreased viral DNA and mRNA. These results imply that the activation of TLR7 by imiquimod induces Th1 cytokine production to clear HPV infection[127].
However, such strong innate immune activations induced by TLRs may be deleterious to host immune homeostasis. Sustained TLR activation is associated with the overproduction of proinflammatory cytokines and can lead to a systemic inflammatory response syndrome. Excessive TLR3 expression or triggering is associated with several inflammatory diseases, such as inflammation-associated lupus nephritis, and viral or autoimmune diseases. Moreover, LPS regulates the innate immune system through TLR4, resulting in the excessive production of proinflammatory cytokines such as TNFalpha and type I IFNs followed by lethal septic shock[128]. Other TLR ligands, when pretreated with sensitizing agents such as heat killed Propionibacterium acnes, lead to death in a similar way[129]. The TLR9 ligand has been implicated in triggering autoimmune responses including systemic lupus erythematosus or rheumatoid arthritis[77, 130]. A simple and direct strategy for preventing or treating TLR mediated pathogeneses would be to use TLR antagonists to block TLRs. An antagonistic monoclonal antibody against TLR2 was shown to block TLR2 stimulation and inflammation following lethal shock[131]. In addition to direct targeting of TLRs by specific TLR antagonists, targeting the negative regulators for intracellular TLR signaling will be another promising therapeutic target for the prevention and treatment of infectious or inflammatory diseases.
Thus, TLR agonists and antagonists have therapeutic potential as anti-infectious or anti-inflammatory agents. The modulation of immune responses holds promise for the development of new therapeutic options for infectious/inflammatory diseases in humans. The discovery of TLRs and the identification of both their cognate ligands and their downstream signaling pathways have provided a new set of targets for drug development, with the advantage of regulating the first steps of the disease[20, 132–136]. Therapeutic strategies for both reducing and enhancing TLR pathways are being developed by means of the use of agonists, antagonists and other mediators. Although TLRs are involved in the pathogenesis of a multitude of diseases, the modulation of TLRs as a therapeutic approach is very complex. TLR mediated innate immune activation in response to either infection or inflammation, may result in a deleterious outcome. Inhibition of TLR signaling, on the other hand, may cause innate and /or adaptive immune deficiency (immunological tolerance or impaired Th1 responses) to some extent, which could be lethal for certain patient populations. Moreover, blocking TLR may lead to “inappropriate” immune responses such as allergic Th2 responses, or immunological tolerance. Therefore, it is clear that the risks and benefits manipulation of TLR mediated immune responses need to be balanced, being restricted to the safer side of their potential uses.
References
- 1.Akira S, Takeda K, Kaisho T. Nat. Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Uematsu S, Takeuchi O. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann JA. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 6.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 7.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda , Akira S. J. Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 8.Takeda K, Akira S. Int. Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 10.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. Nat. Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 11.Leifer CA, Kennedy MN, Mazzoni A, Lee C, Kruhlak MJ, Segal DM. J. Immunol. 2004;173:1179–1183. doi: 10.4049/jimmunol.173.2.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto M, Funami K, Tanabe M, Oshiumi H, Shingai M, Seto Y, Yamamoto A, Seya T. J. Immunol. 2003;171:3154–3162. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- 13.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. J. Biol. Chem. 2004;279:17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 14.Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL. J. Biol. Chem. 2007;282:18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 15.Bowie A, O'Neill LA. J. Leukoc. Biol. 2000;67:508–514. doi: 10.1002/jlb.67.4.508. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, Bird TA, O'Neill LA. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 17.Horng T, Barton GM, Medzhitov R. Nat. Immunol. 2001;2:835–841. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- 18.Bin LH, Xu LG, Shu HB. J. Biol. Chem. 2003;278:24526–24532. doi: 10.1074/jbc.M303451200. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. J. Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 20.Ishii KJ, Uematsu S, Akira S. Curr. Pharm. Des. 2006;12:4135–4142. doi: 10.2174/138161206778743484. [DOI] [PubMed] [Google Scholar]
- 21.Oshiumi H, Sasai M, Shida K, Fujita T, Matsumoto M, Seya T. J. Biol. Chem. 2003;278:49751–49762. doi: 10.1074/jbc.M305820200. [DOI] [PubMed] [Google Scholar]
- 22.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. Nat. Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, Monks B, Pitha PM, Golenbock DT. J. Exp. Med. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Neill LA, Fitzgerald KA, Bowie AG. Trends Immunol. 2003;24:286–290. doi: 10.1016/s1471-4906(03)00115-7. [DOI] [PubMed] [Google Scholar]
- 25.Sato S, Nomura F, Kawai T, Takeuchi O, Muhlradt PF, Takeda K, Akira S. J. Immunol. 2000;165:7096–7101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- 26.Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, Hoshino K, Akira S. J. Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 27.Honda K, Yanai H, Mizutani T, Negishi H, Shimada N, Suzuki N, Ohba Y, Takaoka A, Yeh WC, Taniguchi T. Proc. Natl. Acad. Sci. U S A. 2004;101:15416–15421. doi: 10.1073/pnas.0406933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Strelow A, Fontana EJ, Wesche H. Proc. Natl. Acad. Sci. U S A. 2002;99:5567–5572. doi: 10.1073/pnas.082100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki N, Suzuki S, Duncan GS, Millar DG, Wada T, Mirtsos C, Takada H, Wakeham A, Itie A, Li S, Penninger JM, Wesche H, Ohashi PS, Mak TW, Yeh WC. Nature. 2002;416:750–756. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- 32.Qian Y, Commane M, Ninomiya-Tsuji J, Matsumoto K, Li X. J. Biol. Chem. 2001;276:41661–41667. doi: 10.1074/jbc.M102262200. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Z, Ninomiya-Tsuji J, Qian Y, Matsumoto K, Li X. Mol. Cell. Biol. 2002;22:7158–7167. doi: 10.1128/MCB.22.20.7158-7167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karin M, Ben-Neriah Y. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 35.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 36.Regnier CH, Song HY, Gao X, Goeddel DV, Cao Z, Rothe M. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 37.Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 38.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 39.Sato S, Sugiyama M, Yamamoto M, Watanabe Y, Kawai T, Takeda K, Akira S. J. Immunol. 2003;171:4304–4310. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Z, Zamanian-Daryoush M, Nie H, Silva AM, Williams BR, Li X. J. Biol. Chem. 2003;278:16713–16719. doi: 10.1074/jbc.M300562200. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Z, Mak TW, Sen G, Li X. Proc. Natl. Acad. Sci. U S A. 2004;101:3533–3538. doi: 10.1073/pnas.0308496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J. Nat. Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 43.Gohda J, Matsumura T, Inoue J. J. Immunol. 2004;173:2913–2917. doi: 10.4049/jimmunol.173.5.2913. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 45.Sharma RP, He Q, Johnson VJ. J. Interferon Cytokine Res. 2003;23:13–23. doi: 10.1089/10799900360520414. [DOI] [PubMed] [Google Scholar]
- 46.Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Br. J. Dermatol. 2003;148:670–679. doi: 10.1046/j.1365-2133.2003.05287.x. [DOI] [PubMed] [Google Scholar]
- 47.Lebre MC, van der Aar AM, van Baarsen L, van Capel TM, Schuitemaker JH, Kapsenberg ML, de Jong EC. J. Invest. Dermatol. 2007;127:331–341. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- 48.Miller LS, Modlin RL. Semin. Immunopathol. 2007;29:15–26. doi: 10.1007/s00281-007-0061-8. [DOI] [PubMed] [Google Scholar]
- 49.Kollisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J, Bauer S, Jakob T, Mempel M, Ollert M. Immunology. 2005;114:531–541. doi: 10.1111/j.1365-2567.2005.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerold G, Zychlinsky A, de Diego JL. Semin. Immunol. 2007;19:41–47. doi: 10.1016/j.smim.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Blander JM, Medzhitov R. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 52.Krutzik SR, Ochoa MT, Sieling PA, Uematsu S, Ng YW, Legaspi A, Liu PT, Cole ST, Godowski PJ, Maeda Y, Sarno EN, Norgard MV, Brennan PJ, Akira S, Rea TH, Modlin RL. Nat. Med. 2003;9:525–532. doi: 10.1038/nm864. [DOI] [PubMed] [Google Scholar]
- 53.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Nat. Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 54.Zasloff M. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 55.Ganz T. Science. 1999;286:420–421. doi: 10.1126/science.286.5439.420. [DOI] [PubMed] [Google Scholar]
- 56.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Annu. Rev. Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 57.Brown KL, Hancock RE. Curr. Opin. Immunol. 2006;18:24–30. doi: 10.1016/j.coi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RI. J. Clin. Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang D, Chertov O, Oppenheim JJ. J. Leukoc. Biol. 2001;69:691–697. [PubMed] [Google Scholar]
- 60.Zaiou M, Gallo RL. J. Mol. Med. 2002;80:549–561. doi: 10.1007/s00109-002-0350-6. [DOI] [PubMed] [Google Scholar]
- 61.Abtin A, Eckhart L, Mildner M, Gruber F, Schroder JM, Tschachler E. Faseb J. 2008 doi: 10.1096/fj.07-104117. [DOI] [PubMed] [Google Scholar]
- 62.Sumikawa Y, Asada H, Hoshino K, Azukizawa H, Katayama I, Akira S, Itami S. Microbes Infect. 2006;8:1513–1521. doi: 10.1016/j.micinf.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 63.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 64.Schauber J, Oda Y, Buchau AS, Yun QC, Steinmeyer A, Zugel U, Bikle DD, Gallo RL. J. Invest. Dermatol. 2008;128:816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- 65.Elssner A, Duncan M, Gavrilin M, Wewers MD. J. Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 66.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. J. Exp. Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Funderburg N, Lederman MM, Feng Z, Drage MG, Jadlowsky J, Harding CV, Weinberg A, Sieg SF. Proc. Natl. Acad. Sci. U S A. 2007;104:18631–18635. doi: 10.1073/PNAS.0702130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Nardo A, Braff MH, Taylor KR, Na C, Granstein RD, McInturff JE, Krutzik S, Modlin RL, Gallo RL. J. Immunol. 2007;178:1829–1834. doi: 10.4049/jimmunol.178.3.1829. [DOI] [PubMed] [Google Scholar]
- 69.Banchereau J, Steinman RM. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 70.Medzhitov R. Nat. Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 71.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 72.Takeda K, Kaisho T, Akira S. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 73.Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM, Vogel SN. Infect. Immun. 2001;69:1477–1482. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Re F, Strominger JL. J. Biol. Chem. 2001;276:37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- 75.Jotwani R, Pulendran B, Agrawal S, Cutler CW. Eur. J. Immunol. 2003;33:2980–2986. doi: 10.1002/eji.200324392. [DOI] [PubMed] [Google Scholar]
- 76.Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. Blood. 2006;107:610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. J. Exp. Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pestka S, Krause CD, Walter MR. Immunol. Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 79.Coccia EM, Severa M, Giacomini E, Monneron D, Remoli ME, Julkunen I, Cella M, Lande R, Uze G. Eur. J. Immunol. 2004;34:796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- 80.Homma T, Kato A, Hashimoto N, Batchelor J, Yoshikawa M, Imai S, Wakiguchi H, Saito H, Matsumoto K. Am J Respir Cell. Mol. Biol. 2004;31:463–469. doi: 10.1165/rcmb.2004-0161OC. [DOI] [PubMed] [Google Scholar]
- 81.Lotz M, Ebert S, Esselmann H, Iliev AI, Prinz M, Wiazewicz N, Wiltfang J, Gerber J, Nau R. J. Neurochem. 2005;94:289–298. doi: 10.1111/j.1471-4159.2005.03188.x. [DOI] [PubMed] [Google Scholar]
- 82.Tao X, Xu Y, Zheng Y, Beg AA, Tong L. Biochem. Biophys. Res. Commun. 2002;299:216–221. doi: 10.1016/s0006-291x(02)02581-0. [DOI] [PubMed] [Google Scholar]
- 83.Underhill DM, Ozinsky A. Curr. Opin. Immunol. 2002;14:103–110. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 84.Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M, Akira S, Norgard MV, Belisle JT, Godowski PJ, Bloom BR, Modlin RL. Science. 2001;291:1544–1547. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 85.Gorski KS, Waller EL, Bjornton-Severson J, Hanten JA, Riter CL, Kieper WC, Gorden KB, Miller JS, Vasilakos JP, Tomai MA, Alkan SS. Int. Immunol. 2006;18:1115–1126. doi: 10.1093/intimm/dxl046. [DOI] [PubMed] [Google Scholar]
- 86.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Nat. Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matsukura S, Kokubu F, Kurokawa M, Kawaguchi M, Ieki K, Kuga H, Odaka M, Suzuki S, Watanabe S, Takeuchi H, Kasama T, Adachi M. Clin. Exp. Allergy. 2006;36:1049–1062. doi: 10.1111/j.1365-2222.2006.02530.x. [DOI] [PubMed] [Google Scholar]
- 88.Iwasaki A, Medzhitov R. Nat. Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 89.Cook DN, Pisetsky DS, Schwartz DA. Nat. Immunol. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- 90.Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. J. Exp. Med. 1998;188:985–990. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alonzi T, Fattori E, Lazzaro D, Costa P, Probert L, Kollias G, De Benedetti F, Poli V, Ciliberto G. J. Exp. Med. 1998;187:461–468. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kobayashi H, Ohshima S, Nishioka K, Yamaguchi N, Umeshita-Sasai M, Ishii T, Mima T, Kishimoto T, Kawase I, Saeki Y. J. Rheumatol. 2002;29:1176–1182. [PubMed] [Google Scholar]
- 93.Ohshima S, Saeki Y, Mima T, Sasai M, Nishioka K, Nomura S, Kopf M, Katada Y, Tanaka T, Suemura M, Kishimoto T. Proc. Natl. Acad. Sci. U S A. 1998;95:8222–8226. doi: 10.1073/pnas.95.14.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Foster SL, Hargreaves DC, Medzhitov R. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 95.Wei XQ, Leung BP, Niedbala W, Piedrafita D, Feng GJ, Sweet M, Dobbie L, Smith AJ, Liew FY. J. Immunol. 1999;163:2821–2828. [PubMed] [Google Scholar]
- 96.Kurt-Jones EA, Belko J, Yu C, Newburger PE, Wang J, Chan M, Knipe DM, Finberg RW. J. Infect. Dis. 2005;191:746–748. doi: 10.1086/427339. [DOI] [PubMed] [Google Scholar]
- 97.Kawai T, Akira S. Semin. Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 98.Su X, Li S, Meng M, Qian W, Xie W, Chen D, Zhai Z, Shu HB. Eur. J. Immunol. 2006;36:199–206. doi: 10.1002/eji.200535415. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G. Nat. Immunol. 2006;7:139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- 100.Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A. Nat. Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 101.van 't Veer C, van den Pangaart PS, van Zoelen MA, de Kruif M, Birjmohun RS, Stroes ES, de Vos AF, van der Poll T. J. Immunol. 2007;179:7110–7120. doi: 10.4049/jimmunol.179.10.7110. [DOI] [PubMed] [Google Scholar]
- 102.Lowy FD. N. Engl. J. Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 103.Hashimoto M, Tawaratsumida K, Kariya H, Aoyama K, Tamura T, Suda Y. Int. Immunol. 2006;18:355–362. doi: 10.1093/intimm/dxh374. [DOI] [PubMed] [Google Scholar]
- 104.Bubeck Wardenburg J, Williams WA, Missiakas D. Proc. Natl. Acad. Sci. U S A. 2006;103:13831–13836. doi: 10.1073/pnas.0603072103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 106.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. J. Biol. Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 107.Girardin SE, Travassos LH, Herve M, Blanot D, Boneca IG, Philpott DJ, Sansonetti PJ, Mengin-Lecreulx D. J. Biol. Chem. 2003;278:41702–41708. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- 108.Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 109.Takeuchi O, Hoshino K, Akira S. J. Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 110.Lorenz E, Mira JP, Cornish KL, Arbour NC, Schwartz DA. Infect. Immun. 2000;68:6398–6401. doi: 10.1128/iai.68.11.6398-6401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kang SS, Kauls LS, Gaspari AA. J. Am. Acad. Dermatol. 2006;54:951–983. doi: 10.1016/j.jaad.2005.05.004. quiz 983–956. [DOI] [PubMed] [Google Scholar]
- 112.McInturff JE, Kim J. Semin. Cutan. Med. Surg. 2005;24:73–78. doi: 10.1016/j.sder.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 113.Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, Brightbill HD, Holland D, Cunliffe WJ, Akira S, Sieling PA, Godowski PJ, Modlin RL. J. Immunol. 2002;169:1535–1541. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jugeau S, Tenaud I, Knol AC, Jarrousse V, Quereux G, Khammari , Dreno B. Br. J. Dermatol. 2005;153:1105–1113. doi: 10.1111/j.1365-2133.2005.06933.x. [DOI] [PubMed] [Google Scholar]
- 115.Job CK. Int. J. Lepr. 1965;33 Suppl:533–541. [PubMed] [Google Scholar]
- 116.Wang T, Lafuse WP, Zwilling BS. J. Immunol. 2000;165:6308–6313. doi: 10.4049/jimmunol.165.11.6308. [DOI] [PubMed] [Google Scholar]
- 117.Baker BS. Clin. Exp. Immunol. 2006;144:1–9. doi: 10.1111/j.1365-2249.2005.02980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. N. Engl. J. Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 119.Birchler T, Seibl R, Buchner K, Loeliger S, Seger R, Hossle JP, Aguzzi A, Lauener RP. Eur. J. Immunol. 2001;31:3131–3137. doi: 10.1002/1521-4141(200111)31:11<3131::aid-immu3131>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 120.Curry JL, Qin JZ, Bonish B, Carrick R, Bacon P, Panella J, Robinson J, Nickoloff BJ. Arch. Pathol. Lab Med. 2003;127:178–186. doi: 10.5858/2003-127-178-IIRRIN. [DOI] [PubMed] [Google Scholar]
- 121.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Wang YH, Su B, Nestle FO, Zal T, Mellman I, Schroder JM, Liu YJ, Gilliet M. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 122.Baroni A, Orlando M, Donnarumma G, Farro P, Iovene MR, Tufano MA, Buommino E. Arch. Dermatol. Res. 2006;297:280–288. doi: 10.1007/s00403-005-0594-4. [DOI] [PubMed] [Google Scholar]
- 123.Vercammen E, Staal J, Beyaert R. Clin. Microbiol. Rev. 2008;21:13–25. doi: 10.1128/CMR.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ichinohe T, Watanabe I, Ito S, Fujii H, Moriyama M, Tamura S, Takahashi H, Sawa H, Chiba J, Kurata T, Sata T, Hasegawa H. J. Virol. 2005;79:2910–2919. doi: 10.1128/JVI.79.5.2910-2919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dong L, Ito S, Ishii KJ, Klinman DM. Arthritis Rheum. 2004;50:1686–1689. doi: 10.1002/art.20263. [DOI] [PubMed] [Google Scholar]
- 126.Imbertson LM, Beaurline JM, Couture AM, Gibson SJ, Smith RM, Miller RL, Reiter MJ, Wagner TL, Tomai MA. J. Invest. Dermatol. 1998;110:734–739. doi: 10.1046/j.1523-1747.1998.00174.x. [DOI] [PubMed] [Google Scholar]
- 127.Tyring SK, Arany I, Stanley MA, Tomai MA, Miller RL, Smith MH, McDermott DJ, Slade HB. J. Infect. Dis. 1998;178:551–555. doi: 10.1086/517472. [DOI] [PubMed] [Google Scholar]
- 128.Beutler B, Rietschel ET. Nat. Rev. Immunol. 2003;3:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 129.Tsutsui H, Matsui K, Okamura H, Nakanishi K. Immunol. Rev. 2000;174:192–209. doi: 10.1034/j.1600-0528.2002.017418.x. [DOI] [PubMed] [Google Scholar]
- 130.Deng GM, Nilsson IM, Verdrengh M, Collins LV, Tarkowski A. Nat. Med. 1999;5:702–705. doi: 10.1038/9554. [DOI] [PubMed] [Google Scholar]
- 131.Meng G, Rutz M, Schiemann M, Metzger J, Grabiec A, Schwandner R, Luppa PB, Ebel F, Busch DH, Bauer S, Wagner H, Kirschning CJ. J. Clin. Invest. 2004;113:1473–1481. doi: 10.1172/JCI20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Harris G, KuoLee R, Chen W. World J. Gastroenterol. 2006;12:2149–2160. doi: 10.3748/wjg.v12.i14.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hoebe K, Jiang Z, Georgel P, Tabeta K, Janssen E, Du X, Beutler B. Curr. Pharm. Des. 2006;12:4123–4134. doi: 10.2174/138161206778743466. [DOI] [PubMed] [Google Scholar]
- 134.Ishihara S, Rumi MA, Ortega-Cava CF, Kazumori H, Kadowaki Y, Ishimura N, Kinoshita Y. Curr. Pharm. Des. 2006;12:4215–4228. doi: 10.2174/138161206778743448. [DOI] [PubMed] [Google Scholar]
- 135.Romagne F. Drug Discov. Today. 2007;12:80–87. doi: 10.1016/j.drudis.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 136.Ulevitch RJ. Nat. Rev. Immunol. 2004;4:512–520. doi: 10.1038/nri1396. [DOI] [PubMed] [Google Scholar]