Abstract
Lipid species change for SV40 transformed fibroblasts from wild-type or from diacylglycerol kinase-ε (DGKε) or diacylglycerol kinase-α (DGKα) knock out mice, were determined for glycerophospholipids, poly-phosphatidylinositides (GPInsPn), and diacylglycerol (DAG) using direct infusion mass spectrometry. Dramatic differences in arachidonate (20:4 fatty acid)-containing lipids were observed for multiple classes of glycerophospholipids and polyphosphatidylinositides between wild-type and DGKε knock out cells. However, no difference was observed in either the amount or the acyl chain composition of DAG between DGKε knock out and wild-type cells, suggesting that DGKε catalyzed the phosphorylation of a minor fraction of the DAG in these cells. The differences in arachidonate content between the two cell lines were greatest for the GPInsPn lipids and least for DAG. These findings indicate that DGKε plays a significant role in determining the enrichment of GPInsPn with 20:4 and that there is a pathway for the selective translocation of arachidonoyl-phosphatidic acid from the plasma membrane to the endoplasmic reticulum. In contrast, no substantial difference was observed in the acyl chain composition of any class of glycerophospholipid or diacylglycerol between lipid extracts from fibroblasts from wild-type mice or from DGKα knock out mice. However, the cells from the DGKα knock out mice had a higher concentration of DAG, consistent with the lack of down regulation of the major fraction of DAG by DGKα, in contrast with DGKε that is primarily responsible for enrichment of GPInsPn with arachidonoyl acyl chains.
Eukaryotic diacylglycerol kinases (DGKs)1 are a family of 10 known isoforms playing important roles in signal transduction (1–3). In this work we compare the properties of two isoforms of DGK, DGKα and DGKε. Although these two isoforms catalyze the same reaction, they have very different modes of interaction with membranes and different mechanism of regulation. DGKα contains two E-F hands and is a Ca2+-dependent enzyme (4,5), although it can also exhibit Ca2+-independent activity when binding to membranes rich in phosphatidylethanolamine (GPEtn) or cholesterol (6). DGKα is an amphitropic enzyme that binds to membranes only upon activation (7,8). In contrast DGKε is the only isoform with a hydrophobic segment that promotes attachment of the protein to membranes (9). In addition, DGKε is also unique in being the only isoform of DGK that has specificity for DAG containing arachidonoyl chains (10,11) as well as other polyunsaturated acyl chains (12). Hence, the DAG derived from GPIns(4,5)P2 hydrolysis by GPIns(4,5)P2-specific phospholipase C (PLC) will also be arachidonoyl-rich as will the phosphatidic acid (GPA) formed as a product of DGKε-catalyzed phosphorylation. This process is cyclical and the DAG that is phosphorylated by DGK to produce GPA, in turn can be converted to GPIns(4,5)P2 by reaction with CDP-inositol. We studied the contribution of DGKε in determining the enrichment of GPIns(4,5)P2 with arachidonic acid.
Studies using DGKε-deficient mice have demonstrated a role for this isoform in regulating seizure susceptibility and long-term potentiation (13,14). It has also been shown that upon electroconvulsive shock there was a reduced accumulation of arachidonoyl-DAG as well as free arachidonic acid (14). There is also a smaller reduction in the level of GPIns(4,5)P2 in the knock out mice upon electroconvulsive shock (14). In the present work we study the role of DGKε in the distribution of arachidonoyl side chains among different lipids in the basal state without stimulation. Another isoform, DGKα, is a negative regulator of antigen-mediated triggering of the T-cell receptor (15). This isoform has chemotactic, proliferative and angiogenic activity (16). Studies with DGKα knock out mice demonstrated an impairment of anergy induction (17). T-cells from these knock out mice produce more interleukin-2 and show increased proliferation in response to T-cell receptor activation (17). DGKα is expressed in several human melanoma cell lines but not in noncancerous melanocytes (18). It has also recently been found that this isoform can play an important role in the metastasis of certain breast cancers (19). Hence DGKα and DGKε appear to have different biological functions. We have undertaken a lipidomics study to determine if there are also differences in lipid processing resulting from the deletion of each of these two DGK isoforms.
Experimental Procedures
Tissue culture
Mouse fibroblasts were obtained from embryos of mice that were made deficient in DGKε (−/−) (14) or in DGKα (−/−) (17). In each experiment these cells were compared with wild-type embryonic fibroblasts obtained from siblings of the (−/−) mice. These cells derived from DGKε (+/+) or DGKα (+/+) embryos, respectively, are designated WT. All cells were immortalized by transfection with the SV40 large T antigen. Cells were cultured in DMEM supplemented with 10% fetal bovine serum, 25 mM HEPES, and 1% Penicillin/Streptomycin at 37°C in a humidified atmosphere with 5% CO2.
Expression levels of DGK in cell lines
We compared the level of mRNA of DGKε or DGKα from the mouse embryonic fibroblast cell lines described above using reverse transcriptase (RT) PCR. These levels were compared with those of actin mRNA. Briefly, total RNA was isolated from each of the four cell lines and 1µg of RNA was reverse transcribed using Moloney-Murine Leukemia Virus (MMLV) reverse transcriptase. Using 3% of the cDNA, DGKα, DGKε, and actin were amplified for 36 cycles by annealing at 64 °C for 1 min and extending at 72 °C for 1min. The primers used for DGKα were 5’-ATGGCCAAAGAGAAGGGCCTC + 5’-GCAGTGGCACTGTGGTAGCCC and those for DGKε were 5’-CTCAGTTCGAGTCCTTGTTTG + 5’-ATAGACGCCAACGATTTCCAG. Equal aliquot of the resulting reaction was subjected to agarose gel electrophoresis.
Glycerophospholipids analysis
Global lipid extracts were prepared via a modified Bligh/Dyer extraction procedure as described previously (20). Briefly, cells were scraped in 800 µL of ice-cold 0.1N HCl/methanol (1:1) and 400 µL of CHCl3 was added to the suspension. The samples were vortexed for 1 min, layers separated by centrifugation at 18000g for 5 min at 4°C and 20 µL of an internal standard containing equimolar amounts of 22:0 GPCho (1,2-diundecanoyl-sn-glycero-3-phosphocholine) and 28:0 GPA (1,2-dimyristoyl-sn-glycero-3-phosphate) were added to the organic layer before drying in vacuo. Dried lipids were redissolved in 80 µL of CH3OH:CHCl3 (9:1). Mass spectral analysis was performed on a Finnigan TSQ Quantum triple quadrupole mass spectrometer (ThermoFinnigan, San Jose, CA) equipped with a Harvard Apparatus syringe pump and electrospray source. Samples were analyzed at an infusion rate of 10 µL/min in both positive and negative ionization modes over the range of m/z 350–1200. Data were collected with the Excalibur software package (ThermoFinnigan) and analyzed with software developed in our laboratory as described in (21). Identification of individual glycerophospholipids was accomplished by tandem mass spectrometry (ESI/LC/MS/MS). Extracted lipids were dissolved in 100 µl of isopropanol (IPA):hexane:100 mM HCOONH4(aq) 58:40:2 (mobile phase A). We utilized an Applied Biosystems/ MDS SCIEX 4000 Q TRAP hybrid triple quadrupole/ linear ion trap mass spectrometer (Applied Biosystems, Foster City, CA). Coupled to this instrument were a Shimadzu (Shimadzu Scientific Instruments, Inc., Columbia, MD) HPLC system consisting of a SCL 10 AVP controller, two LC 10 ADVP pumps and a CTC HTC PAL autosampler (Leap Technologies, Carrboro, NC). All samples were separated on a Phenomenex (Phenomenex, Torrance, CA) Luna Silica column (2 × 250 mm, 5 µ particle size) using a 20 µl sample injection. Lipids were separated using a binary gradient program consisting of IPA: hexane: 100 mM HCOONH4(aq) 58:40:2 (mobile phase A), and IPA:hexane:100 mM HCOONH4(aq) 50:40:10 (mobile phase B). The following LC gradient was used: 0–5min, B=50%; 5–30min, B=50%-100%; 30–40min, B=100%; 40–41min, B=100%-50%; 41–50min, B=50%, delivered at a flow rate of 0.3ml/min. This analysis results in class separation and fragmentation of the individual species within the class thus allowing for a more precise identification (20,22).
Phosphatidylinositol phosphate (GPInsPn) Determination
Polyphosphatidylinositides extraction was performed as described previously (23), with the following modifications: dried lipids were rapidly redissolved in 55 µL of 1:1:0.3 CHCl3:CH3OH:H2O. Before analysis, 5 µL of 300 mM piperidine(aq) and 1 µL of (0.11 M) internal standard 1,2-dioctanoyl-sn-glycero-3-phosphoinositol-4,5-bisphosphate was added to each sample, and the sample was vortexed and centrifuged briefly prior to mass spectral analysis by direct infusion on a MDS SCIEX 4000 Q TRAP hybrid triple quadrupole/linear ion trap mass spectrometer (Applied Biosystems, Foster City, CA).
DAG analysis
Analysis and quantification of DAG species from cell extracts was performed according to the procedure described in (24). After phospholipids extraction by the modified Bligh/Dyer procedure diacylglycerols were isolated by separation using normal phase column chromatography and isocratic elution with 65:35:0.7 CHCl3:CH3OH:H2O. 1,2-dilauroyl-sn-glycerol (100 ng) was used as an internal standard and added to each sample before column separation.
Results
RT-PCR of DGK
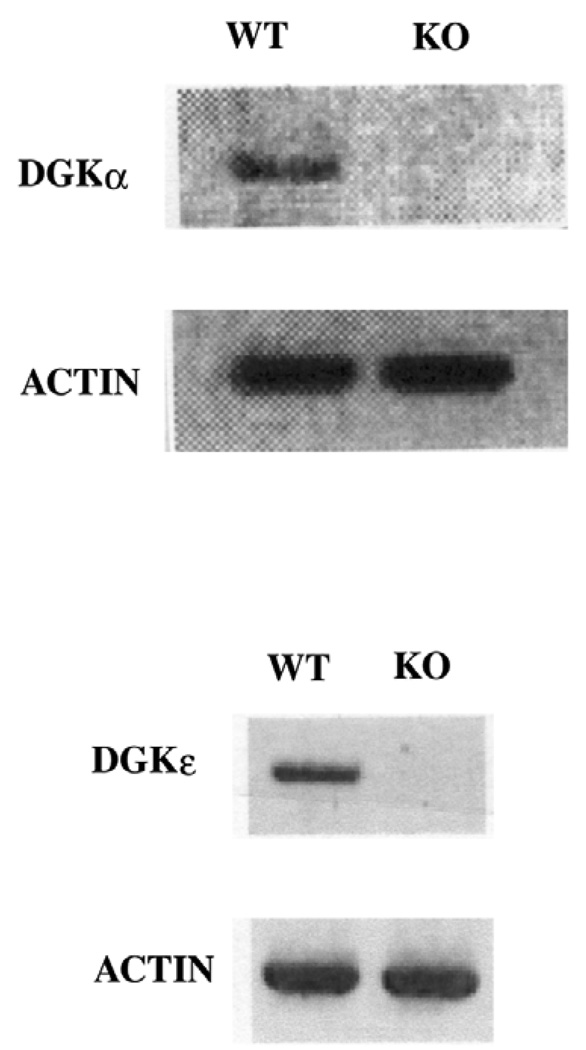
The results of the RT-PCR analysis confirm that the wild type (WT) cells express both DGKα as well as DGKε. However, no mRNA for DGKα or for DGKε was detected in the respective knock out (KO) cell lines (Fig. 1). As a positive control we show that mRNA for actin is detected in comparable amounts in the KO and WT cell lines.
Fig. 1.
RT-PCR detection of mRNA for DGKα and actin in DGKα WT and KO cells and of mRNA for DGKε and actin in DGKε WT and KO cells.
Phospholipid composition of wild type (WT) versus DGKε knockout (KO) cells
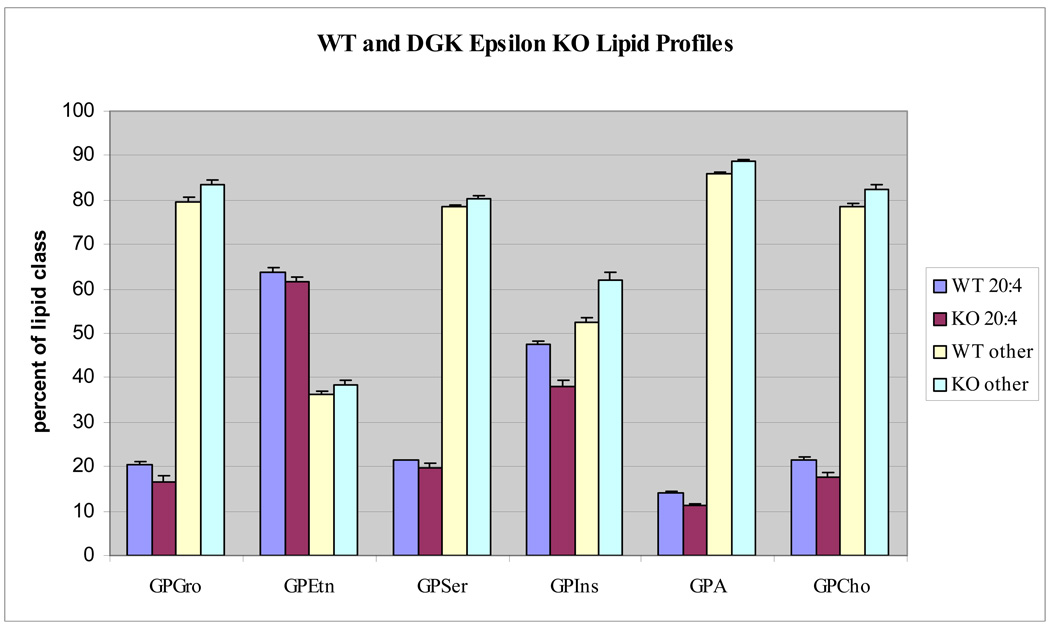
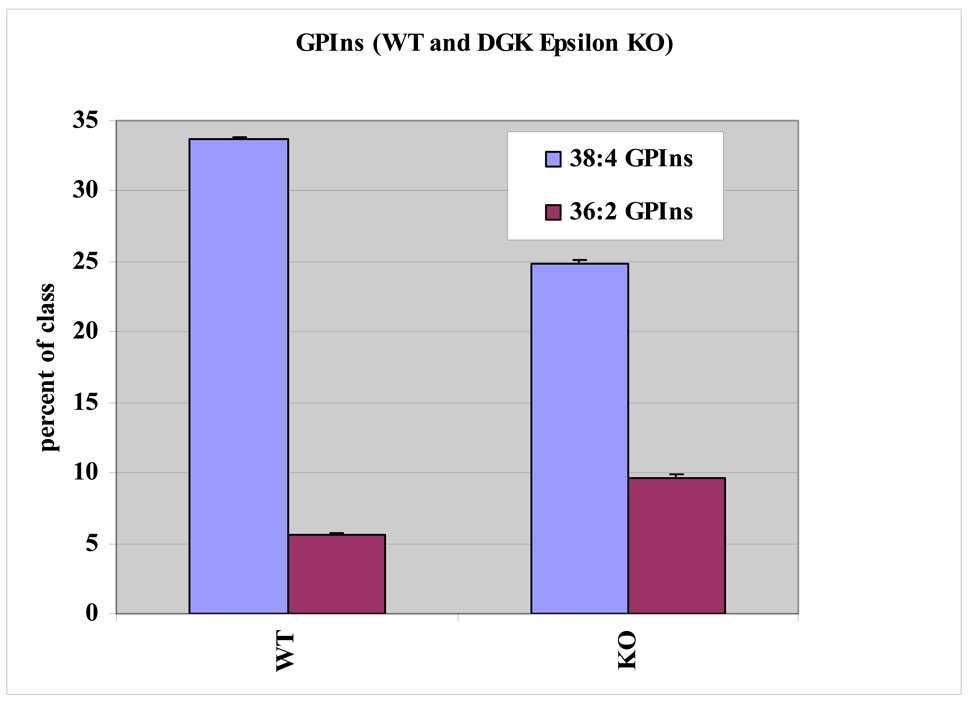
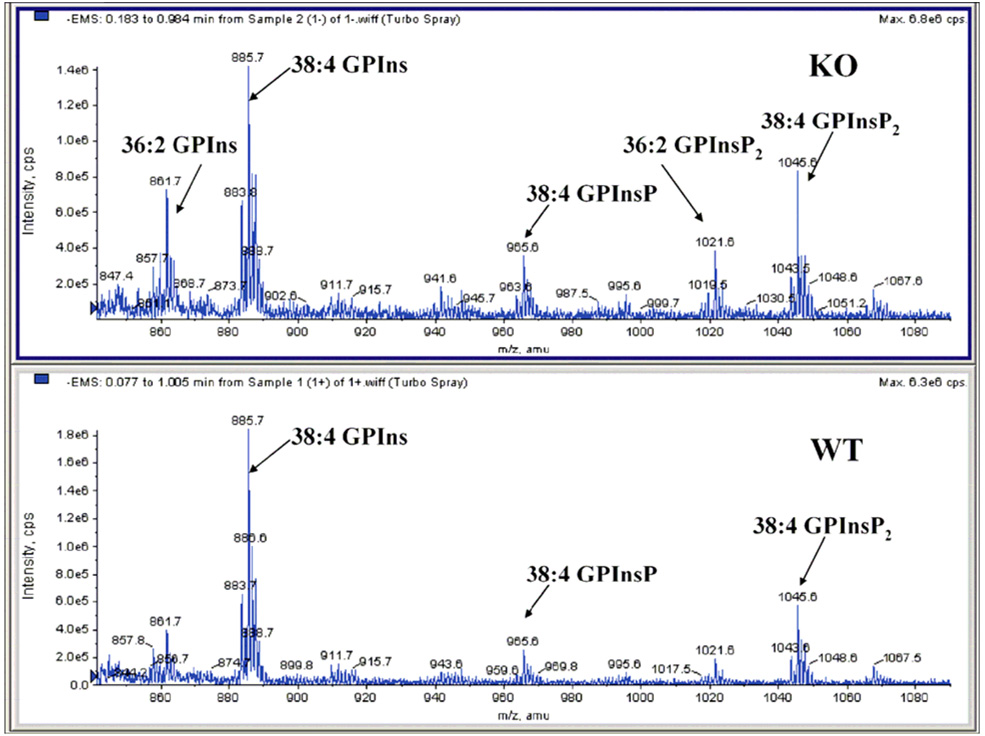
Glycerophospholipid profiles for six major classes show significant differences in arachidonate containing species between the WT and KO cells. The arachidonate-containing lipids are compared to all others that do not possess this acyl substituent between WT and KO cells (Fig. 2). The lipid class showing the greatest difference in arachidonoyl content between WT and KO cells is polyphosphoinositides (GPInsPn), which is the sum of all phosphatidylinositols including GPIns, GPInsP, GPIns(4,5)P2 and GPIns(3,4,5)P3. More specifically, the deletion of DGKε decreases the 38:4 GPIns content from approximately 33% to 24% of the GPIns lipid pool. To compensate for this, it appears that the KO cells increase the incorporation of oleate (18:1 fatty acid) into the inositol lipid fraction (Fig. 3). The identity of each glycerophospholipid component was resolved by tandem mass spectrometry and their fatty acyl composition was determined. These data are compiled in the supplementary Tables available at http://pubs.acs.org. In the case of 36:2 GPIns, for example, 18:1/18:1 fatty acids are the predominant species. The 36:2 GPIns seems to have a significantly higher content in the KO fibroblasts with an increase from around 6 to 11% of the class. Within the GPInsPn lipids, a separate analysis showed that there was a marked change in arachidonate- and oleate-containing lipids following knockout of DGKε for GPIns, GPInsP, GPIns(4,5)P2 (Fig. 4). Due to the low abundance of basal GPIns(3,4,5)P3, neither 36:2 nor 38:4 GPIns(3,4,5)P3 were detectable in these experiments.
Fig. 2.
Lipid profiles for the six major glycerophospholipid classes. DGKε-knockout and WT cells have statistically significant differences in arachidonate containing lipids.
Fig. 3.
Lipid profiles for two major phosphatidylinositol lipids from triplicate samples of WT and KO cells. DGKε-knockout and WT cells have statistically significant differences in arachidonate containing 38:4 GPIns. WT 38:4 GPIns comprises a higher percentage of the GPIns lipid class than does DGKε-knockout 38:4 GPIns. The opposite trend is observed for 36:2 GPIns. The data shown are from one of three representative experiments performed. All three experiments had statistically significant differences in lipid profiles similar to those shown.
Fig. 4.
Phosphatidylinositol polyphosphates mass spectral region of KO and WT mouse fibroblast extracts. DGKε-knockout leads to a dramatic increase in non-arachidonate containing lipid species.
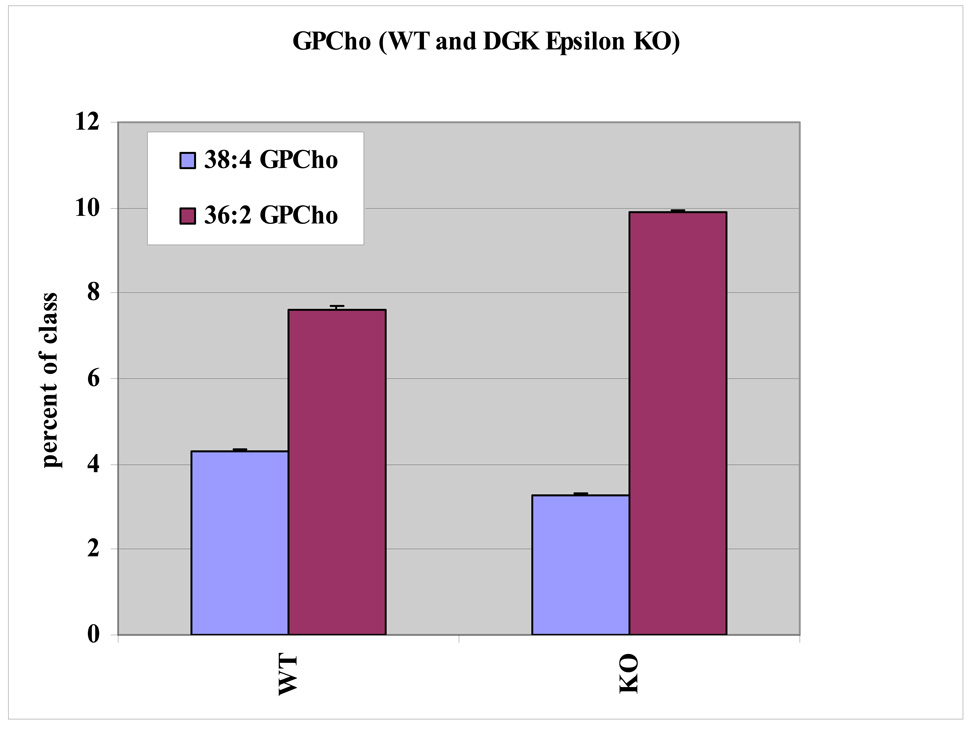
The glycerophosphatidic acid (GPA) lipids had a similar pattern to that observed in the GPIns lipid pool, although the differences between KO and WT were somewhat reduced. Again in this class, 38:4 GPA was the most affected by the deletion of DGKε, with the KO cells having reduced levels 38:4 GPA. To balance this loss, the KO cells have increased amounts of saturated and monounsaturated fatty acid containing species such as 36:2 GPA. Glycerophosphatidylethanolamine (GPEtn) lipids have a more complex lipid pattern due to the presence of plasmanyl and plasmenyl (ether and vinyl ether) containing lipids in addition to the usual diacyl lipids. Numerous arachidonate containing GPEtn lipids (38:4, 38:5, 38:6, as well as the 38:4 and 38:5 plasmenyl/plasmanyl GPEtn for example) all contribute less to the KO fibroblast GPEtn lipid pool compared to the WT cells. However, overall the difference in arachidonoyl-containing GPEtn between KO and WT cells is much less than the differences observed for GPIns. Glycerophosphatidylcholines (GPCho) also had easily observable differences in arachidonate-containing lipid profiles following DGKε knockout. The 38:4 GPCho contributes more to the total GPCho lipid pool in the WT cells compared to the KO cell type. To compensate for the loss of 38:4 GPCho, it appears that the KO cells had a higher fraction of saturated and monounsaturated fatty acid containing GPCho (Fig. 5). Thus, in summary, most lipid classes show greater 38:4 than 36:2 lipids in the WT compared with KO cells, indicating a loss of arachidonoyl-containing lipids.
Fig. 5.
Lipid profiles for two major phosphatidylcholine lipids from one experiment (triplicate samples of WT and KO). DGKε-knockout and WT cells have statistically significant differences in arachidonate containing 38:4 GPCho. WT 38:4 GPCho comprises a higher percentage of the GPCho lipid class than does DGKε-knockout 38:4 GPCho. The opposite trend is observed for 36:2 GPCho. Two additional experimental sets were also analyzed (both of which had statistically significant differences in lipid levels similar to those show).
We have extensively characterized the glycerophospholipid species from both WT and DGKε-KO cell extracts by ESI/LC/MS/MS (Supplemental Table 1&Supplemental Table 2). As a result of this analysis 366 species were identified in WT and 405 species in DGKε-KO cell extracts. Only two of the phospholipid classes show marked differences in terms of the number and variety of the identified species. More phosphatidylserine (GPSer) (50 species vs. 33) and a rare phospholipid, phosphatidylthreonine (GPThr) (25), (20 species vs. 7) species were ascertained in the DGKε-KO cell extracts.
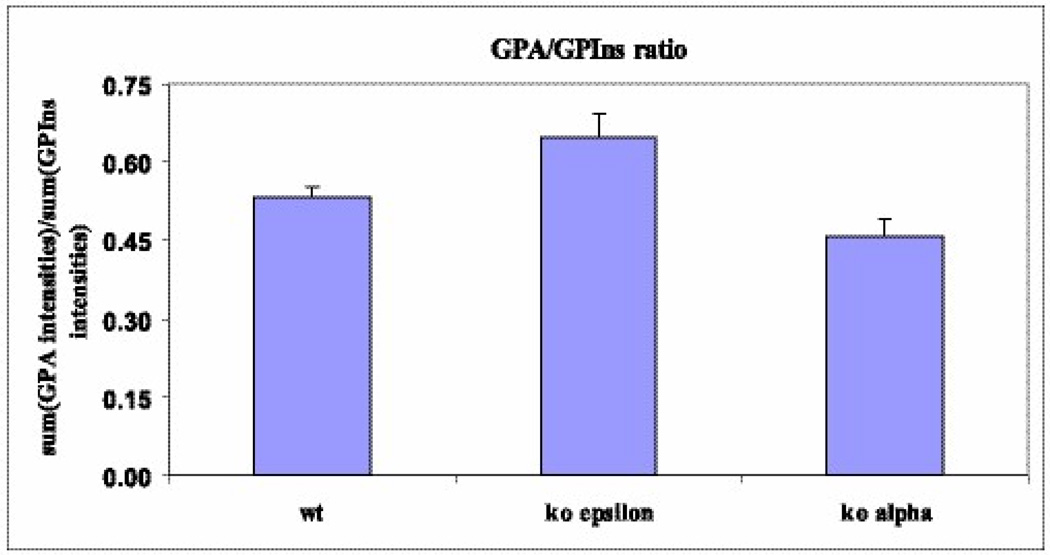
We also determined the ratio of GPA to GPIns for both WT and DGKε-KO cells (Fig. 6). This ratio was significantly higher for the DGKε-KO cells (p=0.0014 by ANOVA with random effects). The analysis of DAG species from both genotypes reveals no significant differences. The lack of measured changes in DAG might have resulted because there were only trace levels of 38:4 DAG in both WT and KO cell lines. The majority of the lipids present were from the 32, 34, and 36 series of DAGs.
Fig. 6.
Ratio of GPA to GPIns lipids in WT, DGKα, and DGKε knockout cells. The DGKε GPA/GPIns ratio was higher than that of the WT samples. Knockout of DGKα led to a decrease in GPA/GPIns compared to the WT cells.
Phospholipid composition of wild type (WT) versus DGKα knockout (KO) cells
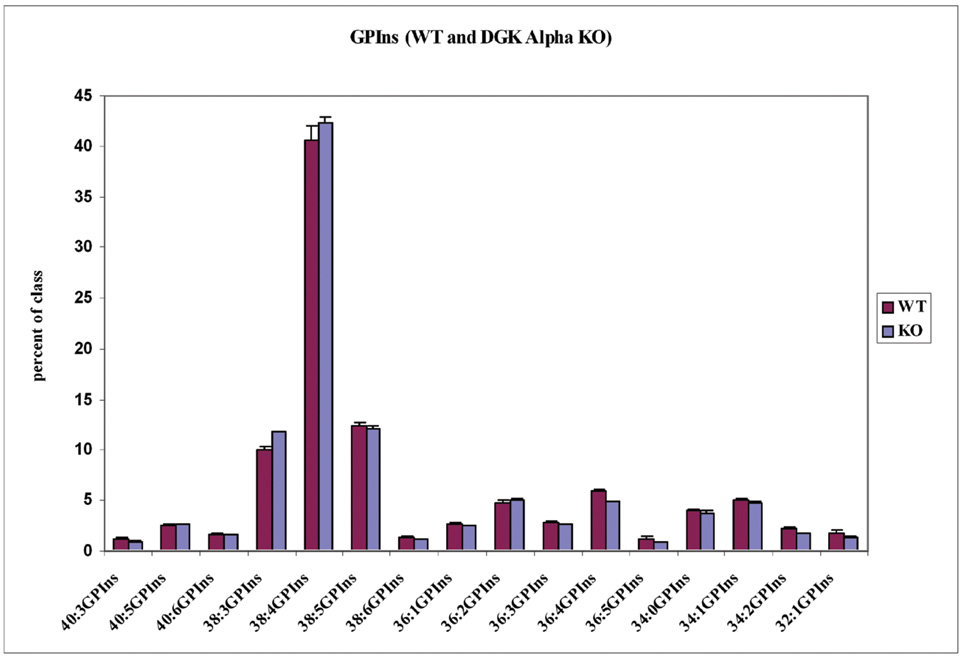
Analysis of the glycerophospholipids from WT and DGKα KO cells was also performed. The cellular phospholipid pattern did not have broad, statistically significant differences in glycerophospholipid species between the WT and the KO. In GPIns only 36:4 does have a modest but significant decrease in class percentage (Fig. 7). The difference in the ratio of GPA to GPIns between the DGKα KO and WT cells was marginally significant (p=0.0498 by ANOVA with random effects). See Fig. 6.
Fig. 7.
Lipid profiles for the major phosphatidylinositol lipids. DGKα-knockout and WT cells have virtually no statistically significant differences in any GPIns lipids. The same is true for almost all glycerophospholipids.
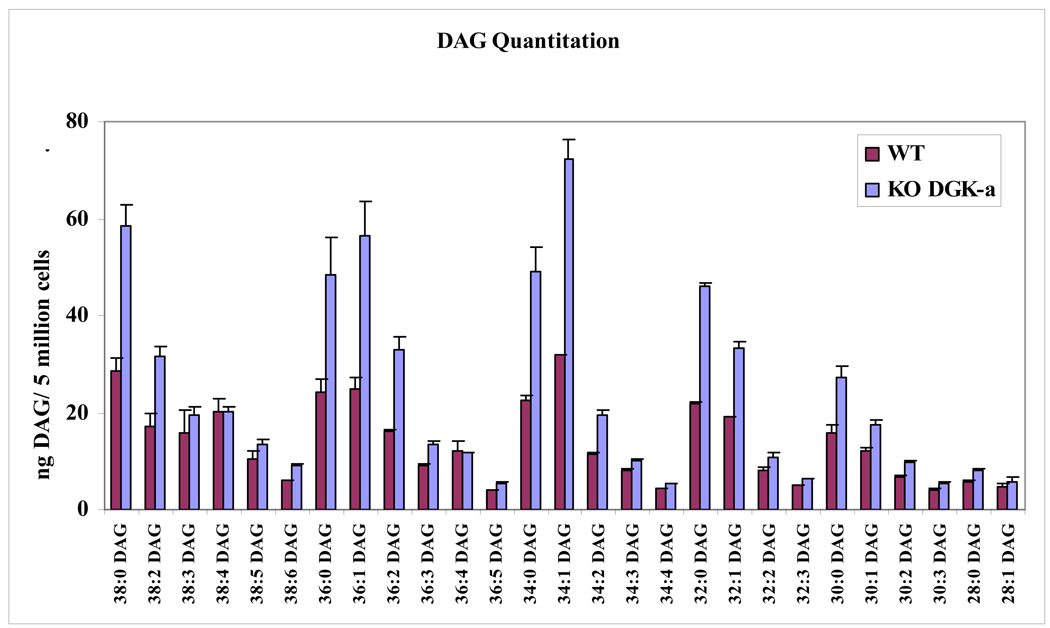
Comprehensive ESI/LC/MS/MS analysis of the glycerophospholipid species in these two phenotypes did not reveal any significant differences. 407 phospholipid species were identified in WT and 348 species in DGKα KO cell extracts (Supplemental table 3&Supplemental table 4). In contrast, the analysis of DAG species revealed some interesting differences between WT and KO. Here too the majority of the DAG species were from the 32, 34 and 36 series but unlike in the DGKε KO case, there were marked changes in the quantity of DAG species in the extracts from DGKα KO. The amount of DAGs is almost double in the KO samples compared to WT. The largest differences occur in saturated, mono- and di- unsaturated DAG species (Fig. 8).
Fig. 8.
Lipid profiles for the major diacylglycerol lipids. DGKα-knockout and WT cells had statistically significant differences in multiple DAG lipids. No significant differences were observed in the DGKε-knockout experiments.
Discussion
The two isoforms of DGK that we studied in this work are known to have different biological functions. One reason for this is that these enzymes are more highly expressed in different organs. DGKε is found principally in brain, retina and cardiac muscle, while DGKα is found mainly in white blood cells, spleen and thymus. Both DGKα and DGKε are expressed in the WT cell lines that we used, but they are not expressed in the corresponding KO cell line (Fig. 1). The present work demonstrates that these two isoforms of DGK also have very different properties on a biochemical level. We suggest that DGKε is involved more specifically in GPInsPn cycling, while DGKα contributes to regulating the bulk concentration of DAG in the cell from all sources.
DGKε contributes significantly to the arachidonoyl enrichment of GPIns(4,5)P2 since the KO cells have less arachidonoyl-GPIns(4,5)P2 (Fig. 3 and Fig. 4). This is the case in spite of the fact that the specificity of DGKε for arachidonoyl-containing forms of DAG is only about 10-fold using in vitro assays (9). Even more surprising is the increase in arachidonoyl-enrichment between the formation of GPA by DGKε and its conversion to GPIns(4,5)Pn (Table 1). In the present work we observed that the arachidonoyl content of DAG is only about 3% and there is little difference between KO and WT cells. This increases to slightly over 10% arachidonoyl content of GPA. The increase is less for KO cells than for WT, indicating that DGKε contributes to the arachidonoyl-enrichment of GPA as would be expected because of the arachidonoyl-specificity for substrates phosphorylated by this DGK isoform. There are also other sources of GPA, in addition to DGKε, including other DGK isoforms as well as from phospholipase D-catalyzed reactions. In the case of phospholipase D, substrates with an arachidonoyl group on the sn-2 of the glycerol backbone are not favored by the enzyme (26), indicating that this is not a source of arachidonoyl enrichment.
Table 1.
Percent of Lipid with Arachidonoyl Chains in DGKε Cells
| Lipid | % PLa | KO | WT |
|---|---|---|---|
| DAG | - | 3 | 3 |
| GPA | 1 | 11 | 13 |
| GPInsPn | 5–10 | 38 | 48 |
Approximate % of total lipid for each of the phospholipid classes taken from values given in Gennis (35). The DAG concentration in a non-stimulated cell is expected to be below 1%. KO and WT columns indicate the % of each lipid class found to contain arachidonic acid.
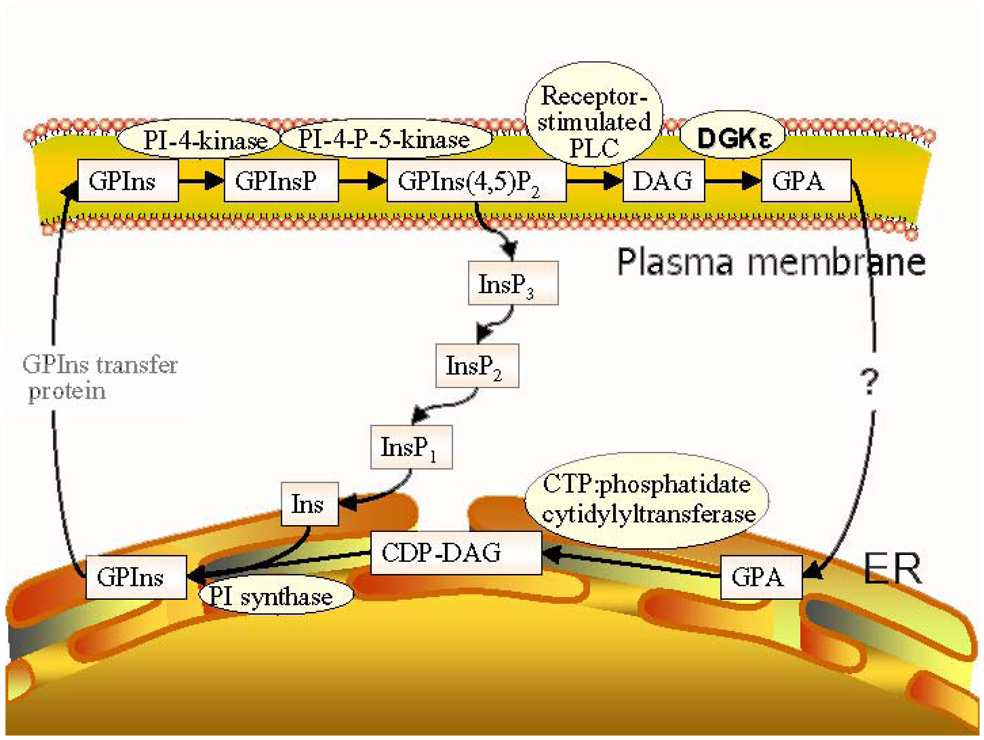
The GPA that is formed can be converted back into GPIns(4,5)P2. The first step in this conversion is the synthesis of GPIns, catalyzed by CTP:phosphatidate cytidylyltransferase. This is the rate limiting step in the synthesis of GPIns(4,5)P2 (Scheme 1). This step takes place in the endoplasmic reticulum and not in the plasma membrane. The enzyme exhibits some specificity for 1-stearoyl-2-arachidonoyl-phosphatidic acid as substrate under certain in vitro conditions (27). However, in order to explain the large increase we observe in arachidonoyl content in going from GPA to GPIns(4,5)P2 (Table 1) there must be some segregation of arachidonyl-GPA from other forms of GPA. In other words, the lipids involved in inositol cycling must be segregated from other lipids. There are two membranes involved in this process, the endoplasmic reticulum and the plasma membrane (Scheme 1). The process starts in the plasma membrane, as a consequence of hormone stimulation, activating a GPIns(4,5)P2-specific phospholipase C that will convert an arachidonoyl-rich GPIns(4,5)P2 to an arachidonoyl-rich DAG. The arachidonoyl-rich DAG is phosphorylated preferentially by DGKε to form an arachidonoyl-rich GPA. The arachidonoyl-GPA must then be selectively transformed into GPIns lipids. This requires the transfer of GPA transfer between the two membranes, a process that is not dependent on soluble lipid carriers or on vesicular transport (28) but may occur at membrane contact sites formed between the plasma membrane and endoplasmic reticulum (29). There is evidence in yeast that such contact sites are enriched in synthases for GPSer and GPIns lipids (31). The close approach of these two membranes is also indicated by the fact that the tyrosine phosphatase, PTP-1B, in the endoplasmic reticulum acts on protein substrates of the plasma membrane (32). A possible explanation of our results is that DGKε at these contact sites would preferentially transfer arachidonoyl-containing GPA. This transfer occurs more readily in the presence of DGKε since the increase in arachidonoyl content between GPA and GPIns lipids is greater for the WT cells than for the KO cells. The process may also involve the DGKε that is present in the endoplasmic reticulum (not shown in Scheme 1) (30), possibly at contact sites where it can directly transfer the arachidonoyl-containing GPA to GPIns synthetase, also known to be present at contact sites (31). We find that the ratio of GPA to GPIns is higher for DGKε KO cells than for either WT or DGKα KO cells (Fig. 6). This is consistent with the suggested promotion of GPA transfer between membranes by DGKε. In the DGKε-KO cells transfer of GPA to the endoplasmic reticulum is slower and hence the concentration of this lipid increases, resulting in the change in the observed GPA/GPIns ratio. The transferred GPA would then be converted into arachidonoyl-rich GPIns and returned to the plasma membrane. The cycle would also be driven by GPA itself since this lipid regulates the transcription of GPIns synthase as well as being an allosteric regulator of type I phosphatidylinositol phosphate kinase (33). Once formed, it has been found that the GPInsPn do not undergo remodeling (34). Thus a combination of the selective phosphorylation of arachidonoyl-DAG by DGKε together with the preferential transfer of arachidonoyl-GPA from the plasma membrane to the endoplasmic reticulum, in which DGKε participates in a non-catalytic role, determines the enrichment of GPIns(4,5)P2 with arachidonoyl groups. A fraction of the arachidonoyl chains are also transferred to other phospholipids, resulting in some arachidonoyl-enrichment of several lipid classes (Fig. 2), but the enrichment of the GPInsPn lipids is clearly the greatest. Thus, in addition to its arachidonoyl specificity in forming GPA, DGKε must also have a non-catalytic role in increasing the arachidonoyl content of GPInsPn.
Scheme 1.
The role of DGKα is quite different. This isoform causes little change in the acyl chain composition of any class of phospholipid, including the GPInsPn (Fig. 7) or the DAG. This result is consistent with the lack of acyl chain specificity observed when DGKα is tested in in vitro DAG kinase assays. However, the DAG content of DGKα KO cells increases significantly (Fig. 8). Thus, unlike DGKε, DGKα plays an important role in regulating the total DAG in the cells since the absence of this isoform in DGKα KO cells leads to an increase in the DGK substrate, DAG.
Supplementary Material
We present as supplementary Tables, listings of identified lipid species for DGKε WT (Table 1), DGKε KO (Table 2), DGKα WT (Table 3) and DGKα KO (Table 4). This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgements
We wish to acknowledge the assistance of Ms Armela O. Dicu and Jyoti Bhardwaj.
Footnotes
This work was supported in part by a grant from the Natural Sciences and Engineering Research Council of Canada, grant 9848 (to R.M.E.) and from the National Institutes of Health Grants R01-CA95463 (to M.K.T.) and U54 GM069338 (to H.A.B.).
Abbreviations: DGK, diacylglycerol kinase; RT, reverse transcriptase; DAG, diacylglycerol; GPA, phosphatidic acid; GPEtn, phosphatidylethanolamine; GPCho, phosphatidylcholine; GPIns, phosphatidylinositol; GPGro, phosphatidylglycerol; GPSer, phosphatidylserine; PLC, phospholipase C; GPIns(4,5)P2, phosphatidylinositol (4,5) bisphosphate; WT, wild type; KO, DGKε-knockout or DGKα-knockout; GPInsPn, polyphosphatidylinositides; ESI/LC/MS/MS, electrospray ionization/liquid chromatography/tandem mass spectrometry.
REFERENCES
- 1.Luo B, Regier DS, Prescott SM, Topham MK. Diacylglycerol kinases. Cell Signal. 2004;16:983–989. doi: 10.1016/j.cellsig.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Topham MK. Signaling roles of diacylglycerol kinases. J. Cell. Biochem. 2006;97:474–484. doi: 10.1002/jcb.20704. [DOI] [PubMed] [Google Scholar]
- 3.Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem. J. 2008;409:1–18. doi: 10.1042/BJ20071040. [DOI] [PubMed] [Google Scholar]
- 4.Abe T, Lu X, Jiang Y, Boccone CE, Qian S, Vattem KM, Wek RC, Walsh JP. Site-directed mutagenesis of the active site of diacylglycerol kinase alpha: calcium and phosphatidylserine stimulate enzyme activity via distinct mechanisms. Biochem. J. 2003;375:673–680. doi: 10.1042/BJ20031052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang Y, Qian W, Hawes JW, Walsh JP. A domain with homology to neuronal calcium sensors is required for calcium-dependent activation of diacylglycerol kinase alpha. J. Biol. Chem. 2000;275:34092–34099. doi: 10.1074/jbc.M004914200. [DOI] [PubMed] [Google Scholar]
- 6.Fanani ML, Topham MK, Walsh JP, Epand RM. Lipid modulation of the activity of diacylglycerol kinase alpha- and zeta-isoforms: activation by phosphatidylethanolamine and cholesterol. Biochemistry. 2004;43:14767–14777. doi: 10.1021/bi049145z. [DOI] [PubMed] [Google Scholar]
- 7.Merino E, Sanjuan MA, Moraga I, Cipres A, Merida I. Role of the Diacylglycerol Kinase {alpha}-Conserved Domains in Membrane Targeting in Intact T Cells. J. Biol. Chem. 2007;282:35396–35404. doi: 10.1074/jbc.M702085200. [DOI] [PubMed] [Google Scholar]
- 8.Sanjuan MA, Pradet-Balade B, Jones DR, Martinez A, Stone JC, Garcia-Sanz JA, Merida I. T Cell Activation In Vivo Targets Diacylglycerol Kinase {alpha} to the Membrane: A Novel Mechanism for Ras Attenuation. J Immunol. 2003;170:2877–2883. doi: 10.4049/jimmunol.170.6.2877. [DOI] [PubMed] [Google Scholar]
- 9.Dicu AO, Topham MK, Ottaway L, Epand RM. Role of the Hydrophobic Segment of Diacylglycerol Kinase epsilon. Biochemistry. 2007;46:6109–6117. doi: 10.1021/bi6024726. [DOI] [PubMed] [Google Scholar]
- 10.Tang W, Bunting M, Zimmerman GA, McIntyre TM, Prescott SM. Molecular cloning of a novel human diacylglycerol kinase highly selective for arachidonate-containing substrates. J. Biol. Chem. 1996;271:10237–10241. [PubMed] [Google Scholar]
- 11.Walsh JP, Suen R, Lemaitre RN, Glomset JA. Arachidonoyl-diacylglycerol kinase from bovine testis. Purification and properties. J. Biol. Chem. 1994;269:21155–21164. [PubMed] [Google Scholar]
- 12.Pettitt TR, Wakelam MJ. Diacylglycerol kinase epsilon, but not zeta, selectively removes polyunsaturated diacylglycerol, inducing altered protein kinase C distribution in vivo. J. Biol. Chem. 1999;274:36181–36186. doi: 10.1074/jbc.274.51.36181. [DOI] [PubMed] [Google Scholar]
- 13.Musto A, Bazan NG. Diacylglycerol kinase epsilon modulates rapid kindling epileptogenesis. Epilepsia. 2006;47:267–276. doi: 10.1111/j.1528-1167.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez de Turco EB, Tang W, Topham MK, Sakane F, Marcheselli VL, Chen C, Taketomi A, Prescott SM, Bazan NG. Diacylglycerol kinase epsilon regulates seizure susceptibility and long-term potentiation through arachidonoyl-inositol lipid signaling. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4740–4745. doi: 10.1073/pnas.081536298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanjuan MA, Jones DR, Izquierdo M, Merida I. Role of diacylglycerol kinase alpha in the attenuation of receptor signaling. J. Cell Biol. 2001;153:207–220. doi: 10.1083/jcb.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chianale F, Cutrupi S, Rainero E, Baldanzi G, Porporato PE, Traini S, Filigheddu N, Gnocchi VF, Santoro MM, Parolini O, Van Blitterswijk WJ, Sinigaglia F, Graziani A. Diacylglycerol Kinase-{alpha} Mediates Hepatocyte Growth Factor-induced Epithelial Cell Scatter by Regulating Rac Activation and Membrane Ruffling. Mol Biol Cell. 2007;18:4859–4871. doi: 10.1091/mbc.E07-02-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 18.Yanagisawa K, Yasuda S, Kai M, Imai S, Yamada K, Yamashita T, Jimbow K, Kanoh H, Sakane F. Diacylglycerol kinase alpha suppresses tumor necrosis factor-alpha-induced apoptosis of human melanoma cells through NF-kappaB activation. Biochim. Biophys. Acta. 2007;1771:462–474. doi: 10.1016/j.bbalip.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Filigheddu N, Cutrupi S, Porporato PE, Riboni F, Baldanzi G, Chianale F, Fortina E, Piantanida P, De BM, Vacca G, Graziani A, Surico N. Diacylglycerol kinase is required for HGF-induced invasiveness and anchorage-independent growth of MDA-MB-231 breast cancer cells. Anticancer Res. 2007;27:1489–1492. [PubMed] [Google Scholar]
- 20.Ivanova PT, Milne SB, Byrne MO, Xiang Y, Brown HA. Glycerophospholipid identification and quantitation by electrospray ionization mass spectrometry. Methods Enzymol. 2007;432:21–57. doi: 10.1016/S0076-6879(07)32002-8. [DOI] [PubMed] [Google Scholar]
- 21.Rouzer CA, Ivanova PT, Byrne MO, Milne SB, Marnett LJ, Brown HA. Lipid Profiling Reveals Arachidonate Deficiency in RAW264.7 Cells: Structural and Functional Implications. Biochemistry. 2006;45:14795–14808. doi: 10.1021/bi061723j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milne S, Ivanova P, Forrester J, Brown HA. Lipidomics: An analysis of cellular lipids by ESI-MS. Methods. 2006;39:92–103. doi: 10.1016/j.ymeth.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Milne SB, Ivanova PT, DeCamp D, Hsueh RC, Brown HA. A targeted mass spectrometric analysis of phosphatidylinositol phosphate species. J Lipid Res. 2005;46:1796–1802. doi: 10.1194/jlr.D500010-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Callender HL, Forrester JS, Ivanova P, Preininger A, Milne S, Brown HA. Quantification of diacylglycerol species from cellular extracts by electrospray ionization mass spectrometry using a linear regression algorithm. Anal. Chem. 2007;79:263–272. doi: 10.1021/ac061083q. [DOI] [PubMed] [Google Scholar]
- 25.Heikinheimo L, Somerharju P. Translocation of phosphatidylthreonine and -serine to mitohondria diminishes exponentially with increasing molecular hydrophobicity. Traffic. 2002;3:367–377. doi: 10.1034/j.1600-0854.2002.30506.x. [DOI] [PubMed] [Google Scholar]
- 26.Mohn H, Chalifa V, Liscovitch M. Substrate specificity of neutral phospholipase D from rat brain studied by selective labeling of endogenous synaptic membrane phospholipids in vitro. J Biol Chem. 1992;267:11131–11136. [PubMed] [Google Scholar]
- 27.Lin CH, Lin J, Strickland KP. Bovine brain microsomal CDP-diacylglycerol synthetase: solubilization and properties. Biochem. Int. 1991;25:299–306. [PubMed] [Google Scholar]
- 28.Whatmore J, Wiedemann C, Somerharju P, Swigart P, Cockcroft S. Resynthesis of phosphatidylinositol in permeabilized neutrophils following phospholipase Cbeta activation: transport of the intermediate, phosphatidic acid, from the plasma membrane to the endoplasmic reticulum for phosphatidylinositol resynthesis is not dependent on soluble lipid carriers or vesicular transport. Biochem. J. 1999;341(Pt 2):435–444. [PMC free article] [PubMed] [Google Scholar]
- 29.Levine T, Loewen C. Inter-organelle membrane contact sites: through a glass, darkly. Curr. Opin. Cell Biol. 2006;18:371–378. doi: 10.1016/j.ceb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi N, Hozumi Y, Ito T, Hosoya T, Kondo H, Goto K. Differential subcellular targeting and activity-dependent subcellular localization of diacylglycerol kinase isozymes in transfected cells. Eur. J. Cell Biol. 2007;86:433–444. doi: 10.1016/j.ejcb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Pichler H, Gaigg B, Hrastnik C, Achleitner G, Kohlwein SD, Zellnig G, Perktold A, Daum G. A subfraction of the yeast endoplasmic reticulum associates with the plasma membrane and has a high capacity to synthesize lipids. Eur. J Biochem. 2001;268:2351–2361. doi: 10.1046/j.1432-1327.2001.02116.x. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein BJ, Bittner-Kowalczyk A, White MF, Harbeck M. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J Biol Chem. 2000;275:4283–4289. doi: 10.1074/jbc.275.6.4283. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Murillas I, Pettitt T, Macdonald E, Okkenhaug H, Georgiev P, Trivedi D, Hassan B, Wakelam M, Raghu P. lazaro encodes a lipid phosphate phosphohydrolase that regulates phosphatidylinositol turnover during Drosophila phototransduction. Neuron. 2006;49:533–546. doi: 10.1016/j.neuron.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Postle AD, Dombrowsky H, Clarke H, Pynn CJ, Koster G, Hunt AN. Mass spectroscopic analysis of phosphatidylinositol synthesis using 6-deuteriatedmyo-inositol: comparison of the molecular specificities and acyl remodeling mechanisms in mouse tissues and cultured cells. Biochem. Soc. Trans. 2004;32:1057–1059. doi: 10.1042/BST0321057. [DOI] [PubMed] [Google Scholar]
- 35.Gennis RB. Biomembranes: Molecular Structure and Function. New York: Springer-Verlag; 1989. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
We present as supplementary Tables, listings of identified lipid species for DGKε WT (Table 1), DGKε KO (Table 2), DGKα WT (Table 3) and DGKα KO (Table 4). This material is available free of charge via the Internet at http://pubs.acs.org.