Abstract
The Hedgehog (Hh) signaling pathway plays an evolutionarily conserved role in patterning fields of cells during metazoan development, and is inappropriately activated in cancer1,2. Hh pathway activity is absolutely dependent upon signaling by the seven-transmembrane protein Smoothened (Smo), which is regulated by the Hh receptor Patched (Ptc). Smo signals to an intracellular multi-protein complex containing the Kinesin related protein Costal2 (Cos2), the protein kinase Fused (Fu) and the transcription factor Cubitus interruptus (Ci)3. In the absence of Hh, this complex regulates the cleavage of full length Ci to a truncated repressor protein, Ci75 in a process that is dependent upon the proteosome and priming phosphorylations by Protein Kinase A (PKA)4. Binding of Hh to Ptc blocks Ptc-mediated Smo inhibition, allowing Smo to signal to the intracellular components to attenuate Ci cleavage. Because of its homology with the Frizzled family of G protein coupled receptors (GPCR)5, a likely candidate for an immediate Smo effector would be a heterotrimeric G protein. However, the role G proteins may play in Hh signal transduction is unclear and quite controversial6-10, which has led to widespread speculation that Smo signals through a variety of novel G protein independent mechanisms. Here, we present in vitro and in vivo evidence that Smo activates a G protein to modulate intracellular cyclic AMP (cAMP) levels in response to Hh. Our results demonstrate that Smo functions as a canonical GPCR, which signals through Gαi to regulate Hh pathway activation.
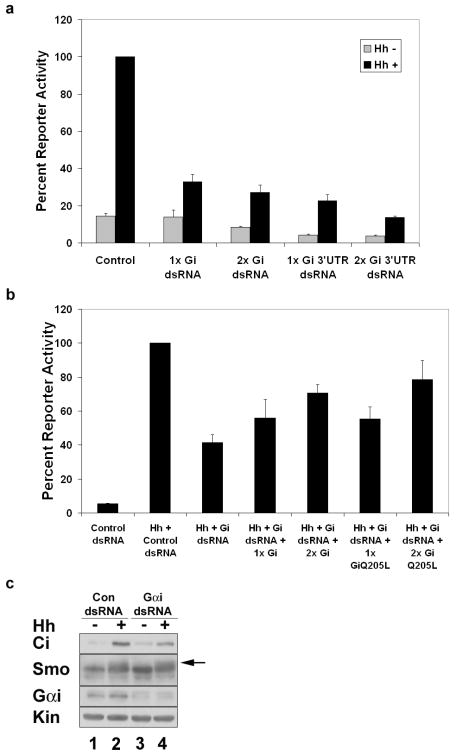
In order to examine whether a G protein is involved in Hh signaling, we targeted a series of G proteins by double stranded RNA (dsRNA)-mediated knockdown. Clone-8 (Cl8) cells were treated with control or Gα subunit specific dsRNA and assayed for changes in Hh-mediated induction of a ptc-luciferase reporter construct 11. We find that while Gαs and Gαo dsRNAs do not significantly alter Hh-induced reporter activation (Sup. Fig. 1a-b), Gαi knockdown is able to trigger a decrease in Hh dependent reporter gene expression (Fig. 1a). While not as effective as Smo knockdown in silencing Hh reporter gene activation (Sup. Fig. 1c), Gαi dsRNA specific to the coding sequence, or 3′ UTR, reduces Hh-induced reporter activity by approximately 70% (Fig. 1a), supporting a role for Gαi in the Hh pathway. To confirm the specificity of Gαi dsRNA effects, we established the functional IC50 of Gαi 3′ UTR dsRNA (Sup. Fig. 1e), then attempted to rescue reporter activity through ectopic expression of wild type Gαi or constitutively active GαiQ205L. Hh-stimulated reporter activity can be restored by both wild type and constitutively active Gαi (Fig. 1b), confirming the specificity of our Gαi dsRNA-mediated effects. Western blot analyses of Cl8 lysates reveal that cells treated with Gαi dsRNA show attenuated stabilization of Ci (Fig. 1c, compare lanes 2 and 4) and decreased Fu phosphorylation (Sup. Fig. 1d, compare lanes 2 and 4) in response to Hh. Hh-induced Smo phosphorylation is maintained in the presence of Gαi dsRNA (Fig. 1c, arrow), supporting that Gαi functions downstream of Smo and upstream of Fu and Ci.
Figure 1.
Gαi is required for Hh signaling. a-b. Cl8 cells were transfected with ptc-luciferase, act-renilla, hh expression vector or empty vector control, and the indicated dsRNA and/or Gαi expression vectors. Percent reporter expression relative to maximal Hh activity for control dsRNA is shown. c. Cl8 cells were transfected with control or Gαi dsRNA and hh expression vector or empty vector control. Cell lysates were analyzed by immunoblotting with the indicated antibodies. The arrow marks the phosphorylation-induced mobility shift. Kinesin (Kin) serves as a loading control. Error bars indicate s.e.m.
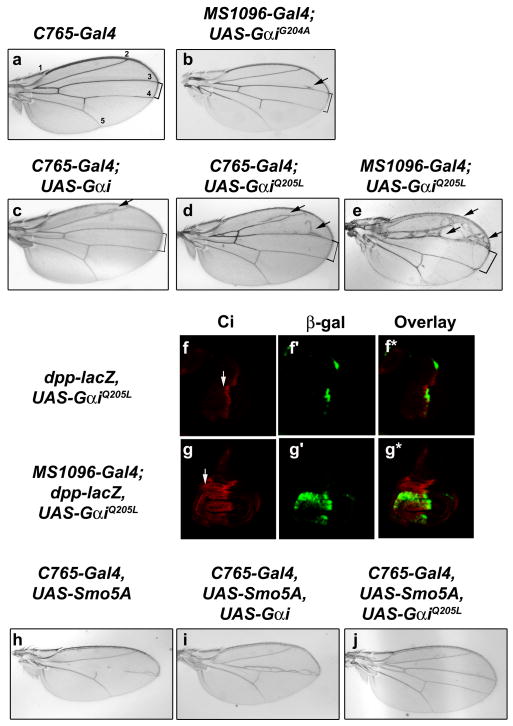
To determine whether Gαi can modulate Hh pathway activity in vivo, Gαi constructs were expressed in wing imaginal discs using MS1096-Gal4 or C765-Gal4. Expression of an inactive Gαi mutant (GαiG204A) or wild type Gαi has little effect on wing vein patterning (Fig 2a-c). However, expression of constitutively active GαiQ205L 12 results in widening of longitudinal vein LV3-LV4 spacing and ectopic vein material on LV2 and LV3 (Fig. 2d compared to 2a-c). The severity of this phenotype is dose-dependent, as higher level expression of UAS-GαiQ205L triggers more severe ectopic vein material anterior to LV3, and further widening of LV3-LV4 spacing (Fig. 2e). Expression of GαiQ205L in wing imaginal discs also results in over-growth of the wing pouch, along with expansion of full length Ci (Fig. 2g, arrow, compared to 2f, no driver). This Ci expansion triggers ectopic expression of the Hh target gene decapentaplegic (dpp)13,14 in the wing pouch, as evidenced by a dpp-lacZ reporter gene (Fig. 2g′ compared to 2f′). Gαi-mediated ectopic expression of dpp is consistent with the ectopic veins observed in wings expressing GαiQ205L (Fig. 2e, arrows)15. Taken together, these results support a role for activation of Gαi in regulating the stability of Ci, and link Gαi to regulation of a known Hh target gene.
Figure 2.
Gαi expression results in ectopic Hh signaling. a-g. Longitudinal veins are numbered (a). Wings from (b) UAS-GαiG204A, (c) UAS-Gαi (d, e) UAS-GαiQ205L demonstrate ectopic veins (arrows) and LV3-4 widening (brackets), as compared to control (a). Wing discs from (f) dpp-lacZ, UAS-GαiQ205L and (g) dpp-lacZ, UAS-GαiQ205L, MS1096-Gal4 larvae were immunostained for Ci (red) and β-galactosidase (green). Overlays are shown in f* and g*. Anterior is left and dorsal is up. h-j. Expression of wild type Gαi partially rescues, and GαiQ205L fully rescues, the phenotype of induced by dominant negative UAS-Smo5A (compare i and j to h).
To determine whether Gαi functions downstream of Smo in vivo, we analyzed the ability of Gαi to modulate Hh pathway activity in a smo sensitized background. As previously demonstrated, expression of a dominant negative smo transgene, UAS-Smo5A, results in severe disruption of LV3-LV4 wing patterning (Fig. 2h and 16). Expression of wild type Gαi in this smo sensitized background allows for partial rescue of wing vein structures in the LV3/LV4 zone (Fig. 2i). Expression of constitutively active GαiQ205L results in a more complete rescue of the Hh loss of function phenotype, allowing for near total restoration of LV3/LV4 patterning (Fig. 2j). As a control, UAS-GFP was co-expressed with Smo5A, and found to have no effect on the Smo5A-induced phenotype (data not shown).
To examine the ability of GαiQ205L to modulate Ci stability and Hh target gene activation in the smo sensitized background, wing imaginal discs were immunostained with antibodies that recognize full length Ci and the target gene product Ptc. UAS-Smo5A expression results in decreased ptc expression and disruption of the Ci gradient (Sup. Fig. 2b compared to 2a). Expression of constitutively active GαiQ205L in this smo sensitized background results in partial restoration of the Ci gradient and a near-complete rescue of ptc expression at the anterior/posterior (A/P) border (Sup Fig. 2c). These results support the model that Gαi contributes to the regulation of Hh target gene expression and Ci stability. Further, that this regulation occurs when Smo function is compromised suggests that Gαi affects Hh signaling at a level downstream of Smo.
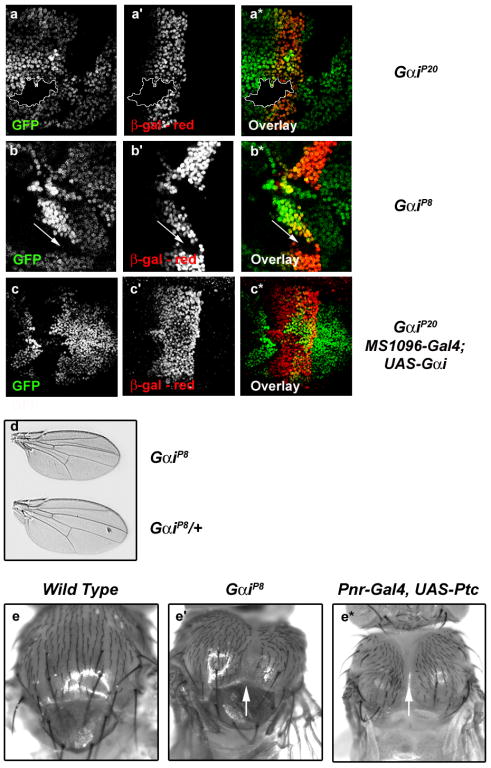
To determine whether Gαi is required for Hh signaling in vivo, we examined Hh target gene expression in clones of cells homozygous for Gαi mutation. The null allele GαiP20 removes the entire coding region of the Gαi gene, and is homozygous lethal17. GαiP8 is a putative hypomorph, which removes the bulk of exons 1 and 2, but leaves the transcriptional start site intact and produces a transcript (Sup. Fig. 3b). Flies that are homozygous for the GαiP8 mutation are viable, but unhealthy17. Mosaic analysis reveals that expression of the Hh target gene dpp is decreased in both GαiP20 (Fig. 3a) and GαiP8 (Fig. 3b) mutant clones, supporting a role for Gαi in activation of Hh target genes in vivo. To confirm that effects on dpp expression are due to loss of Gαi, we attempted to rescue GαiP20 null clones with UAS-Gαi. We find that ectopic expression of Gαi is able to rescue dpp reporter gene expression in GαiP20 clones (Fig. 3c-c*), consistent with decreased dpp expression resulting from disruption of Gαi.
Figure 3.
Gαi is required for Hh signaling in vivo. a-c. Mitotic clones were generated with the GαiP20 null (a, c) or GαiP8 hypomorphic (b) alleles. Wing discs were stained for the dpp-LacZ gene product β-gal (red). Loss of GFP expression (green) marks Gαi mutant clones. c. GαiP20 null clones were generated in MS1096-Gal4, UAS-Gαi wing discs. d-e. Gαi phenotypes are consistent with decreased Hh signaling. d. Homozygous GαiP8 mutant flies exhibit a small wing phenotype. e′. GαiP8 and e* pnr-Gal4, UAS-ptc flies have thoracic clefts (arrows), as compared to (e) wild type.
To determine whether compromised Gαi activity alters Hh-dependent patterning, we utilized the viable mutant allele GαiP8, and an additional viable allele described to be a null or strong hypomorph18, Gαi57. We find that whereas homozygous GαiP8 and Gαi57 mutants do not exhibit vein fusions that are typical of strong Hh loss of function, their wings are smaller than wild type wings (Fig 3d and Sup. Fig. 3c). Small wing size might result from altered dpp expression in anterior cells of the wing pouch, as Dpp regulates wing blade size19,20. Additionally, we find that both GαiP8 and Gαi57 mutant flies demonstrate varying degrees of incomplete thorax closure, as evidenced by mild to severe thoracic clefts (Fig. 3e′, arrow, compared to 3e and Sup. Fig. 3a-a*,arrows). This phenotype is also consistent with decreased dpp expression, in that Dpp, in conjunction with JNK signaling, controls spreading of the anterior edge of wing imaginal discs to initiate thorax closure21,22. To confirm that this phenotype results from decreased Hh signaling, we expressed ptc in the notum and dorsal compartment of the wing imaginal disc. ptc expression triggers the formation of a thoracic cleft when expressed under control of pannier and apterous promoters (Fig. 3e*, arrow, compared to 3e′ and 3e and data not shown), suggesting that the thoracic phenotype we observe in Gαi flies results from compromised Hh signaling. Because GαiP20 null mutant animals are not viable, we could not examine their wings or thoraces. However, we find that attenuation of Hh signaling by expressing dominant negative Smo5A is enhanced in GαiP20 heterozygotes, as evidenced by disruption of LV3 (Sup. Fig. 3d compared to d′).
Our in vitro and in vivo data suggest that loss of Gαi might compromise Ci stabilization in Hh-receiving cells. Interestingly, when we examine Ci and Smo levels in Gαi mutant clones, we find that both appear to be increased in a cell autonomous manner (Sup. Fig. 3e and f). These results are consistent with the modest stabilization of Smo and Ci that we observe upon in vitro Gαi knock-down in non-Hh treated cells (Fig. 1c, compare lane 1 with 3). While these results are unexpected, since Gαi loss is predicted to increase PKA activity and Ci degradation, previous studies have demonstrated that PKA functions to both positively and negatively regulate Hh signaling 23. Phosphorylation of Smo by PKA plays a positive role in pathway activation24, and might account for the modest stabilization of Ci we observe.
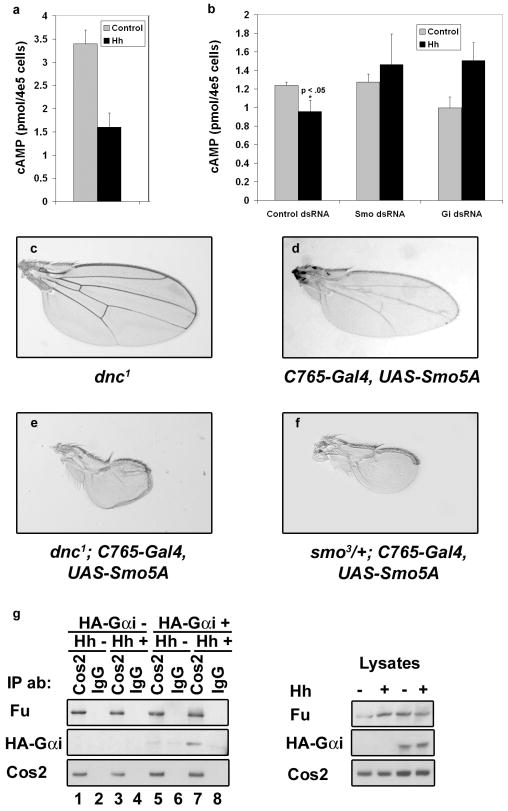
If Smo signals through Gαi, it should be able to induce Gαi activation rapidly in response to Hh stimulation. To assay for Hh-mediated activation of Gαi, we treated Cl8 cells with conditioned media containing the amino-terminal Hh signaling molecule (HhN) or control conditioned media, then assayed for Hh-induced changes in intracellular cAMP. We find that within 5-10 minutes, HhN treatment reduces the basal intracellular cAMP concentration by approximately 50% (Fig. 4a). To confirm that the Hh-induced decrease in intracellular cAMP is dependent upon Hh signaling through Smo and Gαi, we treated cells with Smo, Gαi or control dsRNA, then assayed for a Hh-induced decrease in cAMP (Fig. 4b). We find that whereas cells transfected with control dsRNA maintain the ability to decrease intracellular cAMP in response to HhN, cells transfected with either Smo or Gαi dsRNA are attenuated in their ability to do so. Taken together, these results support that Gαi is activated rapidly, in a Smo-dependent manner, in order to modulate cAMP levels in response to Hh.
Figure 4.
Hh regulates Gαi activity and association with Cos2. a. Cl8 cells were treated with control or HhN conditioned media. Error bars indicate s.e.m. b. Cl8 cells were transfected with control, Smo or Gαi dsRNA, and treated with HhN or control conditioned media. Wings from (c) hemizygous dnc1 (d) UAS-Smo5A, (e) dnc1, UAS-Smo5A and (f) smo3, UAS-Smo5A flies are shown. Introduction of dnc1 enhances the Smo5A phenotype in approximately 50% of flies (n=57). g. Cl8 cells were transfected with HA-Gαi and hh expression vectors, as indicated. Immunoprecipitations were performed from cell lysates using anti-Cos2 or IgG control antibodies.
To determine whether modulation of cAMP can alter Hh signaling in vivo, we utilized a hypomorphic mutant allele of the cAMP-specific phosphodiesterase dunce (dnc1)25 to raise intracellular cAMP levels in a Hh independent manner. Hemizygous dnc1 animals are viable with no obvious Hh defects (Fig. 4c). However, introduction of the dnc1 mutation into a smo sensitized background enhances the Smo loss of function phenotype to result in wings with near complete elimination of wing vein patterning (Fig. 4e compared to d). This enhanced Hh loss of function phenotype is similar to the phenotype obtained upon decreasing smo gene dosage by one-half in the same smo sensitized background (compare Fig. 4f with e and d). Along with our in vitro cAMP assays, these results support that Hh activates Smo to modulate intracellular cAMP, via Gαi, and that this function is important for proper pathway activity in vivo.
Cos2 associates with membranes, microtubules, PKA, Smo, Fu and Ci26,27. To determine whether Cos2 facilitates the coupling of Gαi with these Hh signaling components, we prepared lysates from cells expressing HA-Gαi, and then immunoprecipitated Cos2 (Fig. 4g). We find that Gαi associates with the Cos2 complex, and that this association is enriched in response to Hh (Fig. 4g, left panel, compare lane 7 to 5). The binding of Fu to Cos2 is not altered by Hh, suggesting that the recruitment of Gαi to this protein complex is regulated. This result suggests that Cos2 facilitates the coupling of Smo with Gαi and additional downstream effectors necessary to transduce the Hh signal.
Here, we have shown a requirement for Gαi in the Hh signaling pathway. We have demonstrated Hh-mediated recruitment and activation of Gαi that results in decreased intracellular cAMP, suggesting that Hh may regulate PKA through modulation of intracellular cAMP concentration. We have also demonstrated that Gαi can modulate Hh pathway activity in vitro and in vivo, and appears to do so at a level downstream of Smo. Furthermore, loss of Gαi alters Hh signaling in vivo, supporting that Gαi is a requisite member of the Hh pathway.
Methods Summary
All cell-based assays described here were performed in Clone-8 (Cl8) cells, which are a Hh responsive wing imaginal disc cell line. T7 DNA templates were generated from Gα cDNAs by PCR using Vent polymerase (NEB) as described 28. Cells were lysed 72 hours post dsRNA transfection 29 and post-nuclear lysates analyzed by immunoblotting, using the indicated antibodies. Reporter assays were performed in cells transfected with the indicated agents, along with a ptc-luciferase reporter construct 11 and act-renilla transfection control 29. cAMP levels were determined from cells treated with or without HhN conditioned media for 5-10 minutes, using a [3H]-cAMP receptor competition assay 30. Measured cAMP levels were then compared to a standard curve to determine intracellular cAMP. For Gαi and Smo knockdown effects on cAMP production, cells were transfected with control, Smo or Gαi specific dsRNA 72 hours prior to treatment with HhN or control conditioned media. Student's T test was used to determine p values. Error bars indicate standard error of the mean.
Fly stocks were maintained on standard yeast-cornmeal molasses media. For dnc1 enhancement of the Smo5A phenotype, crosses were begun at 25° C, then moved to 22° C for pupation. All other crosses were performed at 25° C with the exception of mosaic analyses, which were performed at room temperature. Mutant clones were marked by loss of GFP expression. Imaginal discs were dissected and immunostained as described previously29. Adult wings were mounted on glass microscope slides using DPX mounting media. Wing images were collected using a Zeiss Axioskop2 microscope. All images were processed using Adobe Photoshop 6.0. The GαiP8 breakpoint, as well as expression of the other Gαi alleles, was determined using standard molecular biology techniques.
Methods
RNA interference
T7 DNA templates were generated from Gα cDNAs by PCR using Vent polymerase (NEB) as described 28. dsRNA was generated from these T7 templates using the Megascript T7 kit (Ambion) per manufacturer's instructions. Gα subunit specific dsRNAs were generated from the following regions of the indicated G protein cDNAs: Gαi, basepairs 219-966 or 14-538 of the 3′ UTR, Gαo, basepairs 213-964 and Gαs, basepairs 208-1059. BLAST queries were performed to confirm that designed dsRNAs were subunit specific for the intended α subunit. Gαi cDNA was obtained from the Drosophila Genomics Resource Center (DGRC), Gαo cDNAs were provided by the DGRC and T. Kornberg (UCSF), and Gαs cDNA was provided by E. Lee (Vanderbilt). Semi-quantitative rtPCR was performed to confirm Gαo and Gαs knockdown. RNA was purified using TriReagent (MRC) per manufacturer's instructions. cDNA was generated from 5 μg total RNA using random primers and Superscript II reverse transcriptase (Invitrogen). One-twentieth of the cDNA was used for PCR of G proteins and Actin loading control. Reverse transcriptase minus reactions were used as a control.
To examine the effects of Gαi and/or Smo knockdown on the Hh signaling pathway, Cl8 cells were lysed 72 hours post dsRNA transfection as previously described 29. Post-nuclear lysates were analyzed by SDS-PAGE and western blot using the following published antibodies: Ci 31, Cos2 32, Fu33, Gαi12 and Kinesin as a loading control (Cytoskeleton, Inc.). To generate the Smo antibody used for western blot analysis, rats were injected with a peptide corresponding to amino acids 1010-1030 of the Smo carboxyl-terminal tail. Antibodies were then affinity purified from sera over a peptide column. Peptides and antibodies were produced and purified using the Covance custom antibody service.
Reporter assays
Clone-8 (Cl8) cells were transfected with Gαi dsRNA, ptc-luciferase reporter construct 11 and act-renilla transfection control 29 using Cellfectin transfection reagent (Invitrogen). Relative luciferase activity was determined 72 hours post transfection using the Dual Luciferase Assay System (Promega). Each assay was performed a minimum of three times, in duplicate. Values were averaged to determine ptc-luciferase activity relative to renilla transfection control. Error bars indicate standard error of the mean.
Fly crosses and transgenes
Fly stocks were maintained on standard yeast-cornmeal molasses media. For dnc1 enhancement of the Smo5A phenotype, crosses were begun at 25° C, then moved to 22° C for pupation. All other crosses were performed at 25° C with the exception of mosaic analyses, which were performed at room temperature. The following genotypes were used for generation of GαiP20 and GαiP8 mitotic clones: w hs-flp;GαiP20 FRT2A/Ubi-GFP FRT2A and w hs-flp;GαiP8 FRT2A/Ubi-GFP FRT2A. The following genotype was used for GαiP20 clone rescue experiments: MS1096-Gal4/yw hs-flp; dpp-lacZ/UAS-Gαi; GαiP20 FRT2A/Ubi-GFP FRT 2A. Recombination was induced by incubation at 37°C for 1 hour, 48 hours post egg laying34. Null mutant clones were marked by loss of GFP expression. Generation of UAS-GαiG204A transgenic flies was performed by the Duke Model Systems Genomics Core Injection service. UAS-Gαi and UAS-GαiQ205L flies were provided by J. Hooper (UCHSC). C765-Gal4, UAS-Smo5A, flies were provided by D. Casso and T. Kornberg (UCSF). smo alleles were provided by D. Kalderon, K. Basler and P. Ingham. Gαi alleles were provided by X. Yang, W. Chia and J. Knoblich. dnc1, dpp-lacZ, hsFLP; Ubi-GFP, FRT2A, pannier-Gal4 and MS1096-Gal4 flies were obtained from the Bloomington Drosophila Stock Center.
PCR and sequencing
To sequence the GαiP8 breakpoint, one adult fly was homogenized in 50 μl SB buffer (10mM Tris (pH 8.2), 25mM NaCl, 1mM EDTA, 0.2mg/ml protease K) and incubated for 30 minutes at 37°C, followed by an additional 10 minutes at 85°C. Lysates were centrifuged for 5 minutes at 2000 × g. Four μl of the supernatant was used as template for PCR with forward primer (GGATCATATGAGTGGCATTCAAGC) located -245 to -222, relative to the Gαi transcription start site, and reverse primer (CTGATAGCGCGACGCAGAAG), basepairs + 6197 to + 6216 relative to the transcription start site. Ten ng of PCR product was used for sequencing. The GαiP8 mutation deletes basepairs +29 to +6104 with respect to the Gαi transcription start site. For semi-quantitative RT-PCR analysis, 3 adult wild type or GαiP8 flies were homogenized in 200μl Tri Reagent and RNA was extracted per manufacturer's instructions (Molecular Research Center). Approximately 2 μg of RNA was used for reverse transcription reactions, using random hexamer oligos and SuperScript II reverse transcriptase (Invitrogen), or RT-minus control reactions.
Immunostaining and microscopy
Imaginal discs were dissected and immunostained as described previously29. Discs were stained using Ci (2A1)31, Ptc15, En35, Gαi 12, Smo36 and β-galactosidase (Promega) primary antibodies and Alexa-fluor conjugated secondary antibodies (Molecular Probes), as described29. Adult wings were mounted on glass microscope slides using DPX mounting media. Wing images were collected using a Zeiss Axioskop2 microscope and processed with Adobe Photoshop 6.0.
Immunoprecipitation
Cl8 cells were transfected with pAct-HA-Gαi, pAct-hh or empty vector control. Cells were lysed 48 hours post transfection and lysates were immunoprecipitated using Cos2 antisera as previously described32,37. HA antibody was obtained from GeneTex, Inc.
cAMP assay
Cl8 cells were treated with HhN or control S2 conditioned media for 10 minutes prior to lysis by boiling in 50 mM Tris-HCl, 4 mM EDTA, pH 7.9. Cell lysates were cleared of nuclei and cellular debris by centrifugation at 2000 × g for 10 minutes at 4° C. cAMP levels in cell lysates were determined by a radio-receptor competition assay as previously described30. Lysates were compared against a known standard curve similar to Sup. Fig. 4 to determine intracellular cAMP. For Hh-mediated effects on intracellular cAMP, the assay was performed two times in duplicate, and all data were averaged. For Gαi and Smo knockdown effects on cAMP production, cells were transfected with control, Smo or Gαi specific dsRNA 72 hours prior to treatment with HhN or control conditioned media. Following treatment with conditioned media for 5-10 minutes, cells were lysed and analyzed as described above. Assays were performed 2 times in triplicate for Gαi and 3 times in triplicate for Smo. A representative assay is shown. Student's T test was used to determine p values. Error bars indicate standard error of the mean.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants CA82628 (DJR) and HL074190 (JH). We are grateful to J. Knoblich for the Gαi antibody and Gαi flies, X. Yang and W. Chia for Gαi flies, D. Casso and T. Kornberg for Ptc antibody and UAS-Smo5A flies, J. Hooper for UAS-Gαi and UAS-GαiQ205L flies, K. Basler, D. Kalderon and P. Ingham for smo alleles, and L. Lum for Smo antibody. All additional fly stocks were provided by the Bloomington Stock Center. Gαi and Gαo cDNAs were obtained from the Drosophila Genomics Resource Center, Bloomington. E. Lee provided Gαs cDNA. We thank the Dartmouth microscopy core for their expert assistance. We would also like to thank K. Black for technical assistance and members of the Robbins laboratory, E. Lee, J. Hutchinson, R. Taussig, R. Ostrom and C. Pikielny for thoughtful discussion during the course of this work.
Footnotes
Author contributions. S.K.O was involved in the design, execution and analysis of experiments, and in writing the manuscript. D.L.F assisted in design and execution of dsRNA experiments and sequencing of Gαi alleles. J.H. was involved in design and execution of cAMP assays. N.S.S. assisted in execution of cAMP assays. Y.F.A. consulted on design and interpretation of genetic experiments. D.J.R. was involved in design and analysis of experiments and writing the manuscript.
References
- 1.Mullor JL, Sanchez P, Altaba AR. Pathways and consequences: Hedgehog signaling in human disease. Trends Cell Biol. 2002;12:562–9. doi: 10.1016/s0962-8924(02)02405-4. [DOI] [PubMed] [Google Scholar]
- 2.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 3.Robbins DJ, et al. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell. 1997;90:225–34. doi: 10.1016/s0092-8674(00)80331-1. [DOI] [PubMed] [Google Scholar]
- 4.Price MA, Kalderon D. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell. 2002;108:823–35. doi: 10.1016/s0092-8674(02)00664-5. [DOI] [PubMed] [Google Scholar]
- 5.Alcedo J, Ayzenzon M, Von Ohlen T, Noll M, Hooper JE. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell. 1996;86:221–32. doi: 10.1016/s0092-8674(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 6.DeCamp DL, Thompson TM, de Sauvage FJ, Lerner MR. Smoothened activates Galphai-mediated signaling in frog melanophores. J Biol Chem. 2000;275:26322–7. doi: 10.1074/jbc.M004055200. [DOI] [PubMed] [Google Scholar]
- 7.Low WC, et al. The decoupling of Smoothened from Galphai proteins has little effect on Gli3 protein processing and Hedgehog-regulated chick neural tube patterning. Dev Biol. 2008;321:188–96. doi: 10.1016/j.ydbio.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasai K, et al. The G12 family of heterotrimeric G proteins and Rho GTPase mediate Sonic hedgehog signalling. Genes Cells. 2004;9:49–58. doi: 10.1111/j.1356-9597.2004.00701.x. [DOI] [PubMed] [Google Scholar]
- 9.Riobo NA, Saucy B, Dilizio C, Manning DR. Activation of heterotrimeric G proteins by Smoothened. Proc Natl Acad Sci U S A. 2006;103:12607–12. doi: 10.1073/pnas.0600880103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murone M, Rosenthal A, de Sauvage FJ. Sonic hedgehog signaling by the patched-smoothened receptor complex. Curr Biol. 1999;9:76–84. doi: 10.1016/s0960-9822(99)80018-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen CH, et al. Nuclear trafficking of Cubitus interruptus in the transcriptional regulation of Hedgehog target gene expression. Cell. 1999;98:305–16. doi: 10.1016/s0092-8674(00)81960-1. [DOI] [PubMed] [Google Scholar]
- 12.Schaefer M, Petronczki M, Dorner D, Forte M, Knoblich JA. Heterotrimeric G proteins direct two modes of asymmetric cell division in the Drosophila nervous system. Cell. 2001;107:183–94. doi: 10.1016/s0092-8674(01)00521-9. [DOI] [PubMed] [Google Scholar]
- 13.Muller B, Basler K. The repressor and activator forms of Cubitus interruptus control Hedgehog target genes through common generic gli-binding sites. Development. 2000;127:2999–3007. doi: 10.1242/dev.127.14.2999. [DOI] [PubMed] [Google Scholar]
- 14.Methot N, Basler K. Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell. 1999;96:819–31. doi: 10.1016/s0092-8674(00)80592-9. [DOI] [PubMed] [Google Scholar]
- 15.Capdevila J, Guerrero I. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. Embo J. 1994;13:4459–68. doi: 10.1002/j.1460-2075.1994.tb06768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins RT, Cohen SM. A genetic screen in Drosophila for identifying novel components of the hedgehog signaling pathway. Genetics. 2005;170:173–84. doi: 10.1534/genetics.104.039420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu F, Cai Y, Kaushik R, Yang X, Chia W. Distinct roles of Galphai and Gbeta13F subunits of the heterotrimeric G protein complex in the mediation of Drosophila neuroblast asymmetric divisions. J Cell Biol. 2003;162:623–33. doi: 10.1083/jcb.200303174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampoelz B, Hoeller O, Bowman SK, Dunican D, Knoblich JA. Drosophila Ric-8 is essential for plasma-membrane localization of heterotrimeric G proteins. Nat Cell Biol. 2005;7:1099–105. doi: 10.1038/ncb1318. [DOI] [PubMed] [Google Scholar]
- 19.Affolter M, Basler K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat Rev Genet. 2007;8:663–74. doi: 10.1038/nrg2166. [DOI] [PubMed] [Google Scholar]
- 20.Aegerter-Wilmsen T, Aegerter CM, Hafen E, Basler K. Model for the regulation of size in the wing imaginal disc of Drosophila. Mech Dev. 2007;124:318–26. doi: 10.1016/j.mod.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Martin-Blanco E, Pastor-Pareja JC, Garcia-Bellido A. JNK and decapentaplegic signaling control adhesiveness and cytoskeleton dynamics during thorax closure in Drosophila. Proc Natl Acad Sci U S A. 2000;97:7888–93. doi: 10.1073/pnas.97.14.7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pena-Rangel MT, Rodriguez I, Riesgo-Escovar JR. A misexpression study examining dorsal thorax formation in Drosophila melanogaster. Genetics. 2002;160:1035–50. doi: 10.1093/genetics/160.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohlmeyer JT, Kalderon D. Dual pathways for induction of wingless expression by protein kinase A and Hedgehog in Drosophila embryos. Genes Dev. 1997;11:2250–8. doi: 10.1101/gad.11.17.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Apionishev S, Katanayeva NM, Marks SA, Kalderon D, Tomlinson A. Drosophila Smoothened phosphorylation sites essential for Hedgehog signal transduction. Nat Cell Biol. 2005;7:86–92. doi: 10.1038/ncb1210. [DOI] [PubMed] [Google Scholar]
- 25.Davis RL, Kiger JA., Jr Dunce mutants of Drosophila melanogaster: mutants defective in the cyclic AMP phosphodiesterase enzyme system. J Cell Biol. 1981;90:101–7. doi: 10.1083/jcb.90.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogden SK, Ascano M, Jr, Stegman MA, Robbins DJ. Regulation of Hedgehog signaling: a complex story. Biochem Pharmacol. 2004;67:805–14. doi: 10.1016/j.bcp.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia J, Jiang J. Decoding the Hedgehog signal in animal development. Cell Mol Life Sci. 2006 doi: 10.1007/s00018-005-5519-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stegman MA, et al. The Kinesin-related protein Costal2 associates with membranes in a Hedgehog-sensitive, Smoothened-independent manner. J Biol Chem. 2004;279:7064–71. doi: 10.1074/jbc.M311794200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogden SK, et al. Smoothened regulates activator and repressor functions of Hedgehog signaling via two distinct mechanisms. J Biol Chem. 2006;281:7237–43. doi: 10.1074/jbc.M510169200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stitham J, Stojanovic A, Ross LA, Blount AC, Jr, Hwa J. Clusters of transmembrane residues are critical for human prostacyclin receptor activation. Biochemistry. 2004;43:8974–86. doi: 10.1021/bi0496788. [DOI] [PubMed] [Google Scholar]
- 31.Motzny CK, Holmgren R. The Drosophila cubitus interruptus protein and its role in the wingless and hedgehog signal transduction pathways. Mech Dev. 1995;52:137–50. doi: 10.1016/0925-4773(95)00397-j. [DOI] [PubMed] [Google Scholar]
- 32.Stegman MA, et al. Identification of a tetrameric hedgehog signaling complex. J Biol Chem. 2000;275:21809–12. doi: 10.1074/jbc.C000043200. [DOI] [PubMed] [Google Scholar]
- 33.Ascano M, Jr, Nybakken KE, Sosinski J, Stegman MA, Robbins DJ. The carboxyl-terminal domain of the protein kinase fused can function as a dominant inhibitor of hedgehog signaling. Mol Cell Biol. 2002;22:1555–66. doi: 10.1128/mcb.22.5.1555-1566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 35.Patel NH, Kornberg TB, Goodman CS. Expression of engrailed during segmentation in grasshopper and crayfish. Development. 1989;107:201–12. doi: 10.1242/dev.107.2.201. [DOI] [PubMed] [Google Scholar]
- 36.Lum L, et al. Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Mol Cell. 2003;12:1261–74. doi: 10.1016/s1097-2765(03)00426-x. [DOI] [PubMed] [Google Scholar]
- 37.Ogden SK, et al. Identification of a functional interaction between the transmembrane protein Smoothened and the kinesin-related protein Costal2. Curr Biol. 2003;13:1998–2003. doi: 10.1016/j.cub.2003.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.