To the Editor: We read with interest “Dermatologic symptoms associated with the multikinase inhibitor sorafenib” by Robert et al1 in the February 2009 issue of the Journal. The frequency of sorafenib-associated dermatologic side effects and their impact on quality of life highlight the important role of dermatologists in caring for these patients. We wish to share our experience with oncology patients receiving sorafenib at the National Cancer Institute. We found skin changes similar to those described by the authors in addition to some not included in their report.
Sixty-five patients on sorafenib therapy for solid tumors were evaluated in the Dermatology Clinic from August 2005 - December 2007: 24 individuals were examined at baseline and followed for the development of dermatologic side effects (prospective cohort) and 41 individuals were examined after developing cutaneous lesions (consultation cohort). The cutaneous adverse effects discussed by the authors were similarly encountered in both of our cohorts: hand-foot skin reaction/HFSR (63% prospective cohort; 78% consultation cohort), facial/scalp erythema/dysesthesias (63%; 68%), nail changes (33%; 32%), alopecia (21%; 39%), rash/exanthems (21%; 10%), cysts (8%; 27%), eruptive keratoacanthomas (4%; 7%), and eruptive nevi2 (0%; 2%).
In comparison with the review by Robert et al, our cohorts had a much higher incidence of HFSR.3 Dermatologic adverse effects identified in our patients but not reported in Robert’s review include the development of a generalized keratosis pilaris-like eruption (21% prospective cohort; 41% consultation cohort); stomatitis (17%; 22%), inflamed seborrheic keratoses (13%; 10%), and leukocytoclastic vasculitis4 (0%; 2%).
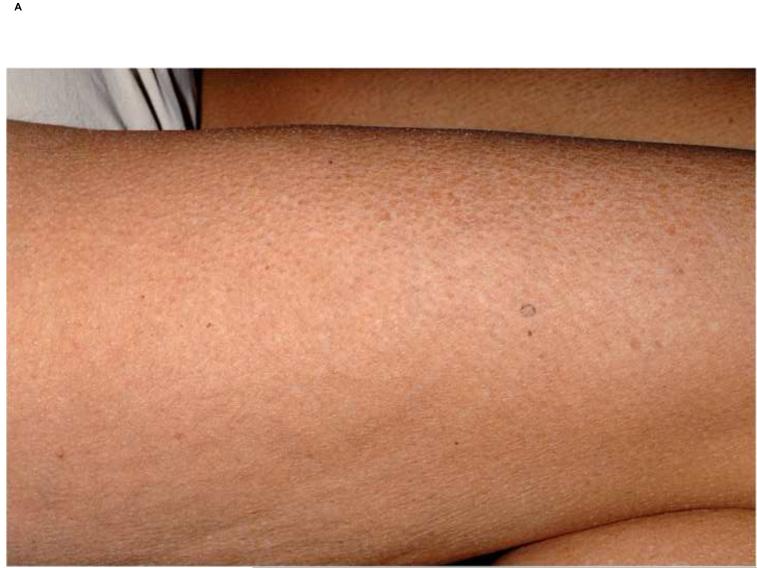
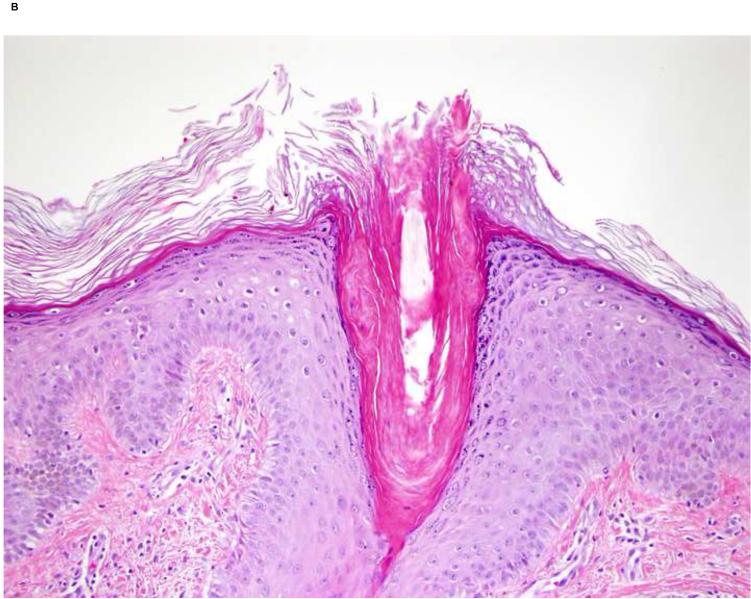
The histology of the generalized keratosis pilaris-like eruption was typical for keratosis pilaris (Fig 1). The findings of a keratosis pilaris-like eruption, eruptive keratoacanthomas and multiple cysts support the hypothesis that sorafenib causes alterations in the keratinocyte differentiation/proliferation pathways.
Fig 1.
Keratosis pilaris-like lesions. A, Fine keratotic papules on thigh of a patient on sorafenib. B, Hyperkeratotic plug within hair follicle (Original magnification: x20.)
While some of the exanthematous eruptions experienced by our patients resembled erythema multiforme (EM) clinically, multiple biopsies demonstrated only mild superficial perivascular lymphocytic infiltrates with no evidence of basal vacuolization nor necrotic keratinocytes as would be expected in EM. A previously reported patient, who presented with erythema multiforme-like lesions, was demonstrated to have leukocytoclastic vasculitis on histologic examinaton. We recommend biopsies from these skin lesions for more precise diagnoses. Sorafenib was successfully re-initiated at reduced doses after temporary rest periods despite these eruptions.
We concur with the assessment of Robert et al 1 5 that the dermatologic side effects of sorafenib are often manageable with topical therapies and/or dose modifications. Increased awareness within the dermatologic community of the diversity, frequency, and treatment of sorafenib-induced cutaneous adverse reactions will be helpful to patients who require chronic therapy with this medication for their cancers.
Acknowledgments
Funding Sources: This research was supported by the Intramural Research Program of the NIH, Center for Cancer Research, National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Disclosure: The authors have no conflict of interest to disclose.
References
- 1.Robert C, Mateus C, Spatz A, Wechsler J, Escudier B. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. J Am Acad Dermatol. 2009;60(2):299–305. doi: 10.1016/j.jaad.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 2.Kong HH, Sibaud V, Chanco Turner ML, Fojo T, Hornyak TJ, Chevreau C. Sorafenib-induced eruptive melanocytic lesions. Arch Dermatol. 2008;144(6):820–2. doi: 10.1001/archderm.144.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azad NS, Aragon-Ching JB, Dahut WL, Gutierrez M, Figg WD, Jain L, et al. Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-VEGF therapy. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-08-1141. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung NM, Gutierrez M, Turner ML. Leukocytoclastic vasculitis masquerading as hand-foot syndrome in a patient treated with sorafenib. Arch Dermatol. 2006;142(11):1510–1. doi: 10.1001/archderm.142.11.1510. [DOI] [PubMed] [Google Scholar]
- 5.Autier J, Escudier B, Wechsler J, Spatz A, Robert C. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008;144(7):886–92. doi: 10.1001/archderm.144.7.886. [DOI] [PubMed] [Google Scholar]