Abstract
Objectives
To develop a 3D computer model of the anterior vaginal wall and its supports, validate that model, and then use it to determine the combinations of muscle and connective tissue impairments that result in cystocele formation, as observed on dynamic magnetic resonance imaging (MRI).
Methods
A subject-specific 3D model of the anterior vaginal wall and its supports was developed based on MRI geometry from a healthy nulliparous woman. It included simplified representations of the anterior vaginal wall, levator muscle, cardinal and uterosacral ligaments, arcus tendineus fascia pelvis and levator ani, paravaginal attachments, and the posterior compartment. This model was then imported into ABAQUS™ and tissue properties were assigned from the literature. An iterative process was used to refine anatomical assumptions until convergence was obtained between model behavior under increases of abdominal pressure up to 168 cmH2O and deformations observed on dynamic MRI.
Results
Cystocele size was sensitive to abdominal pressure and impairment of connective tissue and muscle. Larger cystocele formed in the presence of impairments in muscular and apical connective tissue support compared to either support element alone. Apical impairment resulted in a larger cystocele than paravaginal impairment. Levator ani muscle impairment caused a larger urogenital hiatus size, longer length of the distal vagina exposed to a pressure differential, larger apical descent and resulted in a larger cystocele size.
Conclusions
Development of a cystocele requires a levator muscle impairment, an increase in abdominal pressure, and apical and paravaginal support defects.
Keywords: Female, pelvic floor, genital prolapse, biomechanical model
Introduction
The anterior vaginal wall prolapse, clinically known as cystocele, is the most common form of pelvic organ prolapse (Hendrix et al., 2002). It is also the site with the highest rate of persistent and recurrent support defects (Shull et al., 2000).
A growing number of studies have sought to improve our understanding of normal anterior vaginal support mechanisms as well as how these supports ordinarily prevent cystocele. Early studies were focused primarily on the anatomy and failure of ‘paravaginal support’, defined as the connective tissue attaching the mid-portion of the vagina laterally to the pelvic sidewalls (Richardson, 1976, DeLancey et al., 1992, 2002). Subsequent investigations have revealed levator ani muscle damage in women with anterior vaginal wall prolapse specifically (DeLancey et al., 2002) and prolapse in general (Tunn et al., 1998, Singh et al., 2003, Hoyte et al., 2001 & 2004, DeLancey et al., 2002). The anterior vaginal wall is supported at its apex by the cardinal and uterosacral ligaments which connect the uterus and cervix to the posterior boney pelvis (DeLancey 1992). Dynamic MRI studies have revealed that anterior compartment prolapse is also highly correlated (r=0.73) with loss of apical support (Summers et al., 2006) and vaginal length (Hsu et al., 2008).
Each of these observations concerns a particular aspect of anterior vaginal wall support and failure. To begin to integrate these observations into a single disease model, we first developed a simple planar model (Chen et al., 2006). This model simulated anterior vaginal wall deformation in the mid-sagittal plane and provided an important insight on the interaction between muscular support to the anterior vaginal wall provided by the levator ani and apical connective tissue support provided by the cardinal and uterosacral ligaments. However, this model had many limitations, most notably the lack of any paravaginal attachments to the pelvic side wall. Furthermore, the levator plate and rectum were modeled as rigid bodies.
To address those limitations, we developed a 3D finite element model to test the hypothesis that factors such as muscular support, apical defect, and paravaginal defects, affect cystocele formation.
Methods
MRI scans of a 34-year-old Caucasian woman were selected from an institutional review board-approved study of healthy nulliparous pelvic anatomy. Axial, sagittal and coronal proton density magnetic resonance images of the pelvic floor region were taken at 5-mm intervals, as previously described (Chou, et al., 2001). The geometry of the woman's bony pelvic floor attachment points lay within one standard deviation of the mean size and shape of a group of 278 women (Lien et al., 2004)
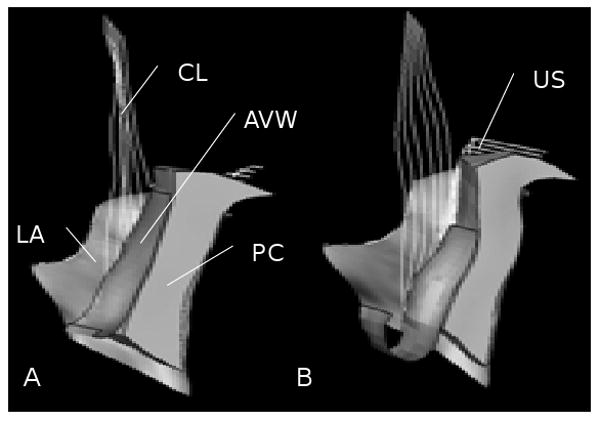
A 3-D volumetric model was rendered by lofting serial axial magnetic resonance sections acquired at 5 mm intervals to establish the geometry of the anterior vaginal wall and surrounding support tissues using 3D Slicer 2.1b1 (Slicer 2b1; Massachusetts Institute of Technology, Cambridge, MA), as described by Hsu et al. (2005) & Chen et al. (2006). The geometric model included the following anatomic structures: the anterior vaginal wall (AVW), levator muscles (LA), cardinal ligaments (CL), uterosacral ligaments (US), arcus tendineus fascia pelvis (ATFP), arcus tendineus levator ani (ATLA), paravaginal support (PS), and the posterior compartment (PC) as shown in Figures 1A and B.
Figure 1.
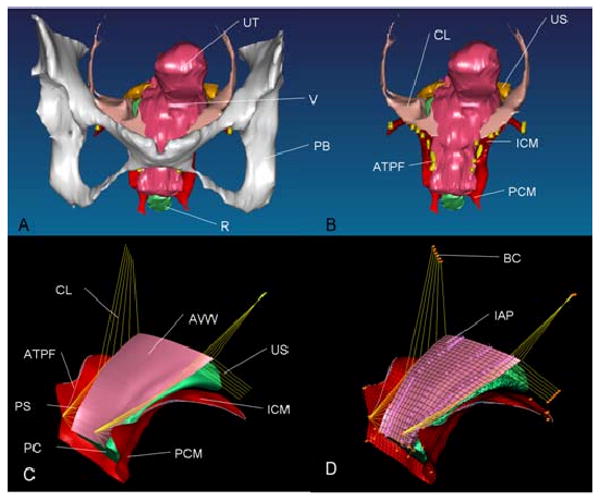
Model development. A: 3D volume-rendered model of anterior support system including pubic bone; B: 3D volume-rendered model without pubic bone; C: Geometrically simplified surface model; D: 3D finite element model with mesh, boundary condition (orange pin representing ligaments and muscle origin is fixed to pubic bone and pelvic sidewall), and abdominal pressure loading. PB denotes pubic bone; UT: uterus (not included in surface model); V: vagina; R: rectum; CL: cardinal ligament; US: uterosacral ligament; ATFP: arcus tendineus fascia pelvis; ICM: iliococcygeus muscle; PCM: pubococcygeus muscle; AVW: anterior vaginal wall; PC: posterior compartment; PS: paravaginal support; and IAP: abdominal pressure
The volumetric model was imported into I-DEAS (Version 9.0, EDS, Hook, UK), an engineering modeling software program. 4th-order B-spline curves were used to smooth the digitized ventral margin of the anterior vaginal wall as well as the margins of each structure before lofting to smooth surface (Figure 1C). The simplified model was then imported into ABAQUS (Version 6.6-1 ABAQUS, Inc) to create the finite element mesh that was used to represent the anterior vaginal wall, levator ani muscles and posterior compartment, and then to predict how the structures would deform under increases in abdominal pressure. The program also predicted the resulting distributions in tissue stresses and strains (Figure 1D). The anterior vaginal wall and levator ani muscles were modeled in the program as deformable shell elements using a total of 416 linear quadrilateral shell elements to represent the anterior vaginal wall with two millimeter thickness, and 1,863 quadrilateral and 17 triangular elements to represent the levator ani muscle using elements varying from two to four millimeter thickness. The posterior compartment, including the posterior vaginal wall and rectum, were combined as a deformable solid and represented using 2,285 tetrahedral elements. Lastly, we used a total of 7,387 3D truss elements to represent the ligamentous connections (CL, US, PS, ATFP, ATLA) to the vaginal wall; the origins and insertions of these ligaments were determined from published anatomic descriptions (DeLancey 1992, 1994, 2002).
The soft tissue attachments to pelvis and stationary structures (known as “boundary conditions”) are shown in Figure 1D. The origin of levator ani muscle from the pubic bone and ATLA, and the points of attachments of the ligaments to the pelvic sidewall, and distal end of the vagina were each assumed to allow rotational, but not translational, movements.
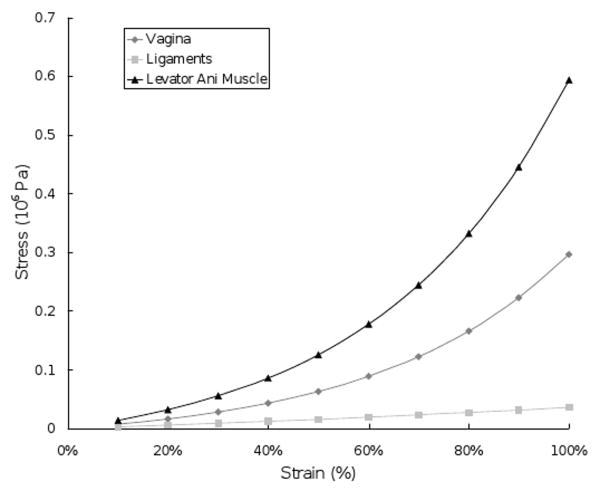
All the model elements were assigned hyperelastic material properties based on values from existing literature (Yamada et al., 1970, Bartscht et al., 1988) (Figure 2). Because this model was only used to simulate cystocele development during straining, when patients were asked to increase abdominal pressure with their pelvic muscles relaxed, the levator ani muscles were only considered to exhibit passive stretch and therefore passive stiffness, without active contractile behavior.
Figure 2.
The material properties of the vagina, ligaments and levator ani muscle used in the model simulation. Note the visceral “ligaments” in this context are mesenteric in nature thereby differing considerably from skeletal ligaments.
The functional anatomy of the paravaginal supports is presently poorly understood. Therefore, an iterative process was used to refine the representation of the paravaginal supports based on the senior author's extensive dissection, clinical examination and surgical experience and dynamic MR imaging observation (DeLancey et al., 1994 & 2002, Summers et al., 2006). The deformation behavior of the model anterior vaginal wall under increasing abdominal pressure was examined and compared to that observed on dynamic MRI during Valsalva (Summers et al., 2006). When paravaginal supports were represented by direct lateral ligamentous connections to the pelvic sidewall, the model vaginal wall deformation pattern did not match the observed behavior on MRI. So, the assumption that the paravaginal support directly attaches to the pelvic sidewall was reevaluated. After several iterations with different origins and insertions, we found that a fan-shaped pattern of fascia originating from the ventral insertion of the arcus tendineus fascia pelvis and inserting onto equidistant points spaced along the lateral aspect of the anterior vaginal wall (Figure 1C & D) resulted in model anterior vaginal wall deformations that matched those observed those in dynamic MRI.
To simulate cystocele formation, that is, anterior wall descent under load, we set model parameters to simulate impairments in the various supportive components (i.e., CL, US, PS and PCM). Impairment in the ligaments was simulated as a 20, 40, 60, or 80% decrease in material property stiffness (Chen et al., 2006). Impairment in levator ani muscle was simulated by decreasing its stiffness by 60% from normal values, consistent with the 60% decrease in maximum muscle strength observed in women with prolapse and identified levator muscle impairment on MR, as compared to those with normal support and intact muscle (DeLancey et al., 2007). This assumption reflects the linear relationship that has been found between the striated muscle contractile force and tensile stiffness (Blanpied et al., 1993).
Simulations of the effects of abdominal pressure on model structures were in quasi-static state and solved using ABAQUS explicit because of its complicate contact conditions. The abdominal pressure was considered to act perpendicular to the ventral surface of the model's anterior vaginal wall. In a previous study of 314 patients with prolapse, the 5th percentile, 50th and 95th percentile values of maximum Valsalva pressure were determined to be 60, 99 and 168 cmH2O, respectively (DeLancey et al., 2007). Therefore, the increase in abdominal pressure loading of the anterior vaginal wall was simulated using 20 increments in pressure from 0 to 168 cmH2O.
The primary model outcome variables included the descent of the most dependent point of the anterior vagina wall (d), apex descent (a) and hiatus size (h) as well as the length of vagina exposed to the pressure differential between maximum and atmospheric abdominal pressure (Figure 3). The maximum stress in the levator ani muscle and vaginal wall were also calculated.
Figure 3.
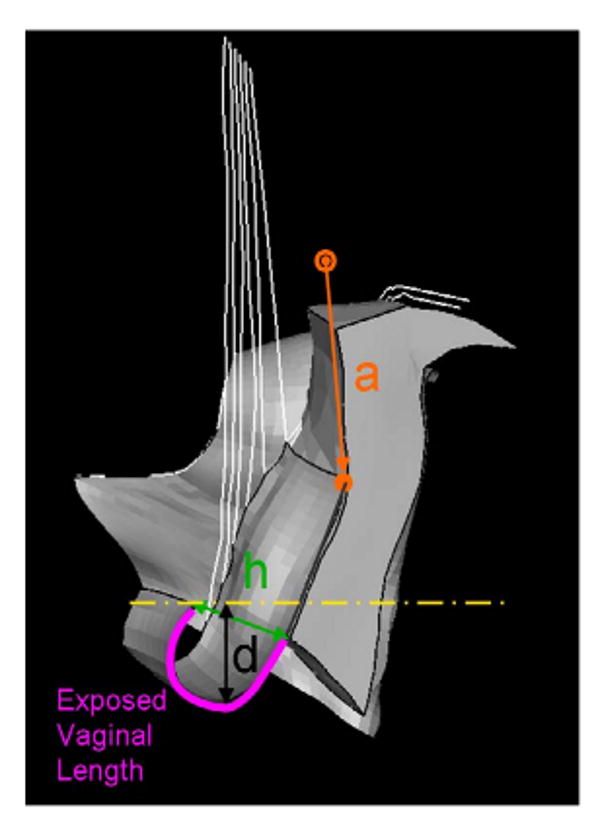
The left, three-quarter view of the model mid-sagittal configuration at maximum abdominal pressure showing the four outcome measurements: a, h, d, and length of exposed vaginal length.
The models with normal apical connective tissue support (cardinal and uterosacral ligmanent) and varying degree of impairments in the pubic portion of the levator ani muscle (i.e., 20, 40, 60, and 80%) were loaded with up to 168 cmH2O intra-abdominal pressure. The model was validated by comparing the mid-sagittal anterior vaginal wall deformation under increasing abdominal pressure with that in the dynamic MRI of a patient with Stage III cystocele performing the same maneuver (Figure 4).
Figure 4.
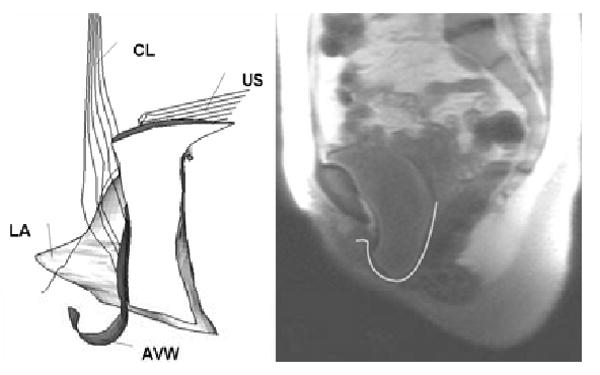
Model validation. On the left is a model-generated simulation result showing a cystocele formed in the manner of that seen clinically in one frame of a dynamic MRI on right side of the figure.
Results
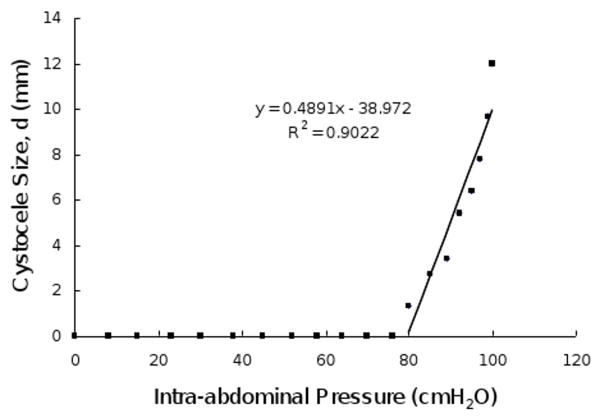
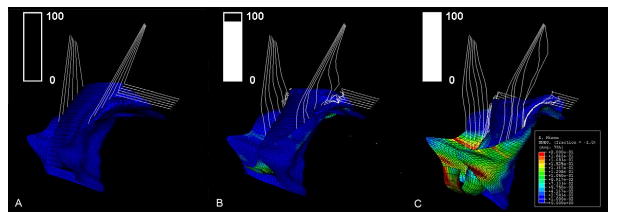
When the intact model (without defects in the levator ani muscle, apical and paravaginal support) was loaded with up to 100 cmH2O (50th percentile), a slight cystocele occurred (Figure 5A). This can be compared with a more typical simulated cystocele formation (Figure 5B), in which cardinal, uterosacral ligament, and paravaginal support was set to 50% impairment and the PCM was set to have a 60% impairment. The sequential development of this cystocele with increasing abdominal pressure is shown in Figure 6. The relationship between simulated cystocele size and intra-abdominal pressure for this simulation can be seen in Figure 7. The best-fit line for the non-zero cystocele size data points was estimated. From the slope of the line the compliance of the anterior support system was estimated to be 0.49 mm/cmH2O.
Figure 5.
Left, three-quarter, view of a mid-sagittal section of the 3D finite element model. Panel A: All support elements (levator ani muscle, cardinal uterosacral ligament, paravaginal support) have normal material properties. Panel B: The levator ani muscle was set to have a 60% reduction in its properties, and a 50% reduction in cardinal and uterosacral ligament and paravaginal support properties. LA denotes levator ani muscle, CL: cardinal ligament, AVW: arterial vaginal wall, PC: posterior compartment, and US: uterosacral ligament
Figure 6.
(Left-to-right) a three-quarter, left, anterior view of the sequential development of a typical simulated cystocele during a gradually increasing load. In this simulation, the levator ani muscle had a 60% impairment, and apical and paravaginal support properties were set to having a 50% impairment. The color map shows the stress distributions in the different regions, with blue indicating a low stress region and red indicating a high stress region. The box on the up left coner shows the abdominal pressure (ordinate), increasing from zero to 100 cmH2O, over a time course (abscissa) corresponding to the three scenarios above.
Figure 7.
Relationship between cystocele size and intra-abdominal pressure in a simulated cystocele. In this simulation, the levator ani muscle had a 60% impairment, apical and paravaginal support were set to having a 50% impairment. The best fit line for non-zero cystocele size data points is plotted. The linear regression equation and coefficient of determination are shown in the illustration.
The size of the cystocele was found to vary depending on the maximal abdominal pressure applied to the model (Figure 8). Cystocele size was sensitive to structure impairments. At a 168 cmH2O maximum intra-abdominal pressure, a levator ani muscle impairment alone resulted in slightly larger cystocele than a apical connective tissue impairment alone. The model with the combined impairment had the largest simulated cystocele size, as hypothesized.
Figure 8.
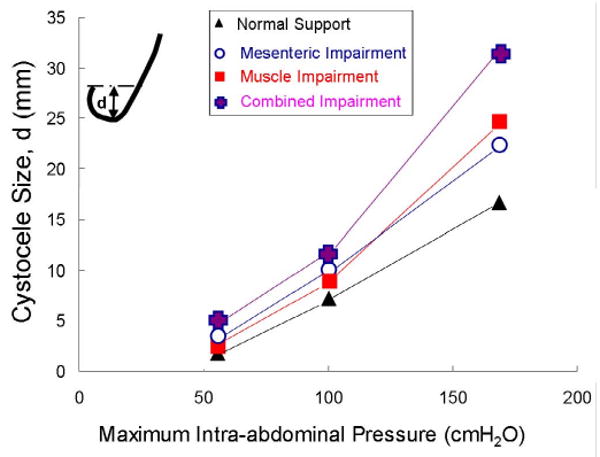
Simulated cystocele size for models with different impairment patterns at increasing values of maximum intra-abdominal pressure loading.
Figure 9 shows the effect of levator ani muscle impairment alone on cystocele formation. With increasing muscle impairment, there is a systematic increase in hiatus size, which allows a longer length of the vaginal wall to be exposed to a pressure differential (with intra-abdominal pressure acting on the “proximal” surface and atmospheric pressure acting on the “distal” surface). As tension builds in the vaginal wall, causing a larger apical descent (Figure 9), this in turn results in larger cystocele size.
Figure 9.
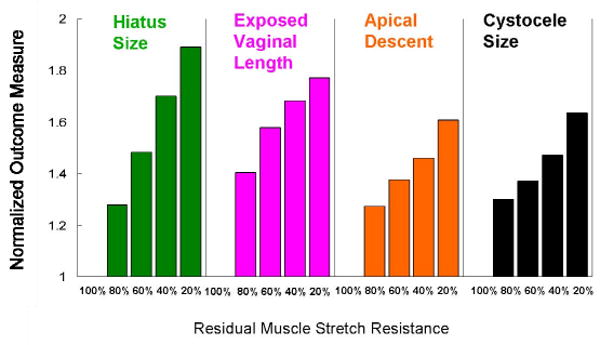
Effects of muscle impairment on cystocele formation. The outcome measurement was normalized to model outcome with intact levator ani muscle resistance to stretch.
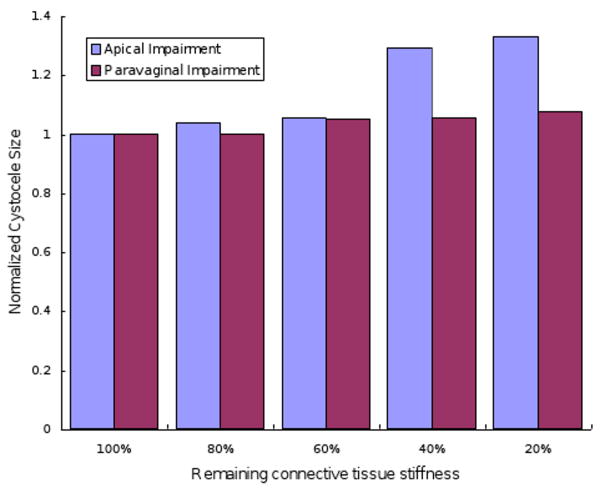
The results of the effects of apical or paravaginal impairment on cystocele formation are shown in Figure 10. Predominant apical impairment (varying apical impairment at 0%, 20%, 40%, 60%, 80% but with normal paravaginal support), predominant paravaginal impairment (varying paravaginal impairment at 0%, 20%, 40%, 60%, 80% but with normal apical support) are shown for 168 cmH2O maximal abdominal pressures and 60% levator ani muscle impairment. All cystocele sizes were normalized to cystocele size at normal apical and normal paravaginal support. The cystocele size was sensitive to the degree of apical impairment with 20% remaining apical support stiffness; the cystocele size increased 33% compared to the scenario with normal apical support. However there was only a slight change in cystocele size with increasing degrees of paravaginal impairment.
Figure 10.
Effects of apical and paravaginal impairments on cystocele formation. Simulations had 60% levator ani muscle impairments and were loaded with 168cmH2O maximum intra-abdominal pressure. For apical impairment simulations, models had normal paravaginal support, but a varying degree of apical support impairment with remaining apical connective tissue stiffness ranging from 20% to 100% of the normal value. For paravaginal impairment simulations, models had normal apical support but varying degree of paravaginal support impairment with remaining paravaginal connective tissue stiffness ranging from 20% to 100% of normal values.
Discussion
In this first 3D simulation of cystocele formation, the hypothesis was supported that levator impairment and apical support impairment can result in cystocele. The model had realistic anatomical geometry from a living normal woman with both connective tissue and muscular supports. It reproduces realistic cystocele similar to those seen clinically, under physiological abdominal pressure loading (Figure 4). Also the estimated anterior compartment compliance from model simulation was about 0.49 mm/cmH2O, which is close to that measured in living women with prolapse (Hsu, Y., personal communication).
The magnitude of the cystocele was found to be sensitive to maximum abdominal pressure, the presence of impaired structures (levator ani muscle, apical connective tissue, paravaginal connective tissue, vaginal wall) and single vs. multiple site impairments. The finding that impairments in multiple domains (muscle impairment and mesenteric connective tissue impairment, including both apical and paravaginal) result in larger cystocele is similar to the result we found in a simpler 2-D model (Chen et al., 2006). In that model we noted that when both muscle and connective tissue impairments were implemented in the model, larger cystoceles resulted than when only one or the other impairment was present.
The present model suggests that levator ani muscle impairment plays an important role in cystocele formation, consistent with clinical findings (DeLancey et al., 2007). A decrease in levator ani muscle resistance to stretch caused a larger hiatus size, and allowed a longer length of vaginal wall to be exposed to the pressure differential between high intra-abdominal pressure and low atmosphere pressure. This caused tension to build up in the vaginal wall tissue, drawing the apex down and thereby resulted in larger cystocele size.
The models also help to explain the importance of apical support. With muscle impairment present and the anterior vaginal wall subjected to a pressure differential loading, this tensile load has to be resisted by both apical and paravaginal connective tissue supports. Our model suggested that an 80% impairment in apical support can result in a 33% larger cystocele size than that with normal apical support and also apical impairment resulted in a larger cystocele size compared to paravaginal impairment. This agrees with our earlier clinical findings that variation in apical support explained 60% of variation in prolapse size in a group of women with a wide spectrum of pelvic floor support (Summer et al., 2006).
Based on the results in this paper we have developed a conceptual model to help provide a framework to systematically investigate the interaction between muscle, connective tissue and anterior vaginal wall structures in the presence and absence of structural defects (Figure 11).
Figure 11.
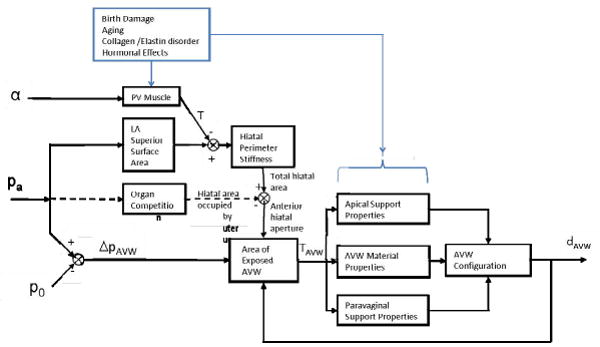
A conceptual model of the pathomechanics of cystocele. Inputs to the support system include the intensity (α) of the pubovisceral (“PV”) muscle contraction (yielding tension, T), intraabdominal pressure (pa) and atmospheric pressure (po). ‘pa’ acts on the surface of the levator ani muscle so as to increase T and help determine total hiatal area. It can also drive the uterus partially into the hiatus (dashed line), leaving the remaining hiatal aperture to be spanned by the distal anterior vaginal wall (AVW) and exposed to the pressure differential (ΔpAVW). The resulting tension (TAVW) in the AVW then applies tension to its Apical and Paravaginal Supports helping to determine the size of the resulting cyctocele (dAVW). Birth damage, aging, collagen/elastin disorders and hormonal effects are all postulated to affect the properties of given structures.
Positive feedback mechanisms in this conceptual model provide insights that help to explain how cystocele can gradually develop over the years. Once a portion of the vaginal wall descends below the support offered by the rectum and levator ani muscle, the exposed anterior vaginal wall comes to lie between abdominal and atmospheric pressure. The force that results from this pressure differential acting on the anterior vaginal wall (ΔpAVW) causes tension in the vaginal tissue. This tension loads the apical support system and causes apical descent. Then this apical descent causes a longer length of vagina to be exposed to the pressure differential, which in turn causes larger tension in vaginal wall and larger loading on apical support, until a new equilibrium is again established in the system due to the increasing resistance to descent of the apex. A second positive feedback mechanism is caused by the anterior vaginal wall having a trapezoidal shape - narrower at distal end than at the apex. As increasing distal vaginal length is exposed to the pressure differential, the area of vagina exposed to the pressure differential will increase non-linearly due to this trapezoidal shape, thereby leading to a non-linear increase in the tensile force in the vagina dragging down the apex. Thirdly, an impairment to the mother during vaginal birth or intrabdominal pressure overloading during activities like lifting, tripping or slipping, could injure the apical support system and thereby decrease the stiffness of apical support system material properties; this will cause an even larger apical descent, and eventually a larger cystocele.
Restoring normal anatomy and function are common goals of reconstructive surgery. At present, our ability to specifically determine the location and type of impairment responsible for cystocele has been limited. The findings of this study can form the framework for new ultrasound or magnetic resonance imaging studies of vaginal wall behavior in living women. It also provides the unique ability to experimentally induce specific impairments and see what their effects are in ways that would not be ethical in humans. This should help direct targeted investigations into anatomical changes seen in women with cystourethrocele. Eventually, once we can determine the exact nature of the structural impairments, operations can be customized for each patient to maximize surgical success while minimizing the risk of compensatory tissue distortion that can lead to postoperative problems with function.
There are several limitations to this model in its current form. An active contraction of levator ani muscle was not represented. This may not be a serious limitation because the model simulates the clinical examination used to detect cystocele whereby patients are instructed to relax their levator ani muscles during the Valsalva maneuver. Second, the model lacks a perineal body, so the constraint at the distal end of the vagina did not permit the downward movement that is sometimes observed during Valsalva. Cystocele size was found not to be particularly sensitive to paravaginal impairment. This might have resulted from gaps in our understanding of paravaginal support anatomy. Third, the model has simplified material properties in that all elements were assumed to be hyperelastic; the next step would be to model the effect of anisotropy, as well as viscous, plastic and remodeling behaviors. While the quantitative values of predicted tissue stresses are unlikely to be accurate, the qualitative behavior of the model should be reasonable. One needs to examine how the number and magnitude of loading cycles and tissue adaptation rates interact to affect cystocele formation. Finally, the effects of abnormal starting geometry and geometric variations in pelvic size and shape need to be further explored.
Acknowledgments
We gratefully acknowledge the support of PHS Grant R01 HD038665-07
Footnotes
Conflict of interest statement: Dr. Ashton-Miller and Dr. DeLancey are consultants to American Medical Systems, Inc. and Johnson & Johnson, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartscht KD, DeLancey JOL. A technique to study the passivesupports of the uterus. Obstet Gynecol. 1988;72:940–3. doi: 10.1097/00006250-198812000-00028. [DOI] [PubMed] [Google Scholar]
- Blanpied P, Smidt GL. The difference in stiffness of the active plantarflexors between young and elderly human females. J Gerontol. 1993;48:M58–M63. doi: 10.1093/geronj/48.2.m58. [DOI] [PubMed] [Google Scholar]
- Chen L, Hsu Y, Ashton-Miller JA, Delancey JOL. Measurement of the pubic portion of the levator ani muscle in women with unilateral defects in 3-D models from MR images. Int J Gynaecol Obstet. 2006;92:234–41. doi: 10.1016/j.ijgo.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Q, DeLancey JOL. A structured system to evaluate urethral support anatomy in magnetic resonance images. Am J Obstet Gynecol. 2001;185(1):44–50. doi: 10.1067/mob.2001.116368. [DOI] [PubMed] [Google Scholar]
- DeLancey JOL. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717–24. doi: 10.1016/0002-9378(92)91562-o. [DOI] [PubMed] [Google Scholar]
- DeLancey JOL. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994;170(6):1713–20. doi: 10.1016/s0002-9378(94)70346-9. [DOI] [PubMed] [Google Scholar]
- DeLancey JOL. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187(1):93–8. doi: 10.1067/mob.2002.125733. [DOI] [PubMed] [Google Scholar]
- DeLancey JOL, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, Hussain H, Umek W, Hsu Y, Ashton-Miller JA. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109:295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160–6. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- Hsu Y, Chen L, DeLancey JOL, Ashton-Miller JA. Vaginal thickness, cross-sectional area, and perimeter in women with and those without prolapse. Obstet Gynecol. 2005;105:1012–7. doi: 10.1097/01.AOG.0000158127.97690.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyte L, Schierlitz L, Zou K, Flesh G, Fielding JR. Two- and 3-dimensional MRI comparison of levator ani structure, volume, and integrity in women with stress incontinence and prolapse. Am J Obstet Gynecol. 2001;185:11–9. doi: 10.1067/mob.2001.116365. [DOI] [PubMed] [Google Scholar]
- Hoyte L, Jakab M, Warfield SK, Shott S, Flesh G, Fielding JR. Levator ani thickness variations in symptomatic and asymptomatic women using magnetic resonance-based 3-dimensional color mapping. Am J Obstet Gynecol. 2004;191:856–61. doi: 10.1016/j.ajog.2004.06.067. [DOI] [PubMed] [Google Scholar]
- Hoyte L, Thomas J, Foster RT, Shott S, Jakab M, Weidner AC. Racial differences in pelvic morphology among asymptomatic nulliparous women as seen on three-dimensional magnetic resonance images. Am J Obstet Gynecol. 2005;193(6):2035–40. doi: 10.1016/j.ajog.2005.06.060. [DOI] [PubMed] [Google Scholar]
- Hsu Y, Summers A, Hussain HK, Guire KE, DeLancey JOL. Levator plate angle in women with pelvic organ prolapse compared to women with normal support using dynamic MR imaging. Am J Obstet Gynecol. 2006;194(5):1427–33. doi: 10.1016/j.ajog.2006.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y, Chen L, Summers A, Ashton-Miller JA, DeLancey JO. Anterior vaginal wall length and degree of anterior compartment prolapse seen on dynamic MRI. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(1):137–42. doi: 10.1007/s00192-007-0405-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien KC, Mooney B, DeLancey JOL, Ashton-Miller JA. Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol. 2004;103(1):31–40. doi: 10.1097/01.AOG.0000109207.22354.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568–73. doi: 10.1016/0002-9378(76)90751-1. [DOI] [PubMed] [Google Scholar]
- Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183(6):1365–73. doi: 10.1067/mob.2000.110910. [DOI] [PubMed] [Google Scholar]
- Singh K, Jakab M, Reid WM, Berger LA, Hoyte L. Three-dimensional magnetic resonance imaging assessment of levator ani morphologic features in different grades of prolapse. Am J Obstet Gynecol. 2003;188:910–5. doi: 10.1067/mob.2003.254. [DOI] [PubMed] [Google Scholar]
- Strohbehn K, Ellis JH, Ashton-Miller JA, DeLancey JOL. Magnetic resonance imaging of the levator ani with anatomic correlation. Obstet Gynecol. 1996;87:277–85. doi: 10.1016/0029-7844(95)00410-6. [DOI] [PubMed] [Google Scholar]
- Summers A, Winkel LA, Hussain HK, DeLancey JOL. The Relationship between Anterior and Apical Compartment Support. Am J Obstet Gynecol. 2006;194:1438–43. doi: 10.1016/j.ajog.2006.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunn R, Paris S, Fischer W, Hamm B, Kuchinke J. Static magnetic resonance imaging of the pelvic floor muscle morphology in women with stress urinary incontinence and pelvic prolapse. Neurourol Urodyn. 1998;17:579–89. doi: 10.1002/(sici)1520-6777(1998)17:6<579::aid-nau2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Yamada H. Strength of Biological Materials. Baltimore (MD): Williams & Wilkins; 1970. pp. 205–70. [Google Scholar]