Abstract
The human female reproductive tract (FRT) must balance the requirements of procreation with the demands of protection from pathogen invasion. We hypothesize that the FRT expresses functional pattern recognition receptors (PRRs), including Toll-like Receptors (TLRs) and nucleotide-binding oligomerization domain (NOD) proteins that may mediate these tasks. Expression of PRRs was evaluated in FRT tissues by RT-PCR. PRR function within FRT tissue cells was determined by CXCL8 (IL-8) production in response to treatment with PRR agonists. We now report that TLRs 7–9 are expressed in Fallopian tube, uterine endometrium, cervix and ectocervix, while TLR10 expression is restricted to Fallopian tube. NOD1 and NOD2 and the signal transducer RICK were detected in all FRT tissues. Stimulation of FRT tissue cells with PRR ligands resulted in secretion of CXCL8. Results of these studies indicate that PRR are functionally expressed in FRT tissues, and suggest that these receptors mediate microbial recognition and immune defense in the reproductive tract.
Keywords: Human, Female, Reproductive, TLR, NOD
1. Introduction
The human female reproductive tract (FRT) is charged with simultaneously ensuring maternal immune protection from pathogenic challenge and maintaining an environment compatible with implantation and fetal development. To mediate these disparate tasks, the FRT must recognize and react to a broad diversity of microbial ligands. Two key families of pattern recognition receptors (PRRs), the Toll-like receptors (TLRs) and the nucleotide-binding oligomerization domain (NOD) proteins, bind conserved pathogen associated molecular patterns (PAMPs) synthesized by microbes (Gordon, 2002). We hypothesize that the human FRT functionally expresses PRRs to enable recognition of PAMPs by TLRs and NODs.
To date, at least 10 mammalian TLRs have been cloned (Takeda et al., 2003). PAMPs have been identified for TLRs 1–9, but the ligand for TLR 10 has not yet been determined (Akira and Takeda, 2004). TLRs 1, 2–6 and 10 are localized to the cell surface and detect bacterial ligands, while TLRs 3 and 7–9 are targeted to endosomal membranes and sense endocytosed viral or bacterial DNA (Janeway and Medzhitov, 2002). TLR3 recognizes double-stranded viral RNA; TLRs 7 and 8 detect single-stranded viral RNA; and TLR9 detects microbial CpG DNA (Barton, 2007). Subsequent TLR signal transduction proceeds via two well-defined pathways that are mediated by either the MyD88 or the toll-receptor associated activator of interferon (TRIF) adaptor proteins (Akira and Takeda, 2004). MyD88-dependent signaling is utilized by all TLRs except TLR3, and relies on the IL-1R-associated kinase (IRAK) pathway to propagate downstream signals (Akira and Takeda, 2004). MyD88-independent signaling occurs via TRIF and is employed by TLR3 and TLR4. This pathway signals through the interferon regulatory factor 3 (IRF3) (Miggin and O’Neill, 2006).
In addition to the TLR family of PRRS, NOD receptors also serve as microbial sensors. Unlike TLRs, NODs are located within the cytosolic compartment of host cells (Rosenstiel et al., 2008). NOD1 (which is encoded by the caspase-recruitment domain 4 gene, CARD4) and NOD2 (which is encoded by CARD15) recognize muropeptides derived from the degradation of bacterial peptidoglycan (PGN) (Manon et al., 2007). NOD2 binds muramyl dipeptide (MDP), which is a component of PGN from both gram-negative and gram-positive bacteria (Beynon et al., 2008). MDP is recognized by NOD2 but not TLR2, TLR1/6 or TLR2/6 (Girardin et al., 2003a). In contrast, NOD1 recognizes diaminopimelate-containing PGN, which is restricted to the cell walls of gram-negative bacteria (Girardin et al., 2003a). Downstream signaling following NOD1 and NOD2 recognition of their respective ligands is mediated through association of these receptors with the signaling effector protein RIP-like interacting CLARP kinase (RICK) (Park et al., 2007).
Although TLRs and NODs use different cellular adaptor proteins to activate signal transduction pathways, engagement of TLRs and NODs result in the activation of NFκB and the production of pro-inflammatory chemokines and cytokines (Creagh and O’Neill, 2006). Because of the important role these receptors play in mediating innate immune defense, the goal of this study was to determine the expression and function of TLRs and NODs in tissues of the human FRT. Because we and others have previously documented expression of TLRs 1–6 in tissues of the human female reproductive tract (Fazeli et al., 2005, Hirata et al., 2005, Pioli et al., 2004, Young et al., 2004), we limited our current investigation to characterization of TLRs 7–10 and NOD1 and 2. In this work, we now demonstrate for the first time functional expression of TLRs 7–9 and NODs 1 and 2 in tissues of the human FRT.
2. Materials and Methods
2.1 Reproductive Tract Tissues
Human tissues were obtained immediately after surgery from patients who had given informed consent. Donors ranged in age from 32 to 74, and were treated for a range of gynecologic problems. All tissues used in this study were distal to sites of pathology and were anatomically unaffected by disease. Tissues on ice were transported from the Department of Pathology and procedures to prepare the FRT cells began within 1 hr of surgery. Approval to use human tissues was obtained from the Human Experimentation Committee in accordance with the human experimentation guidelines established by the U.S. Department of Health and Human Services. Matched sets of tissues (Fallopian tube, uterine endometrium, cervix and ectocervix) were compared from 10 donors. The tissue samples used in these studies were whole cross sections of the reproductive tract. A portion of each tissue was used for RNA isolation, and the remaining tissue was digested as described in 2.4 and utilized in functional assays. A summary of the patient population is provided in Table 2.
Table 2.
Summary of Patient Population
| Patient no. | Age | Menstrual Stage | Diagnosis |
|---|---|---|---|
| 3116 | 48 | secretory | fibroids |
| 3219 | 48 | secretory | menorrhagia |
| 3610 | 47 | secretory | fibroids |
| 3645 | 44 | secretory | fibroids |
| 3238 | 46 | proliferative | fibroids |
| 3679 | 32 | proliferative | prolapse |
| 4017 | 42 | proliferative | fibroids |
| 4096 | 48 | proliferative | pelvic mass |
| 3641 | 44 | postmenopausal | fibroids |
| 3929 | 74 | postmenopausal | prolapse |
2.2 RNA Isolation
RNA was isolated from human FRT tissues as previously described (Pioli et al., 2004). Briefly, tissue samples were homogenized using a roto-stator and total RNA was sequentially extracted using Trizol reagent (Invitrogen), followed by purification with RNeasy mini-columns (Qiagen). All RNA samples were treated with DNase to eliminate genomic DNA contamination. Integrity and quantity of isolated RNA was determined using the RNA 6000 Nano LabChip on an Agilent 2100 Bioanalyzer (Agilent Technologies). RNA was extracted from 10 Fallopian tube, uterine endometrial, cervical and ectocervical tissues derived from the patients described in Table 2.
2.3 Reverse Transcription PCR
First strand cDNA was synthesized from 500 ng of template RNA using random hexamers and Superscript II Moloney murine leukemia virus reverse transcriptase (Invitrogen). PCR was performed with primer pairs listed in Table 1, using Taq DNA polymerase (Invitrogen) in a PTC 100 Thermal Cycler (MJ Research). PCR cycling conditions were as follows: 2 minutes of initial denaturation at 95C, followed by 35 cycles of 30s at 94C, 30s at 57C, 45s at 72C and a final extension for 5 minutes at 72C. PCR reactions were carried out in the absence of reverse transcriptase as negative controls. Non-template water controls were also included to ensure lack of reagent DNA contamination. 10 μl of each amplicon was electrophoresed on a 2.5% agarose gel with 0.5% ethidium bromide and photographed under UV light. Primers were designed to specifically amplify each PRR. We further verified the specificity of each amplification by isolating each PCR band and sequencing. All reactions depicted in this manuscript were specific for their intended target.
Table 1.
List of primers used for amplification of PRRs and Signaling Adapters
| Primer | Forward primer 5-3′ | Reverse primer 5-3′ |
|---|---|---|
| TLR7 | ATGGGCAGACCTTGGATCTAA | GGAGTAAATCAAGCCGGTTGT |
| TLR8 | TTGACCCAACTTCGATACCTA | AATGCAATGCCCGTAGAGA |
| TLR9 | GGCCTGCGCTACGTGGAC | CACCAGGTTGTTCCGTGACAG |
| TLR10 | TCACAGGTACACCGGAATGG | ATTCATCTGATGGCCTTACGA |
| RICK | ACGTCTGCAGCCTGGTATAGC | AGGGCATCTAGCGACTGGTTAA |
| NOD1 | CTCGCAGATGCCTACGTGGAC | GGGCATAGCACAGCACGAAC |
| NOD2 | GGTACTGAGGAAGCGAGACTG | CCACGGTGAAAGCGAATG |
2.4 FRT tissue cell isolation and culture
To isolate FRT tissue cells, tissues were minced and treated with an enzyme mixture of collagenase, pancreatin and hyaluronidase for two hours as previously described (White et al., 2000). Single cell suspensions of these digested tissues were obtained by filtering through a 100-μm screen, and cells were incubated overnight in complete RPMI 1640 media supplemented with 10% fetal bovine serum. Cells were cultured in six well tissue culture dishes (Falcon) at a density of 1.5 × 106/ml.
2.5 Limulus amoebocyte lysate (LAL) assay for detection of LPS contamination
To ensure that PRR agonists were not contaminated with LPS, all reagents were tested using the QCL-1000® Chromogenic limulus amoebocyte lysate (LAL) Endpoint assay (Cambrex, Cottonwood, AZ) as previously described (Weaver et al., 2006). Briefly, protein-free, phenol-water-extracted E. coli K235 LPS was used to create a linear standard curve from 10 to 100 pg/ml. LAL reactions were performed at multiple concentrations of agonist within the linear range of the standard curve to rule out product inhibition of the LAL reaction.
2.6 PAMP stimulation of FRT cells
To screen for CXCL8 production, 5 × 106 isolated FRT tissue cells (derived from patients listed in Table 2) were stimulated for 24 hours with the following PRR agonists: imiquimod (10 μg/ml) for TLR7 (Weaver et al., 2006), CL075 (0.4 μM) for TLR8 (Qin et al., 2006), CpG DNA (2 μM) for TLR9 (Weaver et al., 2006), iE-DAP (10 μg/ml) for NOD1 (Chamaillard et al., 2003) and MDP-1 (10 μg/ml) for NOD2 (Girardin et al., 2003b). Supernatants were harvested from these cultures after 0, 2, 4 and 6 hours of treatment for analysis of CXCL8 production by ELISA. Cells were also incubated in the absence of agonist to control for constitutive CXCL8 secretion. Tissues from all three phases (secretory, proliferative and postmenopausal) were incubated with PAMPs and analyzed for CXCL8 production by ELISA. All tissues were responsive to PAMP stimulation, as indicated in Table 4. Figure 3 specifically shows results for tissues in the secretory phase.
Table 4.
CXCL8 production by reproductive tract tissues in response to PRR stimulation
| imiquimod | CL075 | CpG | iE-DAP | MDP-1 | |
|---|---|---|---|---|---|
| Fallopian tube | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 |
| Endometrium | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 |
| Cervix | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 |
| Ectocervix | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 |
Figure 3.
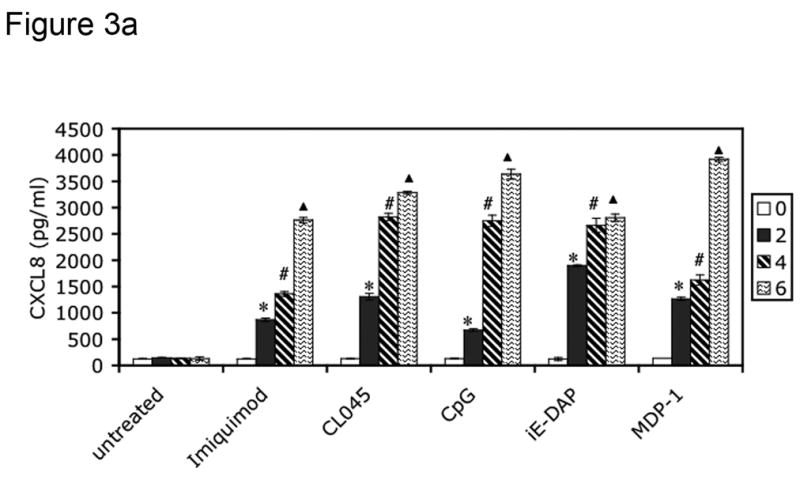
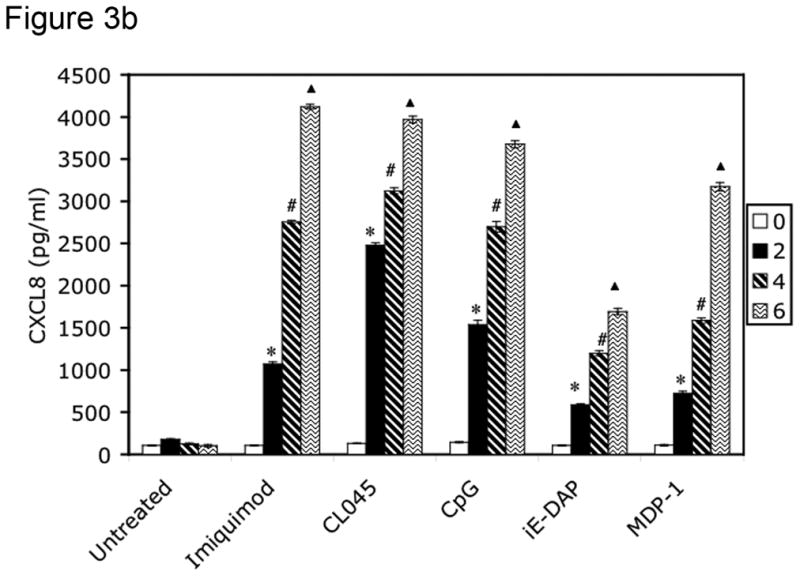
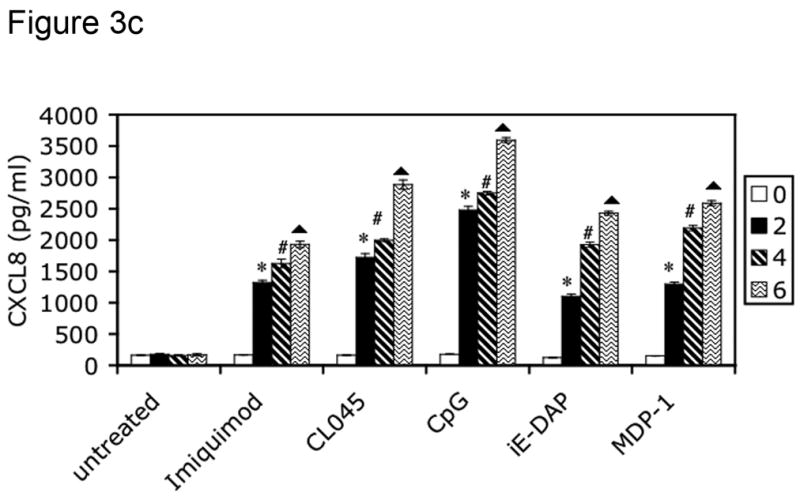
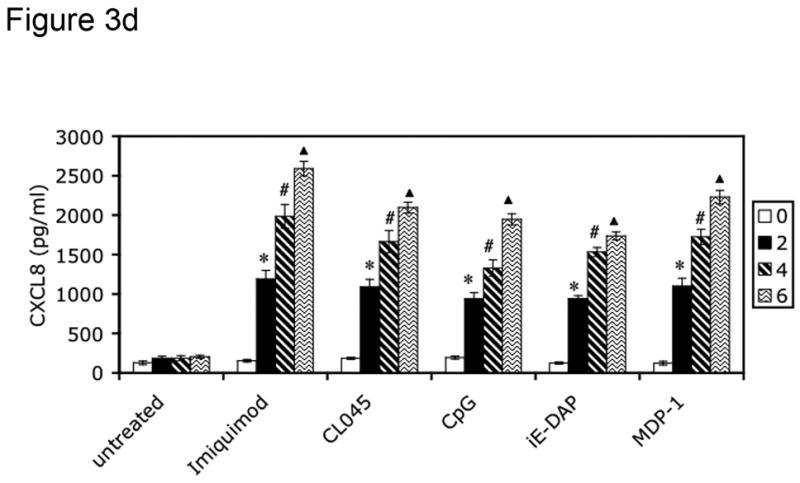
PRRs are functionally expressed in human female reproductive tract tissues. FRT tissue cells were isolated and untreated or incubated with indicated PAMPs for 2, 4 or 6 hours. Following treatment, cell-free supernatants were analyzed for CXCL8 secretion by ELISA. Figure 3a shows CXCL8 secretion from Fallopian tube tissue cells, 3b from uterine endometrial tissue cell, 3c from cervical tissue cells and 3d from ectocervical tissue cells. Results are shown as mean +/− SEM. Statistical significance was achieved at p<0.05. *denotes statistically significant differences in CXCL8 production by tissues following 2 hours of stimulation compared with unstimulated samples, # indicates differences after 4 hours of treatment, and indicates differences following 6 hours of stimulation. Data are representative of three independent experiments for each tissue.
2.7 ELISA
Supernatants were collected from FRT tissue cells, and expression of CXCL8 was quantified using the human CXCL8/IL-8 Quantikine ELISA kit (R and D Systems). Prior to analysis, supernatants were diluted 1:100 in calibrator diluent in accordance with manufacturer’s instructions. The detection limit of this assay is 0.28 pg/ml.
2.8 Statistical Analysis
All experiments were repeated at least three times. Data were analyzed using one-way analysis of variance (ANOVA) with Dunnett’s post test and are represented as mean +/− SEM. Statistical significance was achieved at P<0.05.
3. Results
3.1 Tissues of the human FRT express TLR 7, 8, 9 and 10
In previous work, we demonstrated that TLR 1–6 are constitutively expressed in tissues of the human FRT (Pioli et al., 2004). Although expression of TLRs 7–10 has been investigated in uterine epithelial cells and endometrial tissues (Aflatoonian et al., 2007, Schaefer et al., 2004), expression of TLRs 7–10 in other FRT tissues has not been determined. To assess TLR expression in these tissues, total RNA was isolated from Fallopian tubes, uterine endometrium, cervix and ectocervix, reverse-transcribed with random hexamers, and analyzed by PCR. As demonstrated in Figure 1, mRNA expression of TLRs 7, 8 and 9 was detected in all four FRT tissues. However, although TLR 10 expression was identified in Fallopian tube cells, we failed to detect expression of TLR 10 in uterine endometrium, cervix, and ectocervix (Figure 1, Table 3). RNA from primary human monocytes was analyzed as positive control for TLRs 7–9 (not shown), and from the human B cell lymphoma Ramos cell line as positive control for expression of TLR10 (Figure 1).
Figure 1.
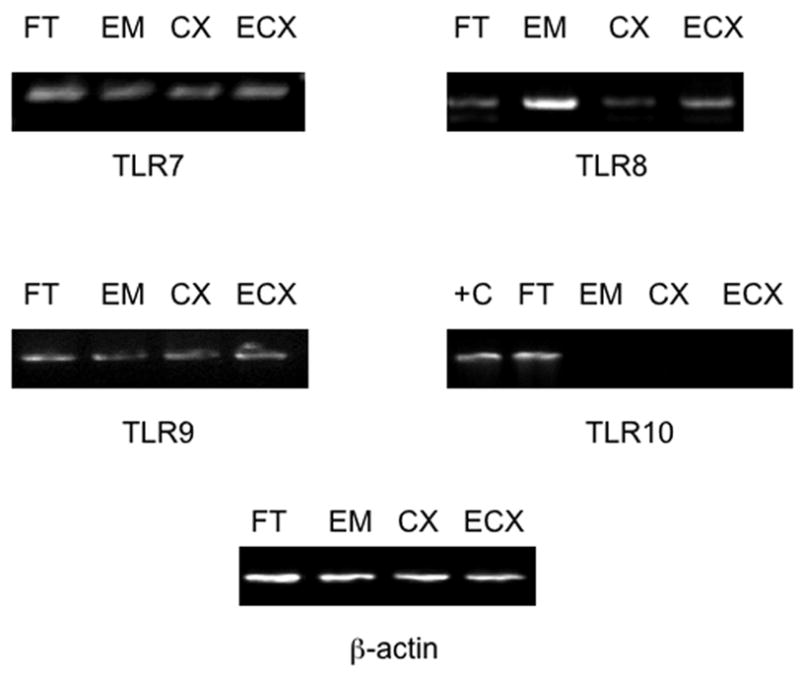
mRNA expression of TLRs 7–10 in tissues of the human female reproductive tract. Total RNA was extracted from Fallopian tube, uterine endometrial, cervical and ectocervical tissues, reverse transcribed and amplified with the primers described in Table 1 for expression of pattern recognition receptors. RNA from the Ramos B cell line was used as positive control (+C) for TLR10 mRNA expression. For each tissue, the data shown are representative of gene expression observed in samples from 10 different donors.
Table 3.
Expression of PRR and RICK mRNA by tissues of the human female reproductive tract
| TLR7 | TLR8 | TLR9 | TLR10 | NOD1 | NOD2 | RICK | |
|---|---|---|---|---|---|---|---|
| Fallopian tube | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 |
| Endometrium | 10/10 | 10/10 | 10/10 | 0/10 | 10/10 | 10/10 | 10/10 |
| Cervix | 10/10 | 10/10 | 10/10 | 0/10 | 10/10 | 10/10 | 10/10 |
| Ectocervix | 10/10 | 10/10 | 10/10 | 0/10 | 10/10 | 10/10 | 10/10 |
3.2 FRT Tissues Express NOD family members 1 and 2
In addition to the innate immune system receptors comprising the TLR family, two additional PRRs, NOD1 and NOD2, have been identified as important cytoplasmic sensors of bacterial infection (Kufer et al., 2006). Given the microbial diversity encountered by the reproductive tract and the importance of innate immune responses in the elimination of pathogens, we examined mRNA expression of NOD1 and NOD2 in tissues of the FRT. Expression of NOD1 and NOD2 message was observed in Fallopian tubes, uterine endometrium, cervix and ectocervix by RT-PCR (Figure 2, Table 3).
Figure 2.
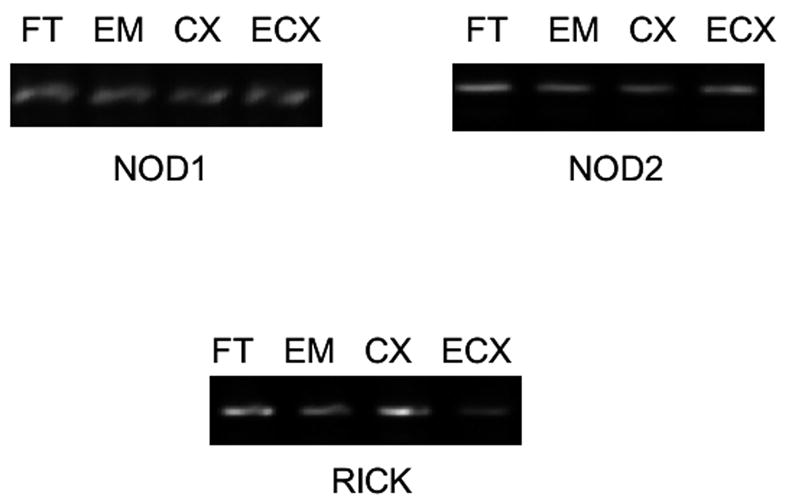
mRNA expression of NOD receptors and the NOD signaling effector RICK by tissues of the human female reproductive tract. Total RNA was isolated from FRT tissues, and expression of NOD1, NOD2 and RICK was demonstrated using primers delineated in Table 1. Data shown are representative of gene expression in tissues derived from 10 different donors.
NOD receptors transduce signals using the adapter protein RICK, which is a serine/threonine kinase that physically associates with the CARD domains of NOD1 and NOD2 (Ogura et al., 2001). Binding of NODs to RICK initiates a signaling cascade that culminates in the activation of NFκB (Kobayashi et al., 2002). Because of the importance of this molecule in mediating NOD responses to pathogenic challenge, we also characterized the expression of RICK in FRT tissues. As demonstrated in Figure 2 and Table 3, RICK mRNA is present in cells from Fallopian tube, uterine endometrium, cervix and ectocervix.
3.3 Functional expression of TLR and NOD receptors in the tissues of the human FRT
To assess the ability of the TLR and NOD proteins present in the FRT to trigger an innate immune response, cells cultured from primary tissue samples were treated with TLR and NOD ligands, and cell-free supernatants were analyzed for CXCL8 secretion every two hours for a period of six hours. Primary cells from Fallopian tube (Figure 3a), uterine endometrium (Figure 3b), cervix (Figure 3c) and ectocervix (Figure 3d) secreted increasing amounts of CXCL8 in response to imiquimod (TLR7), CL045 (TLR8), CpG (TLR9), iE-DAP (NOD1) and MDP-1 (NOD2) stimulation during the 6 hour treatment (Figure 3a–3d). All tissues tested were responsive to PRR ligand stimulation, as summarized in Table 4. Figure 3 specifically shows results for tissues in the secretory phase.
4. Discussion
The mucosal immune system of the human female reproductive tract (FRT) is unique. It is the only immune site that must balance the demands of protection from pathogen invasion with the requirements of fetal implantation and procreation. This task is accomplished by microbial recognition mediated by PRRs, which include members of the TLR and NOD families (reviewed in (Mitchell et al., 2007). In this study, we report that TLRs 7–9 are constitutively expressed in FRT tissues including Fallopian tubes, uterine endometrium, cervix and ectocervix, while expression of TLR10 is restricted to the Fallopian tubes. NOD1 and NOD2 as well as the signal transducer RICK are detectable in all FRT tissues. Moreover, these receptors are functional, as treatment of FRT tissue cells with ligands for TLR and NOD induces production of pro-inflammatory CXCL8.
Immune surveillance in the FRT is critically influenced by the interplay of many factors including hormones, resident leukocyte populations, and the distinct microenvironment of each component of the reproductive tract. The non-sterile lower portions of the FRT harbor a variety of commensal bacteria, and are exposed to a wide diversity of sexually transmitted microbes (Quayle, 2002). While the upper regions of the tract, notably the Fallopian tubes and uterine endometrium, have been considered sterile sites, emerging evidence demonstrates that these regions of the FRT are subject to challenge by ascending pathogens, such as Neisseria gonorrhoeae and Chlamydia trachomatis (Quayle, 2002). Given the diversity of microbes to which the tract is exposed, it is imperative that tissues of the FRT are responsive to pathogenic challenge. In this regard, PRRs play a critical role in mediating microbial recognition. Previous studies indicate that TLRs 1–6 are present in tissues of the human FRT (Fazeli et al., 2005, Hirata et al., 2005, Pioli et al., 2004, Young et al., 2004). We now extend these findings to demonstrate constitutive expression of TLRs 7–9 and NOD1 and 2 in these tissues as well. Thus, cells of the human FRT are capable of mediating responses to a broad range of microbes.
This is particularly important with respect to NOD receptor expression, as these PRRs recognize intracellular cytoplasmic bacterial PAMPs. Because Chlamydia trachomatis is an intracellular pathogen that can infect both the lower and upper FRT (den Hartog et al., 2006), NODs may play a pivotal role in mediating immune responses to this microbe. Indeed, recent work has shown that functional NOD1 expression is important for the activation of NFκB during chlamydial infection (Welter-Stahl et al., 2006). Clearance of C. trachomatis infections is contingent on recognition of this microbe and stimulation of the inflammatory immune response. The consequences of persistent chlamydial infection include endosalpingeal tissue damage and tubal factor subfertility (den Hartog et al., 2006). Moreover, increasing evidence suggests that chlamydial infections may be causally linked to pregnancy complications, particularly preeclampsia and preterm labor (Hossain et al., 1990, Jain et al., 1991). In this regard, studies conducted by Costello et al have demonstrated functional NOD expression in first trimester trophoblasts (Costello et al., 2007). Here we are now show for the first time the presence of functional NOD receptors in non-pregnant tissues of the human FRT.
It is intriguing that TLR10 was only detected in the Fallopian tubes. Our determination that human uterine endometrium lacks expression of TLR10 is consistent with studies conducted by Young et al and Schaefer et al (Schaefer et al., 2004, Young et al., 2004). Moreover, it is not surprising that TLR10 expression is restricted to the Fallopian tubes, as TLR10 is mainly expressed in B lymphocytes (Hornung et al., 2002). Previous work has shown that human FRT tissues in general contain low numbers of B cells, with the exception of the Fallopian tubes (Givan et al., 1997). However, our data are discordant with the findings of Aflatoonian et al, in which human endometrium was shown to express TLR10 mRNA (Aflatoonian et al., 2007). To attempt to reconcile this disparity, we performed PCR using the primers used to identify TLR10 in the study above, but failed to observe expression of TLR10 (data not shown). As we utilized appropriate positive and negative controls, the explanation for the variance in these results is unclear, but clearly merits further investigation. It will be important to determine if this receptor is functional in FRT tissues after a ligand has been identified for this receptor.
In addition to the characterization of NOD receptors, we also found that human FRT tissues express the signaling adapter RICK. Analysis of the signal transduction pathway initiated by NOD-ligand engagement demonstrates that RICK mediates NFκB activation through the polyubiquitylation of the inhibitor of NFκB (IκB)-kinase-γ (Abbott et al., 2004). In this work, we have identified expression of RICK throughout the human FRT. Therefore, FRT tissues contain the necessary signaling machinery for inducing inflammation and immune responses.
Signaling through NODs has also been shown to activate caspase-1 and MAPK pathways and TLR engagement also triggers activation of MAPK signaling cascades and NFκB (Akira and Takeda, 2004, Strober et al., 2006). Stimulation of these signaling pathways leads to the generation of an inflammatory response that is characterized by the synthesis and secretion of pro-inflammatory cytokines. CXCL8 is a key mediator of inflammation that is rapidly synthesized by a broad variety of innate effector cells, including epithelial cells, uNK cells, monocytes and macrophages (Akahoshi et al., 1994). For this reason, we utilized CXCL8 as an indicator of TLR and NOD activation. However, signaling through any TLR or NOD would be expected to result in production and secretion of CXCL8, and a recent report has shown that many TLR agonists are contaminated with the TLR4 agonist lipopolysaccharide (LPS) (Weaver et al., 2006), To ensure the specificity of PRR activation, we tested all TLR and NOD agonists for LPS contamination using the Limulus ameobocyte lysate (LAL) assay (data not shown). This assay is capable of detecting as little as 10 pg/ml LPS. Using this measure, all reagents were determined to be free of LPS contamination. As demonstrated in this study, stimulation of FRT tissue cells with specific TLR and NOD agonists leads to secretion of CXCL8. These data demonstrate that TLRs and NODs are functionally expressed in FRT tissues.
In summary, we have demonstrated for the first time functional expression of TLRs 7–9 and NOD1 and NOD2 in human Fallopian tubes, uterine endometrium, cervix and ectocervix. Expression of TLR10 was restricted to Fallopian tubes. In addition, we have also shown that each of the four FRT tissues expresses the NOD signaling adapter protein RICK. Significantly, treatment of human FRT tissue cells with TLR and NOD agonists induced secretion of the chemokine CXCL8, indicating these receptors are functional. Collectively, these data establish TLR and NOD expression in the female genital tract and implicate TLR and NOD-regulated immune surveillance as an important defense mechanism in the human FRT.
Acknowledgments
This work was funded by NIH grant AI51877.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ABBOTT DW, WILKINS A, ASARA JM, CANTLEY LC. The Crohn’s disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol. 2004;14:2217–27. doi: 10.1016/j.cub.2004.12.032. [DOI] [PubMed] [Google Scholar]
- AFLATOONIAN R, TUCKERMAN E, ELLIOTT SL, BRUCE C, AFLATOONIAN A, LI TC, FAZELI A. Menstrual cycle-dependent changes of Toll-like receptors in endometrium. Hum Reprod. 2007;22:586–93. doi: 10.1093/humrep/del388. [DOI] [PubMed] [Google Scholar]
- AKAHOSHI T, ENDO H, KONDO H, KASHIWAZAKI S, KASAHARA T, MUKAIDA N, HARADA A, MATSUSHIMA K. Essential involvement of interleukin-8 in neutrophil recruitment in rabbits with acute experimental arthritis induced by lipopolysaccharide and interleukin-1. Lymphokine Cytokine Res. 1994;13:113–6. [PubMed] [Google Scholar]
- AKIRA S, TAKEDA K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- BARTON GM. Viral recognition by Toll-like receptors. Semin Immunol. 2007;19:33–40. doi: 10.1016/j.smim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- BEYNON V, COTOFANA S, BRAND S, LOHSE P, MAIR A, WAGNER S, MUSSACK T, OCHSENKUHN T, FOLWACZNY M, FOLWACZNY C, GLAS J, TOROK HP. NOD2/CARD15 genotype influences MDP-induced cytokine release and basal IL-12p40 levels in primary isolated peripheral blood monocytes. Inflamm Bowel Dis. 2008 doi: 10.1002/ibd.20441. [DOI] [PubMed] [Google Scholar]
- CHAMAILLARD M, HASHIMOTO M, HORIE Y, MASUMOTO J, QIU S, SAAB L, OGURA Y, KAWASAKI A, FUKASE K, KUSUMOTO S, VALVANO MA, FOSTER SJ, MAK TW, NUNEZ G, INOHARA N. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–7. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- COSTELLO MJ, JOYCE SK, ABRAHAMS VM. NOD protein expression and function in first trimester trophoblast cells. Am J Reprod Immunol. 2007;57:67–80. doi: 10.1111/j.1600-0897.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- CREAGH EM, O’NEILL LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–7. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- DEN HARTOG JE, MORRE SA, LAND JA. Chlamydia trachomatis-associated tubal factor subfertility: Immunogenetic aspects and serological screening. Hum Reprod Update. 2006;12:719–30. doi: 10.1093/humupd/dml030. [DOI] [PubMed] [Google Scholar]
- FAZELI A, BRUCE C, ANUMBA DO. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum Reprod. 2005;20:1372–8. doi: 10.1093/humrep/deh775. [DOI] [PubMed] [Google Scholar]
- GIRARDIN SE, BONECA IG, CARNEIRO LA, ANTIGNAC A, JEHANNO M, VIALA J, TEDIN K, TAHA MK, LABIGNE A, ZAHRINGER U, COYLE AJ, DISTEFANO PS, BERTIN J, SANSONETTI PJ, PHILPOTT DJ. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003a;300:1584–7. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- GIRARDIN SE, TRAVASSOS LH, HERVE M, BLANOT D, BONECA IG, PHILPOTT DJ, SANSONETTI PJ, MENGIN-LECREULX D. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003b;278:41702–8. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- GIVAN AL, WHITE HD, STERN JE, COLBY E, GOSSELIN EJ, GUYRE PM, WIRA CR. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350–9. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- GORDON S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–30. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- HIRATA T, OSUGA Y, HIROTA Y, KOGA K, YOSHINO O, HARADA M, MORIMOTO C, YANO T, NISHII O, TSUTSUMI O, TAKETANI Y. Evidence for the presence of toll-like receptor 4 system in the human endometrium. J Clin Endocrinol Metab. 2005;90:548–56. doi: 10.1210/jc.2004-0241. [DOI] [PubMed] [Google Scholar]
- HORNUNG V, ROTHENFUSSER S, BRITSCH S, KRUG A, JAHRSDORFER B, GIESE T, ENDRES S, HARTMANN G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- HOSSAIN A, ARIF M, RAMIA S, BAKIR TF. Chlamydia trachomatis as a cause of abortion. J Hyg Epidemiol Microbiol Immunol. 1990;34:53–5. [PubMed] [Google Scholar]
- JAIN A, NAG VL, GOEL MM, CHANDRAWATI &, CHATURVEDI UC. Adverse foetal outcome in specific IgM positive Chlamydia trachomatis infection in pregnancy. Indian J Med Res. 1991;94:420–3. [PubMed] [Google Scholar]
- JANEWAY CA, JR, MEDZHITOV R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI K, INOHARA N, HERNANDEZ LD, GALAN JE, NUNEZ G, JANEWAY CA, MEDZHITOV R, FLAVELL RA. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–9. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- KUFER TA, BANKS DJ, PHILPOTT DJ. Innate immune sensing of microbes by Nod proteins. Inflammatory Bowel Disease: Genetics, Barrier Function, Immunologic Mechanisms, and Microbial Pathways. 2006 doi: 10.1196/annals.1326.020. [DOI] [PubMed] [Google Scholar]
- MANON F, FAVIER A, NUNEZ G, SIMORRE JP, CUSACK S. Solution structure of NOD1 CARD and mutational analysis of its interaction with the CARD of downstream kinase RICK. J Mol Biol. 2007;365:160–74. doi: 10.1016/j.jmb.2006.09.067. [DOI] [PubMed] [Google Scholar]
- MIGGIN SM, O’NEILL LA. New insights into the regulation of TLR signaling. J Leukoc Biol. 2006;80:220–6. doi: 10.1189/jlb.1105672. [DOI] [PubMed] [Google Scholar]
- MITCHELL JA, PAUL-CLARK MJ, CLARKE GW, MCMASTER SK, CARTWRIGHT N. Critical role of toll-like receptors and nucleotide oligomerisation domain in the regulation of health and disease. J Endocrinol. 2007;193:323–30. doi: 10.1677/JOE-07-0067. [DOI] [PubMed] [Google Scholar]
- OGURA Y, INOHARA N, BENITO A, CHEN FF, YAMAOKA S, NUNEZ G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–8. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- PARK JH, KIM YG, MCDONALD C, KANNEGANTI TD, HASEGAWA M, BODY-MALAPEL M, INOHARA N, NUNEZ G. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380–6. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- PIOLI PA, AMIEL E, SCHAEFER TM, CONNOLLY JE, WIRA CR, GUYRE PM. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect Immun. 2004;72:5799–806. doi: 10.1128/IAI.72.10.5799-5806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QIN J, YAO J, CUI G, XIAO H, KIM TW, FRACZEK J, WIGHTMAN P, SATO S, AKIRA S, PUEL A, CASANOVA JL, SU B, LI X. TLR8-mediated NF-kappaB and JNK activation are TAK1-independent and MEKK3-dependent. J Biol Chem. 2006;281:21013–21. doi: 10.1074/jbc.M512908200. [DOI] [PubMed] [Google Scholar]
- QUAYLE AJ. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J Reprod Immunol. 2002;57:61–79. doi: 10.1016/s0165-0378(02)00019-0. [DOI] [PubMed] [Google Scholar]
- ROSENSTIEL P, JACOBS G, TILL A, SCHREIBER S. NOD-like receptors: Ancient sentinels of the innate immune system. Cell Mol Life Sci. 2008 doi: 10.1007/s00018-008-7502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEFER TM, DESOUZA K, FAHEY JV, BEAGLEY KW, WIRA CR. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112:428–36. doi: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROBER W, MURRAY PJ, KITANI A, WATANABE T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- TAKEDA K, KAISHO T, AKIRA S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- WEAVER LK, HINTZ-GOLDSTEIN KA, PIOLI PA, WARDWELL K, QURESHI N, VOGEL SN, GUYRE PM. Pivotal Advance: Activation of cell surface Toll-like receptors causes shedding of the hemoglobin scavenger receptor CD163. J Leukoc Biol. 2006;80:26–35. doi: 10.1189/jlb.1205756. [DOI] [PubMed] [Google Scholar]
- WELTER-STAHL L, OJCIUS DM, VIALA J, GIRARDIN S, LIU W, DELARBRE C, PHILPOTT D, KELLY KA, DARVILLE T. Stimulation of the cytosolic receptor for peptidoglycan, Nod1, by infection with Chlamydia trachomatis or Chlamydia muridarum. Cell Microbiol. 2006;8:1047–57. doi: 10.1111/j.1462-5822.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- WHITE HD, PRABHALA RH, HUMPHREY SL, CRASSI KM, RICHARDSON JM, WIRA CR. A method for the dispersal and characterization of leukocytes from the human female reproductive tract. Am J Reprod Immunol. 2000;44:96–103. doi: 10.1111/j.8755-8920.2000.440205.x. [DOI] [PubMed] [Google Scholar]
- YOUNG SL, LYDDON TD, JORGENSON RL, MISFELDT ML. Expression of Toll-like receptors in human endometrial epithelial cells and cell lines. Am J Reprod Immunol. 2004;52:67–73. doi: 10.1111/j.1600-0897.2004.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


