Abstract
The mammalian circadian system has been implicated in the regulation of various biological processes including those involved in genotoxic stress responses and tumor suppression. Here we report that mice with the functional deficiency in circadian transcription factor CLOCK (Clock/Clock mutant mice) do not display predisposition to tumor formation both during their normal lifespan or when challenged by γ-radiation. This phenotype is consistent with high apoptotic and low proliferation rate in lymphoid tissues of Clock mutant mice and is supported by the gene expression profiling of a number of apoptosis and cell cycle-related genes, as well as by growth inhibition of cells with CLOCK downregulation. At the same time, Clock mutant mice respond to low-dose irradiation by accelerating their aging program, and develop phenotypes that are reminiscent of those in Bmal1-deficient mice. Taken together, our results demonstrate the dichotomy in biological consequences of the disruption of the circadian clock with respect to ageing and cancer. They also highlight the existence of a complex interconnection between ageing, carcinogenesis and individual components of the circadian clock machinery.
Keywords: circadian, CLOCK/BMAL1 transcriptional complex, aging, carcinogenesis, ionizing radiation
Introduction
The circadian clock is a universal intrinsic timekeeping system, which regulates many vital physiological and biochemical processes ensuring their proper adaptation to a constantly changing environment. In mammals, it is organized as a hierarchical network of molecular clocks that are operative in all tissues, with the master clock residing in the hypothalamic suprachiasmatic nucleus (SCN). The master clock is synchronized with the environment by daily changes in the light:dark cycles and transmits information regarding its phase to multiple tissue-specific clocks.1 At the molecular level, circadian clocks are comprised of interlocked transcription/translation feedback loops, in which the positive components (in the major loop—bHLH-PAS domain transcription factors CLOCK and BMAL1) drive the expression of their own repressors (PERIODs and CRYPTOCHROMEs). Since CLOCK/BMAL1-dependent transcription is mediated through the widely represented E box elements in the promoter regions of target genes, in addition to transcriptional regulation of the core components of molecular circadian machinery, the CLOCK/BMAL1 complex regulates the expression of multiple clock-controlled genes (CCGs). It is believed that rhythmic expression of CCGs underlies circadian output in physiology and metabolism.2
Given such complex multilevel organization of the circadian system, one could predict that de-regulation of any of its components (desynchronization with the environment, desynchronization of tissue-specific clocks with each other, or disruption of clock function at the cellular level) might lead to the development of various pathological conditions including carcinogenesis. In addition to this, the growing amount of evidence suggests that core clock proteins may have distinct non-circadian functions important for maintaining tissue homeostasis under normal and stress conditions, and their malfunction may contribute to disease development.3,4
The interconnection between the circadian clock and carcinogenesis was first demonstrated in a series of epidemiological studies reporting a high incidence of tumor formation in shift workers5 and an increased incidence of breast cancer among flight attendants.6,7 Epidemiological data were supported by mouse model studies that showed that disruption of normal rhythmicity either by surgical ablation of the SCN or by chronic exposure to frequent changes in light: dark cycle, resulted in faster rates of implanted tumor growth.8
A number of studies using genetic models including mice with mutations or targeted disruption of individual components of molecular clock machinery shed light on complex functional interconnection between the circadian clock, the cell cycle and genotoxic stress response pathways thereby linking disruption of circadian rhythms to carcinogenesis. Thus, it has been demonstrated that a number of key regulators of cell cycle progression and genotoxic stress responses (Mdm2, Gadd45, Cyclin D1, Cyclin B1, Cyclin E, Cylin A, p53, Wee1, c-Myc and others) display circadian patterns of expression at either the mRNA or protein level.9,10 Moreover, some of them, such as the Wee1 kinase and c-Myc transcription factor, have been identified as direct transcriptional targets of the circadian CLOCK-BMAL1 transactivation complex.10,11 As expected, the expression pattern of many of these genes is altered in the tissues of circadian mutant mice or in cells with modulated expression of circadian proteins.10–13 In addition to circadian variations in the expression of genes involved in cell cycle regulation and genotoxic stress responses, core circadian proteins PERIOD1 (PER1) and TIMELESS (TIM) interact with components of the cell cycle checkpoint system (reviewed in ref. 14). Altogether, deregulation of the cell cycle and genotoxic stress response could affect the process of carcinogenesis. However, when challenged with ionizing radiation, circadian mutants that are deficient in negative regulators of the circadian autoregulatory loop, PERs and CRYs, demonstrate contrasting responses. Animals deficient in Per2 gene are reported to be more cancer-prone whereas “clock-less” mice with targeted disruption of both Cryptochrome genes did not differ from their wild type littermates in the frequency of tumor development. 12 Until now, nothing has been reported on the possible roles of functional deficiencies in components of the positive limb of the circadian transcription/translation loop, CLOCK and BMAL1, in carcinogenesis.
The CLOCK protein is the first core circadian component discovered in mammals.15 It is a bHLH-PAS domain transcription factor, which functions in a complex with its partner, BMAL1. Clock mutant mice are behaviorally arrhythmic,15 display pronounced defects in light-responses and metabolic activities,16,17 and, similar to Bmal1−/− mice, demonstrate higher sensitivity to chemotherapeutic drug cyclophosphamide.18 In order to test whether the disruption of the circadian clock due to deficiency of functional CLOCK protein affects the rate of carcinogenesis, we exposed a large group of age and sex-matched Clock/Clock and wild-type mice to a single dose of ionizing radiation and monitored their health and body weight for 80 weeks. Interestingly, Clock/Clock mice did not show higher predisposition to neoplasia but responded to long-term effects of ionizing irradiation by accelerating their ageing program. Thus, disruption of the circadian clock per se does not make animals more cancer-prone, suggesting that the final outcome of the exposure to genotoxic stress depends on which particular component of the molecular clock is altered in its function.
Results
Effects of ionizing radiation on survival and body weight of Clock mutant mice
Despite several examples that link the disruption of the circadian system to carcinogenesis, previous studies of Clock mutant mice did not identify higher incidence of spontaneous tumor development (Antoch MP, unpublished observation). Therefore, in order to explore the potential role of the CLOCK protein in predisposition to tumor formation, we exposed WT and Clock/Clock mice of both sexes to ionizing radiation—a well-known carcinogen that is able to initiate and promote neoplastic progression. After the treatment, animals were weighed weekly and were closely monitored for any changes in their gross appearance for 80 weeks after the treatment.
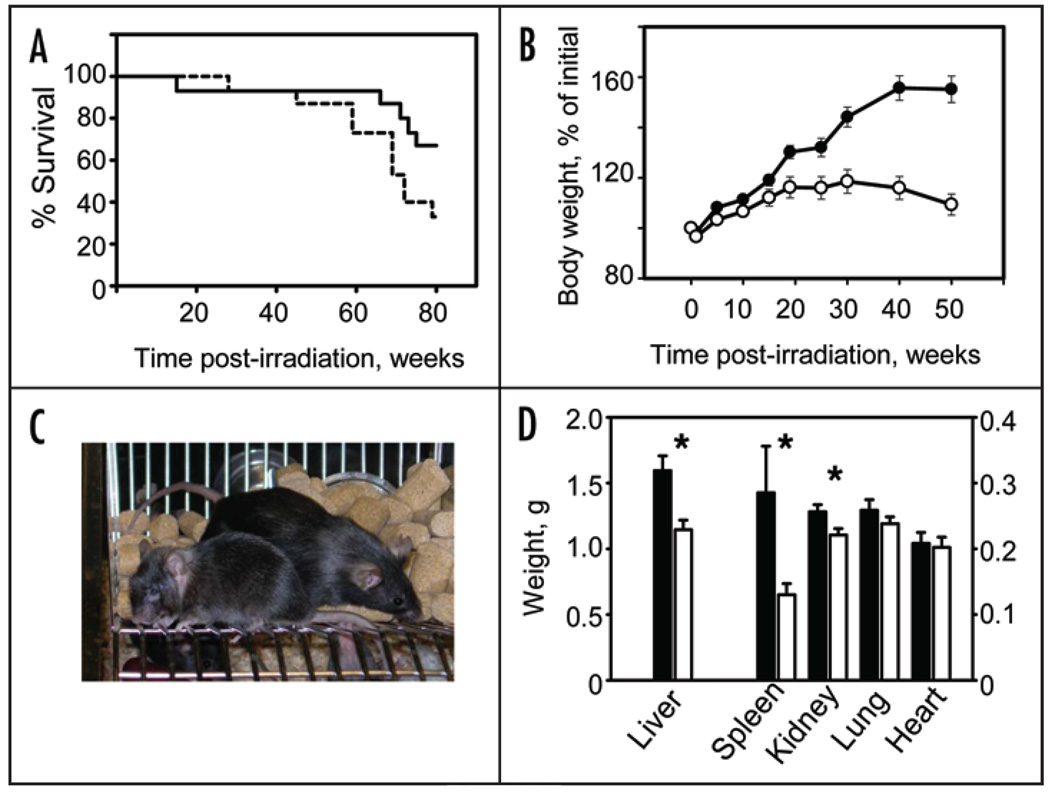
Interestingly, long-term effects of low-dose radiation demonstrated pronounced sex-dependent variations. While the survival rate of Clock/Clock female mice was significantly lower than in their WT littermates, male mice did not demonstrate any considerable allele-specific difference in mortality (Fig. 1A and Suppl. Fig. 1). Such sex-dependent variations have been described before and could be attributed to molecular differences in acute radiation response in male and female mice.19,20
Figure 1.
Increased mortality and body weight loss of Clock/Clock female mice after low-dose irradiation. (A) Kaplan-Meyer survival curve (mean survival for WT and Clock/Clock mice are 73.2 and 63.6 weeks respectively, p = 0.022). Solid line—WT mice, dotted line—Clock/Clock mice. All survived animals were sacrificed 80 weeks post irradiation (B) Changes in body weight of WT and Clock mutant mice; t = 0 corresponds to the time of irradiation. Fifteen WT and fifteen Clock/Clock mice were weighed weekly. Starting at 20 weeks after irradiation Clock mutant mice demonstrate progressive weight loss (p < 0.001). Data shown as mean ± s.e.m. Closed circles—WT; open circles—Clock/Clock. (C) Gross appearance of WT (right) and Clock/Clock (left) female mice 80 weeks after TBI. Note significant reduction in weight, kyphosis and cataract in Clock mutant female. (D) Weight of major organs from female mice after 80 weeks of TBI (mean ± s.e.m.; *p < 0.01; filled bars—WT, open bars—Clock mutant mice. Liver, spleen and kidney of Clock/Clock female mice showed significant reduction when compared to age-matched WT mice.
At the same time, morbidity associated with the treatment (manifested by the loss of the body weight) was more pronounced in Clock/Clock mice of both sexes. Starting at ~20 weeks after TBI, both male and female mice stopped gaining weight and then started progressively loose it (Fig. 1B and Suppl. Fig. 1). As a result, at 80 weeks after TBI, Clock/Clock mice that normally weigh 10–20% more than their WT littermates, showed significant reduction in their body weight (Fig. 1C and Suppl. Fig. 2).
To determine whether decreased body weight correlates with an increased incidence of neoplasia in the Clock mutant mice, we performed detailed necropsies on 13 WT and 12 Clock/Clock female mice, because females demonstrated more pronounced genotype-related differences in their responses to ionizing radiation. Since lymphomas are the most common spontaneous and irradiation-induced malignancies in C57Bl/6J mice,21 particular attention was paid to lymphoid organs including spleen, thymus and lymph nodes.
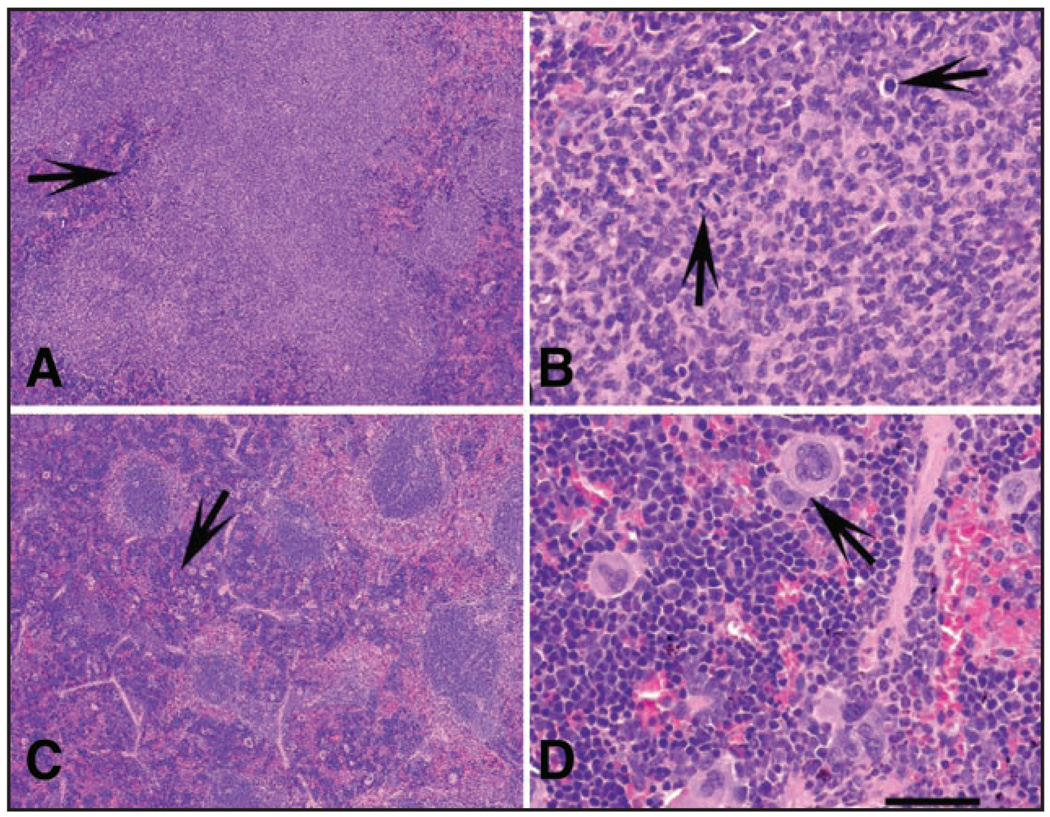
None of the animals tested demonstrated thymic enlargement. In fact, in most of the animals thymus was not detectable. In contrast, the size of the spleen in WT mice was significantly increased in ten WT but only in one Clock/Clock female (Fig. 1D, Table 1), This increase histologically correlated with the signs of extramedullary hematopoiesis (EMH) of the spleen, which was detected in 10 out of 12 WT mice but only in 4 out of 12 Clock/Clock mice (Fisher’s exact test p = 0.0361; Fig. 2, Table 1). Only 2 WT and 1 Clock mutant mouse developed follicular lymphoma (Fig. 2, Table 1).
Table 1.
Histopathological evaluation of wild-type (WT) and clock/clock female mice
| WT (lesion/total) | Clock/clock (lesion/total) | Fisher’s exact test p (*significant) | |
|---|---|---|---|
| Spleen | |||
| Splenomegaly (>200 mg) | 10/13 | 1/12 | 0.0010* |
| Hyperplasia of the white pulp | 2/12 | 4/12 | 0.6404 |
| Follicular lymphoma | 2/12 | 1/12 | 1.0000 |
| Extramedullary hematopoiesis | 10/12 | 4/12 | 0.0361* |
| Hemangioma | 1/12 | 1/12 | 1.5217 |
| Mandibular lymph nodes | |||
| Visually enlarged | 1/13 | 9/12 | 0.0010* |
| Hyperplasia/medullary plasmacytosis | 4/12 | 9/11 | 0.0361* |
| Angiectasis | 6/12 | 1/11 | 0.0686 |
| Other lesions: | |||
| Liver, ductal cystadenoma | 2/13 | 2/12 | 1.0000 |
| Liver, hemangioma | 0/13 | 1/12 | 0.4800 |
| Liver, hepatocellular adenoma | 1/13 | 0/12 | 1.0000 |
| Ovary, cystadenoma | 1/13 | 1/12 | 1.0000 |
| Ovary, granulosa cell tumor | 0/13 | 1/12 | 0.4800 |
| Salivary gland, ductal cystadenoma | 2/13 | 1/12 | 1.0000 |
Figure 2.
Major pathological changes in spleens of Clock/Clock and WT mice at 80 weeks after TBI. (A and B) Lymphoma with loss of normal architecture in the white pulp (A, arrow) and numerous mitotic figures (B, arrows). Two wild-type and one Clock/Clock mouse were diagnosed with lymphoma. (C and D). Extensive extramedullary hematopoiesis (EMH, C, arrow) in the red pulp of the spleen. Note large number of megakaryocytes (arrow) as well as myeloid and erythroid precursors, (D) EMH was diagnosed in 9 WT mice, but in only 4 Clock mutants. Calibration Bar: (A and C) 400 μm; (B and D) 50 µm.
Although more Clock/Clock mice showed enlargement of mandibular lymph nodes (9 out of 12 compared to 1 out of 13 in WT females), histopathological analysis suggested that such an increase in size was due to hyperplastic lesions (follicular hyperplasia and medullary plasmacytosis, Suppl. Fig. 3, Table 1) and most likely reflected reactive response rather than neoplasia. Such non-neoplastic lesions are most common in ageing mice (reviewed in ref. 22) and increase in their frequency is well consistent with observed accelerated ageing of Clock/Clock mice (see below).
No allele-specific differences in frequencies of lesions were observed in other organs and systems (Table 1). Taken together, the histological data suggest that WT and Clock mutant mice show similar incidence of tumor formation in response to ionizing radiation. Consistent with our histopathological evaluation, no differences in total blood cell counts were detected in peripheral blood of mice of both genotypes (Suppl. Table 1). Thus, disruption of the circadian function due to the mutation in the Clock gene does not make animals more cancer-prone.
The Clock mutation affects cell proliferation and apoptosis in vivo
The process of lymphomagenesis, which involves a combination of various genetic and epigenetic factors, is often associated with the enhancement of proliferation and inhibition of differentiation and anti-apoptotic activities.
It has been reported that CLOCK regulates cell growth and proliferation. Gene profiling studies performed on liver and muscle tissues of WT and Clock mutant mice showed that the Clock mutation results in upregulation of several cell cycle inhibitory genes and downregulation of proliferative genes.13 This expression pattern correlated with in vivo studies, in which both proliferation and cell growth were measured in several independently obtained primary cultures of WT and Clock/Clock fibroblasts.13
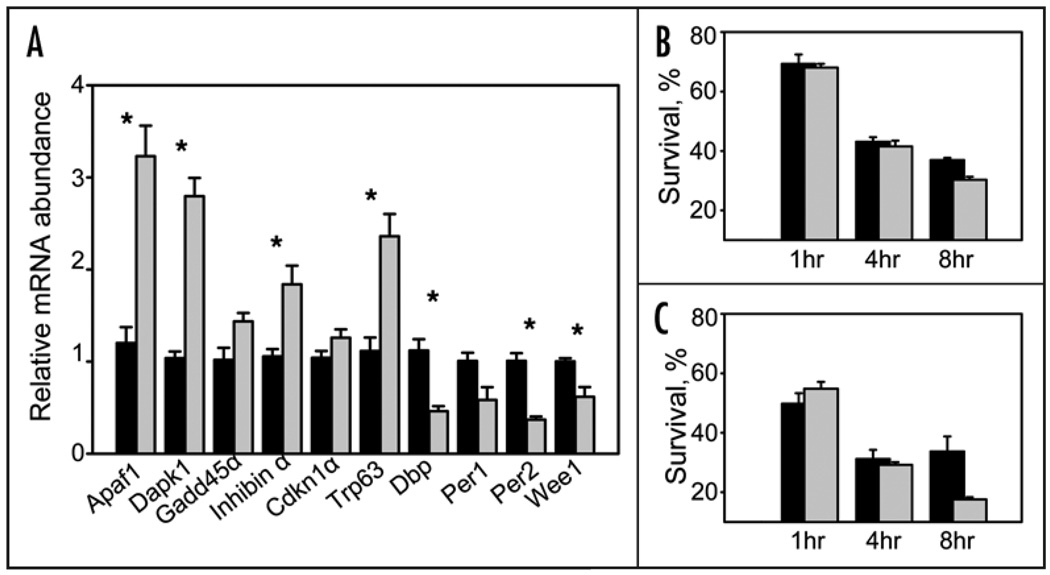
Since circadian regulation shows a high level of tissue specificity, in order to address possible deregulation of these genes in relation to lymphoma-agenesis, we performed quantitative analysis of a set of cell cycle- and apoptosis-related genes in the spleen of WT and Clock mutant mice using Pathway Focused RT2 Profiler PCR Array System (SuperArray Bioscience Corp.). Out of 84 cell cycle-related genes represented on Mouse Cell Cycle Array, 9 were upregulated at least 1.5-fold and 5 were downregulated in tissue of Clock/Clock mice (Suppl. Table 2). Importantly, the majority of genes that were upregulated are the ones that are associated with growth arrest whereas three out of five that are downregulated are associated with proliferation. Analysis of the gene expression level for the transcripts present on Mouse Apoptosis Array demonstrated from 2 to 5-fold upregulation of several genes with pro-apoptotic activities (Suppl. Table 3). These results were confirmed by real-time RT-PCR for individual genes performed on individual RNA samples isolated from spleens of WT and Clock/Clock mice (Fig. 3A).
Figure 3.
The Clock mutation affects the level of radiation-induced apoptosis in radiation-sensitive lymphoid tissues. (A) Upregulation of several key proapoptotic and growth arrest-specific genes in the spleen of Clock/Clock mutant mice. Data presented as mean ± s.e.m., n = 18; *p < 0.001. WT—black bars, Clock/Clock— grey bars. mRNA expression levels were determined by real-time RT-PCR. Expression of the proproliferative gene Wee1 was significantly (p = 0.025) reduced in Clock mice. Consistent with previously reported data, spleens of Clock/Clock mice also showed significantly reduced levels of expression of direct transcriptional targets of the CLOCK protein, Dbp and Per2 (p = 0.002). (B) Percentage of viable (annexin V-negative) CD3- positive cells in thymuses of WT (black bars) and Clock/Clock (grey bars) mice at different times after 8.5 Gy of TBI. Data presented as mean ± s.e.m., n = 4, p = 0.005. (C) Percentage of viable (annexin V-negative) B220-positive cells in spleens of WT (black bars) and Clock/Clock (grey bars) mice after 8.5Gy of TBI. Data presented as mean ± s.e.m., n = 4, p = 0.05.
Based on gene expression analysis, one would expect that the Clock mutation should result in a low proliferation and high apoptotic rate in vivo. To test this prediction, we first estimated the rate of apoptosis in radiation-sensitive tissues (thymus and spleen) of WT and Clock/Clock mice after exposing them to 8.5 Gy of ionizing radiation. Quantitative flow cytometry analysis of different populations of T and B cells did not reveal any differences in their basal levels in thymuses and spleens of untreated animals of both genotypes (data not shown). However, after TBI, a number of viable (Annexin V-negative) cells in tissues of Clock/Clock mice significantly decreased when compared to irradiated WT animals (Fig. 3B and C). Thus, the elevated levels of expression of several key pro-apoptotic genes detected in our experiments correlated with a higher rate of radiation-induced apoptosis in both types of lymphoid tissues tested.
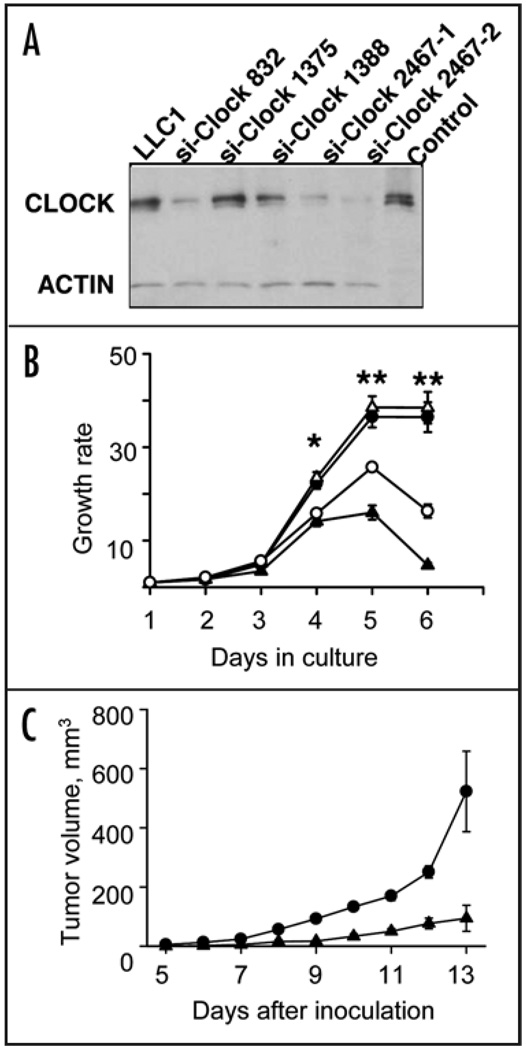
To demonstrate the direct involvement of CLOCK in regulation of cell growth, we generated several stably transfected LLC1 cell lines, in which the endogenous CLOCK protein is suppressed by specific small interfering RNA (siRNA). Western blot analysis showed that two of these cell lines (LLC1/siClock-835 and LLC1/siClock-2467) efficiently suppressed CLOCK protein (Fig. 4A), whereas two other lines (LLC1/siClock-1375 and LLC1/siClock-1388) had no effect on the abundance of the endogenous protein. Consistently, suppression of the CLOCK protein in line LLC1/siClock-835 and LLC1/siClock-2467 resulted in decrease in mRNA abundance of key transcriptional targets of the CLOCK/BMAL1 complex (Periods and Cryptochromes; data not shown).
Figure 4.
Suppression of CLOCK protein reduces cell proliferation. (A) CLOCK suppression by siRNA. Western blot of lysates from LLC1 cells infected with four different siClock lentiviral constructs and probed with a CLOCK-specific antibody. Three independently obtained clones (si-Clock-832, siClock-2467-1 and siClock-2467-2) showed significant reduction in CLOCK abundance. (B) CLOCK suppression by siClock-832 (open circles) or 2467-1 (closed triangles) results in a decrease in cell growth rate when compared to parental LLC1 (closed circles) or inactive LLC1-siClock-1375 (open trianges) cell lines, presented as mean ± s.e.m., n = 3, *p < 0.05, **p < 0.01; (C) CLOCK suppression by siClock-2467 reduces the tumor growth rate. LLC1 cells infected by inactive (siClock-1375, circles) or functional (siClock-2467, trianges) lentiviral constructs were inoculated s.c. into C57BL/6J mice. Tumor volumes were measured starting day 5 after inoculation. Data shown as mean ± s.e.m., n = 20, at all days p < 0.01.
To test whether the suppression of CLOCK affects the growth characteristics of the cells, we compared the growth rate of the parental LLC1 cell line with the lines transfected with siRNA. As shown in Figure 4B, the growth rate of LLC1 that was infected with lentivirus expressing an inactive siRNA construct (siClock-1375) was indistinguishable from the parental line. In contrast, two clones that efficiently suppress CLOCK (siClock-835 and siClock-2467 were growing much slower and never reached confluency (Fig. 4B). When the cells LLC1/siClock-2467 were inoculated into C57BL/6J mice, they formed tumors; however, the rate of tumor growth was significantly slower than in parental line (Fig. 4C). Taken together, the gene expression data and in vivo functional assays demonstrate that the deficiency in CLOCK protein function indeed results in the increase of apoptotic response and decrease in cell proliferation. This pattern is consistent with histopathological analyses showing no predisposition for tumor formation in response to ionizing radiation. Consistently, the higher incidence of such proliferative lesion as EMH in the spleen of WT mice may result from growth suppressive effects of CLOCK deficiency.
TBI accelerates the ageing process in Clock/Clock mice
Although Clock mutant mice did not demonstrate a higher incidence of tumor formation, they still show elevated sensitivity to TBI. Most obviously this was manifested by higher mortality (demonstrated by female mice) and significant weight loss (both in males and females) that started not immediately after the treatment but 20–25 weeks later (Fig. 1 and Suppl. Fig. 1). Necropsy results suggest that this reduction could be explained by a size reduction in several major organs (liver, spleen and kidney) as well as the loss of adipose tissue.
In addition to body weight reduction, Clock/Clock mice showed signs of hair graying significantly earlier. In Clock/Clock females hair graying started as early as 6 weeks after TBI, while in WT females graying occurred only after 30 weeks. Radiation-induced alopecia was also more pronounced in Clock/Clock females where it could be detected at 13 weeks after TBI (compared to 37 weeks in WT females).
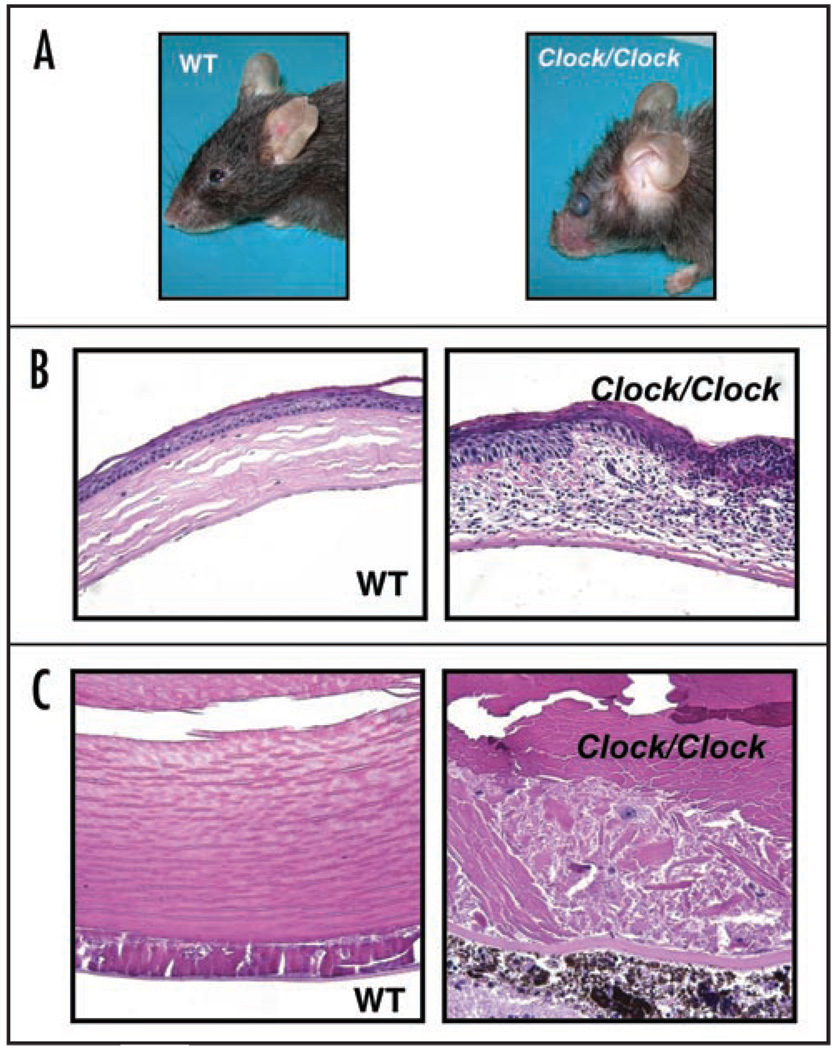
The higher level of radiation-induced morbidity in Clock/Clock mice was also manifested by increased incidents of eye pathologies that were observed in 8 Clock mutants but only in 3 WT animals. The Clock/Clock mice started developing cataracts and eye inflammation at 40 weeks after radiation at the age of 52–54 weeks. Untreated mice and irradiated WT mice showed no signs of eye inflammation or cataract development at this age (Fig. 5A–C). Histological examination of H&E stained lens posterior zone in mice of both genotypes after 80 weeks of TBI showed that the severity of cataracts is more pronounced in Clock mutants (Suppl. Fig. 4). Together, all pathological conditions that developed in Clock/Clock mutant mice after low doses of TBI were reminiscent of the manifestation of the premature aging phenotype previously described for Bmal1−/− mice.23 Thus, although under normal conditions Clock/Clock mice do not display signs of premature aging, exposure to genotoxic treatment such as ionizing radiation, accelerates the ageing program without affecting the rate of carcinogenesis.
Figure 5.
Eye pathologies in Clock/Clock mice after 4Gy of TBI. (A) Gross appearance of wild type and Clock/Clock female mice at 80 weeks after TBI. Eye inflammation and opacity of lens can be observed in Clock/Clock animals. (B and C) Representative example of eye histology. (B) Cornea inflammation. Infiltration by immune cells and abundant changes in the structure of cornea in Clock/Clock mice as compared to WT animals. (C) Cataract development. Posterior cataract (this cataract was graded as grade III in Suppl. Fig. 4) developed in the lens of the Clock/Clock mutant mouse. Only minor changes can be observed in posterior zone of wild type lens.
Discussion
The circadian system represents a complex temporal regulatory network that synchronizes multiple biological processes with the environment. It has been recognized that deregulation of such synchronization may be detrimental for an organism’s well-being and may result in various pathological conditions, including development of tumors. The first potential link between circadian rhythms and cancer biology came from studies in the field of chronotherapy of tumors, which demonstrated (both in animal model and in clinical studies) that the efficacy of many antitumor drugs strongly depended on the time of their administration (reviewed in refs. 24 and 25). These data were supported by a number of epidemiological studies that correlated higher incidence of tumor development in shift workers and flight attendants with the disruption of their circadian clock due to irregular working schedule.5 Manipulations with the light/dark regimes may also affect the rate of tumor growth in the experimental systems.8 However, molecular mechanisms underlying the clock-cancer connection are still poorly understood. Moreover, recent studies using various circadian mutant mouse models indicate that the relation between carcinogenesis and the circadian clock is more complex than initially proposed.
Thus, none of the circadian mutant mice demonstrate a predisposition to the development of spontaneous tumors. When challenged by γ-radiation, mPer2 deficient mice exhibited a higher rate of tumor incidence as well as some characteristics of aging (i.e., earlier hair graying).10 The cancer-prone phenotype of Per2-deficient mice was attributed to defects in DNA damage responses associated with changes in the expression patterns of several oncogenes and tumor suppressor genes.10 Independent lines of evidence linking PERIOD proteins and carcinogenesis came from the study of PER expression in human tumor cell lines and tumors. The Per1 expression was changed in primary colorectal tumors,26 in endometrial carcinomas27 and in non-small cell lung cancer.28 In addition, intratumoral delivery of Per2 inhibited PCNA expression and induced apoptosis in Lewis lung carcinoma tumors, suggesting that the PER2 protein may act as a tumor suppressor.29 However, these characteristics may be unique for Per2 deficiency. Indeed, identical treatment of the animals deficient in both Cryptochromes with the DNA-damaging agents does not result in an increase in carcinogenesis suggesting that cancer predisposition in “clock-less” mice is dependent on which particular way clock is disrupted.12 Consistent with this, the lifespan and the incidence of tumor development in Clock mutant mice under normal conditions are indistinguishable from their WT littermates. Moreover, as we demonstrate here, using ionizing radiation as a carcinogen does not increase the rate of tumor formation in Clock/Clock mice compared to WT. Instead, when challenged by γ-radiation, mice with a functional deficiency in CLOCK protein demonstrate acceleration of the aging process. This phenotype is consistent with a high apoptotic and a low cell proliferation rate in lymphoid tissues of Clock mutant mice, and is supported by gene expression profiling of a number of apoptosis and cell cycle-related genes. Thus, after exposure to low doses of ionizing radiation, Clock/Clock mice start developing a phenotype of premature aging, which is reminiscent of that normally occurring in Bmal1−/− animals.
CLOCK is a transcription factor and a component of the molecular circadian oscillator, which acts in complex with the circadian protein BMAL1.30 The heterodimerization of these two proteins is important for their posttranslational regulation and transcriptional activity.31 The activity of the CLOCK/BMAL1 complex is impaired in both Clock mutant and Bmal1−/− animals, resulting in a partial overlap in changes in the expression of the CLOCK/BMAL1 target genes in tissues of mutant animals, however, the phenotypes are not completely identical.32 Recently we have reported that mice deficient in the core circadian gene Bmal1 are characterized with reduced lifespan and various symptoms of premature aging, including sarcopenia, cataracts, organ shrinkage and others.23 Although aging is often associated with increased carcinogenesis, surprisingly, none of the Bmal1−/− mice developed tumors in the course of their lifespan. Thus, despite similar behavioral phenotype displayed by various circadian mutants, only Per2 mutation is associated with an increased rate of carcinogenesis. While accelerated aging occurs spontaneously in Bmal1−/− animals, it can be provoked in Clock/Clock and Per2 mutants. There are currently no reports describing accelerated aging in Cry1−/−Cry2−/− double knockout animals. Altogether, our results provide additional evidence of diverse roles for the circadian proteins in various physiological processes, which are not completely understood at the mechanistic level.
These data also present an intriguing example of the complex relationship between such fundamental processes as cancer and aging and their interconnection with the molecular clock. Based on the link that exists between advanced age and increased incidence of cancer, cancer has always been considered a major age-related disease (reviewed in ref. 33). It has been proposed that aged tissues create an environment that stimulates the growth of tumor cells.34 At the same time, recent data suggest that at least some tumor suppressor mechanisms can promote aging phenotypes35,36 further suggesting more antagonistic interconnection between the two processes. Thus, mice with chronically activated p53 (a critically important tumor suppressor) demonstrate exceptional protection against cancer, which is accompanied by acceleration of a number of degenerative processes associated with aging. Thus, aging may result as a consequence of the same mechanisms that serve to prevent tumor formation at an early age. The results obtained for Clock mutant and Bmal1-deficient animals are within the framework of this concept.
In summary, our results suggest the existence of a complex interconnection between aging, carcinogenesis and the circadian clock. Future study of physiological activities of core circadian proteins will contribute to our understanding of the mechanisms of aging and cancer progression.
Materials and Methods
Animals
Clock mutant mice15,37,38 were backcrossed to C57Bl/6J mice for 16 generations. Twenty wild type (WT) and Clock/Clock males (10 of each genotype) and 30 WT and Clock/Clock females (15 of each genotype) received 4 Gy of total body irradiation (TBI) (Cs-137 source; 2.5 Gy/min) at 12–14 weeks of age. After irradiation, mice were monitored by visual inspection and weighed weekly. Animals were sacrificed 80 weeks post irradiation or earlier if they lost more than 20% of their original body weight or displayed any other signs of morbidity. All animals were evaluated for their gross appearance (including body weight and hair graying), gross pathological changes in major organs, whole blood cell analysis and pathohistology of lymphoid organs (spleen, lymph nodes and thymus). All animal studies were conducted in accordance with the regulations of the Committee of Animal Care and Use at the Cleveland Clinic Foundation.
Tissue collection, histology and whole blood cell analysis
For histological analysis, tissue samples were fixed in 10% neutral-buffered formalin, dehydrated, embedded in paraffin, sectioned at 5 µm, and stained with H&E. Histological sections were masked and interpreted independently by two investigators. Total blood cell analysis was performed on samples obtained through retro-orbital bleeding as previously described.18 For mRNA expression analysis, untreated animals (18 WT and 18 Clock/Clock) were maintained on a 12 h: 12 h light:dark cycle. Three animals of each genotype were sacrificed every 4 hours though the course of the 24-hr cycle, spleens were removed, immediately frozen on dry ice and stored at -80°C until RNA extraction.
RNA isolation and real-time RT-PCR
RNA was isolated from individual samples using TriZol reagent (Invitrogen) according to manufacturer’s protocol. In order to estimate relative abundance of cell cycle- and apoptosis-related genes in spleens of animals of the two genotypes, 18 individual WT and 18 Clock/Clock RNA samples collected around the clock were pooled in equimolar ratio to obtain a single representative sample for each genotype. One microgram of each pooled RNA sample was converted to cDNA with RT2 First Strand kit (SuperArray Biosience Corp.) and used in real-time PCR performed on Mouse Cell Cycle and Mouse Apoptosis RT2 Profiler PCR Arrays (SuperArray Bioscience Corp.) according to manufacturer’s protocol. Data analysis was performed using ΔΔCt method and Gapdh expression for normalization. TaqMan RT-PCR technology (Applied Biosystems, Foster City, CA) was employed to validate array results and measure expression of genes in the spleen using pre-made TaqMan gene expression assays and EZ RT-PCR kit (Applied Biosystems).
Flow cytometry and annexin assay
WT and Clock/Clock animals received 8.5 Gy of TBI and were sacrificed 1, 4 and 8 hrs post irradiation. Spleens and thymuses were removed, dispersed by passing through the cell strainer (Beckton Dickinson), washed from red blood cells by 1-min incubation in hypotonic solution, and resuspended in PBS. Detection of apoptotic cells was performed using Annexin V/FITC Apoptosis Detection Kit (Alexis Biochemicals) as suggested by the manufacturer. Data was analyzed using a FACSCalibur and FACSComp Software (BD Biosciences, San Jose, CA). Sample data were collected on 10,000 ungated cells. In each experiment, thymocytes and spleenocytes from untreated mice were used to determine the fluorescence window specific for the B (B220-positive) and T (CD3-positive) cells, and these windows were used to gate the cell population when analyzing the experimental samples to determine the percentage of B220- (or CD3)-expressing cells stained with Annexin. FITC-labeled anti-mouse B220 PE-labeled CD3 antibodies (Pharmingen) were used according to manufacturer’s protocol.
Cell culture, growth assay, siRNA constructs and western blotting
Lewis Lung carcinoma (LLC1) cells were maintained in DMEM supplemented with 10% fetal bovine serum. For stable expression of siRNAs targeting Clock we used the pLSLP lentiviral vector that contains a siRNA-expression cassette within the U3 region of the right LTR as previously described.23 The following sequences, representing 19 bp stretches of the Clock mRNA were present in the hairpin transcripts: siClock-832, 5'TCTTGTGGATCAAAGTATA; siClock-1375, 5'TTTGCCTTTTCCATATTGC; siClock-2467, 5'AAATCTTATCTGTCTGTCC. Generation of recombinant lentiviruses and cell infection were performed as described.39 The efficiency of inhibition of CLOCK expression was monitored by Western blot analysis with anti-CLOCK antibody. LLC1 cells infected with siClock-1375 construct showed no suppression of CLOCK protein and were used as control. To address the effect of suppression of CLOCK protein on the growth rate of cells in culture, parental LLC1 line, LLC1 expressing siClock-1375 and two independently obtained lines expressing siClock-2467 were plated on 24-well plates at the density of 2 × 104 cells/well. Three wells of each line were fixed with 50% ethanol daily for 7 days and quantitated for cell density using methylene blue calorimetric assay at the end of the experiment.
Tumorigenicity assay
Parental LLC1 cells and cells expressing inactive (siClock-1375) or functional (siClock-2467) si-constructs were inoculated subcutaneously into 40 C57BL/6J male mice at 106 cells/site. Each animal received two injections: LLC1 or siClock- 1375 on one side and siClock-2467—on the other side. In order to eliminate the effect of position of inoculation site, the left and the right sides were alternated. Tumor size was measured with a caliper and volumes were calculated as L × W2 × 0.52.
Statistical analyses
All statistical analyses in this study were done with SigmaStat 3.5 software (Systat Software, Inc., CA). Survival fractions were calculated using the Kaplan-Meier method and survival curves were compared by log-rank Mantel-Haenszel tests. P values <0.05 were considered as significant.
Supplementary Material
Acknowledgements
We would like to thank Karen Kuropatwinski for the help with real-time PCR, Mary Spengler for critical reading of the manuscript and helpful discussions. This work is supported by NCI grant CA102522 (to M.P.A) and NCRR/NIH Midcareer Award in Mouse Pathobiology RR17595 (to A.Y.N.).
Footnotes
Note
Supplementary materials can be found at: www.landesbioscience.com/supplement/AntochCC7-9-Sup.pdf
References
- 1.Schibler U, Sassone Corsi P. A web of circadian pacemakers. Cell. 2002;111:919–922. doi: 10.1016/s0092-8674(02)01225-4. [DOI] [PubMed] [Google Scholar]
- 2.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 3.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Human molecular genetics. 2006;15:271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 4.Kondratov RV, Gorbacheva VY, Antoch MP. The role of mammalian circadian proteins in normal physiology and genotoxic stress responses. Current topics in developmental biology. 2007;78:173–216. doi: 10.1016/S0070-2153(06)78005-X. [DOI] [PubMed] [Google Scholar]
- 5.Haus E, Smolensky M. Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control. 2006;17:489–500. doi: 10.1007/s10552-005-9015-4. [DOI] [PubMed] [Google Scholar]
- 6.Rafnsson V, Sulem P, Tulinius H, Hrafnkelsson J. Breast cancer risk in airline cabin attendants: a nested case-control study in Iceland. Occup Environ Med. 2003;60:807–809. doi: 10.1136/oem.60.11.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rafnsson V, Tulinius H, Jonasson JG, Hrafnkelsson J. Risk of breast cancer in female flight attendants: a population-based study (Iceland) Cancer Causes Control. 2001;12:95–101. doi: 10.1023/a:1008983416836. [DOI] [PubMed] [Google Scholar]
- 8.Filipski E, Li XM, Levi F. Disruption of circadian coordination and malignant growth. Cancer Causes Control. 2006;17:509–514. doi: 10.1007/s10552-005-9007-4. [DOI] [PubMed] [Google Scholar]
- 9.Bjarnason GA, Jordan RC, Wood PA, Li Q, Lincoln DW, Sothern RB, Hrushesky WJ, Ben David Y. Circadian expression of clock genes in human oral mucosa and skin: association with specific cell cycle phases. The American journal of pathology. 2001;158:1793–1801. doi: 10.1016/S0002-9440(10)64135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science (New York, NY) 2003;302:255–259. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- 12.Gauger MA, Sancar A. Cryptochrome, circadian cycle, cell cycle checkpoints, and cancer. Cancer Res. 2005;65:6828–6834. doi: 10.1158/0008-5472.CAN-05-1119. [DOI] [PubMed] [Google Scholar]
- 13.Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, Takahashi JS. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci USA. 2007;104:3342–3347. doi: 10.1073/pnas.0611724104. Epub 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondratov RV, Antoch MP. Circadian proteins in the regulation of cell cycle and genotoxic stress responses. Trends in cell biology. 2007;17:311–317. doi: 10.1016/j.tcb.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science (New York, NY) 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitaterna MH, Ko CH, Chang AM, Buhr ED, Fruechte EM, Schook A, Antoch MP, Turek FW, Takahashi JS. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9327–9332. doi: 10.1073/pnas.0603601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorbacheva VY, Kondratov RV, Zhang R, Cherukuri S, Gudkov AV, Takahashi JS, Antoch MP. Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3407–3412. doi: 10.1073/pnas.0409897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Besplug J, Burke P, Ponton A, Filkowski J, Titov V, Kovalchuk I, Kovalchuk O. Sex and tissue-specific differences in low-dose radiation-induced oncogenic signaling. Int J Radiat Biol. 2005;81:157–168. doi: 10.1080/09553000500103512. [DOI] [PubMed] [Google Scholar]
- 20.Kovalchuk O, Ponton A, Filkowski J, Kovalchuk I. Dissimilar genome response to acute and chronic low-dose radiation in male and female mice. Mutat Res. 2004;550:59–72. doi: 10.1016/j.mrfmmm.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Ward J, Anver M, Mahler J, Devor Henneman D. Pathology of mice commonly used in genetic engineering (C57BL/6; 129; B6,129; and FVB/N) In: Ward J, Mahler J, Maronpot R, Sundberg J, editors. Pathology of genetically engineered mice. Ames: Iowa State University Press; 2000. pp. 161–179. [Google Scholar]
- 22.Mohr U. Pathobiology of Aging Mouse. In: Mohr U, Dungworth D, Capen C, Carlton W, Sundberg J, Ward J, editors. Washington: ILSI Press; 1996. [Google Scholar]
- 23.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi M, Wood PA, Hrushesky WJ. Circadian chemotherapy for gynecological and genitourinary cancers. Chronobiol Int. 2002;19:237–251. doi: 10.1081/cbi-120002600. [DOI] [PubMed] [Google Scholar]
- 25.Levi F. Handbook of Experimental Pharmacology. Berlin: Springer-Verlag; 1997. Chronopharmacology of anticancer agents; pp. 299–331. [Google Scholar]
- 26.Krugluger W, Brandstaetter A, Kallay E, Schueller J, Krexner E, Kriwanek S, Bonner E, Cross HS. Regulation of genes of the circadian clock in human colon cancer: reduced period-1 and dihydropyrimidine dehydrogenase transcription correlates in high-grade tumors. Cancer research. 2007;67:7917–7922. doi: 10.1158/0008-5472.CAN-07-0133. [DOI] [PubMed] [Google Scholar]
- 27.Yeh KT, Yang MY, Liu TC, Chen JC, Chan WL, Lin SF, Chang JG. Abnormal expression of period 1 (PER1) in endometrial carcinoma. The Journal of pathology. 2005;206:111–120. doi: 10.1002/path.1756. [DOI] [PubMed] [Google Scholar]
- 28.Gery S, Komatsu N, Kawamata N, Miller CW, Desmond J, Virk RK, Marchevsky A, McKenna R, Taguchi H, Koeffler HP. Epigenetic silencing of the candidate tumor suppressor gene Per1 in non-small cell lung cancer. Clin Cancer Res. 2007;13:1399–1404. doi: 10.1158/1078-0432.CCR-06-1730. [DOI] [PubMed] [Google Scholar]
- 29.Hua H, Wang Y, Wan C, Liu Y, Zhu B, Wang X, Wang Z, Ding JM. Inhibition of tumorigenesis by intratumoral delivery of the circadian gene mPer2 in C57BL/6 mice. Cancer gene therapy. 2007;14:815–818. doi: 10.1038/sj.cgt.7701061. [DOI] [PubMed] [Google Scholar]
- 30.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science (New York, NY) 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 31.Kondratov RV, Chernov MV, Kondratova AA, Gorbacheva VY, Gudkov AV, Antoch MP. BMAL1-dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes & development. 2003;17:1921–1932. doi: 10.1101/gad.1099503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kondratov RV, Shamanna RK, Kondratova AA, Gorbacheva VY, Antoch MP. Dual role of the CLOCK/BMAL1 circadian complex in transcriptional regulation. Faseb J. 2006;20:530–532. doi: 10.1096/fj.05-5321fje. [DOI] [PubMed] [Google Scholar]
- 33.DePinho RA. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- 34.Krtolica A, Campisi J. Cancer and aging: a model for the cancer promoting effects of the aging stroma. The international journal of biochemistry & cell biology. 2002;34:1401–1414. doi: 10.1016/s1357-2725(02)00053-5. [DOI] [PubMed] [Google Scholar]
- 35.Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, Sutherland A, Thorner M, Scrable H. Modulation of mammalian life span by the short isoform of p53. Genes & development. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Hee Park S, Thompson T, Karsenty G, Bradley A, Donehower LA. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 37.Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD, Sangoram AM, King DP, Pinto LH, Takahashi JS. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell. 1997;89:655–667. doi: 10.1016/s0092-8674(00)80246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science (New York, NY) 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.