Abstract
Signaling via endothelial nitric oxide synthase (NOS3) limits the heart’s response to β-adrenergic (β-AR) stimulation, which may be protective against arrhythmias. However, mechanistic data are limited. Therefore, we performed simultaneous measurements of action potential (AP, using patch clamp), Ca2+ transients (fluo 4), and myocyte shortening (edge detection). L-type Ca2+ current (ICa) was directly measured by the whole cell ruptured patchclamp technique. Myocytes were isolated from wild-type (WT) and NOS3 knockout (NOS3−/−) mice. NOS3−/− myocytes exhibited a larger incidence of β-AR (isoproterenol, 1 µM)-induced early afterdepolarizations (EADs) and spontaneous activity (defined as aftercontractions). We also examined ICa, a major trigger for EADs. NOS3−/− myocytes had a significantly larger β-AR- stimulated increase in ICa compared with WT myocytes. In addition, NOS3−/− myocytes had a larger response to β-AR stimulation compared with WT myocytes in Ca2+ transient amplitude, shortening amplitude, and AP duration (APD). We observed similar effects with specific NOS3 inhibition [l-N5-(1-iminoethyl)-ornithine (l-NIO), 10 µM] in WT myocytes as with NOS3 knockout. Specifically, l-NIO further increased isoprot-erenol-stimulated EADs and aftercontractions. l-NIO also further increased the isoproterenol-stimulated ICa, Ca2+ transient amplitude, shortening amplitude, and APD (all P < 0.05 vs isoproterenol alone). l-NIO had no effect in NOS3−/− myocytes. These results indicate that NOS3 signaling inhibits the β-AR response by reducing ICa and protects against arrhythmias. This mechanism may play an important role in heart failure, where arrhythmias are increased and NOS3 expression is decreased.
Keywords: endothelial nitric oxide synthase, cardiac myocytes, early afterdepo-larizations, action potential
Stimulation of the β-adrenergic (β-AR) pathway is an important regulator of cardiac contractility, leading to positive inotropic and lusitropic effects (7). Activation of the cAMP-dependent protein kinase (PKA) leads to phosphorylation of several myocyte proteins, including the L-type Ca2+ channel, phospholamban, the ryanodine receptor, myosin-binding protein C, and troponin I. PKA-dependent phosphorylation of the L-type Ca2+ channel causes an increase in Ca2+ influx, which contributes to the enhanced Ca2+ cycling and the positive inotropic effect.
Nitric oxide (NO), produced via NO synthase (NOS), is also an important regulator of cardiac contractility (54). Cardiac myocytes constitutively express neuronal NOS (nNOS, NOS1) and endothelial NOS (eNOS, NOS3). Recent studies have shown that NOS1 and NOS3 differentially regulate the response to β-AR stimulation (4). NOS1 signaling has been found to potentiate the response to β-AR stimulation (4). The observed effects of NOS3 signaling on the β-AR response are the opposite of NOS1 (30), that is, studies have shown that NOS3 signaling depresses the functional response to β-AR stimulation. For example, mice with specific knockout of NOS3 (NOS3−/−) have an increased response to β-AR stimulation (4, 11, 19, 20, 47). Similarly, transgenic mice with cardiac myocyte-specific NOS3 overexpression have a decreased response to β-AR stimulation (9, 23). Although the vast majority of studies found the above effects, it should be noted that a few studies observed dissimilar results (29, 46). In addition, there are limited studies investigating the mechanism of the NOS3-induced reduction of the β-AR response.
The regulation of β-AR stimulation by NOS3 may be via modulation of the L-type Ca2+ channel, since one study has shown that nonspecific NOS inhibition can further increase the cAMP-stimulated L-type Ca2+ current (ICa) (33). However, specific NOS isoforms were not examined in this study. Exogenous NO (i.e., NO donors) has also been found to decrease β-AR-stimulated ICa (48). NOS3 is localized to the caveolae, along with the L-type Ca2+ channel and the β2-AR receptor (3, 17). In addition, a study showed that myocytes from female mouse hearts have smaller β-AR -induced ICa and a higher association between NOS3 and caveolin-3 compared with myocytes from male mouse hearts (43). Indirect evidence also suggests that NOS3 can regulate ICa via activation of the β3-AR receptor (4, 47, 53). These studies have led groups to hypothesize that NOS3 regulates ICa (4, 28, 43). However, this has yet to be observed in NOS3−/− myocytes (21, 46).
High sympathetic activation and specifically increased ICa can be detrimental to cardiac myocytes, leading to arrhythmias (24). Interestingly, transgenic mice overexpressing NOS3 demonstrated a lower incidence of spontaneous arrhythmic contractions in cultured neonatal myocytes (32). In addition, NOS3−/− mice had a higher incidence of arrhythmias (28, 39). However, the mechanism of the antiarrhythmic effect of NOS3 is unknown. The regulation of ICa by NOS3 would also affect the action potential (AP) waveform, and AP prolongation may play a role in reentrant arrhythmias (37). There are no studies that we are aware of examining the AP waveform in isolated myocytes from NOS3−/− mice. Therefore, the purpose of this study is to examine the effects of NOS3 knockout or acute inhibition on AP, Ca2+ transients, and myocyte shortening. We also directly measured ICa. We hypothesize that NO produced via NOS3 is protective against arrhythmias (defined as early afterdepolarizations and aftercontractions) by modulation of the β-AR-stimulated Ica. This reduction in ICa will also result in a decrease in action potential duration (APD) measured as time to 90% repolarization (APD90), Ca2+ transients, and myocyte shortening amplitude.
MATERIALS AND METHODS
Isolation of ventricular myocytes
Ventricular myocytes were isolated from NOS3−/− mice (42, 52; Jackson Laboratories, Bar Harbor, ME) and corresponding wild-type (WT) mice (C57BL/6J) as previously described (27). Briefly, the heart was mounted on a Langendorff apparatus and perfused with modified MEM (37°C, bubbled with 95% O2-5% CO2; Sigma, St. Louis, MO). Blendzyme type IV (0.077 mg/ml; Roche Applied Science, Indianapolis, IN) was then added to the perfusate. After 10–15 min, the heart was taken down, the ventricles were minced, and myocytes were dissociated by trituration. Subsequently, the myocytes were filtered, centrifuged, and resus-pended in MEM containing 200 µM Ca2+. Myocytes were used within 6 h after isolation. All animal protocols and procedures were performed in accordance with National Institutes of Health guidelines and approved by the Institutional Laboratory Animal Care and Use Committee at The Ohio State University.
Measurement of ICa
Whole cell voltage-clamp was used to measure Ica using an Axopatch-200B amplifier and pClamp 8.1 software (Axon Instrument, Foster City, CA), as described previously (56). Electrodes (borosilicate glass tubing), with a resistance of 1.5–3 MΩ, were filled with (in mM): 120 CsCl, 6 MgCl2, 10 EGTA, 10 HEPES, and 2 MgATP, pH 7.2 adjusted with CsOH. The bath solution consisted of (in mM): 120 NaCl, 4 CsCl, 1 MgCl2, 1 CaCl2, 10 glucose, 5 HEPES, and 1 L-arginine, pH 7.4 adjusted with CsOH or HCl. lCa was elicited by 200-ms pulses to 0 mV from a holding potential of −80 mV (following a prepulse to −40 mV) at a frequency of 0.2 Hz. This procedure isolates the Ica by inactivation of the Na+ current with the prepulse, and replacement of K+ with Cs+ eliminates the K+ current. Measurements were performed at room temperature.
Simultaneous measurement of Ca2+ transients, cell shortening, and AP
Myocytes were loaded at 22°C with fluo 4-AM (10 µM; Molecular Probes, Eugene, OR) for 30 min and washed out, and then 30 min were allowed for intracellular deesterification. The instrumentation used for cell fluorescence measurement was a Cairn Research Limited (Faversham, UK) epifluorescence system. Intracellular Ca2+ concentration was measured by fluo 4 epifluorescence with excitation at 480 ± 20 nm and emission at 535 ± 25 nm. The illumination field was restricted to collect the emission of a single cell. Data were expressed as ΔF/F0, where F is the fluorescence intensity and F0 is the intensity at rest. Uneven indicator loading, photobleaching, and motion artifact errors may be introduced by using the singal wavelength Ca2+ indicator, fluo 4. However, since each myocyte served as its own control, these errors should be minimized. Simultaneous measurement of shortening was also performed using an edge detection system (Crescent Electronics, Sandy, UT). Data were expressed as cell shortening (µm). AP were recorded with the whole cell current-clamp technique using an Axopatch-200B amplifier and pClamp 8.1 software (Axon Instrument). The pipettes, resistance of 9–11 MΩ, were filled with (in mM): 8 NaCl, 10 KCl, 140 potassium aspartate, 5 HEPES, and 2 MgATP, pH 7.2 adjusted with KOH or HCl. A Grass S48 stimulator gated the amplifier for current injection to activate the AP, triggered by a 1- to 5-ms, 2-nA current injection. Measurements were performed at room temperature.
Solution and drugs
Normal Tyrode solution consisted of (in mM): 140 NaCl, 4 KCl, 1 MgCl2, 1 CaCl2, 10 glucose, 5 HEPES, and 1 L-arginine, pH 7.4 adjusted with NaOH or HCl. Isoproterenol (ISO, 1 µM, Sigma), a nonselective β-AR agonist, and l-N5-(1-iminoethyl)-ornithine (l-NIO, 10 µM, Sigma), a specific NOS3 inhibitor (50) were prepared fresh each experimental day.
Statistics
Myocyte data were averaged per heart and presented as means ± SE. Differences between multiple groups were evaluated for statistical significance using an ANOVA (followed by Neuman-Keuls test) or by paired or unpaired Student’s t-test for two groups. Statistical significance was accepted at the level of P < 0.05.
RESULTS
NOS3 and arrhythmogenesis
Previous studies have shown that NOS3 knockout leads to increased arrhythmias in ouabaintreated myocytes or with digoxin in vivo (28, 39). Thus we investigated if NOS3 knockout or inhibition increases the β-AR-stimulated arrhythmogenesis at the level of the myocyte. Figure 1 shows representative examples, in the presence of β-AR stimulation (ISO, 1 µM), of early afterdepolarizations (EADs) in NOS3−/− and WT myocytes with NOS3 inhibition (l-NIO, 10 µM) (Fig. 1A). Summary data are shown in Fig. 1B. No EADs were observed in WT or NOS3−/− myocytes during control (basal) stimulation. In the presence of ISO (1 µM), 57 ± 12% of NOS3−/− myocytes per heart vs only 8 ± 5% (P < 0.05 vs NOS3−/−) of WT myocytes per heart displayed EADs. Acute inhibition of NOS3, in the presence of ISO, increased the incidence of EADs in WT myocytes per heart (37 ± 11%, P < 0.05 vs. ISO alone). Spontaneous activity (defined as aftercontractions, ACs) was also observed in NOS3−/− myocytes during β-AR stimulation (Fig. 1C). Summary data are shown in Fig. 1D. No ACs were observed in WT or NOS3−/− myocytes during basal stimulation. However, during β-AR stimulation, 69 ± 10% of NOS3−/− myocytes per heart vs. only 6 ± 6% (P < 0.05 vs NOS3−/−) of WT myocytes per heart had ACs. Acute inhibition of NOS3, in the presence of ISO, increased the incidence of ACs in WT myocytes per heart (35 ± 9%, P < 0.05 vs. ISO alone). During β-AR stimulation, we observed EADs and ACs in myocytes isolated from all NOS3−/− hearts (100%), whereas myocytes from only 25% of WT hearts exhibited spontaneous activity. These data suggest that NOS3 signaling can protect cardiac myocytes from β-AR-stimulated arrhythmias.
Figure 1.
A: top, example of an action potential (AP) that shows early afterdepolarizations (EADs) in the presence of isoproterenol (ISO, 1 µM) and l-N5-(1-iminoethyl)-ornithine (L-NIO, 10 µM, specific NOS3 inhibitor) in a wild-type (WT) myocyte. Bottom, example of an AP that shows EADs in the presence of ISO in a endothelial nitric oxide synthase knockout (NOS3−/−) myocyte. B: summary data, %myocytes/ heart that showed EADs. C: example of a NOS3−/− myocyte that shows spontaneous activity in AP (top) and shortening (bottom) after ISO stimulation. S, spontaneous AP; AC, aftercontraction. D: %myo-cytes/heart that showed aftercontractions. *P < 0.05 vs. WT ISO; n = 8 hearts for WT, n = 5 hearts for NOS3−/−.
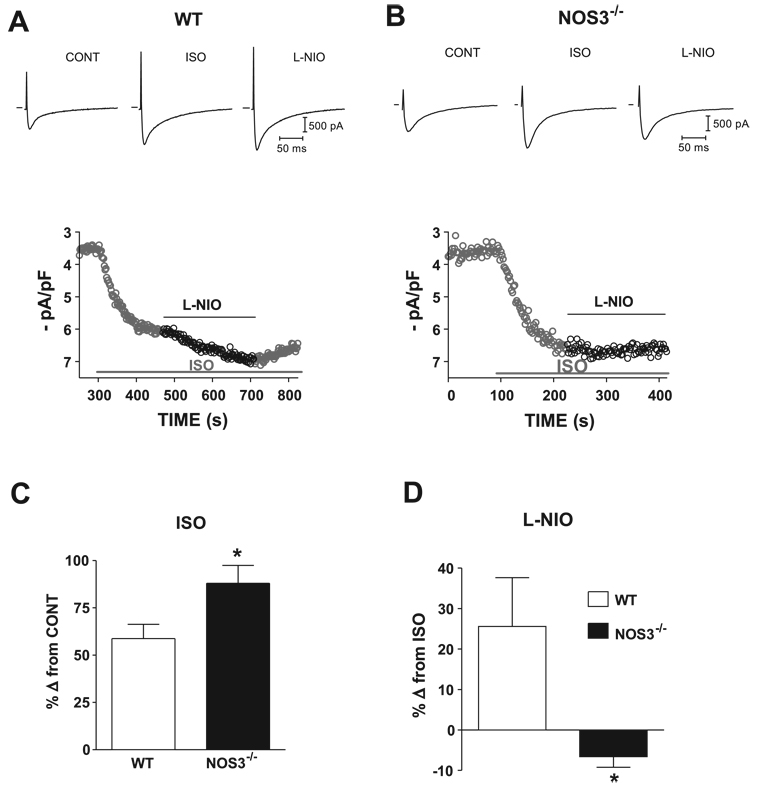
Effects of NOS3 on ICa
Abnormal L-type Ca2+ channel activity can cause EADs (24). Thus we examined the effects of NOS3 signaling on the ICa in myocytes from WT and NOS3−/− mice. Our data showed that there was no statistical difference in basal ICa between the two groups (WT: 3.5 ± 0.2 pA/pF, NOS3−/−: 3.8 ± 0.4 pA/pF). Figure 2, A and B, shows representative current traces and time plots of the effects of β-AR stimulation (ISO, 1 µM) and NOS3 inhibition (l-NIO) in a WT myocyte and a NOS3−/− myocyte. Figure 2C shows NOS3−/− myocytes had a significantly larger response to β-AR stimulation compared with WT (increased 88 ± 10% of control in NOS3−/− vs. 58 ± 7% of control in WT, P <0.05). In addition, specific NOS3 inhibition with l-NIO, in the presence of ISO, further increased ICa in WT myocytes (26 ± 12% from ISO alone) but had no effect in NOS3−/− myocytes (decreased 7 ± 3% from ISO alone, P < 0.05 vs. WT; Fig. 2D). This small decrease was most likely due to the rundown of Ca2+ current. These data indicate that the L-type Ca2+ channel is an important end target of NOS3 and are consistent with a NOS3-mediated reduction in ICa being protective against arrhythmias.
Figure 2.
A and B: representative traces (top) and time plot (bottom) of ICa with ISO (1 µM) and L-NIO (10 µM), selective NOS3 inhibitor, in a WT (A) and NOS3−/− (B) myocyte. C: summary data (means ± SE) of the effects of ISO in WT and NOS3−/− myocytes. D: summary data (means ± SE) of the effects of L-NIO in WT and NOS3−/− myocytes. *P < 0.05 vs. WT (n = 5 hearts for WT; n = 5 hearts for NOS3−/−).
Effects of NOS3-derived NO on myocyte function
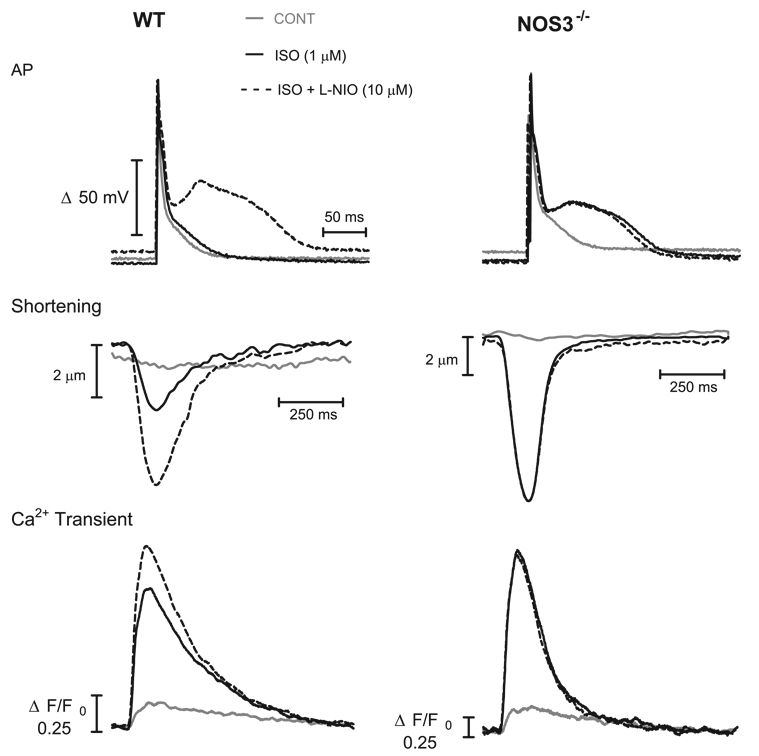
We also tested the effects of NOS3 on myocyte function. Functional experiments were performed on isolated myocytes from NOS3−/− and WT mice in which AP, Ca2+ transients, and shortening were simultaneously measured at a stimulation frequency of 1 Hz. Representative examples of AP (top), myocyte shortening (middle), and Ca2+ transient (bottom) traces from a WT and NOS3−/− myocyte are shown in Fig. 3 and summarized in Fig. 4 and Fig. 5 .
Figure 3.
Representative examples of AP (top), shortening (middle), and Ca2+ transients (bottom) from a WT (left) and a NOS3−/− (right) myocyte.
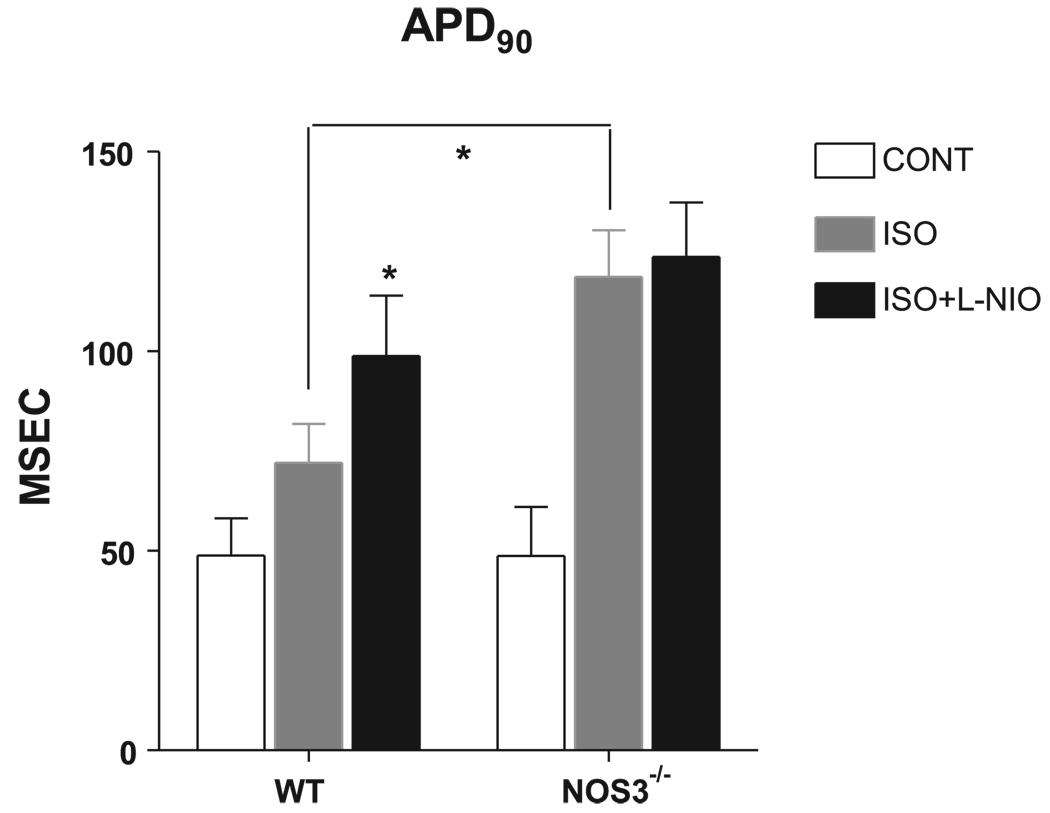
Figure 4.
Summary data (means ± SE) of the effects of ISO (1 µM) and L-NIO (selective NOS3 inhibitor, 10 µM) on action potential duration measured as time to 90% repolarization (APD90). *P < 0.05 vs. WT ISO; n = 8 hearts for WT, n = 5 hearts for NOS3−/−.
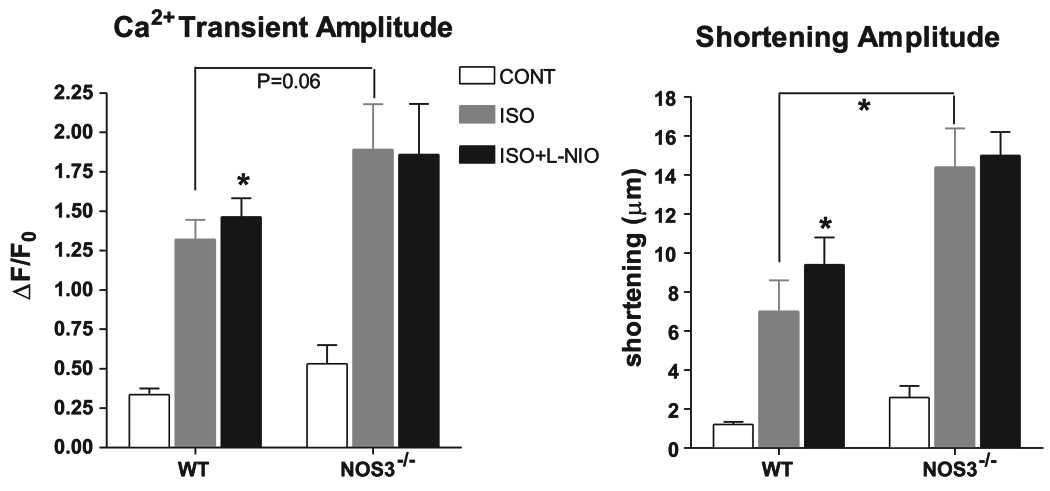
Figure 5.
Summary data (means ± SE) of the effects of ISO (1 µM) and L-NIO (10 µM), a selective NOS3 inhibitor, on Ca2+ transients and shortening amplitude. *P < 0.05 vs. WT ISO; n = 8 hearts for WT, n = 5 hearts for NOS3−/−.
After reaching steady state, the myocytes were perfused with ISO (1 µM). In WT myocytes, β-AR stimulation increased APD, measured as time to 90% repolarization (APD90) (49 ± 9 vs. 72 ± 10 ms), shortening amplitude (1.2 ± 0.1 vs. 7 ± 2 µm), and Ca2+ transient amplitude (0.34 ± 0.04 vs. 1.3 ± 0.1 ΔF/F0) and hastened the rate of Ca2+ decline, measured as time to 50% relaxation (RT50) (267 ± 20 vs. 147 ± 8 ms) (all P < 0.05 vs. control). In NOS3−/− myocytes, ISO also increased APD90 (49 ± 12 vs. 119 ± 12 ms), shortening amplitude (2.6 ± 0.6 vs. 14 ± 2 µm), and Ca2+ transient amplitude (0.5 ± 0.1 vs. 1.9 ± 0.3 ΔF/F0) and hastened the rate of Ca2+ decline (Ca2+ transient RT50: 276 ± 39 vs. 124 ± 7 ms; all P < 0.05 vs. control). However, there was a larger response to β-AR stimulation in myocytes from NOS3−/− compared with WT mice in APD90 (P < 0.05), shortening amplitude (P < 0.05), and a trend in Ca2+ transient amplitude (P = 0.06). These data suggest that myocytes from NOS3−/− mice have a larger response to β-AR stimulation compared with WT myocytes.
After steady state, the bath solution was switched to ISO (1 µM) plus l-NIO (10 µM). In NOS3−/− myocytes, there was no effect of l-NIO on APD90 (124 ± 14 ms), Ca2+ transient amplitude (1.9 ± 0.3 ΔF/F0), or shortening amplitude (15 ± 1 µm) compared with ISO alone. Thus l-NIO had no effect on these aspects of NOS3−/− myocyte function. However, l-NIO further increased β-AR-stimulated APD90 (99 ± 15 ms), Ca2+ transient amplitude (1.5 ± 0.1 ΔF/F0), and shortening amplitude (9 ± 1 µm) in WT myocytes (all P < 0.05 vs. ISO alone). These data demonstrate that the increased response to β-AR stimulation in myocytes from NOS3−/− mice is due to the deletion of NOS3 and not to phenotypic adaptation.
We also observed no change in the response to β-AR stimulation in the Ca2+ transient RT50 between NOS3−/− and WT myocytes (WT: decreased 56 ± 4% from control with ISO; NOS3−/−: decreased 52 ± 10% from control with ISO). In addition, in β-AR-stimulated WT myocytes, l-NIO had no effect on Ca2+ transient RT50 (decreased 5 ± 5% from ISO alone). Thus these data suggest that NOS3 signaling does not modulate sarcoplasmic reticulum (SR) Ca2+ uptake. Overall, our myocyte functional data suggest that NOS3 deletion or inhibition leads to an increased response to β-AR stimulation in terms of APD90, Ca2+ transient amplitude, and shortening amplitude.
DISCUSSION
It is known that NOS3 signaling leads to antiadrenergic effects and is protective against arrhythmias. However, the mechanism(s) of this effect is unknown. Spontaneous activity (EADs and aftercontractions) in our study was observed at the level of NOS3−/− myocytes (NOS3 gene deficiency was confirmed by Western Blot, data not shown) or with acute NOS3 inhibition in WT myocytes. We also investigated ICa in NOS3−/− myocytes. We are the first to report that NOS3−/− myocytes have an increased ICa in response to β-AR stimulation. We further demonstrated that acute NOS3 inhibition in WT myocytes also caused a further increase in the β-AR-stimulated ICa. We simultaneously measured AP, Ca2+ transients, and myocyte shortening. We observed that NOS3−/− myocytes had a significantly prolonged APD, increased myocyte shortening, and a trend toward increased Ca2+ transient amplitude with β-AR stimulation compared with WT myocytes. These same results were observed with acute NOS3 inhibition in WT myocytes (i.e., significantly prolonged APD, increased Ca2+ transient amplitude, and myocyte shortening). Therefore, it is likely that NOS3 signaling modulates ICa to limit the β-AR response and protect against arrhythmias.
NOS3 and arrhythmogenesis
Previous studies have observed that NOS3−/− mice have an increased incidence of arrhythmias. This was observed as increased ouabain-induced aftercontractions in isolated NOS3−/− myocytes due to an increase in a transient inward current (most likely Na+/Ca2+ exchanger) (28). Similar effects were demonstrated in a study observing electrocardiograms in NOS3−/− and WT mice. Digoxin induced more premature ventricular beats and ventricular tachycardia in the NOS3−/− mice (39). These premature ventricular beats are analogous to the afterdepolarizations observed in their isolated myocyte studies. Our data show that myocytes with NOS3 knockout or acute NOS3 inhibition had an increased incidence of EADs and aftercontractions in response to β-AR stimulation (Fig. 1). Previous work has shown that EADs can result from abnormal ICa activity (24). In addition to EADs, increased Ca2+ influx via ICa can also lead to aftercontractions. This increased Ca2+ influx can lead to SR Ca2+ overload and spontaneous release, resulting in aftercontractions (45). Therefore, NOS3 signaling is protective against arrhythmias by inhibiting β-AR stimulated ICa.
NOS3 and the ICa
Studies have shown that EADs can be induced using the L-type Ca2+ channel agonist BAY K 8644 (15, 24). Additionally, studies have shown that L-type Ca2+ channel antagonists can significantly decrease the occurrence of EADs (24, 44). Exogenous NO (i.e., NO donors) and nonspecific NOS inhibitors have also been shown to regulate β-AR-stimulated ICa (33, 48), and it was hypothesized that this effect was via NOS3. Within cardiac myocytes, NOS3 is localized with the L-type Ca2+ channel and the β2-AR receptor to the caveolae (3, 17). However, studies investigating β-AR-stimulated ICa in NOS3−/− myocytes observed no difference compared with WT myocytes (21, 46). We observed an increase in the β-AR-stimulated ICa from NOS3−/− myocytes compared with WT myocytes (Fig. 2). We also observed that acute NOS3 inhibition in WT myocytes further increased the β-AR-stimulated ICa (Fig. 2). Thus our data demonstrate that NOS3 is able to modulate the β-AR-stimulated ICa. We believe that the inability of previous studies to observe a difference in the β-AR-stimulated ICa in NOS3−/− myocytes was due to the lack of L-arginine (precursor of NO) in the solutions they used. We (55) and others (40) have found for endogenous NO to affect ICa measured using the ruptured patch-clamp technique, L-arginine must be added to the solution. Additionally, we did not observe a difference in basal ICa between NOS3−/− and WT myocytes, suggesting that NOS3 specifically modulates the β-AR-stimulated ICa. Thus the NOS3-mediated reduction in β-AR-stimulated ICa will reduce the incidence of EADs and aftercontractions and protect the heart from arrhythmias.
In cardiac myocytes, NOS3 is localized in the caveolae with superoxide dismutase (SOD; see Refs. 8 and 17), a superoxide scavenger that which will prevent from reacting with NO. Thus NO generated from NOS3 is more likely to activate guanylate cyclase and subsequently form cGMP. The cGMP pathway in cardiac myocytes is primarily via activation of the cGMP-dependent protein kinase (PKG) (49). Previous work has shown that exogenous NO (48), cGMP analogs (26) or exogenous PKG (34) can decrease β-AR-stimulated ICa. Recent work has shown that PKG can phosphorylate the α1C-subunit of L-type Ca2+ channel at position Ser533 (25) and that this phosphorylation does occur within cardiac myocytes (51).
NOS3 and myocyte function
ICa, rigger leading to Ca2+ release from the SR, is an important contributor to myocyte contraction (5). For example, increased ICa, via transgenesis or adenovirally mediated, increases contraction (13, 35). Because we observed a difference in β-AR-stimulated ICa, we also investigated myocyte function by simultaneously measuring AP, Ca2+ transients, and myocyte shortening (Fig. 3). We observed that β-AR-stimulated Ca2+ transient amplitude (trend) and shortening amplitude (Fig. 5) were significantly greater in NOS3−/− myocytes than WT myocytes. We did not observe any difference in the response to β-AR stimulation in the Ca2+ transient RT50 between NOS3−/− and WT myocytes. These data suggest that NOS3 does not regulate SR Ca2+ uptake. Acute NOS3 inhibition in WT myocytes also led to a further, significant increase in β-AR-stimulated Ca2+ transient amplitude and myocyte shortening amplitude. NOS3 inhibition did not change β-AR-stimulated Ca2+ transient decline, further supporting the idea that NOS3 does not regulate SR Ca2+ uptake. We also observed large increases in APD90 with NOS3 knockout or acute inhibition with β-AR stimulation (Fig. 4). This increase can be contributed to increased ICa leading to increased Na+/Ca2+ exchanger activity. It has also been demonstrated that NOS3 is able to modulate K+ channels. Specifically, activation of NOS3 leads to an enhancement of slow-delayed rectifier K+ current and shortening of APD in guinea pig myocytes (2). However, in normal adult mouse ventricular myocytes, the expression of delayed-rectifier K+ channel is very low (22), and the functional effects of Iks on AP waveform are still undetermined (36). With NOS3 knockout (or inhibition), we could potentially be inhibiting a K+ current that would result in a prolonged APD. It is known that prolonged APD is a contributing factor to reentrant arrhythmias (38). Thus NOS3 signaling is antiadrenergic in limiting the increase in Ca2+ transient and shortening amplitude and also lessens APD. We believe NOS3 signaling may be acting as an endogenous β-blocker (31) protecting against arrhythmias. β-Blockers have been found to reduce sudden cardiac death in heart failure (33a).
l-NIO is a selective NOS3 inhibitor (50) and did not have any effect in NOS3−/− myocytes at 10 µM. Therefore, 10 µM l-NIO did not appear to have any nonspecific (e.g., NOS1 inhibition) effects under our experimental conditions. As discussed, acute NOS3 inhibition with l-NIO in WT myocytes further increased EADs, aftercontractions, ICa, APD90, Ca2+ transient, and shortening amplitude. Because similar results were observed with NOS3 knockout or inhibition, we are confident that NOS3 signaling has antiadrenergic and antiarrhythmic effects.
Although we found significant effects of NOS3 knockout and inhibition on β-AR-stimulated myocyte function, NOS3 signaling may be more important in protecting the heart. A study found that, after chronic pressure overload, NOS3−/− mice had increased hypertrophy, fibrosis, and contractile dysfunction compared with WT mice (41). In addition, mice with cardiac myocyte-specific NOS3 overexpression had limited hypertrophy and contractile dysfunction after pressure overload and myocardial infarction (10, 23). Furthermore, it is known that ICa plays a role in hypertrophy (14). Ca2+ influx via ICa activates calcineurin, leading to nuclear factor of activated T cells dephosphorylation and the activation of hypertrophic signaling. Exogenous NO-mediated inhibition of ICa can inhibit this calcineurin activation (18). Ca2+ influx via ICa also leads to apoptosis (12) and arrhythmias (1). Thus increased Ca2+ influx via β-AR-stimulated ICa can be detrimental to the myocyte. Interestingly, it has been observed that NOS3 expression is decreased in human heart failure (16). Thus we believe that NOS3 plays an important, protective role against toxic sympathetic activation by reducing the L-type Ca2+ current and APD.
Acknowledgments
GRANTS
This work was supported by The American Heart Association [Postdoctoral Fellowship 0725560B (H. Wang); Predoctoral Fellowship 0715159B (M. J. Kohr); Scientist Development Grant 0335385Z (M. T. Ziolo)] and National Heart, Lung, and Blood Institute Gratn R01 HL-079283 (M. T. Ziolo).
REFERENCES
- 1.Anderson ME. Calmodulin kinase and L-type calcium channels; a recipe for arrhythmias? Trends Cardiovasc Med. 2004;14:152–161. doi: 10.1016/j.tcm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Bai CX, Namekata I, Kurokawa J, Tanaka H, Shigenobu K, Furukawa T. Role of nitric oxide in Ca2+ sensitivity of the slowly activating delayed rectifier K+ current in cardiac myocytes. Circ Res. 2005;96:64–72. doi: 10.1161/01.RES.0000151846.19788.E0. [DOI] [PubMed] [Google Scholar]
- 3.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci USA. 2006;103:7500–7505. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O’Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 5.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 7.Bers DM, Ziolo MT. When is cAMP not cAMP? Effects of compartmen-talization. Circ Res. 2001;89:373–375. [PubMed] [Google Scholar]
- 8.Brahmajothi MV, Campbell DL. Heterogeneous basal expression of nitric oxide synthase and superoxide dismutase isoforms in mammalian heart: implications for mechanisms governing indirect and direct nitric oxide-related effects. Circ Res. 1999;85:575–587. doi: 10.1161/01.res.85.7.575. [DOI] [PubMed] [Google Scholar]
- 9.Brunner F, Andrew P, Wolkart G, Zechner R, Mayer B. Myocardial contractile function and heart rate in mice with myocyte-specific overex-pression of endothelial nitric oxide synthase. Circulation. 2001;104:3097–3102. doi: 10.1161/hc5001.101966. [DOI] [PubMed] [Google Scholar]
- 10.Buys ES, Raher MJ, Blake SL, Neilan TG, Graveline AR, Passeri JJ, Llano M, Perez-Sanz TM, Ichinose F, Janssens S, Zapol WM, Picard MH, Bloch KD, Scherrer-Crosbie M. Cardiomyocyte-restricted restoration of nitric oxide synthase 3 attenuates left ventricular remodeling after chronic pressure overload. Am J Physiol Heart Circ Physiol. 2007;293:H620–H627. doi: 10.1152/ajpheart.01236.2006. [DOI] [PubMed] [Google Scholar]
- 11.Champion HC, Georgakopoulos D, Takimoto E, Isoda T, Wang Y, Kass DA. Modulation of in vivo cardiac function by myocyte-specific nitric oxide synthase-3. Circ Res. 2004;94:657–663. doi: 10.1161/01.RES.0000119323.79644.20. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 14.Cingolani E, Ramirez Correa GA, Kizana E, Murata M, Cho HC, Marban E. Gene therapy to inhibit the calcium channel beta subunit: physiological consequences and pathophysiological effects in models of cardiac hypertrophy. Circ Res. 2007;101:166–175. doi: 10.1161/CIRCRESAHA.107.155721. [DOI] [PubMed] [Google Scholar]
- 15.Damiano BP, Rosen MR. Effects of pacing on triggered activity induced by early afterdepolarizations. Circulation. 1984;69:1013–1025. doi: 10.1161/01.cir.69.5.1013. [DOI] [PubMed] [Google Scholar]
- 16.Damy T, Ratajczak P, Shah AM, Camors E, Marty I, Hasenfuss G, Marotte F, Samuel JL, Heymes C. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet. 2004;363:1365–1367. doi: 10.1016/S0140-6736(04)16048-0. [DOI] [PubMed] [Google Scholar]
- 17.Feron O, Dessy C, Opel DJ, Arstall MA, Kelly RA, Michel T. Modulation of the endothelial nitric-oxide synthase-caveolin interaction in cardiac myocytes. Implications for the autonomic regulation of heart rate. J Biol Chem. 1998;273:30249–30254. doi: 10.1074/jbc.273.46.30249. [DOI] [PubMed] [Google Scholar]
- 18.Fiedler B, Lohmann SM, Smolenski A, Linnemuller S, Pieske B, Schroder F, Molkentin JD, Drexler H, Wollert KC. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci USA. 2002;99:11363–11368. doi: 10.1073/pnas.162100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godecke A, Heinicke T, Kamkin A, Kiseleva I, Strasser RH, Decking UK, Stumpe T, Isenberg G, Schrader J. Inotropic response to beta-adrenergic receptor stimulation and anti-adrenergic effect of ACh in endothelial NO synthase-deficient mouse hearts. J Physiol. 2001;532:195–204. doi: 10.1111/j.1469-7793.2001.0195g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gyurko R, Kuhlencordt P, Fishman MC, Huang PL. Modulation of mouse cardiac function in vivo by eNOS and ANP. Am J Physiol Heart Circ Physiol. 2000;278:H971–H981. doi: 10.1152/ajpheart.2000.278.3.H971. [DOI] [PubMed] [Google Scholar]
- 21.Han X, Kubota I, Feron O, Opel DJ, Arstall MA, Zhao YY, Huang P, Fishman MC, Michel T, Kelly RA. Muscarinic cholinergic regulation of cardiac myocyte ICa-L is absent in mice with targeted disruption of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1998;95:6510–6515. doi: 10.1073/pnas.95.11.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honore E, Attali B, Romey G, Heurteaux C, Ricard P, Lesage F, Lazdunski M, Barhanin J. Cloning, expression, pharmacology and regulation of a delayed rectifier K+ channel in mouse heart. Embo J. 1991;10:2805–2811. doi: 10.1002/j.1460-2075.1991.tb07829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssens S, Pokreisz P, Schoonjans L, Pellens M, Vermeersch P, Tjwa M, Jans P, Scherrer-Crosbie M, Picard MH, Szelid Z, Gillijns H, Van de Werf F, Collen D, Bloch KD. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circ Res. 2004;94:1256–1262. doi: 10.1161/01.RES.0000126497.38281.23. [DOI] [PubMed] [Google Scholar]
- 24.January CT, Riddle JM. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res. 1989;64:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- 25.Jiang LH, Gawler DJ, Hodson N, Milligan CJ, Pearson HA, Porter V, Wray D. Regulation of cloned cardiac L-type calcium channels by cGMP-dependent protein kinase. J Biol Chem. 2000;275:6135–6143. doi: 10.1074/jbc.275.9.6135. [DOI] [PubMed] [Google Scholar]
- 26.Klein G, Drexler H, Schroder F. Protein kinase G reverses all isoproterenol induced changes of cardiac single L-type calcium channel gating. Cardiovasc Res. 2000;48:367–374. doi: 10.1016/s0008-6363(00)00194-2. [DOI] [PubMed] [Google Scholar]
- 27.Kohr MJ, Wang H, Wheeler DG, Velayutham M, Zweier JL, Ziolo MT. Targeting of phospholamban by peroxynitrite decreases in β-adren-ergic stimulation in cardiomyocytes. Cardiovasc Res. 2008;77:353–361. doi: 10.1093/cvr/cvm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubota I, Han X, Opel DJ, Zhao YY, Baliga R, Huang P, Fishman MC, Shannon RP, Michel T, Kelly RA. Increased susceptibility to development of triggered activity in myocytes from mice with targeted disruption of endothelial nitric oxide synthase. J Mol Cell Cardiol. 2000;32:1239–1248. doi: 10.1006/jmcc.2000.1158. [DOI] [PubMed] [Google Scholar]
- 29.Martin SR, Emanuel K, Sears CE, Zhang YH, Casadei B. Are myocardial eNOS and nNOS involved in the beta-adrenergic and muscarinic regulation of inotropy? A systematic investigation. Cardiovasc Res. 2006;70:97–106. doi: 10.1016/j.cardiores.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Massion PB, Balligand JL. Modulation of cardiac contraction, relaxation and rate by the endothelial nitric oxide synthase (eNOS): lessons from genetically modified mice. J Physiol. 2003;546:63–75. doi: 10.1113/jphysiol.2002.025973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massion PB, Balligand JL. Relevance of nitric oxide for myocardial remodeling. Curr Heart Fail Rep. 2007;4:18–25. doi: 10.1007/s11897-007-0021-6. [DOI] [PubMed] [Google Scholar]
- 32.Massion PB, Dessy C, Desjardins F, Pelat M, Havaux X, Belge C, Moulin P, Guiot Y, Feron O, Janssens S, Balligand JL. Cardiomyocyte-restricted overexpression of endothelial nitric oxide synthase (NOS3) attenuates beta-adrenergic stimulation and reinforces vagal inhibition of cardiac contraction. Circulation. 2004;110:2666–2672. doi: 10.1161/01.CIR.0000145608.80855.BC. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto S, Takahashi T, Ikeda M, Nishikawa T, Yoshida S, Kawase T. Effects of N(G)-monomethyl-L-arginine on Ca(2+) current and nitric-oxide synthase in rat ventricular myocytes. J Pharmacol Exp Ther. 2000;294:216–223. [PubMed] [Google Scholar]
- 33a.MERIT-HF Study Group. Effect of metoprolol C.R/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 34.Mery PF, Lohmann SM, Walter U, Fischmeister R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proc Natl Acad Sci USA. 1991;88:1197–1201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muth JN, Yamaguchi H, Mikala G, Grupp IL, Lewis W, Cheng H, Song LS, Lakatta EG, Varadi G, Schwartz A. Cardiac-specific over-expression of the alpha(1) subunit of the L-type voltage-dependent Ca(2+) channel in transgenic mice. Loss of isoproterenol-induced contraction. J Biol Chem. 1999;274:21503–21506. doi: 10.1074/jbc.274.31.21503. [DOI] [PubMed] [Google Scholar]
- 36.Nerbonne JM. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J Physiol. 2000;525:285–298. doi: 10.1111/j.1469-7793.2000.t01-1-00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmo-genesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 39.Rakhit A, Maguire CT, Wakimoto H, Gehrmann J, Li GK, Kelly RA, Michel T, Berul CI. In vivo electrophysiologic studies in endothelial nitric oxide synthase (eNOS)-deficient mice. J Cardiovasc Electrophysiol. 2001;12:1295–1301. doi: 10.1046/j.1540-8167.2001.01295.x. [DOI] [PubMed] [Google Scholar]
- 40.Rozanski GJ, Witt RC. IL-1 inhibits beta-adrenergic control of cardiac calcium current: role of L-arginine/nitric oxide pathway. Am J Physiol Heart Circ Physiol. 1994;267:H1753–H1758. doi: 10.1152/ajpheart.1994.267.5.H1753. [DOI] [PubMed] [Google Scholar]
- 41.Ruetten H, Dimmeler S, Gehring D, Ihling C, Zeiher AM. Concentric left ventricular remodeling in endothelial nitric oxide synthase knockout mice by chronic pressure overload. Cardiovasc Res. 2005;66:444–453. doi: 10.1016/j.cardiores.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98:403–411. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- 44.Thomas G, Gurung IS, Killeen MJ, Hakim P, Goddard CA, Mahaut-Smith MP, Colledge WH, Grace AA, Huang CL. Effects of L-type Ca2+ channel antagonism on ventricular arrhythmogenesis in murine hearts containing a modification in the Scn5a gene modelling human long QT syndrome 3. J Physiol. 2007;578:85–97. doi: 10.1113/jphysiol.2006.121921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tweedie D, Harding SE, MacLeod KT. Sarcoplasmic reticulum Ca content, sarcolemmal Ca influx and the genesis of arrhythmias in isolated guinea-pig cardiomyocytes. J Mol Cell Cardiol. 2000;32:261–272. doi: 10.1006/jmcc.1999.1070. [DOI] [PubMed] [Google Scholar]
- 46.Vandecasteele G, Eschenhagen T, Scholz H, Stein B, Verde I, Fisch-meister R. Muscarinic and beta-adrenergic regulation of heart rate, force of contraction and calcium current is preserved in mice lacking endothelial nitric oxide synthase. Nat Med. 1999;5:331–334. doi: 10.1038/6553. [DOI] [PubMed] [Google Scholar]
- 47.Varghese P, Harrison RW, Lofthouse RA, Georgakopoulos D, Berkowitz DE, Hare JM. beta(3)-adrenoceptor deficiency blocks nitric oxide-dependent inhibition of myocardial contractility. J Clin Invest. 2000;106:697–703. doi: 10.1172/JCI9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wahler GM, Dollinger SJ. Nitric oxide donor SIN-1 inhibits mammalian cardiac calcium current through cGMP-dependent protein kinase. Am J Physiol Cell Physiol. 1995;268:C45–C54. doi: 10.1152/ajpcell.1995.268.1.C45. [DOI] [PubMed] [Google Scholar]
- 49.Wegener JW, Nawrath H, Wolfsgruber W, Kuhbandner S, Werner C, Hofmann F, Feil R. cGMP-dependent protein kinase I mediates the negative inotropic effect of cGMP in the murine myocardium. Circ Res. 2002;90:18–20. doi: 10.1161/hh0102.103222. [DOI] [PubMed] [Google Scholar]
- 50.Wolff DJ, Lubeskie A, Gauld DS, Neulander MJ. Inactivation of nitric oxide synthases and cellular nitric oxide formation by N6-iminoethyl-L-lysine and N5-iminoethyl-L-ornithine. Eur J Pharmacol. 1998;350:325–334. doi: 10.1016/s0014-2999(98)00267-2. [DOI] [PubMed] [Google Scholar]
- 51.Yang L, Liu G, Zakharov SI, Bellinger AM, Mongillo M, Marx SO. Protein kinase G phosphorylates Cav1.2 alpha1c and beta2 subunits. Circ Res. 2007;101:465–474. doi: 10.1161/CIRCRESAHA.107.156976. [DOI] [PubMed] [Google Scholar]
- 52.Yang XP, Liu YH, Shesely EG, Bulagannawar M, Liu F, Carretero OA. Endothelial nitric oxide gene knockout mice: cardiac phenotypes and the effect of angiotensin-converting enzyme inhibitor on myocardial ischemia/reperfusion injury. Hypertension. 1999;34:24–30. doi: 10.1161/01.hyp.34.1.24. [DOI] [PubMed] [Google Scholar]
- 53.Zhang ZS, Cheng HJ, Onishi K, Ohte N, Wannenburg T, Cheng CP. Enhanced inhibition of L-type Ca2+ current by beta3-adrenergic stimulation in failing rat heart. J Pharmacol Exp Ther. 2005;315:1203–1211. doi: 10.1124/jpet.105.089672. [DOI] [PubMed] [Google Scholar]
- 54.Ziolo MT, Bers DM. The real estate of NOS signaling: location, location, location. Circ Res. 2003;92:1279–1281. doi: 10.1161/01.RES.0000080783.34092.AF. [DOI] [PubMed] [Google Scholar]
- 55.Ziolo MT, Harshbarger CH, Roycroft KE, Smith JM, Romano FD, Sondgeroth KL, Wahler GM. Myocytes isolated from rejecting transplanted rat hearts exhibit a nitric oxide-mediated reduction in the calcium current. J Mol Cell Cardiol. 2001;33:1691–1699. doi: 10.1006/jmcc.2001.1420. [DOI] [PubMed] [Google Scholar]
- 56.Ziolo MT, Lewandowski SJ, Smith JM, Romano FD, Wahler GM. Inhibition of cyclic GMP hydrolysis with zaprinast reduces basal and cyclic AMP-elevated L-type calcium current in guinea-pig ventricular myocytes. Br J Pharmacol. 2003;138:986–994. doi: 10.1038/sj.bjp.0705112. [DOI] [PMC free article] [PubMed] [Google Scholar]