Abstract
Background
Stroke is the third most common cause of cardiovascular disease death in patients on dialysis; however, characteristics of cerebrovascular disease, including clinical subtypes and subsequent consequences, have not been well described.
Study Design
Prospective national cohort study, the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) study.
Settings & Participants
1,041 incident dialysis patients treated in 81 clinics, enrolled from 10/95–7/98, followed until 12/31/2004.
Predictor
Time from dialysis initiation.
Outcomes & Measurements
Cerebrovascular disease events were defined as non-fatal (hospitalized stroke, carotid endarterectomy) and fatal (stroke death) events after dialysis initiation. Stroke subtypes were classified using standardized criteria from the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) system. Incidence of cerebrovascular event subtypes were analyzed using time-to-event analyses accounting for competing risk of death. Clinical outcomes after stroke were abstracted from medical records.
Results
A total of 165 participants experienced a cerebrovascular event with an overall incidence of 4.9 per 100 person-years. Ischemic stroke was the most common (76% of all 200 events) with cardioembolism subtype accounting for 28% of the 95 abstracted ischemic events. The median time from onset of symptoms to first stroke evaluation was 8.5 hours (25th and 75th percentiles 1, 42 hours), with only 56% of patients successfully escaping death, nursing home, or a skilled nursing facility.
Limitations
Relatively small sample size limits power to determine risk factors.
Conclusions
Cerebrovascular disease is common in dialysis patients, is identified late, and carries a significant risk of morbidity and mortality. Stroke etiologic subtypes on dialysis are multifactorial, suggesting risk factors may change the longer one has ESRD. Further studies are needed to address the poor prognosis through prevention, early identification, and treatment.
Keywords: Cerebrovascular disease, Stroke, Dialysis, Prognosis, Epidemiology
Cardiovascular disease (CVD) is the leading cause of death for patients on dialysis, with stroke as the third leading cause of CVD death.1 In the dialysis population, there have been only a few studies of cerebrovascular disease, including outcomes such as clinical or subclinical stroke, or carotid endarterectomy (CEA).2,3 Even though traditional atherosclerotic risk factors for cerebrovascular disease, such as age, hypertension, diabetes mellitus, and dyslipidemia, are common in dialysis patients, there are also risk factors unique to the uremic process2, 4, 5 and to the dialysis process itself,6 which may predispose individuals on dialysis to either ischemic or hemorrhagic strokes. Given this dynamic accumulation of traditional and non-traditional risk factors on dialysis, the type of stroke, whether it is hemorrhagic or ischemic and particular ischemic subtypes, may change the longer one has ESRD.
Directed therapy, prognosis, and prevention of recurrence require identification of the specific stroke subtypes in order to appropriately prescribe care.7 In the general population, stroke remains a significant cause of morbidity and mortality despite these measures aimed at early treatment.8 For patients on dialysis, the timing of presentation after stroke and immediate clinical outcomes have not been systematically studied. As such, optimal management of patients on dialysis with stroke lacks an evidence base.
The aims of our study were to determine the incidence of cerebrovascular events and stroke subtypes, clinical characteristics of the cerebrovascular events, and post-event outcomes in patients initiating dialysis.
METHODS
Study Design
The study participants were from the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) study.9 This national prospective cohort study of incident dialysis patients was initiated in 1995 to investigate treatment choices and clinical outcomes in dialysis care. Eligibility criteria for enrollment in CHOICE included new onset of chronic dialysis therapy in the preceding 3 months, ability to provide informed consent, age of 18 years or older, and ability to speak English or Spanish. The Johns Hopkins University School of Medicine Institutional Review Board and the clinical centers’ review boards approved the study. A total of 1,041 participants from 19 states were enrolled from October 1995 to June 1998 at 81 dialysis clinics associated with the nonprofit Dialysis Clinic, Inc. (Nashville, Tenn; n=923), New Haven CAPD (New Haven, Conn; n=86), or Saint Raphael’s Hospital (New Haven, Conn; n=32).
Data Collection
The observation period for each patient began on the date of enrollment and continued through transplantation (n=267) or December 31, 2004, the last follow-up date at CHOICE study completion. A main outcome in CHOICE was any cardiovascular event; all possible cerebrovascular events underwent evaluation in CHOICE. The primary outcome of this ancillary investigation was the first cerebrovascular event after dialysis initiation, including ischemic or hemorrhagic stroke, CEA, or stroke death.
Assignment of a non-fatal cerebrovascular event in CHOICE was based upon information from the four data sources by the following process. A) Adjudicated records were considered the primary source of information for a non-fatal event. A Cardiovascular Disease Endpoints committee of trained physicians reviewed hospital records to determine cause of hospitalization using uniformly applied criteria modified from the Cardiovascular Health Study10 and the Hemodialysis (HEMO) study.11 Each chart was independently reviewed by two members of the committee and the cause of hospitalization was defined as either a definite or probable cerebrovascular event and then verified by an independent adjudicator. If the two initial reviews differed in assigned cause of hospitalization, a third independent reviewer resolved the disagreement. B) In the absence of an adjudicated record, any United States Renal Data System (USRDS) or Health Care Financing Administration (HCFA) billing data code for CEA [38.10, 38.11, 38.12] or report from DCI confirmed a procedure event. C) For non-procedure events without an adjudicated record, the algorithm for assigning stroke, based upon the strength of the data source, was as follows: (1) any USRDS or HCFA billing data code for cerebrovascular disease [430–436, 437.0, 437.1, 437.8, 437.9]; (2) a clinic (Dialysis Clinic Inc [DCI]) record, when supported by the corresponding comorbidity record; and (3) subsequent stroke events in the same broad category within 30 days of discharge from a prior hospitalization for an assigned stroke event were not assigned as separate events. Administrative data containing ICD-9 diagnostic and procedural codes were available through 12/31/04. Stroke death was assigned by information from adjudicated death records and National Death Index (NDI) records.
Medical charts from all available strokes were further reviewed by two independent abstractors for this ancillary investigation. Stroke was classified as ischemic or hemorrhagic based primarily on medical chart review. When charts were unavailable, ICD-9 codes 430 (subarachnoid hemorrhage), 431 (intracerebral hemorrhage), and 432 (other and unspecified intracranial hemorrhage) were classified as hemorrhagic stroke while the other cerebrovascular disease ICD-9 codes mentioned above were classified as ischemic stroke, similar to classification performed in other studies of stroke in dialysis.2
Ischemic stroke was further subclassified using the TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria into five etiologic subtypes.12 These subtypes were large-artery atherosclerosis, cardioembolism, small-vessel occlusion, stroke of other determined etiology, and stroke of undetermined etiology. Stroke caused by rare causes (such as nonatherosclerotic vasculopathy, hypercoagulable states, or hematologic disorders) were grouped with unknown causes due to the low frequency. The diagnosis of each subtype required clinical, radiographic, imaging, and laboratory findings. If the two initial reviewers disagreed on the etiologic subtype, a third reviewer reviewed and resolved these discrepancies.
Data on patient demographics and medical history were collected from a baseline self-report questionnaire. Race/ethnicity was self-reported by patients. Dialysis modality at baseline was defined as the modality at four weeks after study enrollment.
Baseline individual comorbid conditions were abstracted from dialysis unit records, hospital discharge summaries, medication lists, consultation notes, diagnostic imaging, and cardiac imaging reports. A comorbidity score, the Index of Coexistent Disease (ICED), was calculated based on the presence and severity of comorbid conditions; scores range from 0 to 3 with 3 as the highest severity level.13 The reliability of data abstraction and ICED severity scoring was assessed with a masked recoding of 45 medical records with high interrater reliability (κ = 0.93).
Baseline nonfasting venous blood specimens were routinely collected at the DCI facilities just before a dialysis session. Laboratory values were obtained from monthly dialysis laboratory tests or sent to Quest Diagnostics (Baltimore, MD).
Information abstracted from the hospitalized records for this ancillary investigation included those items related to presentation and outcomes after CEA and stroke. These included time from symptom onset to presentation at the hospital emergency department, length of hospital stay, and discharge location. Discharge locations were predefined as home, death, an acute rehabilitation center, a nursing home, or a skilled nursing facility. “Successful recovery” from the hospital was defined as discharge to home or to an acute rehabilitation center, as opposed to death, nursing home, or skilled nursing facility. Stroke death and case fatality was defined as those occurring within 30 days of the stroke and related to the stroke itself, defined as either a primary or underlying cause of death obtained from review of adjudicated death records or NDI records.
Statistical Methods
Baseline characteristics between those individuals who suffered a subsequent cerebrovascular event were compared to those individuals who were free of cerebrovascular event at last follow-up using Fisher’s exact test and Mann-Whitney rank-sum test. The crude incidence rate of cerebrovascular events was calculated by dividing the number of patients with an event by the cumulative time at risk from study enrollment to first cerebrovascular event with 95% confidence intervals constructed using a Poisson model. In addition, incidence rates were calculated for several periods after dialysis initiation (0–2, 2–4, 4–6, and >6 years).
Because the occurrence of death from causes other than stroke precludes the occurrence of cerebrovascular events, cumulative incidence curves accounting for the competing risk of non-stroke death were generated for each type of cerebrovascular event using methods described by Coviello and Boggess.14 The cumulative incidence at 2, 4, and 6 years, and end of study period were obtained from these curves. Multivariate Cox cause-specific hazard ratios of cerebrovascular events associated with age, sex, race, ICED, presence of diabetes, and presence of cerebrovascular disease were calculated from these competing risk models using methods of Lunn and McNeil,15 in separate models excluding and including CEA as a cerebrovascular event. Descriptive statistics of each of the clinical outcomes were reported by etiologic stroke subtype.
A two-sided p-value less than 0.05 was used as the level of statistical significance for all tests. Statistical analyses were performed using Stata SE software, version 9.2 (StataCorp, College Station, Texas).
RESULTS
Approximately two-thirds of eligible patients were enrolled in CHOICE from the participating dialysis units. Eligible patients enrolled were similar to eligible but unenrolled patients with regard to age, sex, dialysis modality, albumin, and blood pressure. Baseline characteristics of the 1,041 CHOICE participants are presented in Table 1. Age, sex, race, and dialysis modality distributions were similar to that of the 1997 US dialysis population, as described previously.16 During a median follow-up of 2.7 years (range 0.1–9.5 years), a total of 165 patients experienced an incident cerebrovascular event after dialysis initiation, with 27% of these patients having cerebrovascular disease prior to dialysis initiation (19% prior stroke). Those with cerebrovascular events during the study were more likely to be older, men, have a lower diastolic blood pressure, and have a history of diabetes mellitus, prior cerebrovascular disease, or prior peripheral vascular disease at baseline than those without subsequent cerebrovascular events. Other common risk factors, such as systolic blood pressure, presence of arrhythmias, presence of left ventricular hypertrophy, and cholesterol levels were not statistically significantly different among the two groups. In multivariate Cox proportional hazards regression analysis accounting for the competing risk of death, age, race, ICED, previous diabetes mellitus, and previous cerebrovascular disease were significantly associated with increased risk of cerebrovascular disease events (see Table 2). The results were similar between models that included or excluded CEA as a cerebrovascular event.
Table 1.
Baseline Characteristics of 1,041 Incident Dialysis Patients
| Characteristic* | All Patients N=1,041 | Patients Without Cerebrovascular Event n=876 | Patients With Cerebrovascular Event n=165 | p-value |
|---|---|---|---|---|
| Age (years) | 58 ± 15 | 57 ± 15 | 62 ± 13 | <0.001 |
| Sex: Men | 564 (54) | 490 (56) | 74 (45) | 0.006 |
| Race: | ||||
| White | 695 (67) | 585 (67) | 110 (67) | 0.9 |
| Black | 295 (28) | 249 (28) | 46 (28) | |
| Other | 51 (5) | 42 (5) | 9 (5) | |
| Systolic blood pressure (mm Hg), [n=944] | 149 ± 19 | 148 ± 19 | 150 ± 18 | 0.3 |
| Diastolic blood pressure (mm Hg), [n=944] | 79 ± 10 | 79 ± 11 | 76 ± 9 | <0.001 |
| Modality: hemodialysis | 767 (74) | 637 (73) | 130 (79) | 0.1 |
| Tobacco use: current or former, [n=977] | 592 (61) | 498 (60) | 94 (62) | 0.8 |
| Comorbid conditions: | ||||
| Diabetes mellitus | 561 (54) | 447 (51) | 114 (69) | <0.001 |
| Cerebrovascular disease | 176 (17) | 132 (15) | 44 (27) | 0.001 |
| Coronary heart disease | 457 (44) | 382 (44) | 75 (45) | 0.7 |
| Peripheral vascular disease | 270 (26) | 216 (25) | 54 (33) | 0.03 |
| Left ventricular hypertrophy | 259 (24) | 213 (24) | 46 (28) | 0.4 |
| Arrhythmia | 308 (30) | 258 (30) | 50 (30) | 0.9 |
| Valvular disorder | 189 (18) | 153 (18) | 36 (22) | 0.2 |
| Congestive heart failure | 466 (46) | 383 (45) | 83 (51) | 0.2 |
| Index of Coexistent Disease | 1.94 ± 0.81 | 1.92 ± 0.82 | 2.04 ± 0.77 | 0.08 |
| Total cholesterol (mg/dL), [n=999] | 189 ± 48 | 188 ± 49 | 193 ± 45 | 0.1 |
| LDL cholesterol (mg/dL), [n=872] | 104 ± 40 | 104 ± 40 | 107 ± 41 | 0.3 |
| HDL cholesterol (mg/dL), [n=867] | 44 ± 17 | 43 ± 17 | 45 ± 16 | 0.4 |
| Triglycerides (mg/dL), [n=916] | 206 ± 129 | 203 ± 129 | 217 ± 131 | 0.2 |
| Albumin (mg/dL) | 3.62 ± 0.37 | 3.63 ± 0.38 | 3.59 ± 0.36 | 0.2 |
| Corrected Ca-P product (mg2/dL2) | 48.9 ± 12.6 | 49.0 ± 12.8 | 48.3 ± 12.0 | 0.7 |
| Hematocrit (%) | 32.5 ± 4.1 | 32.5 ± 4.1 | 32.3 ± 3.8 | 0.6 |
Mean ± SD or N(%), < 5% missing unless otherwise noted
LDL: low-density lipoprotein; HDL: high-density lipoprotein, Ca × Phos: calcium phosphate product
p-values by Fisher’s exact test or Mann-Whitney rank-sum test
Table 2.
Association of Risk Factors with Cerebrovascular Disease Events Among the CHOICE Participants*
| Risk Factor | HR (95% CI) of Stroke (without CEA) n=147 | HR (95% CI) of Stroke or CEA n=165 |
|---|---|---|
| Age (per 10 years) | 1.26 (1.19–1.34)§ | 1.26 (1.19–1.34)§ |
| Sex: | ||
| Men | Ref | Ref |
| Women | 1.04 (0.90–1.21) | 1.04 (0.90–1.21) |
| Race: | ||
| White | Ref | Ref |
| Black | 0.64 (0.54–0.77)§ | 0.64 (0.53–0.76)§ |
| Other | 0.68 (0.50–0.91)† | 0.69 (0.51–0.94)† |
| Modality: | ||
| Hemodialysis | Ref | Ref |
| Peritoneal dialysis | 1.12 (0.93–1.35) | 1.14 (0.95–1.36) |
| Comorbid conditions: | ||
| Diabetes mellitus | 1.32 (1.13–1.54)§ | 1.35 (1.16–1.57)§ |
| Cerebrovascular disease | 1.20 (0.99–1.46) | 1.23 (1.02–1.49)† |
| Index of Coexistent Disease | ||
| ICED=0 or 1 | Ref | Ref |
| ICED=2 | 1.40 (1.18–1.65)† | 1.35 (1.14–1.59)† |
| ICED=3 | 1.68 (1.39–2.03)§ | 1.64 (1.36–1.98)§ |
HR, hazard ratio; CI, confidence interval; ICED. Index of Coexistent Disease
Multivariate adjustment accounting for the competing risk of death from causes other than stroke
p<0.05
p<0.001
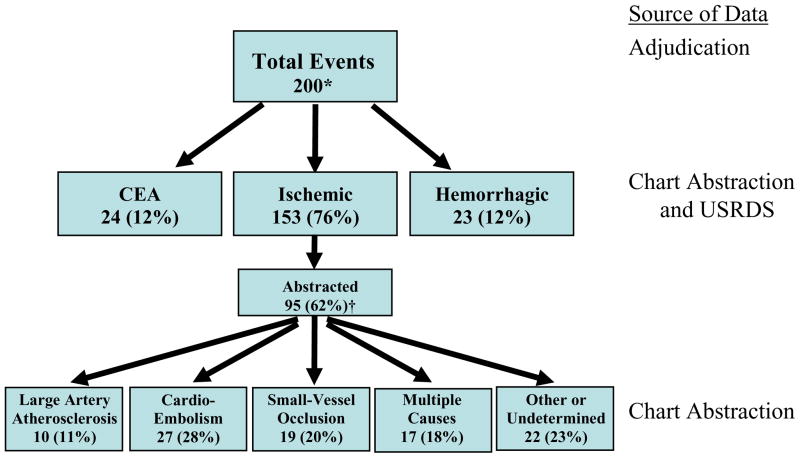
Figure 1 describes the characterization of the cerebrovascular events. From the 165 patients who experienced an incident event during the study, there were 35 additional repeated events in 29 patients, as patients could experience multiple separate events over the course of the study. One-hundred sixteen (58%) of these 200 events were ascertained by chart adjudication (95 ischemic strokes, 10 hemorrhagic strokes, 11 CEAs) with 68% agreement from USRDS or HCFA records and 13% from the combination of DCI and comorbidity records. Seventy-seven (39%) events were assigned using USRDS or HCFA records (8% agreement with the combination of DCI and comorbidity records), and seven (4%) events were assigned solely using the combination of DCI and comorbidity records. Fourteen of the 105 abstracted stroke events were recurrent strokes.
Figure 1. Cerebrovascular Event Classification in 1,041 Incident Dialysis Patients.
The numbers of each cerebrovascular event, including recurrent events, are shown. Event types were determined from ICD-9 codes and chart abstraction. Ischemic stroke subtypes were defined using the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification system by two abstractors.
CEA: Carotid endarterectomy
USRDS: U.S. Renal Data System
*for 165 patients; 35 had repeated events
†No statistically significant differences from non-abstracted patients with regard to demographics, comorbid conditions, or baseline laboratory results
The majority of the 200 total cerebrovascular events were ischemic strokes. Of the 95 (62%) ischemic strokes available for chart abstraction, the largest proportion of events was cardioembolic in origin at 28%, although other ischemic stroke subtypes were also common. Of the 11 CEA’s available for chart abstraction, 55% were preceded by symptoms of stroke or transient ischemic attack, while the remainder were performed for intraluminal stenosis that was greater than 60% but asymptomatic. The median stenosis measured by ultrasonagraphy or angiography was 90% (range 70–99%). The subset of patients with available medical charts for chart abstraction did not differ significantly by demographics, clinical characteristics or comorbid conditions compared to those patients without available medical records (data not shown).
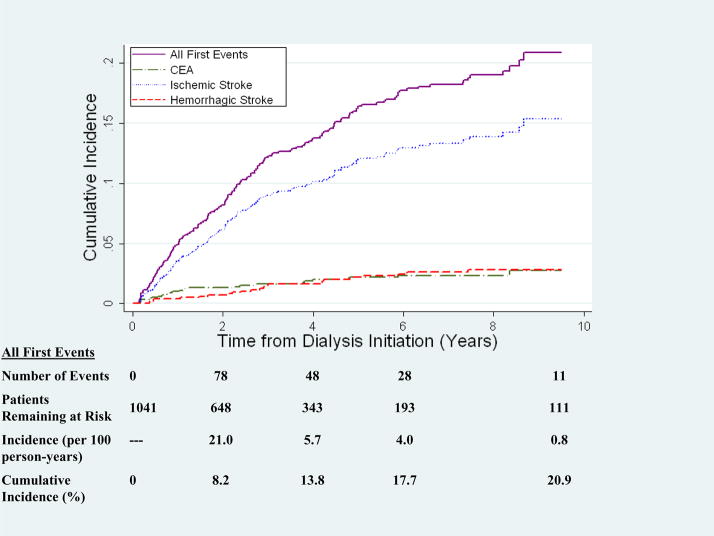
Figure 2 shows the cumulative incidence of first cerebrovascular event type accounting for the competing risk of non-stroke death. Twenty one percent of patients experienced a cerebrovascular event by the end of the study, with 15% of patients experiencing an ischemic stroke, 3% a hemorrhagic stroke, and 3% a CEA. The overall incidence rate of cerebrovascular events was 4.9 per 100 person-years (95% confidence interval (CI) 4.2–5.7 per 100 person-years). While the incidence rate for cerebrovascular events was highest in the first two years after dialysis initiation at 20.9 events per 100 person-years, the majority of incident cerebrovascular events occurred after being on dialysis for over two years. Ischemic stroke accounted for the majority of events at all time points. The incidence across time was similar for CEA and hemorrhagic stroke.
Figure 2. Cumulative Incidence of Cerebrovascular Events in Incident Dialysis Patients.
The cumulative incidence accounts for the competing risk of death from causes other than stroke.
CEA: Carotid Endarterectomy
Nineteen of the 176 strokes (10%) occurred in the setting of a hospitalization for acute myocardial infarction (8) or a CVD procedure (3 coronary artery bypass surgeries, 1 percutaneous transluminal coronary angioplasty, 4 non-traumatic amputations, 2 peripheral bypass surgeries, and 2 carotid endarterectomies). For the 90 abstracted strokes that occurred in hemodialysis patients, 10 occurred during the dialysis session, 17 occurred on the same day as the dialysis procedure, 35 occurred on an intradialytic day[ND1], and 28 occurred at a remote indeterminate time point from last dialysis.
Table 3 shows the presentation and follow-up characteristics of strokes in CHOICE. Overall, the time from stroke symptom onset to presentation was long (median of 8.5 hours [25th and 75th percentiles: 1, 42 hours]), but shortest for cardioembolic and hemorrhagic causes of stroke (median times of 2.5 hours). Stroke from multiple causes and from small-vessel occlusion had the longest time to presentation (medians of 13 and 18 hours, respectively). The median length of hospital stay for all events was six days. Stroke from small-vessel occlusion and hemorrhagic stroke had the shortest lengths of stay at median 3.5 days, while strokes from cardioembolism and large artery atherosclerosis had the longest lengths of stay, at 9 and 10 days, respectively. Thirty-five percent of all events were fatal; 28% of ischemic strokes were fatal, while 90% of hemorrhagic strokes were fatal. Overall, 56% of the patients who suffered a stroke were able to be discharged to home or acute rehabilitation, with the best recovery seen in those with small-vessel occlusion (78% discharge rate). On the other hand, only 10% of patients with hemorrhagic stroke were able to be discharged to home or acute rehabilitation. Baseline dialysis modality was not associated with significant differences in stroke etiologic subtype (p=0.6), time from symptom onset to presentation (p=0.09), length of hospital stay (p=0.2), or discharge location (p=0.5).
Table 3.
Stroke Presentation and Follow-Up Characteristics in Incident Dialysis Patients
| Median Time from Symptoms to Presentation (hours (25th and 75th percentiles)) | Median Length of Hospital Stay (days (25th and 75th percentiles)) | Case-Fatality Percentage (%) | “Successful Recovery” (% Discharged to Home or Acute Rehabilitation) | |
|---|---|---|---|---|
| Stroke Overall (n=105) | 8.5 (1, 42) | 6 (3, 13) | 35 | 56 |
| Ischemic Stroke (n=95) | 11 (1, 48) | 7 (4, 13) | 28 | 54 |
| Large Artery | 7.5 (3, 13) | 10.5 (5, 14) | 29 | 56 |
| Cardioembolism | 2.5 (1, 16) | 9 (6, 17) | 36 | 54 |
| Small-Vessel Occlusion | 18 (6, 48) | 3.5 (3, 7) | 17 | 78 |
| Multiple Causes | 13 (1, 72) | 7 (4, 15) | 41 | 41 |
| Other/Undetermined | 4 (1, 48) | 6 (3, 13) | 19 | 70 |
| Hemorrhagic Stroke (n=10) | 2.5 (1, 19) | 3.5 (1, 9) | 90 | 10 |
Data from strokes that were chart-abstracted
DISCUSSION
In this national prospective cohort study of patients initiating dialysis for end-stage renal disease, cerebrovascular events including fatal and non-fatal clinical stroke and carotid endarterectomy occurred ten times more frequently than in the general population,8, 17 with an incidence rate of 4.9 per 100 person years. The majority of events were related to ischemic stroke, with cardioembolic stroke being the most common form of ischemic stroke among dialysis patients. To our knowledge, this is one of the first studies to classify ischemic stroke in dialysis patients into etiologic subtypes based on the TOAST criteria.12 This allows us to potentially alter our stroke prevention strategies from those used in the general population[ND2]. We found that dialysis patients with stroke present rather late after symptom onset, leaving little room for early interventions. Dialysis patients also have high fatality and low recovery rates. Dialysis patients and their families may need better education about stroke warning signs and symptoms and encouragement to bring these symptoms to their providers’ attention quickly.
We found that ischemic stroke was more common than hemorrhagic stroke in our national US dialysis cohort. This is consistent with a prior study relying on national administrative data.2 Few other studies have characterized stroke types except for a Japanese cohort of 151 patients, in whom hemorrhagic stroke was the most common type of stroke.18 Causes of hemorrhagic stroke may differ from ischemic stroke on long-term dialysis and thus acquired risk factors could account for this later hemorrhagic stroke risk. Reasons could include excess vascular calcification and stiffness,19, 20 leading to worsening hypertension. This, combined with the use of anticoagulation on dialysis, could increase hemorrhagic stroke.
For ischemic stroke, cardioembolism was the most common subtype, though all ischemic stroke subtypes were well represented. In addition to preventing vascular calcification and arterial stiffness, treatment of underlying cardiac disease at dialysis initiation may mitigate future stroke risk in this high-risk population. The use of aspirin in dialysis patients has been associated with a reduced risk of stroke, but the overall strength of this association was modest, as noted in the international Dialysis Outcomes and Practice Patterns Study (DOPPS).21 Baseline cardioembolism risk factors in our study, such as arrhythmias, left ventricular hypertrophy, valvular disease, and congestive heart failure, were not significantly different between individuals that experienced a cerebrovascular event versus those that did not in our cohort, suggesting these aspects may not be correctly identified in dialysis patients. Measurement of cardiac function by echocardiography has been suggested for all patients initiating dialysis22 because of its more accurate assessment than physical examination and chest radiography in identifying cardiac dysfunction and valvular disease. Furthermore, arrhythmias are common among dialysis patients and early recognition and treatment of these arrhythmias may also reduce the risk of stroke. The use of other prevention measures, such as statins, had not been associated with a decreased risk of stroke in prevalent diabetic hemodialysis patients in the 4D (Die Deutsche Diabetes Dialyse) study.23 Whether similar findings hold for incident dialysis patients is unclear.
The median time to presentation was over 8 hours, which is much longer than that observed in a systematic review of the literature on all strokes,24 signifying symptoms of stroke may be missed by providers and/or patients. This is especially problematic since patients on hemodialysis have regular access to the health-care system. With this delay in presentation, any benefit from earlier interventions in this population such as thrombolytic use25 may not be practiced, thus worsening mortality and morbidity after stroke. Improved education about symptoms of transient ischemic attack or stroke itself, such as numbness, weakness, confusion, or difficulty speaking should be given to dialysis patients and their families. One might suspect that stroke is more common during or immediately after a dialysis session due to changes in cerebral blood flow.6 In a study by Toyoda, 34% of ischemic brain infarcts occurred within 30 minutes of the dialysis procedure.18 Thirty percent of abstracted strokes in this study occurred either during the dialysis session or several hours later, though this timing was not statistically different from events that occurred on an intradialytic[ND3] day. A larger study may need to be undertaken to observe any temporal trends.26
Outcomes after stroke, especially hemorrhagic stroke, were poor with a high case-fatality and low “successful recovery” rate. Based on this study, for every 100 dialysis patients who have a stroke, thirty-five of them will die within thirty days, and only fifty-six of the one hundred patients will be able to go home or to an acute rehabilitation facility. While this low “successful recovery” rate is similar to that found in an earlier single-center cohort of prevalent hemodialysis patients followed in New York,27 it is inferior to the 10% adjusted stroke case-fatality rate seen in the Atherosclerosis Risk in Communities (ARIC) cohort28 and the 20% of patients requiring institutional care in the general population.28 This likely reflects the comorbid conditions that our patients on dialysis accumulated before they experienced a stroke. The overall length of hospital stay, surprisingly, was similar to that observed in the general population using the National Hospital Discharge Survey.29 This implies that practice patterns of post-stroke care, including evaluation, management, and disposition, may be similar across populations.
As a national prospective cohort study with characteristics similar to the 1997 US dialysis population, the results of this study may be generalizable to the population of patients initiating dialysis. The follow-up of up to 9.5 years in this study also allowed for evaluation of changing stroke etiologies over time on dialysis. Our ability to have such lengthy follow-up with well-described characteristics and events is a unique aspect of this study.
This study has some limitations which deserve mention. We had a relatively small number of cerebrovascular events, which limited our ability to evaluate specific risk factors for stroke subtypes. We also did not have medical records on all stroke events to classify stroke; however, the subset of the population with medical records of the stroke event did not differ from those with no medical record. In the cases where a chart was not available, the use of administrative data might not necessarily reflect an admission for an acute cerebrovascular event. In addition, CEA could potentially involve a referral bias and thus may not represent an acute event; however, the CEA events which we were able to abstract involved symptomatic disease or significant intraluminal stenosis suggesting these events were indicative of clinically meaningful cerebrovascular disease.
We conclude that cerebrovascular events are common in patients initiating dialysis. Dialysis-related risk factors for all types of cerebrovascular events may differ by the type of event and timing of event after dialysis initiation. This classification of cerebrovascular event types may help focus our efforts to better treat and prevent recurrence of stroke, thereby improving the prognosis of dialysis patients. Further studies to understand the pathophysiology, prevention, and treatment of cerebrovascular disease in ESRD need to focus on subclinical disease, including cognitive function,30 cerebral white matter changes,31 and subclinical strokes,3 as well as imaging techniques with magnetic resonance imaging to earlier identify cerebrovascular disease.
Acknowledgments
We thank the patients, staff, laboratory, and medical directors of the participating clinics at Dialysis Clinic, Inc (DCI), New Haven CAPD, and St. Raphael’s Hospital who contributed to the study.
We thank members of the Cardiovascular Endpoint Committee: Michael J. Choi, MD; Joseph A. Eustace, MD, MHS; Caroline Fox, MD, MPH; Melanie H. Katzman, MD, MHS; Michael J. Klag, MD, MPH; Yongmei Liu, MD, PhD; J. Craig Longenecker, MD, PhD; Michal Melamed, MD, MHS; Renuka Sothinathan, MD, MHS; Richard M. Ugarte, MD, MHS and Gayanne Yenokian, MD.
The manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official view of National Center for Research Resources (NCRR) or National Institutes of Health (NIH). Some of the data reported here have been supplied by the US Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Support: Dr Sozio was supported by KL2RR025006 from NCRR, a component of NIH, and the NIH Roadmap for Medical Research. Dr Coresh was supported by grants R21DK067651 and U01DK067651, Dr Powe was supported by grant K24DK002643, and Dr Parekh was supported by grants U01DK057304 and R01DK072367 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). CHOICE was supported by grant RO1HL62985 from the National Heart, Lung, and Blood Institute, NIDDK grant RO1DK059616, and grant R01HS008365 from the Agency for Health Care Research and Quality.
Footnotes
N section (please place after received/accepted dates line): Because the Editor-in-Chief recused himself from consideration of this manuscript, the Deputy Editor (Daniel E. Weiner, MD, MS) served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Editorial Policies section of the AJKD website.
Financial Disclosure: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Renal Data System. USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2007. [Google Scholar]
- 2.Seliger SL, Gillen DL, Longstreth WT, Jr, et al. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64(2):603. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 3.Nakatani T, Naganuma T, Uchida J, et al. Silent cerebral infarction in hemodialysis patients. Am J Nephrol. 2003;23(2):86. doi: 10.1159/000068034. [DOI] [PubMed] [Google Scholar]
- 4.Abramson JL, Jurkovitz CT, Vaccarino V, et al. Chronic kidney disease, anemia, and incident stroke in a middle-aged, community-based population: the ARIC Study. Kidney Int. 2003;64(2):610. doi: 10.1046/j.1523-1755.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 5.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 6.Ishida I, Hirakata H, Sugimori H, et al. Hemodialysis causes severe orthostatic reduction in cerebral blood flow velocity in diabetic patients. Am J Kidney Dis. 1999;34(6):1096. doi: 10.1016/s0272-6386(99)70016-8. [DOI] [PubMed] [Google Scholar]
- 7.Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 8.Feigin VL, Lawes CM, Bennett DA, et al. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2(1):43. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 9.Powe NR, Klag MJ, Sadler JH, et al. Choices for Healthy Outcomes In Caring for End Stage Renal Disease. Seminars in Dialysis. 1996;9(1):9. [Google Scholar]
- 10.Tracy RP, Lemaitre RN, Psaty BM, et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997;17(6):1121. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 11.Rocco MV, Yan G, Gassman J, et al. Comparison of causes of death using HEMO Study and HCFA end-stage renal disease death notification classification systems. The National Institutes of Health-funded Hemodialysis. Health Care Financing Administration. Am J Kidney Dis. 2002;39(1):146. doi: 10.1053/ajkd.2002.29905. [DOI] [PubMed] [Google Scholar]
- 12.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 13.Athienites NV, Miskulin DC, Fernandez G, et al. Comorbidity assessment in hemodialysis and peritoneal dialysis using the index of coexistent disease. Semin Dial. 2000;13(5):320. doi: 10.1046/j.1525-139x.2000.00095.x. [DOI] [PubMed] [Google Scholar]
- 14.Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. The Stata Journal. 2004;4(2):103. [Google Scholar]
- 15.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524. [PubMed] [Google Scholar]
- 16.Liu Y, Coresh J, Eustace JA, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. 2004;291(4):451. doi: 10.1001/jama.291.4.451. [DOI] [PubMed] [Google Scholar]
- 17.Rothwell PM, Coull AJ, Silver LE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366(9499):1773. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 18.Toyoda K, Fujii K, Fujimi S, et al. Stroke in patients on maintenance hemodialysis: a 22-year single-center study. Am J Kidney Dis. 2005;45(6):1058. doi: 10.1053/j.ajkd.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 19.Blacher J, Guerin AP, Pannier B, et al. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99(18):2434. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 20.Sigrist M, Bungay P, Taal MW, et al. Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol Dial Transplant. 2006;21(3):707. doi: 10.1093/ndt/gfi236. [DOI] [PubMed] [Google Scholar]
- 21.Ethier J, Bragg-Gresham JL, Piera L, et al. Aspirin prescription and outcomes in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2007;50(4):602. doi: 10.1053/j.ajkd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1. [PubMed] [Google Scholar]
- 23.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 24.Evenson KR, Rosamond WD, Morris DL. Prehospital and in-hospital delays in acute stroke care. Neuroepidemiology. 2001;20(2):65. doi: 10.1159/000054763. [DOI] [PubMed] [Google Scholar]
- 25.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 26.Bleyer AJ, Russell GB, Satko SG. Sudden and cardiac death rates in hemodialysis patients. Kidney Int. 1999;55(4):1553. doi: 10.1046/j.1523-1755.1999.00391.x. [DOI] [PubMed] [Google Scholar]
- 27.Mattana J, Effiong C, Gooneratne R, et al. Outcome of stroke in patients undergoing hemodialysis. Arch Intern Med. 1998;158(5):537. doi: 10.1001/archinte.158.5.537. [DOI] [PubMed] [Google Scholar]
- 28.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 29.DeFrances CJ, Hall MJ. National Hospital Discharge Survey. Adv Data. 2005;385:1. 2007. [PubMed] [Google Scholar]
- 30.Pereira AA, Weiner DE, Scott T, et al. Subcortical cognitive impairment in dialysis patients. Hemodial Int. 2007;11(3):309. doi: 10.1111/j.1542-4758.2007.00185.x. [DOI] [PubMed] [Google Scholar]
- 31.Savazzi GM, Cusmano F, Musini S. Cerebral imaging changes in patients with chronic renal failure treated conservatively or in hemodialysis. Nephron. 2001;89(1):31. doi: 10.1159/000046040. [DOI] [PubMed] [Google Scholar]