Abstract
Human adenovirus Ad36 is causally and correlatively associated in animals and humans, respectively, with increased adiposity and altered metabolic profile. We inoculated rats with Ad36, UV-inactivated Ad36 or mock-infected. Four-days later, Ad36-infected rats showed 23% greater epididymal fat pad weight and viral mRNA, the viral DNA could also be detected in tissues viz. the liver, brain, and adipose tissue. Intranasal or intra-peritoneal routes of viral inoculations showed similar tissue affinity. Serum cytokine response was remarkably down regulated. Ad36 acutely suppresses systemic immune response and spreads widely. This information will help to determine Ad36 tissue tropism and its metabolic consequences.
Keywords: obesity, adiposity, adipose tissue, cytokine, immune response, MCP1, IL6, IL18, IL12
Although obesity is associated with several health risks[9], not all obese individuals are adversely affected by it[24]. To better utilize health care resources, it would help immensely to selectively target prevention and treatment efforts by identifying “at risk” individuals from those who are relatively protected from the adverse effects of obesity. Recent emerging evidence from animal models[18] and human studies[17] indicates that some forms of obesity may indeed carry relatively low health risk. We reported that human adenovirus Ad36 infection appears to be one such marker of “low risk” obesity. Experimental infection of animals with Ad36 induces obesity without increasing food intake, and induces relative-hypolipidemia[6–8] and better insulin sensitivity[20]. In-vitro, Ad36 induces adipogenic commitment, differentiation and lipid accumulation in adipocyte progenitors[21, 22, 25], and increases cellular glucose uptake[26].[23] These causal effects of Ad36 infection are mirrored in humans naturally infected with Ad36, who have greater body weight and body fat[1], but relative hypolipidemia[1], greater potential of preadipocyte differentiation[21], better glycemic control and lower inflammatory response of adipose tissue[23], compared to uninfected individuals. Thus, Ad36 induces obesity with metabolically favorable profile in animals and is associated with such obesity in humans. Strong association of Ad36 infection with human obesity and metabolic phenotype makes it an important candidate for evaluating its causative role in human obesity. Also, Ad36 proteins are promising novel candidates[22] as a tool to reveal regulatory controls which could be therapeutically manipulated to alleviate cardiovascular risk factors in humans. Despite the increasing data and significance of Ad36, the fundamental information about the acute spread of Ad36 in a host is unknown, which is critical for determining the host-virus interaction in future.
Ad36 belongs to serotype D of 51 human adenoviruses identified and is antigenically unique[27]. Ad36 was isolated from fecal sample of a girl suffering from enteritis[27] and shows about 15 – 17% prevalence in adult population in the United States[1]. About 30% of the obese, but only 11% of the non-obese adults are naturally infected with the virus[1]. While Ad36 DNA can be isolated from human adipose tissue[21], Ad36 tissue tropism or the host immune response to experimental infection in humans cannot be studied due to ethical considerations. Since Ad36 can successfully infect rats, we used this animal model to study acute host response to the viral infection as described in the following two experiments.
For experiment 1, twenty-two male Wistar rats (4-weeks old) were obtained from Harlan (Madison, WI). After one week of acclimatization to the new surroundings, rats were randomized into 3 weight-matched groups (control-N=8, Ad-36 inactivated-N=7 and Ad-36-N=7) and housed individually in micro-isolator cages under biosafety level-two containment. Rats were inoculated intranasally with media (mock), 8 × 105 particles of either UV inactivated Ad-36 or Ad-36, respectively. Ad-libitum access to water and rat chow was offered. Protective clothing, gloves, shoes, hairnets and masks were used to enter the rooms and care was taken to prevent cross contamination. Four days post inoculation, animals were sacrificed after an overnight fast. Trunk blood was collected and serum was separated. Lung, pancreas, heart, spleen, brain, stomach, epididymal fat, liver and spleen were carefully separated, and flash frozen in liquid nitrogen. Various techniques used and assays that were conducted are described below.
Second experiment determined the effect of a different route of infection on spread of Ad36, 4 wk old male Wistar rats were intraperitoneally (i.p.) inoculated with either 200 μL cell culture media (N = 6) or 107 PFU of Ad36 (N=6). Four days post inoculation, the animals were sacrificed and epididymal, visceral and retroperitoneal fat tissue, lung, liver spleen and skeletal muscle were harvested. DNA was isolated and a nested PCR was conducted as described below.
Ad-36 was obtained from American Type Culture Collection (catalog no. VR-913; American Type Culture Collection, Manassas, VA). The virus was plaque-purified as previously described[6].
Purified virus was UV irradiated with 250 mJ/cm2 using a Stratalinker UV crosslinker (cat# 400079, Stratagene).
RNA was extracted using the RNeasy Mini Kit, as per the manufacturer’s instructions (cat # 74104, Qiagen). Residual DNA was eliminated by using Amplification Grade Deoxyribonuclease I (cat # 18068-015, Invitrogen). One μg of total RNA was reverse-transcribed to cDNA using iScript™ cDNA Synthesis Kit (cat # 170-8890, Bio-Rad) as perthe manufacturer’s protocol. Samples were stored at −80°C untilused for Real Time PCR.
A standard was generated using DNA pooled from the experimental samples. At least 3 data points, representing 10-fold dilutions, were required for generating a standard curve. Non-template control reaction mixtures contained water instead of sample. Real-time quantitative PCR was carried out in optical 96 or 384 well reaction plates using ABI PRISM 7700 sequence detector (Applied Biosystems, Branchburg, NJ) using a SYBER Green detection system (cat # 170-8880, Bio-Rad). Both, samples and standards were run at least in duplicate, and each transcript level was adjusted to the housekeeping gene used (β-actin). The reactions were performed using the following parameters: one cycle of 48°C for 30 min, then 95°C for 10 min, followed by 40 cycles at 95°Cfor 15 s and 60°C for 1 min. For the detection of Ad-36 DNA the following primers were used: forward 5′-GGCATACTAACCCAGTCCGATG-3′ and reverse 5′-TTGCCAGAATCCCACCCATAC-3′.
PCR core system II (cat # M7665, Promega) was used for the amplification of cDNA, obtained as described above. Water was used a negative PCR control. Positive PCR control was DNA from Ad36 infected A549 cells. DNA was denatured for 2 min at 95°C and subjected to 35 cycles of PCR (94°C for 1 min, 58°C for 1 min, 72°C for 2 min) followed by extension at 72°C for 5 min. PCR products were visualized on a 1.2 % agarose gel with a 100 bp DNA ladder (cat # G-2101, Promega). The following primers were used to detect Ad-36 E4Orf1: forward 5′-GGCATACTAACCCAGTCCGATG -3′ and reverse 5′-AATCACTCTCTCCAGCAGCAGG -3′. The expected product size was 138 bp.
Total DNA was extracted from the tissue using DNeasy Tissue Mini Kit (cat # 69504, Qiagen). DNA samples were stored at −80°C until used for amplification. PCR core system II (cat # M7665, Promega) was used for the amplification of DNA. Negative PCR controls were water and DNA from uninfected rats. Positive PCR control was DNA from Ad36 infected A549 cells. DNA was denatured for 2 min at 95°C and subjected to 35 cycles of PCR (94°C for 1 min, 58°C for 1 min, 72°C for 2 min) followed by extension at 72°C for 5 min. PCR products were visualized on a 1.2 % agarose gel with a 100 bp DNA ladder (cat # G-2101, Promega).
The following primers from the fiber protein region were used.
Ad-36 Outer forward primer (5′-GTCTGGAAAACTGAGTGTGGATA),
Ad-36 Outer reverse primer (5′-ATCCAAAATCAAATGTAATAGAGT),
Ad-36 Inner forward primer (5′-TTAACTGGAAAAGGAATAGGTA),
Ad-36 Inner reverse primer (5′-GGTGTTGTTGGTTGGCTTAGGATA).
Expected product size: 650bp
A 14-plex assay using Luminex xMAP technology was used to determine serum cytokine levels (Millipore corporation, cat # RCYTO-80K-PMX). Serum samples from 5 randomly selected animals from each group were used. The lowest detection limit for all assays was 24.5pg/mL.
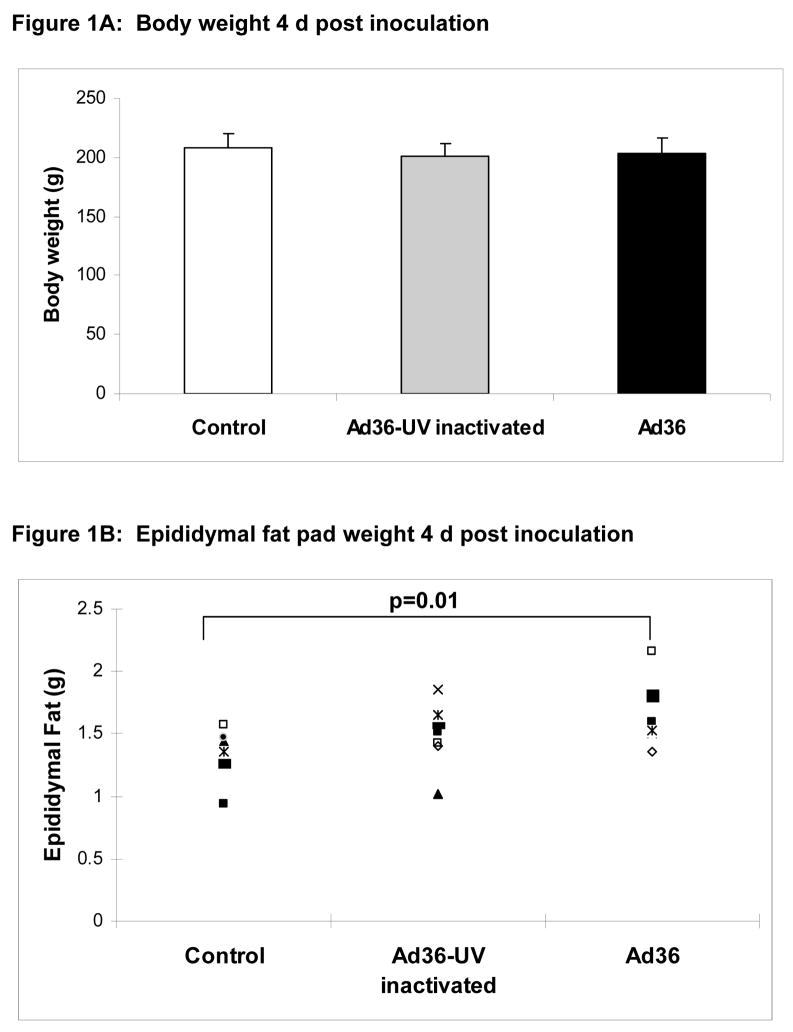
Upon termination of the experiments, we observed that the body weight of rats from all three groups remained almost identical 4 d post inoculation (Figure 1A), but interestingly, epididymal fat pad weight of the Ad36 infected group was 23% heavier (p=.01) compared to that of the uninfected control group (Figure 1B). As expected, UV-inactivated virus did not influence the fat pad weight.
Figure 1.
Figure 1A: Body weight of rats in three groups on day 4 post inoculation was not significantly different.
Figure 1B: Epididymal fat pad weights of rats on day 4 post inoculation.
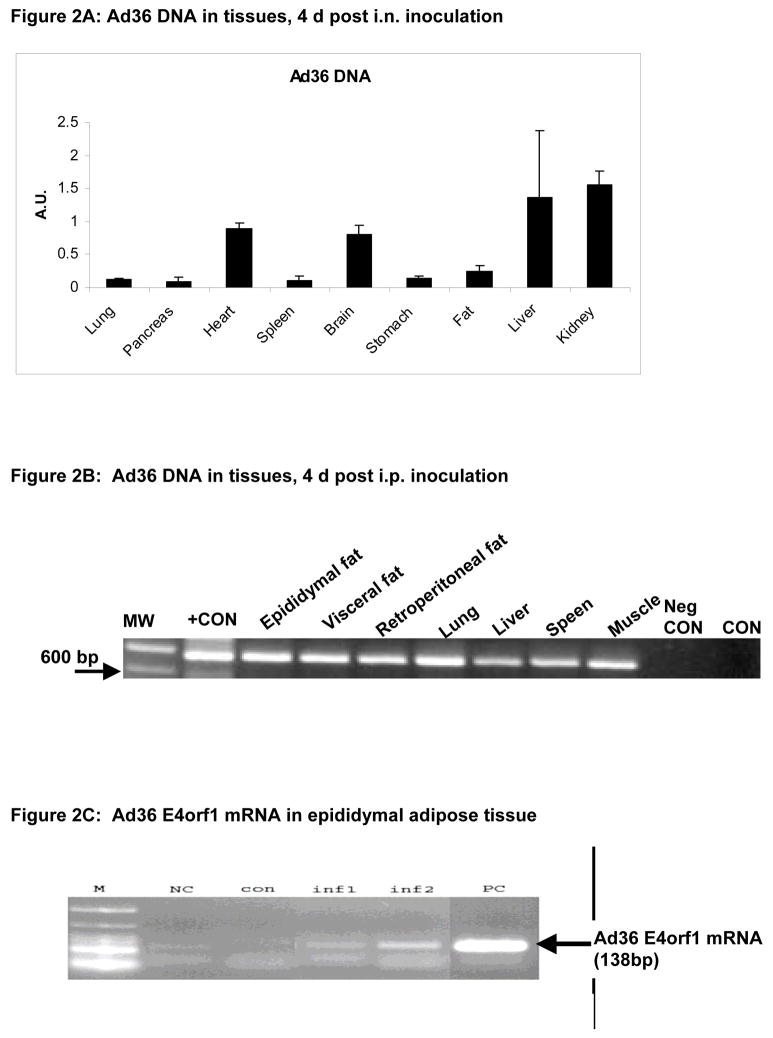
Two experiments determined the spread of Ad36 to body tissues in rats inoculated via two separate routes. Samples from all harvested tissues showed the presence of viral DNA (Figures 2A–B), but not the uninfected control animals (a representative sample shown in Figure 2B). Epididymal fat pad showed Ad36 E4orf1 mRNA, indicative of viral replication process (Figure 2C).
Figure 2.
Figure 2A: Presence of Ad36 DNA in various tissues of rats infected by an intra-nasal inoculation. The quantities were determined by quantitative real time PCR.
Figure 2B: Presence of Ad36 DNA in various tissues of rats infected by intraperitoneal inoculation. The quantities were determined by nested PCR. MW: molecular weight ladder. Neg. CON: Water template, CON: Tissue from uninfected control animal.
Figure 2C: Ad36 E4orf1 mRNA in epididymal fat pad of two representative animals (inf 1 and inf2) from the Ad36 infected group. PC: Positive control mRNA from Ad36 infected A549 cells. NC: negative control water template. M: molecular weight ladder.
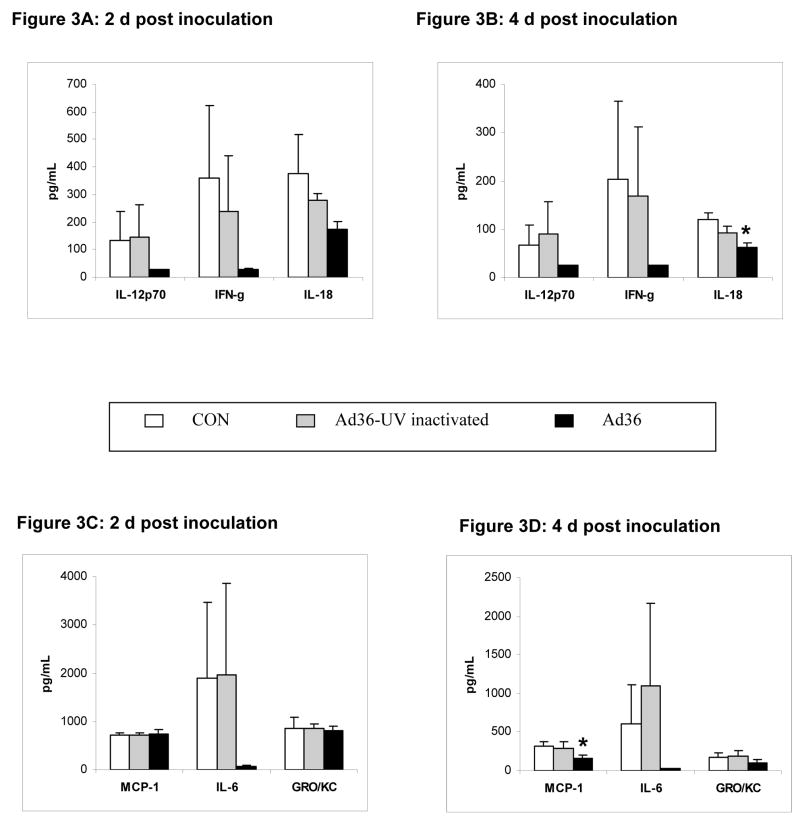
The following cytokines were below the detection level (24.5pg/mL) for all animals in the group: Granulocyte-monocyte-colony-stimulating factor (GMCSF), IL1, IL4, IL18, IL10, IL12, IL5, TNFα and were excluded from the analysis. The rest of the cytokines were analyzed and if values were below the detection levels, they were considered as 24.5 pg/mL. Overall, not the UV-inactivated Ad36, but the wild type virus appears to reduce cytokine response 2d and 4d post inoculation (Figure 3). Although the reduction in cytokine response to Ad36 infected reached statistical significance for only macrophage chemoattractant protein 1 (MCP-1) and IL-18, perhaps due a limited number of animals per group (n = 5 per group), many of the cytokine levels were reduced by 50% to 95% by the virus.
Figure 3.
Figures 3A–D: Serum cytokine levels in 5 randomly selected animals from each group, 2 and 4 days post inoculation. * p < 0.05. GRO/KC: growth regulated oncogene.
In humans, adenovirus infections are common and cause acute upper respiratory tract infections, enteritis or conjunctivitis[10]. Adenoviruses may persist asymptomatically in adult humans[13]. Although adenoviruses are not thought to integrate with host DNA, recent reports suggest this to be a possibility[28]. There are 6 major subgroups (A–F) among the 50 human adenoviruses. Each subgroup has a number of specific serotypes. Ad36 belongs to subgroup D, serotype 36. Ad36 is serologically different compared to other human adenoviruses [27]. Adenoviruses are non-enveloped DNA viruses that replicate in host cell nucleus
Several medium to long-term studies about the adipogenic effect of adenoviruses are published[5–8, 20]. However, surprisingly little is known about the acute host response to these adipogenic adenoviruses, which is fundamental to understanding the role of these viruses in human obesity and for devising treatment or prevention strategies. While Ad36 is a strong candidate for determining its role in human obesity and is relatively most studied virus, sketchy information is available about it’s target tissue and the immediate response of its host. Changes in food intake or activity do not explain the metabolic effects of Ad36 and the animals do not show overt signs of infection or premature mortality even up to 7 mo post infection[6–8, 20]. Animals could be experimentally infected by intra nasal or intra peritoneal inoculation of Ad36. Also, blood-borne, air- or fecal-oral transmission of Ad36 was observed[6–8, 20]. Ad36 DNA appears in the blood of experimentally infected chickens and their uninfected cagemates in about 12 h post inoculation and lasts in the blood for up to 4 wks[7] Also, Ad36 DNA could be detected in the blood and adipose tissue of animals 5 wks post infection[6], by capillary electrophoresis method. Ad36 infected marmosets excreted active virus in feces up to 2 months post infection and harbored viral DNA in adipose tissue up to 7 months[8], and in hamsters up to 4 wks post infection when the experiments were terminated.
In the present study, an increase in epididymal fat pad weight in just 4 d post Ad36 infection was surprising. No change in the fat pad weight of the UV-inactivated group further supports the role of active virus in increasing the adipose tissue. Also, mRNA expression of Ad36 E4orf1, the adipogenic gene of the virus[22] in these fat pads is particularly noteworthy. This is the first demonstration of Ad36 gene expression in adipose tissue of an experimentally infected animal host, suggesting active viral replication and possible adipogenic effect on the tissue.
As determined by the presence of viral DNA, intra-nasally or intra-peritoneally inoculated Ad36 spreads rapidly to several tissues, including some known for adenoviral tropism, such as the liver. Although the spread of the virus to the brain may be somewhat unconventional, it may explain the changes in neurotransmitter levels observed in Ad36 infected rats[20]. Spread of the virus to adipose tissue and skeletal muscle is consistent with our previous findings[6, 8] and important due to our recent in-vitro findings in these tissues[26] [21, 23]. Given the effect of the virus on adipose tissue derived stem cell commitment and differentiation[21], spread of Ad36 to adipose tissue is particularly intriguing. About 27% of the Pima Indians screened harbored Ad36 DNA in their subcutaneous adipose tissue and showed greater adipogenic potential compared to their antibody negative counterparts[21]. Functional effect, if any, of Ad36 viral DNA presence on these tissues remain to be determined.
Many viruses subvert the host immune system for successful infection, spread or persistence and understanding these immune modulations may provide key information for defense against the microbe. Overall, Ad36 appears to down-regulate the host immune response in just 2 to 4 days post inoculation. In response to infection, IL-12 induces IFNγ and along with IL-18 host mounts a Th1 type of immune response. In early stages of infection, many viruses down-regulate the Th1 response by suppressing the production of these cytokines [4]. Down regulation of this cytotoxic immune response may allow Ad36-infected cells to survive, which may be of particular relevance to cells from the adipose tissue, which expands in response to Ad36 infection. A long-term and tissue-specific assessment of cytokine response to Ad36 is required to further understand the interaction.
Inflammatory cytokines are positively associated with obesity and insulin resistance [15, 29] and approaches to transgenically knockout inflammatory cytokines such as MCP1, TNFα (Tumor necrosis factor alpha) show improvement in insulin resistance[14] and knockout of cytokines such as IL-18 or receptor of IL-1 increase adiposity[11, 19]. In-vitro infection of human adipose tissue by Ad36 down regulates inflammatory cytokines (unpublished observations). Thus, the down regulation of inflammatory cytokines by Ad36 may contribute to its adipogenic effect and the paradoxical improvement in glycemic control. Long-term studies are required to test this hypothesis.
Although Ad36 appears to acutely down regulate response of the inflammatory cytokines investigated, other intact human adenoviruses or replication deficient adenoviral vectors are known to elicit a strong and acute inflammatory cytokine response[3, 12], which in fact is a serious limitation of otherwise efficient application of human adenoviruses as vectors[2]. Extensive research has been undertaken to overcome this shortcoming of human adenoviral vector use[2]. One of the alternative strategies is to use an adenovirus that does not elicit strong inflammatory cytokine response. In response to such concerns, Iacobelli-Martinez et al [16] reported human adenoviruses Ad16, Ad35 and Ad37, which down regulate acute inflammatory response of cytokines such as IL-12, IFNγ and IL-6, and suggested the use of these adenoviruses for gene delivery and vaccine development as implications. A pronounced anti-inflammatory cytokine response by Ad36, coupled with its ability to infect several host tissues also suggests its possible role as vector.
In conclusion, acute effects of Ad36 in rats include greater fat pad weight, and attenuation of host immune response, which may help in the widespread tissue distribution observed. This study provides the basis to study tissue tropism and long-term modulation of host immune response and its metabolic consequences. The data also provide candidate host tissues for the virus in humans naturally infected with Ad36.
Supplementary Material
Acknowledgments
This work was funded by NIH1R01DK066164 awarded to NVD.
References
- 1.Atkinson RL, Dhurandhar NV, Allison DB, Bowen RL, Israel BA, Albu JB, Augustus AS. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes (Lond) 2005;29:281–286. doi: 10.1038/sj.ijo.0802830. [DOI] [PubMed] [Google Scholar]
- 2.Bangari DS, Mittal SK. Current strategies and future directions for eluding adenoviral vector immunity. Curr Gene Ther. 2006;6:215–226. doi: 10.2174/156652306776359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen GP, Borgland SL, Lam M, Libermann TA, Wong NC, Muruve DA. Adenovirus vector-induced inflammation: capsid-dependent induction of the C-C chemokine RANTES requires NF-kappa B. Hum Gene Ther. 2002;13:367–379. doi: 10.1089/10430340252792503. [DOI] [PubMed] [Google Scholar]
- 4.Chinen J, Shearer WT. Basic and clinical immunology. J Allergy Clin Immunol. 2005;116:411–418. doi: 10.1016/j.jaci.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Dhurandhar NV, Kulkarni P, Ajinkya SM, Sherikar A. Effect of adenovirus infection on adiposity in chicken. Vet Microbiol. 1992;31:101–107. doi: 10.1016/0378-1135(92)90068-5. [DOI] [PubMed] [Google Scholar]
- 6.Dhurandhar NV, Israel BA, Kolesar JM, Mayhew GF, Cook ME, Atkinson RL. Increased adiposity in animals due to a human virus. Int J Obes Relat Metab Disord. 2000;24:989–996. doi: 10.1038/sj.ijo.0801319. [DOI] [PubMed] [Google Scholar]
- 7.Dhurandhar NV, Israel BA, Kolesar JM, Mayhew G, Cook ME, Atkinson RL. Transmissibility of adenovirus-induced adiposity in a chicken model. Int J Obes Relat Metab Disord. 2001;25:990–996. doi: 10.1038/sj.ijo.0801668. [DOI] [PubMed] [Google Scholar]
- 8.Dhurandhar NV, Whigham LD, Abbott DH, Schultz-Darken NJ, Israel BA, Bradley SM, Kemnitz JW, Allison DB, Atkinson RL. Human adenovirus Ad-36 promotes weight gain in male rhesus and marmoset monkeys. J Nutr. 2002;132:3155–3160. doi: 10.1093/jn/131.10.3155. [DOI] [PubMed] [Google Scholar]
- 9.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. Jama. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 10.Foy HM, Grayston JT. Adenoviruses. In: Evans Alfred S, editor. Viral infections of humans: Epidemiology and control. Plenum Medical; New York: 1976. pp. 53–70. [Google Scholar]
- 11.Garcia MC, Wernstedt I, Berndtsson A, Enge M, Bell M, Hultgren O, Horn M, Ahren B, Enerback S, Ohlsson C, Wallenius V, Jansson JO. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes. 2006;55:1205–1213. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- 12.Higginbotham JN, Seth P, Blaese RM, Ramsey WJ. The release of inflammatory cytokines from human peripheral blood mononuclear cells in vitro following exposure to adenovirus variants and capsid. Hum Gene Ther. 2002;13:129–141. doi: 10.1089/10430340152712683. [DOI] [PubMed] [Google Scholar]
- 13.Horvath J, Palkonyay L, Weber J. Group C adenovirus DNA sequences in human lymphoid cells. J Virol. 1986;59:189–192. doi: 10.1128/jvi.59.1.189-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotamisligil GS. Mechanisms of TNF-alpha-induced insulin resistance. Exp Clin Endocrinol Diabetes. 1999;107:119–125. doi: 10.1055/s-0029-1212086. [DOI] [PubMed] [Google Scholar]
- 15.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 16.Iacobelli-Martinez M, Nepomuceno RR, Connolly J, Nemerow GR. CD46-utilizing adenoviruses inhibit C/EBPbeta-dependent expression of proinflammatory cytokines. J Virol. 2005;79:11259–11268. doi: 10.1128/JVI.79.17.11259-11268.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karelis AD, Messier V, Brochu M, Rabasa-Lhoret R. Metabolically healthy but obese women: effect of an energy-restricted diet. Diabetologia. 2008 doi: 10.1007/s00125-008-1038-4. [DOI] [PubMed] [Google Scholar]
- 18.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007 doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, Tack CJ, van Krieken H, Kim SH, Stalenhoef AF, van de Loo FA, Verschueren I, Pulawa L, Akira S, Eckel RH, Dinarello CA, van den Berg W, van der Meer JW. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 20.Pasarica M, Shin AC, Yu M, Ou Yang HM, Rathod M, Jen KL, MohanKumar S, MohanKumar PS, Markward N, Dhurandhar NV. Human adenovirus 36 induces adiposity, increases insulin sensitivity, and alters hypothalamic monoamines in rats. Obesity (Silver Spring) 2006;14:1905–1913. doi: 10.1038/oby.2006.222. [DOI] [PubMed] [Google Scholar]
- 21.Pasarica M, Mashtalir N, McAllister EJ, Kilroy GE, Koska J, Permana P, de Courten B, Yu M, Ravussin E, Gimble JM, Dhurandhar NV. Adipogenic human adenovirus Ad-36 induces commitment, differentiation and lipid accumulation in human adipose-derived stem cells. Stem Cells. 2008;26:969–978. doi: 10.1634/stemcells.2007-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers PM, Fusinski K, Rathod MA, Loiler S, Pasarica M, Shaw M, Kilroy G, Sutton G, McAllister E, Mashtalir N, Gimble J, Holland TNVD. Human adenovirus Ad-36 induces adipogenesis via its E4 orf-1 gene. International Journal of Obesity. 2007;32:397–406. doi: 10.1038/sj.ijo.0803748. [DOI] [PubMed] [Google Scholar]
- 23.Rogers PMMN, Rathod MA, Dubuisson O, Wang ZQ, Dasuri K, Babin S, Gupta A, Markward N, Cefalu WT, Dhurandhar NV. Metabolically Favorable Remodeling of Human Adipose Tissue by Human Adenovirus Ad-36. Diabetes. 2008 Jul 3; doi: 10.2337/db07-1311. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499–1504. doi: 10.1053/meta.2001.27213. [DOI] [PubMed] [Google Scholar]
- 25.Vangipuram SD, Sheele J, Atkinson RL, Holland TC, Dhurandhar NV. A human adenovirus enhances preadipocyte differentiation. Obes Res. 2004;12:770–777. doi: 10.1038/oby.2004.93. [DOI] [PubMed] [Google Scholar]
- 26.Wang ZQCW, Zhang XH, Yongmei Y, Qin J, Son L, Rogers PM, Mashtalir N, Bordelon JR, Ye J, Dhurandhar NV. Human adenovirus type 36 enhances glucose uptake in diabetic and non-diabetic human skeletal muscle cells independent of insulin signaling. Diabetes. 2008 Apr 16; doi: 10.2337/db07-1313. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wigand R, Gelderblom H, Wadell G. New human adenovirus (candidate adenovirus 36), a novel member of subgroup D. Arch Virol. 1980;64:225–233. doi: 10.1007/BF01322702. [DOI] [PubMed] [Google Scholar]
- 28.Wronka G, Bernardi G, Doerfler W. Localization of integrated adenovirus DNA in the hamster genome. Cell Mol Life Sci. 2004;61:2983–2990. doi: 10.1007/s00018-004-4294-6. [DOI] [PubMed] [Google Scholar]
- 29.Yudkin JS. Inflammation, obesity, and the metabolic syndrome. Horm Metab Res. 2007;39:707–709. doi: 10.1055/s-2007-985898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.