Abstract
Compared to C57BL/6 mice, BALB/c mice exhibit greater “anxiousness” on behavioral tests of anxiety, and can show significantly longer sleep disruptions after exposure to anxiogenic situations. Relative to C57BL/6 mice, BALB/c mice also have reduced benzodiazepine (BZ) receptor densities in the brain and fivefold less BZ receptor density in the amygdala, a region important in anxiety and in the control of arousal. Lorazepam is a BZ receptor full agonist and has been used to treat both anxiety and insomnia. Differences between C57BL/6 and BALB/c mice raise the question of whether BZ agonists would differentially impact sleep and activity in the two strains. We examined the effects of two dosages of lorazepam (0.5 mg/kg; 1.5 mg/kg) or saline alone (0.2 ml) on sleep and activity in C57BL/6 (n=8) and BALB/c (n=8) mice. Compared to saline, both dosages of lorazepam significant increased NREM and reduced activity in both strains. In C57BL/6 mice, REM was increased at both dosages. In BALB/c mice, the 0.5 mg/kg dosage had no significant influence on REM, whereas REM was significantly reduced after the 1.5 mg/kg dosage. The results demonstrate significant differences between C57BL/6 and BALB/c mice in the effects of lorazepam on REM, whereas the effects on NREM and activity were similar. Strain differences in the number BZ receptors in the amygdala, but not other brain regions, suggests possible site specificity in the effects of lorazepam on REM. These differences in BZ binding sites in the amygdala could be a significant factor in differences in the sleep response between C57 and BALB/c mice.
Keywords: activity, benzodiazepine, lorazepam, mouse strains, sleep
Introduction
Results on a variety of behavioral tests have consistently shown that, compared to C57BL/6 mice, BALB/c mice exhibit greater anxiety-like behaviors (Tang et al., 2002b). Indeed, BALB/cJ mice have been suggested to exhibit trait or pathological anxiety (Belzung & Griebel, 2001; Tang et al., 2002a) whereas C57BL/6J mice show an intermediate phenotype in most behaviors (Crawley, 1999) and are often used in strain comparisons. Relative to C57BL/6 mice, BALB/c mice exhibit more persistent reductions in the time spent in sleep, particularly in rapid eye movement sleep (REM) after exposure to mild stressors including an open field (Tang et al., 2004), presentation of a novel object in the home cage and cage change (Tang et al., 2005). In addition, BALB/c mice show greater reductions in REM after footshock training, and after presentation of fearful conditioned cues and contexts (Sanford et al., 2003a; Sanford et al., 2003b). Therefore, comparisons between BALB/c and C57BL/6 mice may be useful for examining the complex interactions between sleep and emotional regulation.
Benzodiazepine (BZ) receptor agonists are the most commonly prescribed clinical antianxiety agents. BZ agonists act by increasing the affinity of the GABAA receptor to endogenously available GABA (Feldman et al., 1997). BZ receptors are widely distributed in the brain, but they are particularly abundant in the limbic system including the amygdala (Niehoff & Kuhar, 1983; Onoe et al., 1996; Zezula et al., 1988), putatively a key site of action for their efficacy in treating anxiety (Yadin et al., 1991; Sanders & Shekhar, 1995). This is suggested by findings that local BZ agonists microinjections into the amygdala decrease “anxiety” (they increase punished responses) in conflict paradigms (Shibata et al., 1989; Davis, 1990), whereas antagonizing GABA increases anxiety behaviors (Sanders & Shekhar, 1995). BZ agonists also have been used for treatment of many forms of insomnia, including those arising from stressful or anxiety producing events (Roehrs et al., 2000).
BALB/c mice have been reported to have more sensitive behavioral reactions to BZ agonists (Griebel et al., 2000) and compared to C57BL/6 mice have reduced binding to BZ receptors in the whole brain (Robertson, 1979; Chapouthier et al., 1991) and fivefold less BZ receptor density in the amygdala (Hode et al., 2000). While there have been various studies that have examined in these or related strains the effects of BZ agonists on anxiety in behavioral paradigms, to our knowledge, there are no reports on whether BZ agonists differentially alter their sleep and activity. Therefore, in this study, we administered lorazepam, a BZ receptor full agonist that has been used to treat both anxiety (e.g., (Chouinard, 2004)) and insomnia (e.g., (Bonnet & Arand, 1999)), to BALB/c and C57BL/6 mice and recorded its effects on electrographically determined sleep and on home cage activity to determine whether the strains show a difference in the effects of a BZ agonist on arousal.
Methods
Subjects
The subjects were 8 C57BL/6J and 7 BALB/cJ male mice purchased from the Jackson Laboratory (Bar Harbor, Maine). The animals were individually housed upon arrival and for the duration of the experiment. The mice were approximately 10 weeks of age at the beginning of the experiment. Food and water were available ad libitum. Ambient room temperature was maintained at 24.5 ± 0.5° C and lights were kept on a 12:12 cycle with lights on from 7:00 A.M. to 7:00 P.M.
Surgery
The mice were surgically implanted with ETA10-F20 telemetry transmitters (DataSciences International, Saint Paul, Minnesota) for recording EEG and activity as previously described (Tang & Sanford, 2002). Surgery was conducted with the mice under isoflurane (as inhalant: 5.0% induction; 2.0% maintenance) anesthesia. Screw electrodes were bilaterally implanted (A: 1.0, L: 1.0; P: 3.0, L: 3.0; relative to Bregma) for recording EEG. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by Eastern Virginia Medical School's Animal Care and Use Committee (Protocol # 02-022).
Experimental Procedure
Twenty four hour sleep recordings were obtained after two weeks of recovery from surgery. Afterwards, I.P. injections of lorazepam (7-Chloro-5-(2-chlorophenyl)-1,3-dihydro-3-hydroxy-2H-1,4-benzodiazepin-2-one) acquired from Sigma-Aldrich (St. Louis, Missouri) were administered. The injections took place over an approximately 15 min period immediately prior to the third h of the light period and sleep was recorded for the following 22 h (10 h in the light period and 12 h in the dark period). The mice received two dosages of lorazepam (LZA: 0.5 mg/kg; LZB: 1.5 mg/kg) or saline alone (SAL, 0.2 ml). Injections were administered in a counterbalanced order at one-week intervals.
Determination of Behavioral State and Activity Levels
For recording sleep and activity, individual home cages were placed on a DataSciences telemetry receiver (RPC-1) and the transmitter was activated with a magnetic switch. Telemetry signals (cortical EEG and TTL (transitor-transitor logic) pulses generated when the animals moved) were processed by a DataSciences analog converter (ART Analog-8 CM) and routed to an A/D board (Eagle PC30) housed in a Pentium class PC. The EEG and TTL activity signals were digitized at 128 Hz. The EEG and activity data were saved to the hard disk for subsequent offline data analyses and for visual scoring of behavioral state. Wakefulness, NREM and REM were visually determined in 10 s epochs on the basis of EEG and gross whole body activity. TTL pulses were counted as a measure of activity.
Data Analyses
Activity and sleep were processed to obtain hourly totals. Afterwards, the data (22-h recording day) were collapsed to obtain two 5-h blocks in the light period and two 6-h blocks in the dark period. Total activity, total NREM (min), total REM (min), total sleep (NREM + REM), latency to NREM (first 10 sec epoch) from start of recording, latency to REM (first 10 sec epoch) from start of recording and latency to REM from sleep onset (latency to REM – latency to NREM) were analyzed across treatments.
All statistical analyses were conducted using SigmaStat software (SPSS, Inc., Chicago, Illinois). Separate two-way repeated measures ANOVAs (3 treatments × 4 blocks) for each strain were conducted. One-way ANOVA was used in the comparisons of sleep latency between treatments. Post hoc comparisons among means were conducted using Tukey tests when the ANOVAs indicated significant differences. In addition, we examined the percentage change relative to control [(treatment)-SAL)/SAL*100] for 10 h light, 12 h dark and total 22 h recording periods in NREM, REM, total sleep and activity count, to compensate for strain differences. Comparisons for this data were made across strain within treatments via independent t-tests.
Results
Sleep Measures
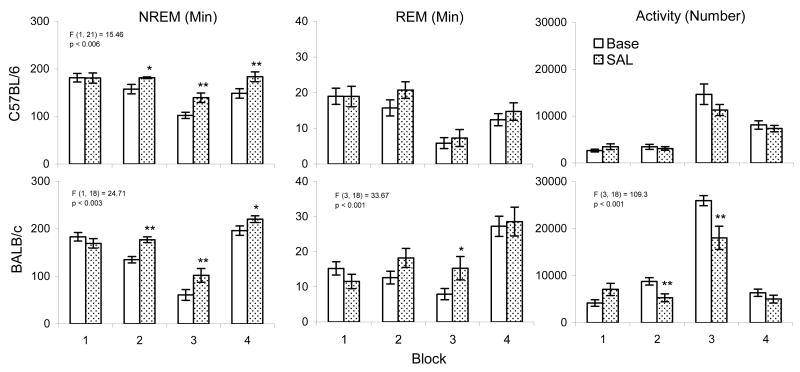
Handling procedures alone can produce significant alterations in sleep compared to undisturbed baseline. In order to determine possible strain differences in sleep in response to procedures necessary for administering the SAL control injection, we compared time spent in NREM and REM between time-matched non-disturbed baseline and after SAL in the two mouse strains. As shown in Figure 1, both strains showed similar increases in NREM time during blocks 2, 3 and 4 after SAL compared to baseline. Amounts of REM did not differ between undisturbed baseline and SAL conditions in C57BL/6 mice, but REM was significantly increased in BALB/c mice during block 3. Figure 1 (right panels) also demonstrates that changes in sleep after injections of SAL were accompanied by decreases in activity that were significant during blocks 2 and 3 in BALB/c mice whereas C57BL/6 mice did not show significant reductions in activity. These results suggest that the effects of lorazepam we observed should be interpreted as occurring in comparison to a control treatment that also could alter sleep and arousal.
Figure 1.
Selected sleep parameters and activity plotted in time-matched blocks for non-disturbed baseline and following injections of SAL in C57BL/6J and BALB/cJ mice. Values are means±SEM. Block data are plotted in two 5-h blocks for the light period and two 6-h blocks for the dark period. The results for significant two-way ANOVAs are indicated above the appropriate bars for entire 22-h recording period. F- and p-values are presented for significant main effect across treatments for NREM and for interactions across treatment and block for REM and activity. Stars indicate significant differences (*p <0.05, **p <0.01) compared to baseline conducted with Tukey test.
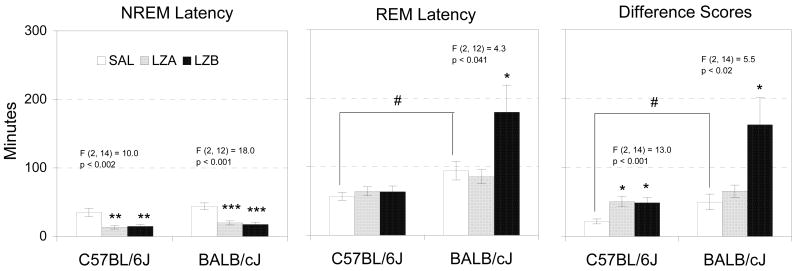
Compared to SAL, the administration of LZA and LZB significantly reduced the latency to NREM in both strains (Figure 2). However, the latency to REM from the start of recording was not altered by either dosage of lorazepam in C57BL/6 mice and was not altered by the low dosage of lorazepam in BALB/c mice. By comparison, the high dosage of lorazepam produced a significant increase in the latency to REM from the start of recording in BALB/c mice. When calculated from the start of sleep, REM latency was increased in C57BL/6 mice at both dosages of lorazepam and in BALB/c mice at the high dosage. Between strains comparisons of latencies to NREM and REM were made after injections of SAL using independent t-tests. BALB/c mice showed significantly longer latency to REM determined either from start of recording or sleep onset.
Figure 2.
Latency to NREM, REM and difference score (REM latency – NREM latency) in minutes following injections of SAL, LZA (0.5 mg/kg) and LZB (1.5 mg/kg) in C57BL/6J and BALB/cJ mice. Values are means±SEM. The results for significant ANOVAs are indicated above the appropriate bars for the analyses between treatments. F- and p-values are presented for main effect across treatments. Stars indicate significant differences (*p <0.05, **p <0.01, ***p <0.001) compared to SAL conducted with Tukey test. # illustrates significant difference (p < 0.05) between strains (t-test).
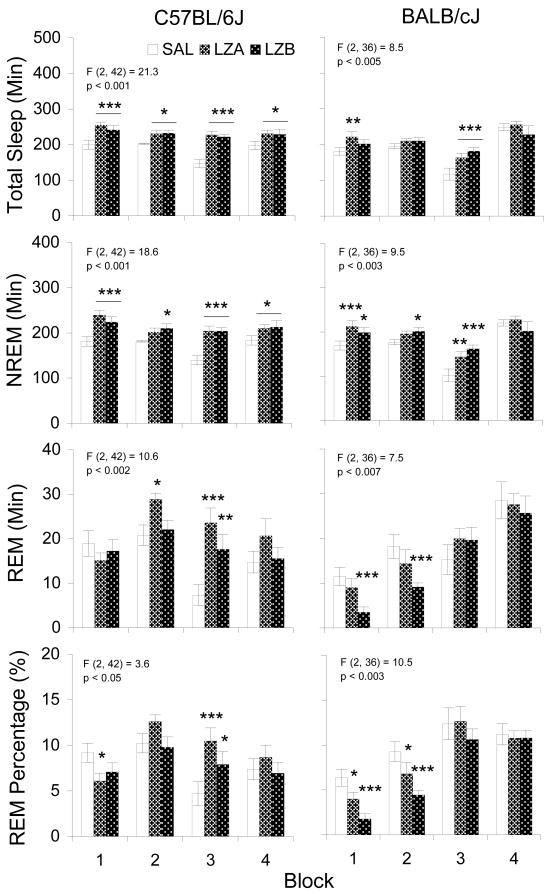
Figure 3 shows selected measures of sleep analyzed in blocks (two 5 h blocks in light period and two 6 h blocks in dark period) for both strains. In C57BL/6 mice (Figure 3, Left Panels), compared to SAL, both dosages of lorazepam produced significant increases in total sleep and NREM time during almost all of the measurement periods. The sole exception was that the increase in NREM did not reach significance during block 2 after LZA. Further analyses of the NREM data with two-way repeated measures ANOVAs (treatments × blocks) followed by post hoc comparisons (data not presented) revealed that increases in NREM time during blocks 1 and 2 were due to increases in the average lengths of NREM episodes [F (2, 42) = 4.7, p < 0.027] whereas increases in NREM time in block 3 was due to increases in the number of NREM episodes [F (2, 42) = 5.9, p < 0.014]. No significant changes were seen in either number or average length for NREM episodes in block 4. C57BL/6 mice also showed increases in REM time during block 2 after LZA and during block 3 after both LZA and LZB. The changes in REM time were associated with increases in both number [F (2, 42) = 4.5, p < 0.032] and average length [F (2, 42) = 6.7, p < 0.009] of REM episodes (data not shown). LZA produced a significant decrease in REM percentage during block 1, whereas both LZA and LZB produced significant increases in REM percentage during block 3.
Figure 3.
Selected sleep measures plotted in blocks after injections of SAL, LZA (0.5 mg/kg) and LZB (1.5 mg/kg) in C57BL/6J and BALB/cJ mice. Values are means±SEM. Block data are plotted in two 5-h blocks for the light period and two 6-h blocks for the dark period. The results for significant two-way ANOVAs are indicated above the appropriate bars for entire 22-h recording period. F- and p-values are presented for main effect across treatments. Stars indicate significant differences (*p <0.05, **p <0.01, ***p <0.001) compared to SAL conducted with Tukey test.
In BALB/c mice (Figure 3, Right Panels), increases in total sleep were found after LZA in block 1 and after LZA and LZB in block 3. Increases in NREM time were found after LZB in blocks 1 through 3 and after LZA during block 1 and block 3. No significant findings were in the number and average lengths of NREM episodes (data not presented). LZB decreased REM time during blocks 1 and 2, and both LZA and LZB decreased REM percentage during blocks 1 and 2. Significant decreases were found in the number of REM episodes [F (2, 36) = 12.1, p < 0.001], but not in the average length of REM episodes (data not shown).
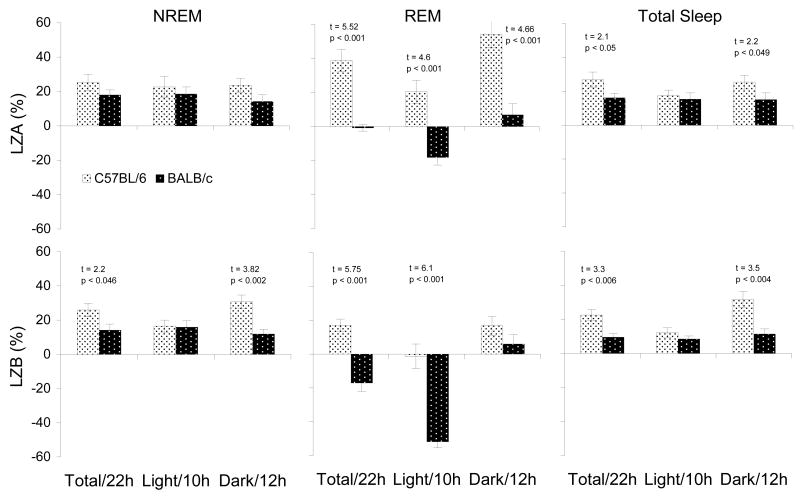
Comparisons between strains for each dosage of lorazepam were made using percentage change after drug administration relative to that after SAL (Figure 4). For LZA, significant strain differences were found in REM time during the total 22 h recording period and for the separate 10 h light and 12 h dark periods (increases in C57BL/6 and no significant changes or decreases in BALB/c mice). Significant strain differences were also found for total sleep time during the total 22 h recording period and during the 12 h dark period. For LZB, significant strain differences were found for NREM time and total sleep during the total 22 h recording period and the 12 h dark period, and significant strain differences in REM time (increases in C57BL/6 and no significant change or decrease in BALB/c mice) were found for the total 22 h recording period and the 10 h light period.
Figure 4.
NREM, REM and total sleep plotted as a percentage change relative to those of control (SAL) for LZA (0.5 mg/kg) and LZB (1.5 mg/kg) injections during total 22-h, 10-h light and 12-h dark periods in C57BL/6J and BALB/cJ mice. Values are means±SEM. t- and p-values for significant differences between strains are indicated above the appropriate bas.
Activity Counts
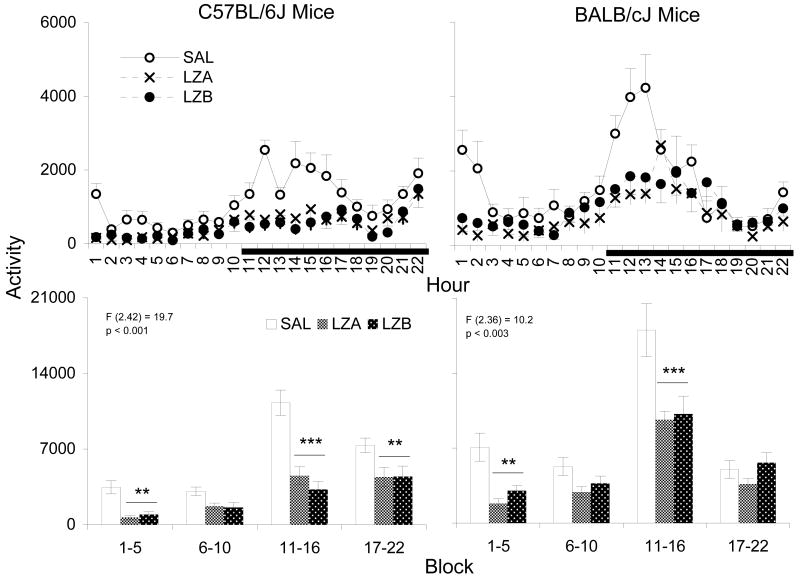
Figure 5 shows activity counts after the administrations of SAL, LZA and LZB in both mouse strains. As shown in the graphs of hourly activity (upper plots), compared to SAL, both LZA and LZB suppressed activity during the light period immediately after drug administration, and during the dark periods. Two-way repeated measures ANOVAs (treatments × blocks) followed by post hoc comparisons revealed that the decreases in activity after both drug dosages were significant during blocks 1 and 3 in both strains, and was also significant during the block 4 in C57BL/6 mice.
Figure 5.
Activity is plotted hourly (Upper) and blocks (Lower) after injections of SAL, LZA (0.5 mg/kg) and LZB (1.5 mg/kg) in C57BL/6J and BALB/cJ mice. Values are means±SEM. Block data are plotted in two 5-h blocks for the light period and two 6-h blocks for the dark period. The results for significant two-way ANOVAs are indicated above the appropriate bars for entire 22-h recording period. F- and p-values are presented for main effect across treatments. Stars indicate significant differences (**p <0.01, ***p <0.001) compared to SAL conducted with Tukey test.
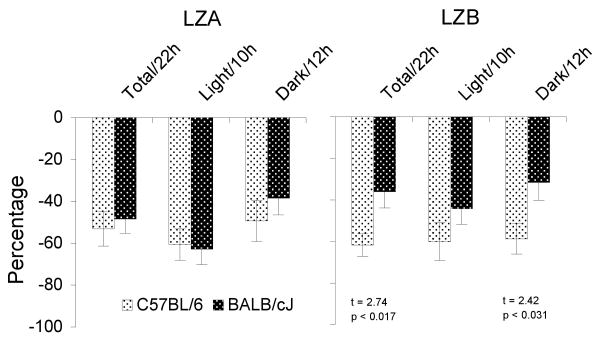
Comparisons of the two strains using percentage change after drug administration relative to that after SAL showed that differences between strains were significant during the total 22 h recording period and during the 12 h dark period for LZB (Figure 6). LZA did not produce significant strain differences using this measure. In general, C57BL/6 mice showed a greater reduction in activity than did BALB/c mice.
Figure 6.
Activity plotted as a percentage change relative to that of control (SAL) for LZA (0.5 mg/kg) and LZB (1.5 mg/kg) injections during total 22-h, 10-h light and 12-h dark periods in C57BL/6J and BALB/cJ mice. Values are means±SEM. t- and p-values for significant differences between strains are indicated under the appropriate bas.
Discussion
This study demonstrated significant differences between C57BL/6 and BALB/c mice in the effects of lorazepam on sleep and activity. BZ agonists have consistently been found to reduce sleep latency and increase total sleep time (Reviewed in (Mendelson, 2005)). In this study, lorazepam produced significant reductions in sleep latency (latency to NREM onset) in both strains that occurred without an apparent dose response. Total sleep was also increased in both strains, but the increase was more consistent in C57BL/6 mice which showed increased total sleep in all analysis blocks and increased total NREM in all blocks except block 2 at the low dosage. By comparison, the effect of lorazepam on total sleep and total NREM in BALB/c mice was more variable across dosages and across analysis blocks.
BZ agonists may produce a mild suppressing effect on REM in humans (Mendelson, 2005) and the initial effect of lorazepam on REM in C57BL/6 mice could be characterized as mild. However, the strain differences we observed in the effects of lorazepam on REM were quite pronounced. In C57BL/6 mice, total REM was not significantly reduced in any measurement period and was significantly increased during block 2 at the low dosage and during block 3 at both low and high dosages. REM considered as a percentage of total sleep time was only significantly reduced at the low dosage during block 1; however, this change resulted from significant increases in total sleep and total NREM during this period. By comparison, significant reductions in total REM were observed during blocks 1 and 2 at the high dosage of lorazepam in BALB/c mice. REM percentage was decreased at both low and high dosages in BALB/c mice during the same time periods. The decrease in REM percentage in BALB/c mice appeared to result from both decreases in total REM and increases in total sleep and total NREM.
Motor activity is a critical component in virtually all behavioral paradigms in mice (Crawley, 1999). BZ agonists produce a sedative effect that is exemplified by decreases in spontaneous motor activity and exploration (File, 1984). In this study, lorazepam produced similar decreases in spontaneous home cage activity in both strains, with the sole exception of the last six h of the dark period where activity was decreased in C57BL/6J mice but not decreased in BALB/cJ mice. Based on percentage change after drug administration relative to that after SAL, C57BL/6 mice showed a greater reduction in activity only after the high dosage of lorazepam than did BALB/c mice. In general, the strains showed less overall differences in the effects of lorazepam on total sleep, total NREM and spontaneous activity and relatively greater differences in the effects on REM. The significant differences in REM suggest that strain comparisons relying solely on performance on standard behavioral paradigms may not reveal the full extent of important strain differences in the effects of BZ agonists.
Strain Comparisons and Behavioral Effects of Benzodiazepines
The C57BL/6 strain is the most commonly studied mouse strain and it ranks higher on most phenotypic indices of inbred mouse strains and exhibits fewer inbred deficits relative to comparison strains (Crawley et al., 1997) including BALB/c mice (Goodrick, 1975; Henderson, 1989). Comparisons between C57BL/6 and BALB/c mice are commonly used to address a variety of genetic and behavioral research questions and their strain differences are of particular interest as models for understanding differential responses to anxiety producing situations. A potential concern in comparing reports of strain differences between C57BL/6 and BALB/c mice is that different substrains have often been used. For example, BALB/cBy and C57BL/6By are frequently used substrains. The divergence of the BALB/c and BALB/cBy substrains occurred in the mid 1930s (Bailey, 1978) whereas the divergence of the C57BL/6 and C57BL/6By occurred in the early 1950s (Bailey, 1978). However, both BALB/c and BALB/cBy have been reported to exhibit behaviors indicative of higher anxiety compared to the C57BL/6 substrains. Compared to C57BL/6By, BALB/cByJ mice are also more reactive to stressors and show larger stressor-provoked hypothalamo-pituitary-adrenal axis responses including greater corticotropin releasing hormone, adrenocorticotropin hormone and corticosterone (Reviewed in (Anisman et al., 2007)).
Studies examining potential strain differences in BZ expression have been conducted on different substrains including BALB/cBy compared to C57BL/6 (Chapouthier et al., 1991), BALB/c compared to C57BL/6 (Hode et al., 2000; Robertson, 1979), and BALB/cBy compared to C57BL/6By (Caldji et al., 2004). The reported strain differences in BZ receptors have been relatively consistent across studies regardless of substrains that are being compared and C57BL/6 and BALB/c mice also show strain differences in the effects of BZ agonists on behavior evoked in stressful or novel situations. For example, diazepam significantly increased time spent in the lit box and number of tunnel crossings in the light-dark box in BALB/c mice but did not alter performance in C57BL/6 mice (Griebel et al., 2000). Diazepam also altered behavior in the elevated plus maze in BALB/c mice and increased the percentage of time spent in open arms and number of open arm entries (Griebel et al., 2000). Significant increases in open arm entries and time spent in the open arm was also found in C57BL/6 mice (Griebel et al., 2000). Other studies have reported that BALB/c mice show minimal effect of BZ agonists on light-dark transitions compared to that seen in C57BL/6 mice [Reviewed in (Crawley et al., 1997)].
Amygdala as a Potential Site of Action for Strain Differences
Compared to C57BL/6 mice, BALB/c mice showed significantly lower density of whole brain binding of 3H-diazepam (Robertson, 1979) and 3H-flunitrazepam (Chapouthier et al., 1991) to BZ membrane receptors. More recent work reported that, compared to C57BL/6 mice, BALB/c mice show significantly less BZ receptor density in the amygdala but not in other regions of the brain that were examined (Hode et al., 2000; Caldji et al., 2004). Diazepam potentiation of the ability of GABA to increase chloride influx into cerebral cortical microsacs was significantly decreased in the BALB/c mice compared to C57BL/6 mice (Mihic et al., 1992). Significantly fewer BZ receptors in the amygdala or whole brain of BALB/c mice would indicate an important alteration in an endogenous modulator of emotion that has been suggested to be involved in the trait anxiety BALB/c mice appear to exhibit (Hode et al., 2000).
Differences in BZ receptor binding in the amygdala were found in the central nucleus of amygdala (CNA), basolateral amygdala (BLA) and lateral amygdala (Caldji et al., 2004; Hode et al., 2000). These regions play roles in fear- and emotion-related behaviors (Davis, 1992; Davis, 1992; Davis & Whalen, 2001) and research that has been conducted thus far on the amygdala and sleep has implicated CNA (Calvo et al., 1996; Sanford et al., 1995; Sanford et al., 1998) and BLA (Zhu et al., 1998) in the regulation of sleep. GABA regulates, along with other inhibitory transmitters, the descending output of CNA (Davis et al., 1994) and a number of studies have reported that functional inactivation of CNA can produce reductions in REM sleep. For example, in rats, microinjections into CNA of the GABAA agonist, muscimol, decreased REM sleep, whereas blocking GABAergic inhibition of CNA with microinjections of the GABAA antagonist, bicuculline, increased REM sleep (Sanford et al., 2002). Neither muscimol nor bicuculline produced significant alterations in NREM sleep. By comparison, bilateral electrolytic and chemical lesions of BLA have been reported to increase NREM and total sleep time whereas microinjections of L-glutamate into BLA, which would produce activation, decreased NREM and total sleep time (Zhu et al., 1998). This suggests that alterations in sleep produced by BZ agonists depend on differential activity in BLA and CNA. Increases in NREM could potentially result from an enhanced inhibitory effect of GABA on BLA, and reductions in REM could arise from an enhanced inhibitory effect of GABA on CNA. Interestingly, CNA and BLA may mediate different aspects of the anxiolytic effects of BZ agonists. In rats, local microinjection of midazolam into BLA impaired avoidance of the open-arms in the elevated maze but did not alter avoidance of a shock probe, whereas administration to CNA produced the opposite effects (Pesold & Treit, 1995).
It should be pointed out that systemically administered drugs affect multiple regions in the brain and the medial preoptic region of the hypothalamus has been implicated in the sleep promoting effects of BZ agonists (e.g., (Mendelson & Monti, 1993; Mendelson & Martin, 1992)). However, the demonstrated strain differences in BZ receptors in the amygdala and its involvement in the control of arousal and sleep suggest it may be a significant locus for understanding the strain differences in the effects of lorazepam and other BZ agents on sleep and activity. Focus on the amygdala as an important site of action is also suggested by functional magnetic resonance imaging findings that bilateral activation of the amygdala and insula induced in an emotion face paradigm (Stein et al., 2007) is attenuated by lorazepam in a dose-dependent fashion (Paulus et al., 2005).
While significant differences in BZ receptor density suggest that the amygdala is an important neural site likely involved in mediating strain differences in the effects of BZs, differences in receptor numbers do not entirely explain the strain differences in the effects of lorazepam on sleep. In humans, clinically effective dosages of lorazepam may occupy only 3% of available receptors (Sybirska et al., 1993); thus, its maximal biological effect may be reached with significantly less than 100% occupancy of the receptors. One possible factor is that “receptor reserve” (spare BZ receptors) may be different between the two strains. In addition, previous authors have suggested that receptor affinity is higher in BALB/c mice, thus accounting for the greater effects of BZ agonists compared to C57BL mice (Chapouthier et al., 1991; Robertson, 1979). It has also been suggested that pharmacokinetic differences in metabolism or transport of BZ receptor ligands into the central nervous system of different mouse strains could produce differential responses to drugs (Mathis et al., 1994; Schweri et al., 1983). Compared to other BZs (e.g., diazepam), lorazepam appears to exit the brain more slowly and to less rapidly diffuse into peripheral tissues (Feldman et al., 1997) which could account for the duration of its effects on sleep. In addition, the BZs act on GABAA receptors to increase their affinity to GABA; thus, their effects depend on the availability of GABA and on activity in GABAergic neurons (Feldman et al., 1997) which could differ between strains and interact with potential differences in drug pharmacokinetics.
The current study demonstrates significant differences in the effects of a systemically administered BZ and sleep and activity in two mouse strains that exhibit different amounts of baseline sleep and activity and that exhibit different levels of responsiveness to stressors. Studies demonstrating significant strain differences in BZ receptors in the amygdala and the role of this region in emotional behaviors as well as in the regulation of sleep provide a potential neural substrate that may underlie strain differences in the effects of BZ agonists on sleep and activity. This may particularly be true for REM which showed the greatest between strain differences in response to lorazepam. Consideration of neural and physiological differences between strains may be of significant aid in understanding differences in responses to pharmacological challenges as wall as strain differences in behavior.
Acknowledgments
This work was supported by supported by NIH research grants MH61716 and MH64827.
References
- Anisman H, Prakash P, Merali Z, Poulter MO. Corticotropin releasing hormone receptor alterations elicited by acute and chronic unpredictable stressor challenges in stressor-susceptible and resilient strains of mice. Behav Brain Res. 2007;181:180–190. doi: 10.1016/j.bbr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Bailey DW. Sources of Subline Divergence and Their Relative Importance for Sublines of Six Major Inbred Strains of Mice. In: Morse HC III, editor. Origins of Inbred Mice. NY: Academic Press, New York; 1978. [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. The use of lorazepam TID for chronic insomnia. Int Clin Psychopharmacol. 1999;14:81–89. doi: 10.1097/00004850-199903000-00004. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Anisman H, Meaney MJ. Maternal behavior regulates benzodiazepine/GABAA receptor subunit expression in brain regions associated with fear in BALB/c and C57BL/6 mice. Neuropsychopharmacology. 2004;29:1344–1352. doi: 10.1038/sj.npp.1300436. [DOI] [PubMed] [Google Scholar]
- Calvo J, Simón-Arceo K, Fernández-Mas R. Prolonged enhancement of REM sleep produced by carbachol microinjection into the amygdala. Neuroreport. 1996;7:577–580. doi: 10.1097/00001756-199601310-00048. [DOI] [PubMed] [Google Scholar]
- Chapouthier G, Bondoux D, Martin B, Desforges C, Launay JM. Genetic difference in sensitivity to beta-carboline: evidence for the involvement of brain benzodiazepine receptors. Brain Res. 1991;553:342–346. doi: 10.1016/0006-8993(91)90847-o. [DOI] [PubMed] [Google Scholar]
- Chouinard G. Issues in the clinical use of benzodiazepines: potency, withdrawal, and rebound. J Clin Psychiatry. 2004;65:7–12. [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Davis M. Animal models of anxiety based on classical conditioning: the conditioned emotional response (CER) and the fear-potentiated startle effect. Pharmaco Ther. 1990;47:147–165. doi: 10.1016/0163-7258(90)90084-f. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned fear. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. NY: Wiley-Liss, Inc, New York; 1992. pp. 255–305. [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Ann Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Feldman R, Meyer J, Quenzer L. Principles of Neuropsychopharmacology. Massachusetts: Sinauer Associates Inc., Sunderland; 1997. [Google Scholar]
- File SE. Behavioural pharmacology of benzodiazepines. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:19–31. doi: 10.1016/0278-5846(84)90132-5. [DOI] [PubMed] [Google Scholar]
- Goodrick CL. Life-span and the inheritance of longevity of inbred mice. J Gerontol. 1975;30:257–263. doi: 10.1093/geronj/30.3.257. [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology. 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Henderson ND. Genetic analysis of an avoidance-avoidance response in Mus domesticus. Behav Genet. 1989;19:387–407. doi: 10.1007/BF01066166. [DOI] [PubMed] [Google Scholar]
- Hode Y, Ratomponirina C, Gobaille S, Maitre M, Kopp C, Misslin R. Hypoexpression of benzodiazepine receptors in the amygdala of neophobic BALB/c mice compared to C57BL/6 mice. Pharmacol Biochem Behav. 2000;65:35–38. doi: 10.1016/s0091-3057(99)00131-8. [DOI] [PubMed] [Google Scholar]
- Mathis C, Paul SM, Crawley JN. Characterization of benzodiazepine-sensitive behaviors in the A/J and C57BL/6J inbred strains of mice. Behav Genet. 1994;24:171–80. doi: 10.1007/BF01067821. [DOI] [PubMed] [Google Scholar]
- Mendelson WB. Hypnotic Medications: Mechanisms of Action and Pharmacological Effects. In: Kryger M, Roth T, Dement W, editors. Principles and Practices of Sleep Medicine. PA: Elsevier Saunders, Philadelphia; 2005. pp. 444–451. [Google Scholar]
- Mendelson WB, Martin JV. Characterization of the hypnotic effects of triazolam microinjections into the medial preoptic area. Life Sci. 1992;50:1117–1128. doi: 10.1016/0024-3205(92)90349-t. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Monti D. Effects of triazolam and nifedipine injections into the medial preoptic area on sleep. Neuropsychopharmacology. 1993;8:227–232. doi: 10.1038/npp.1993.25. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Van Berckel BN, O'Dowd BF, Nguyen T, Wu PH. Effects of sedatives on GABA-mediated chloride flux into cerebral cortical microsacs prepared from emotional and non-emotional mice. Eur J Pharmacol. 1992;218:283–286. doi: 10.1016/0014-2999(92)90180-c. [DOI] [PubMed] [Google Scholar]
- Niehoff D, Kuhar M. Benzodiazepine receptors: localization in rat amygdala. J Neurosci. 1983;3:2091–2097. doi: 10.1523/JNEUROSCI.03-10-02091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoe H, Tsukada H, Nishiyama S, Nakanishi S, Inoue O, Langstrom B, Watanabe Y. A subclass of GABAA/benzodiazepine receptor exclusively localized in the limbic system. Neuroreport. 1996;8:117–122. doi: 10.1097/00001756-199612200-00024. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry. 2005;62:282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Pesold C, Treit D. The central and basolateral amygdala differentially mediate the anxiolytic effects of benzodiazepines. Brain Res. 1995;671:213–221. doi: 10.1016/0006-8993(94)01318-c. [DOI] [PubMed] [Google Scholar]
- Robertson HA. Benzodiazepine receptors in “emotional” and “non-emotional” mice; comparison of four strains. Eur J Pharmacol. 1979;56:163–166. doi: 10.1016/0014-2999(79)90447-3. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Zorick FJ, Roth T. Transient and short-term insomnia. In: Kryger M, Roth T, Dement W, editors. Principles and Practices of Sleep Medicine. PA: Saunders, Philadelphia; 2000. pp. 624–632. [Google Scholar]
- Sanders S, Shekhar A. Anxiolytic effects of chlordiazepoxide blocked by injection of GABAA and benzodiazepine receptor antagonists in the region of the anterior basolateral amygdala of rats. Biol Psychiatry. 1995;37:473–476. doi: 10.1016/0006-3223(94)00183-4. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Nassar P, Ross RJ, Schulkin J, Morrison AR. Prolactin microinjections into the amygdalar central nucleus lead to decreased NREM sleep. Sleep Res Online. 1998;1:109–113. [PubMed] [Google Scholar]
- Sanford LD, Parris B, Tang X. GABAergic regulation of the central nucleus of the amygdala: implications for sleep control. Brain Res. 2002;956:276–284. doi: 10.1016/s0006-8993(02)03552-7. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Tang X, Ross RJ, Morrison AR. Influence of shock training and explicit fear-conditioned cues on sleep architecture in mice: strain comparison. Behav Genet. 2003a;33:43–58. doi: 10.1023/a:1021051516829. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Tejani-Butt SM, Ross RJ, Morrison AR. Amygdaloid control of alerting and behavioral arousal in rats: involvement of serotonergic mechanisms. Arch Ital Biol. 1995;134:81–99. [PubMed] [Google Scholar]
- Sanford LD, Yang L, Tang X. Influence of contextual fear on sleep in mice: a strain comparison. Sleep. 2003b;26:527–540. doi: 10.1093/sleep/26.5.527. [DOI] [PubMed] [Google Scholar]
- Shibata S, Yamashita K, Yamamoto E, Ozaki T, Ueki S. Effects of benzodiazepine and GABA antagonists on anticonflict effects of antianxiety drugs injected into the rat amygdala in a water-lick suppression test. Psychopharm. 1989;98:38–44. doi: 10.1007/BF00442003. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Tang X, Orchard SM, Sanford LD. Home cage activity and behavioral performance in inbred and hybrid mice. Behav Brain Res. 2002a;136:555–569. doi: 10.1016/s0166-4328(02)00228-0. [DOI] [PubMed] [Google Scholar]
- Tang X, Orchard SM, Sanford LD. Home cage activity and behavioral performance in inbred and hybrid mice. Behav Brain Res. 2002b;136:555–569. doi: 10.1016/s0166-4328(02)00228-0. [DOI] [PubMed] [Google Scholar]
- Tang X, Sanford LD. Telemetric recording of sleep and home cage activity in mice. Sleep. 2002c;25:691–699. [PubMed] [Google Scholar]
- Tang X, Xiao J, Liu X, Sanford LD. Strain differences in the influence of open field exposure on sleep in mice. Behav Brain Res. 2004;154:137–147. doi: 10.1016/j.bbr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Tang X, Xiao J, Parris BS, Fang J, Sanford LD. Differential effects of two types of environmental novelty on activity and sleep in BALB/cJ and C57BL/J mice. Physiol Behav. 2005;85:419–429. doi: 10.1016/j.physbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Yadin E, Thomas E, Strickland C, Grishkat H. Anxiolytic effects of benzodiazepines in amygdala-lesioned rats Psychopharm. 1991;103:473–479. doi: 10.1007/BF02244247. [DOI] [PubMed] [Google Scholar]
- Zezula J, Cortes R, Probst A, Palacios JM. Benzodiazepine receptor sites in the human brain: autoradiographic mapping. Neurosci. 1988;25:771–795. doi: 10.1016/0306-4522(88)90036-x. [DOI] [PubMed] [Google Scholar]
- Zhu GQ, Zhong MK, Zhang JX, Zhao LZ, Ke DP, Wang M, Shi L. Role of basolateral amygdaloid nuclei in sleep and wakeful state regulation. Sheng Li Xue Bao. 1998;50:688–692. [PubMed] [Google Scholar]