Abstract
Identification of novel and selective anticancer agents remains an important and challenging goal in pharmacological research. The indole nucleus, frequently encountered as a molecular fragment in natural products and pharmaceutically active compounds, was employed as the initial building block for the synthesis of a series of pyrazino[1,2-a]indoles 1a–k, variably substituted at the 6, 7, 8 and 9-positions. Compound 1e, bearing the methoxy group at the 8-position of the pyrazino[1,2-a]indole nucleus was identified as a novel potent antiproliferative agent against the human chronic myelogenous leukemia K562 cell line, but it was much less active against several other cancer cell lines. Comparison of positional isomers indicated that moving the methoxy group from the 8- to the 7- or 6-position, to furnish compounds 1f and 1g, respectively, yielded inactive compounds. The analysis of structure-activity relationships observed in the series of investigated compounds may represent the basis for the design of more active molecules.
Keywords: Pyrazino[1,2-a]indole; Human leukemia K562 cell line; Anticancer agents
1. INTRODUCTION
Cancer is a major worldwide problem and is the second leading cause of mortality in developed countries [1]. Since many of the current treatments have problems with toxicity and drug resistance, there is a strong demand for the discovery and development of effective new cancer therapies [2]. Cytotoxic drugs continue to play a major role in cancer therapy. Searching for antineoplastic agents with improved selectivity towards malignant cells remains, therefore, a central task for drug development [3]. In particular, great efforts have been made in past years in the search of new compounds for the treatment of leukemias [4]. Leukemia therapy with anticancer compounds is based on cell growth inhibition, induction of cell death through apoptosis or of leukemic blast differentiation. Polycyclic nitrogen heterocycles with a planar structure, such as acridine, phenanthridine and actinomycin derivatives, represent pharmacophores for several classes of antitumor agents [5]. Although the indole ring is found in a wide range of biologically active compounds and is frequently condensed with various heterocycles [6], the synthesis and biological evaluation of the pyrazino[1,2-a]indole skeleton has thus far attracted limited attention.
The pyrazino[1,2-a]indole nucleus was previously prepared by flash vacuum pyrolysis starting from 2-benzyl- pyrazine-N-1-oxide [7]. Although this latter compound was obtained by oxidation of the corresponding pyrazine in satisfactory yield, the reaction gave two isomeric N-1 and N-4 pyrazine N-oxides, and separation of the desired N-1-oxide from the isomeric N-4-oxide was necessary.
As a part of our continuing search for nitrogen heterocycles with an indole moiety that have antineoplastic activity, we developed a short and efficient synthetic approach for the preparation of a series of compounds characterized by the presence of the pyrazino[1,2-a]indole skeleton. We evaluated these compounds for their antiproliferative activity against the human chronic myelogenous leukemia K562 cell line. We will next undertake studies directed at elucidating the mechanism of action of this new class of compounds.
Structure-activity relationships (SAR) were examined with various substitutions at the 6, 7, 8 and 9-positions of the tricyclic pyrazino[1,2-a]indole pharmacophore. Besides hydrogen (compound 1a), in order to define the structural requirements for antiproliferative activity, we first evaluated the effects of electron-withdrawing (F and Cl) and electron-donating (Me and MeO) substituents at the 8-position of the pyrazino[1,2-a]indole nucleus (1b–e) and position effects with three methoxyl group isomers (1e–g). By the synthesis of compounds 1h–k, the effects of two (1h–i) or three (1j–k) methoxy groups at the 6, 7 and 8 positions of the pyrazino[1,2-a]indole moiety were also studied.
2. CHEMISTRY
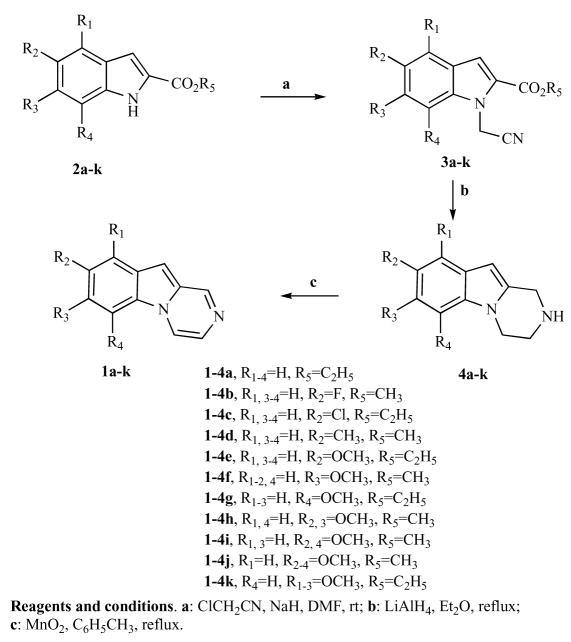
Pyrazino[1,2-a]indoles 1a–k were synthesized following the strategy reported in the Scheme 1.
Scheme 1.
Synthesis of compounds 1a–k.
Condensation of 1H-indole-2-carboxylates 2a–k [8] with chloroacetonitrile in the presence of sodium hydride gave the corresponding 1-cyanomethyl-indole-2-carboxylates 3a–k in good yields (60–75%) [9, 10]. The nitriles 3a–k were transformed by reductive cyclization with lithium aluminium hydride in dry ethyl ether into the desired 1, 2, 3, 4-tetrahydropyrazino[1,2-a]indoles 4a–k in acceptable yields. The subsequent oxidation of 4a–k by treatment with manganese dioxide (MnO2) in toluene under reflux, furnished the pyrazino[1,2-a]indole 1a–k in acceptable yields.
3. RESULTS AND DISCUSSION
Table 1 summarizes the effects of substituted and unsubstituted pyrazino[1,2-a]indoles 1a–k on the growth of human chronic myelogenous K562 leukemia cells. The K562 cell line was used for initial compound screening because of its rapid proliferation, high sensitivity to standard anticancer agents and in order to determine whether these compounds had activity against human transformed cells [11, 12]. With the exception of 1e, all compounds had IC50’s over 35 μM. Incubation of K562 cells with 0.0001% (v/v) DMSO showed that the vehicle did not affect cell proliferation at the concentration used in the experiments presented in Table 1.
Table 1.
In vitro Inhibitory Effects of Compounds 1a–k Against Proliferation of Human K562 Leukemia Cell Lines
 | |||||
|---|---|---|---|---|---|
| Compd | R1 | R2 | R3 | R4 | a IC50 (μM) |
| 1a | H | H | H | H | 161±12 |
| 1b | H | F | H | H | 51±3 |
| 1c | H | Cl | H | H | 70±3 |
| 1d | H | Me | H | H | 80±4 |
| 1e | H | OMe | H | H | 0.07±0.01 |
| 1f | H | H | OMe | H | 67±3 |
| 1g | H | H | H | OMe | 38±3 |
| 1h | H | OMe | OMe | H | 67±4 |
| 1i | H | OMe | H | OMe | 54±2 |
| 1j | H | OMe | OMe | OMe | 83±7 |
| 1k | OMe | OMe | OMe | H | 84±5 |
IC50= compound concentration required to inhibit tumor cell proliferation by 50%. Data are expressed as the mean ± SE from the dose-response curves of at least three independent experiments.
These results indicated that pronounced inhibition of cell growth of this class of compounds required a methoxy group on the pyrazino[1,2-a]indole skeleton at the C-8 position (compound 1e), while only limited activity was observed with the methoxy at the C-6 or C-7 positions (corresponding to derivatives 1g and 1f, respectively). The former compound, however, was almost twice as active as the latter.
SAR studies of 1e indicated that the C-8 methoxy group was critical for activity. Replacing the methoxy group with a methyl (compound 1d) or with electron-withdrawing groups (fluoro and chloro, derivatives 1b and 1c, respectively) led to a dramatic drop in antiproliferative activity. Moreover, there was major loss of activity when additional methoxy groups were added to compound 1e (compounds 1h–i).
Based on the significant antiproliferative activity of compound 1e against the K562 leukemia cell line, this compound was further tested for growth inhibition against murine leukemia (L1210), murine mammary carcinoma (FM3A), human T-lymphoblastoid (Molt/4 and CEM) and human cervical carcinoma (HeLa) cells. Somewhat surprisingly, 1e had little antiproliferative activity against these other cell lines, with IC50 values of 41, 46, 36, 26 and 20 μM, respectively, against the five tumor cell lines.
To investigate whether the antiproliferative activity of compound 1e was caused by an interaction with the microtubule system, compound 1e was evaluated for in vitro inhibition of tubulin polymerization [13, 14]. Compound 1e had no effect on the assembly reaction at concentrations as high as 50μM.
Flow cytometry analysis was performed to determine the effect of 1e on the distribution of K562 cells among the phases of the cell cycle (Table 2). Cells were cultured for 48 h in the presence of compound 1e at the IC50 and examined by flow cytometry [15–17]. The majority of control cells exposed to DMSO of the cell cycle were in the G0–G1 phase (67.8%), with 13.4% of the cells in S phase and 18.8% in G2/M phase. After treatment with 1e for 24 h, there was a partial block of cells in the G2–M phase: 56.4% of cells were in the G0–G1 phase, 14.6% in the S phase and 29% in the G2–M phase.
Table 2.
Effects of Compounds 1e on Cell Cycle Distribution of Treated K562 Cells
| Compd | Cell cycle percentage | ||
|---|---|---|---|
| G0/G1 (%) | S (%) | G2/M (%) | |
| Control | 67.8 | 13.4 | 18.8 |
| 1e | 56.4 | 14.6 | 29.0 |
4. EXPERIMENTAL SECTION
4.1. Chemistry Materials and Methods
1H NMR spectra were recorded on a Bruker AC 200 spectrometer. Chemical shifts (δ) are given in ppm upfield from tetramethylsilane as internal standard, and the spectra were recorded in appropriate deuterated solvents, as indicated. Melting points (mp) were determined on a BuchiTottoli apparatus and are uncorrected. All products reported showed 1H NMR spectra in agreement with the assigned structures. All reactions were carried out under an inert atmosphere of dry nitrogen, unless otherwise indicated. Standard syringe techniques were applied for transferring dry solvents. Reaction courses and product mixtures were routinely monitored by TLC on silica gel (precoated F254 Merck plates) and visualized with aqueous KMnO4. Flash chromatography was performed using 230–400 mesh silica gel and the indicated solvent system. Organic solutions were dried over anhydrous Na2SO4.
4.1.1. General Procedure (A) for the Synthesis of Compounds 3a–k
Sodium hydride (50% dispersion in mineral oil, 744 mg, 15.52 mmol) was slowly added to a solution of 2a–k (5.17 mmol) in dry DMF (7 mL) cooled with an ice bath. The mixture was then stirred for 0.5 h at room temperature, and chloroacetonitrile (1.17 mL, 15.52 mmol) dissolved in DMF (2 mL) was added. The reaction mixture was left at room temperature for 18 h, and ice was added to degrade excess of NaH. The reaction mixture was diluted with ethyl acetate (20 mL), and the organic phase was washed with water (3 × 5 mL) and brine (5 mL), dried over Na2SO4 and concentrated in vacuo. The residue was purified by flash chromatography (ethyl acetate-petroleum ether as eluent) on silica gel.
4.1.2. General Procedure (B) for the Synthesis of Compounds 4a–k
A suspension of 3a–k (11 mmol) in dry ethyl ether (70 mL) was slowly added to a well-stirred suspension of LiAlH4 (1.25 g., 33 mmol) in dry ethyl ether (50 mL) cooled in an ice bath. The mixture was then refluxed for 4 h, and water was added to degrade excess of LiAlH4. The precipitate was removed by filtration through a pad of celite, which was washed three times with ethyl acetate (3 × 20 mL). The combined filtrate was washed with water (30 mL), dried over Na2SO4 and evaporated. The residue was purified by flash chromatography (ethyl acetate-methanol as eluent) on silica gel.
4.1.3. General Procedure (C) for the Synthesis of Compounds 1a–k
A mixture of 4a–k (0.45 mmol), manganese dioxide (800 mg, 9.1 mmol) and toluene (15 mL) was stirred at reflux for 5 h. The insoluble solid was removed by filtration, and the filter was washed with ethyl acetate (3 × 5 mL). The filtrate was concentrated in vacuo. The residue was chromatographed on silica gel.
4.1.3.1. Pyrazino[1,2-a]indole (1a)
Following general procedure C, the crude residue purified by flash chromatography using ethyl acetate as eluent furnished 1a as a yellow solid (54% yield); mp 158–160 °C. 1H-NMR (CDCl3) δ: 7.00 (s, 1H), 7.42 (m 2H), 7.54 (s, 1H), 7.93 (m, 2H), 8.16 (dd, J=5.2 and 2.0 Hz, 1H), 9.01 (d, J=2.0 Hz, 1H).
4.1.3.2. 8-Fluoro-pyrazino[1,2-a]indole (1b)
Following general procedure C, the crude residue purified by flash chromatography using ethyl acetate-petroleum ether 8:2 as eluent furnished 1b as a yellow solid (48% yield); mp 133–135 °C. 1H-NMR (CDCl3) δ: 6.98 (s, 1H), 7.18 (m, 1H), 7.52 (dd, J=9.2 and 2.4 Hz, 1H), 7.59 (d, J=4.8 Hz, 1H), 7.87 (dd, J=9.2 and 4.4 Hz, 1H), 8.14 (m, 1H), 9.01 (d, J=1.0 Hz, 1H).
4.1.3.3. 8-Chloro-pyrazino[1,2-a]indole (1c)
Following general procedure C, the crude residue purified by flash chromatography using ethyl acetate-petroleum ether 9:1 as eluent furnished 1c as a yellow solid (49% yield); mp 136–138 °C. 1H-NMR (CDCl3) δ: 6.95 (s, 1H), 7.36 (dd, J=8.8 and 1.6 Hz, 1H), 7.59 (d, J=5.2 Hz, 1H), 7.87 (m, 2H), 8.1 (d, J=4.8 Hz, 1H), 9.01 (d, J=1.2 Hz, 1H).
4.1.3.4. 8-Methyl-pyrazino[1,2-a]indole (1d)
Following general procedure C, the crude residue purified by flash chromatography using ethyl acetate as eluent furnished 1d as a brown solid (51% yield); mp 148–150 °C. 1H-NMR (CDCl3) δ: 2.55 (s, 3H), 6.94 (s, 1H), 7.27 (d, J=1.5 Hz, 1H), 7.50 (d, J=4.8 Hz, 1H), 7.68 (s, 1H), 7.82 (d, J=8.8 Hz, 1H), 8.1 (d, J=4.8 Hz, 1H), 8.98 (d, J=1.6 Hz, 1H).
4.1.3.5. 8-Methoxy-pyrazino[1,2-a]indole (1e)
Following general procedure C, the crude residue purified by flash chromatography using ethyl acetate as eluent furnished 1e as a yellow solid (44% yield); mp 162–164 °C. 1H-NMR (CDCl3) δ: 3.92 (s, 3H), 6.95 (s, 1H), 7.09 (dd, J=9.2 and 2.8 Hz, 1H), 7.22 (d, J=2.4 Hz, 1H), 7.54 (d, J=4.8 Hz, 1H), 7.84 (d, J=9.2 Hz, 1H), 8.12 (d, J=4.8 Hz, 1H), 8.97 (d, J=1.2 Hz, 1H).
4.1.3.6. 7-Methoxy-pyrazino[1,2-a]indole (1f)
Following general procedure C, the crude residue purified by flash chromatography using ethyl acetate as eluent furnished 1f as a yellow solid (45% yield); mp 118–120 °C.1H-NMR (CDCl3) δ: 3.88 (s, 3H), 6.94 (s, 1H), 7.22 (m, 2H), 7.47 (d, J=5.0 Hz, 1H), 7.84 (d, J=8.8 Hz, 1H), 8.13 (d, J=4.6 Hz, 1H), 8.99 (d, J=1.2 Hz, 1H).
4.1.3.7. 6-Methoxy-pyrazino[1,2-a]indole (1g)
Following general procedure C, the crude residue purified by flash chromatography using ethyl acetate as eluent furnished 1g as a brown oil (44% yield). 1H-NMR (CDCl3) δ: 4.09 (s, 3H), 6.76 (d, J=7.0 Hz, 1H), 6.96 (s, 1H), 7.22 (m, 2H), 7.49 (m, 2H), 8.83 (d, J=4.8 Hz, 1H), 8.96 (d, J=1.2 Hz, 1H).
4.1.3.8. 7,8-Dimethoxy-pyrazino[1,2-a]indole (1h)
Following general procedure C, the crude residue purified by flash chromatography using ethyl acetate-methanol 9.5-0.5 as eluent furnished 1h as a yellow solid (42% yield); mp 156–158 °C. 1H-NMR (CDCl3)δ: 4.00 (s, 3H), 4.03 (s, 3H), 7.22 (m, 3H), 7.52 (d, J=5.2 Hz, 1H), 8.01 (d, J=5.2 Hz, 1H), 8.91 (d, J=1.4 Hz, 1H).
4.1.3.9. 6,8-Dimethoxy-pyrazino[1,2-a]indole (1i)
Following general procedure C, the crude residue purified by flash chromatography using ethyl acetate as eluent furnished 1i as a yellow solid (46% yield); mp 133–135 °C. 1H-NMR (CDCl3) δ 3.90 (s, 3H), 4.05 (s, 3H), 6.46 (d, J=2.0 Hz, 1H), 6.78 (d, J=1.8 Hz, 1H), 6.96 (s, 1H), 7.47 (d, J=5.4 Hz, 1H), 8.75 (d, J=4.4 Hz, 1H), 8.91 (d, J=1.4 Hz, 1H).
4.1.3.10. 6,7,8-Trimethoxy-pyrazino[1,2-a]indole (1j)
Following general procedure C, the crude residue purified by flash chromatography using ethyl acetate as eluent furnished 1j as a yellow solid (46% yield); mp 92–94 °C. 1H-NMR (CDCl3) δ: 3.96 (s, 3H), 3.97 (s, 3H), 4.19 (s, 3H), 6.92 (s, 1H), 6.97(s, 1H), 7.45 (d, J=5.2 Hz, 1H), 8.68 (d, J=4.8 Hz, 1H), 8.92 (d, J=1.4 Hz, 1H).
4.1.3.11. 7,8,9-Trimethoxy-pyrazino[1,2-a]indole (1k)
Following general procedure C, the crude residue purified by flash chromatography using ethyl acetate-methanol 9.5-0.5 as eluent furnished 1k as a brown oil (35% yield). 1H-NMR (CDCl3) δ: 3.94 (s, 3H), 4.00 (s, 3H), 4.17 (s, 3H), 7.00 (s, 1H), 7.08 (s, 1H), 7.46 (d, J=5.2 Hz, 1H), 7.97 (d, J=5.0 Hz, 1H), 8.92 (d, J=1.4 Hz, 1H).
4.2. Biological Assays
4.2.1. Cell Proliferation Analysis
For the evaluation of the effects on cell proliferation, K562 cells were seeded at 20,000 cells/mL in 24-well culture plates. Cell growth was studied by determining the cell number/mL after three days of growth. Cells were counted with a ZF Coulter Counter (Beckman Coulter Electronics, Hialeah, Fla., USA).
Murine leukemia L1210, murine mammary carcinoma FM3A, human T-lymphocyte Molt 4 and CEM and human cervix carcinoma (HeLa) cells were suspended at 300,000–500,000 cells/mL of culture medium, and 100 μL of a cell suspension was added to 100 μL of an appropriate dilution of the test compounds in wells of 96-well microtiter plates. After incubation at 37 °C for two (L1210 and FM3A) or three (Molt 4, CEM and HeLa) days, cell number was determined using a Coulter counter. The IC50 was defined as the compound concentration required to inhibit cell proliferation by 50%.
4.2.2. Effects on Tubulin Polymerization
To evaluate the effect of the compounds on tubulin assembly in vitro [16], varying concentrations were preincubated with 10 μM tubulin in glutamate buffer at 30 °C and then cooled to 0 °C. After addition of GTP, the mixtures were transferred to 0 °C cuvettes in a recording spectrophotometer and warmed to 30 °C, and the assembly of tubulin was observed turbidimetrically. The IC50 was defined as the compound concentration that inhibited the extent of assembly by 50% after 20 min at 30 °C.
4.2.3. Cell Cycle Analysis
For flow cytometric analysis of DNA content, 5×105 K562 cells were centrifuged, fixed in ice-cold ethanol (70%), treated with lysis buffer containing RNAse A, and stained with propidium iodide. Samples were analyzed on a Becton Coulter Epics XL-MCL flow cytometer. For cell cycle analysis, DNA histograms were analyzed using MultiCycle® for Windows (Phoenix Flow Systems, San Diego, CA).
5. CONCLUSIONS
In conclusion, we have discovered and explored the SAR of a new series of compounds characterized by the presence of a pyrazino[1,2-a]indole framework with one, two or three methoxy groups. We found that compound 1e, bearing a methoxy group at the 8-position of the pyrazino[1,2-a]indole system has significant growth inhibitory activity against the K562 cell line. Moreover, it was highly selective for K562 cells in its cytostatic action. From these data, we deduce that methoxy substitution and location plays an important role in affecting antiproliferative activity. Experiments performed to identify a possible interaction of 1e with microtubules failed to demonstrate an interaction with this cell constituent. Flow cytometric analysis on K562 cells indicated that 1e can induce cell cycle arrest in the G2-M phase. These results encourage us to explore the activity of 1e in additional in vitro assay systems to gain insights into its mechanisms of action and to prepare additional analogs in hope of further elucidating important SAR features and identifying other active congeners.
Chart 1.
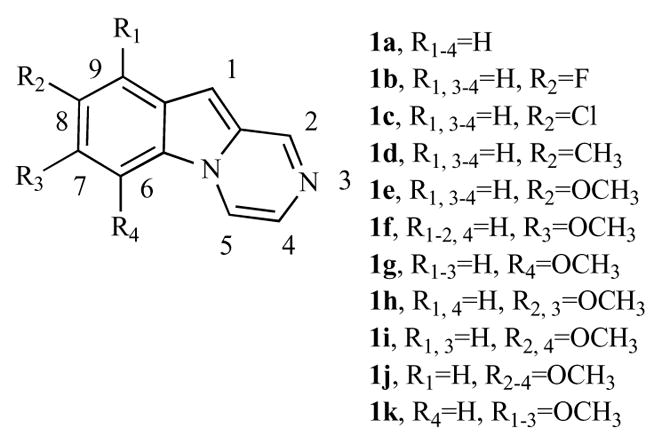
Chemical structures of pyrazino[1,2-a]indoles with general structure 1a–k.
Acknowledgments
RG is supported by grants from the AIRC and the“Fondazione Cassa di Risparmio di Padova e Rovigo”. The financial support (GOA 05/19) was gratefully acknowledged. We thanks Mrs. Lizette van Berckelaer for excellent technical assistance.
References
- 1.a) Adjei AA, Rowinsky EK. Novel anticancer agents in clinical development. Cancer Biol Ther. 2003;2:S5–15. [PubMed] [Google Scholar]; b) Neidle S, Thurston DE. Chemical approaches to the discovery and development of cancer therapies. Nat Rev Cancer. 2005;5:285–296. doi: 10.1038/nrc1587. [DOI] [PubMed] [Google Scholar]
- 2.a) Kamb A, Wee S, Lengauer C. Why is cancer drug discovery so difficult? Nat Rev Drug Discov. 2007;6:115–120. doi: 10.1038/nrd2155. [DOI] [PubMed] [Google Scholar]; b) Pujol MD, Romero M, Sanchez I. Synthesis and biological activity of a new class of deoxygenated anticancer agents. Curr Med Chem Anticancer Agents. 2005;5:215–237. doi: 10.2174/1568011053765930. [DOI] [PubMed] [Google Scholar]
- 3.Alessio E, Mestroni G, Bergamo A, Sava G. Ruthenium antimetastatic agents. Curr Top Med Chem. 2004;4:1525–1535. doi: 10.2174/1568026043387421. [DOI] [PubMed] [Google Scholar]
- 4.Schuler D, Szende B. Apoptosis in acute leukemia. Leukemia Res. 2004;28:661–666. doi: 10.1016/j.leukres.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 5.Wakelin LPG, Waring MJ. In: Comprehensive in Medicinal Chemistry. Sammes PG, editor. Vol. 2. Pergamon; Great Britain: 1990. pp. 702–743. [Google Scholar]
- 6.Brancale A, Silvestri R. Indole, a core nucleus for potent inhibitors of tubulin polymerization. Med Res Rev. 2006;27:209–238. doi: 10.1002/med.20080. [DOI] [PubMed] [Google Scholar]
- 7.Ohsawa A, Kawaguchi T, Igeta H. Application of flash vacuum pyrolysis to the synthesis of 1,2-condensed indoles. Synthesis. 1983:1037–1040. [Google Scholar]
- 8.Rayer SB, Merwade AY, Hendi SB, Basanagoudar LD. Synthesis of 1,2,3,4-tetrahydropyrazino-[1,2-a]indoles and ethyl 1-(2-aminoethyl)indole-2-carboxylates. Indian J Chem. 1989:1065–1068. [Google Scholar]
- 9.Wadia MS, Mali RS, Tilve SG, Yadav VJ. Facile synthesis of ethyl-2-alkylindole-3-carboxylates: reinvestigation of an earlier synthesis of ethyl 3-methylindole-2-carboxylates. Synthesis. 1987:401–404. [Google Scholar]
- 10.For the synthesis of 2a and 2g see: Fagan GP, Chapleo CB, Lane AC, Myers M, Roach AG, Smith CFC, Stillings MR, Welbourn AP. Indoline analogs of idazoxan: potent α2-antagonists and α1-agonists. J Med Chem. 1988;31:944–948. doi: 10.1021/jm00400a009.; for the synthesis of 2b and 2d: Nazare M, Will DW, Matter H, Schreuder H, Ritter K, Urmann M, Essrich M, Bauer A, Wagner M, Czech J, Lorenz M, Laux V, Wehener V. Probing the subpockets of factor Xa reveals two binding modes for inhibitors based on a 2-carboxyindole scaffold: a study combining structure-activity relationship and X-ray crystallography. J Med Chem. 2005;48:4511–4525. doi: 10.1021/jm0490540.; for the synthesis of 2c: Terzioglu N, Rijn R, Bakker RA, Esch IJP, Leurs R. Synthesis and structure-activity relationships of indole and benzoimidazole piperazines as histamine H4 receptor antagonists. Bioorg Med Chem Lett. 2004;14:5251–5256. doi: 10.1016/j.bmcl.2004.08.035.; for the synthesis of 2e: Leon P, Garbay-Jaureguiberry C, Barsi MC, Pecq JB, Le Roques BP. Modulation of the antitumor activity by methyl substitutions in the series of 7H-pyridocarbazole monomers and dimers. J Med Chem. 1987;30:2074–2080. doi: 10.1021/jm00394a024.; for the synthesis of 2f and 2h: Coowar D, Bouissac J, Hanbali M, Paschaki M, Mohier E, Luu B. Effects of indole fatty alcohols on the differentiation of neural stem cell derived neurospheres . J Med Chem. 2004;47:6270–6282. doi: 10.1021/jm0493616.; for the synthesis of 2i: Condie GC, Channon MF, Ivory AJ, Kumar N, Black DC. Regioselective reactivity of some 5,7-dimethoxyindoles. Tetrahedron. 2005;61:4989–5004.; for the synthesis of 2j: Boger DL, Ishizaki T, Zarrinmayeh H, Kitos PA, Suntornwat O. Synthesis and preliminary evaluation of agents incorporating the pharmacophore of the duocarmycin/pyrindamycin alkylation subunit: identification of the CC-1065/duocarmycin common pharmacophore. J Org Chem. 1990;55:4499–4502.
- 11.Suffness M, Douros T. In: Methods of Cancer Research. De Vita VT Jr, Busch H, editors. XVI. Academic Press; New York: 1979. pp. 84–98. Part A. [Google Scholar]
- 12.Lozzio CB, Lozzio BB. Human chronic myelogenous leucemia cell-line with positive Philadelphia chromosome. Blood. 1975;45:321–334. [PubMed] [Google Scholar]
- 13.Hamel E. Evaluation of antimitotic agents by quantitative comparisons of their effects on the polymerization of purified tubulin. Cell Biochem Biophys. 2003;38:1–21. doi: 10.1385/CBB:38:1:1. [DOI] [PubMed] [Google Scholar]
- 14.Verdier-Pinard P, Lai J-Y, Yoo H-D, Yu J, Marquez B, Nagle DG, Nambu M, White JD, Falck JR, Gerwick WH, Day BW, Hamel E. Structure-activity analysis of the interaction of curacin A, the potent colchicine site antimitotic agent, with tubulin and effects of analogs on the growth of MCF-7 breast cancer cells. Mol Pharmacol. 1998;53:62–67. doi: 10.1124/mol.53.1.62. [DOI] [PubMed] [Google Scholar]
- 15.Gambari R, Marks PA, Rifkind RA. Murine erythroleukemia cell differentiation: relationship of globin gene expression and of prolongation of G1 to inducer effects during G1/early S. Proc Natl Acad Sci USA. 1979;76:4511–4515. doi: 10.1073/pnas.76.9.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gambari R, Terada M, Bank A, Rifkind RA, Marks PA. Synthesis of globin mRNA in relation to the cell cycle during induced murine erythroleukemia differentiation. Proc Natl Acad Sci USA. 1978;75:3801–3804. doi: 10.1073/pnas.75.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viola G, Vedaldi D, Dall’Acqua F, Fortunato E, Basso G, Bianchi N, Zuccato C, Borgatti M, Lampronti I, Gambari R. Induction of g-globin mRNA, erythroyd differentiation and apoptosis in UVA-irradiated human erythroyd cells in the presence of furocumarin derivatives. Biochem Pharmacol. 2008;75:810–825. doi: 10.1016/j.bcp.2007.10.007. [DOI] [PubMed] [Google Scholar]