Abstract
The development of an animal model of endometriosis is crucial for the investigation of disease pathogenesis and therapeutic intervention. These models will enhance our ability to evaluate the causes for the subfertility associated with disease and provide a first-line validation of treatment modulators. Currently rodents and non-human primate models have been developed, but each model has their limitations. The aim of this manuscript is to summarize the current findings and theories on the development of endometriosis and disease progression and the effectiveness of therapeutic targets using the experimental induced model of endometriosis in the baboon (Papio anubis).
Keywords: animal model, endometriosis, endometrium, primate, uterine, pathologies
Introduction
Endometriosis is a gynecological condition that affects 1 out of every 10 women of reproductive age (Eskenazi and Warner, 1997). This condition is traditionally defined as the development of endometrial glands and stroma outside of the uterine cavity, but this definition has broadened to include the development of any endometrial cell type (glands or stroma) outside of the uterine cavity (Clement, 2007). Laparoscopic evaluation followed by histological confirmation is the gold standard for diagnosis of endometriosis, but the accuracy of the diagnosis is highly dependent upon surgical and pathological expertise.
Currently there are three theories that describe the etiology of endometriosis. The first is the embryonic rest theory. This theory proposes that at puberty there is activation of cells of Mullerian duct origin at various sites in the pelvic cavity (Batt and Smith, 1989; Batt et al., 1990). This theory supports the finding of rare cases in which endometriosis was found in males (Witz, 1999). The second theory is that of coelomic metaplasia. This theory states that substances in menstrual fluid can induce peritoneal tissues to form endometrial cells, suggesting that there is a factor found in menstrual fluid that is a precursor for the disease (Meyer, 1919). The third theory, and the most widely accepted, is the Sampson hypothesis of retrograde menstruation (Sampson, 1927). This theory states that endometrial fragments are displaced into the peritoneal cavity through a process known as retrograde menstruation. Retrograde menstruation occurs in the majority (70–90%) (Blumenkrantz et al., 1981; Eskenazi and Warner, 1997) of women, but only a small percentage (10%) of these women develop endometriosis, which suggests that the peritoneal environment or the endometrium of women with endometriosis may be altered compared with that of healthy women (Sampson, 1927).
Clinically, endometriosis presents itself with symptoms such as chronic pelvic pain, dysmenorrhea, dyspareunia and subfertility (Verkauf, 1987). The initiation of endometriosis is difficult to evaluate because at the onset of clinical symptoms many women already have significant established diseases (Eskenazi and Warner, 1997). Treatment therapies for endometriosis involve both pharmacological and surgical intervention either individually or combined (Valle, 2002). Medical treatments available for endometriosis include oral contraceptives, GnRH analogs, progesterone analogs and aromatase inhibitors. Surgically, endometriosis has been treated by the excision or ablation of endometriotic lesions and the removal of adhesions in the peritoneal cavity. Several reports have debated the effectiveness of either medical or surgical therapy for the treatment of infertility and chronic pain (Kettel and Murphy, 1989; Barbieri, 1992; Shaw, 1992; Howard, 1993; Hughes et al., 1993; Vercellini et al., 1997; Moghissi, 1999; Harrison and Barry-Kinsella, 2000; Lessey, 2000; Wilson et al., 2000; Chwalisz et al., 2002; Abbott et al., 2003; D'Hooghe, 2003; Donnez et al., 2003; Garry, 2004). Currently, there is no universally acceptable, standard treatment protocol for endometriosis, hence the treatment is individualized for each patient with mixed outcomes.
The identification of molecules involved with the pathogenesis of endometriosis or strategic therapies for treatment is difficult in a spontaneous model of endometriosis because of the many unknown and uncontrollable factors. Furthermore, in women, time from the onset of disease to diagnosis is between 8–11 years (Sinaii et al., 2002). The establishment of an animal model with experimentally inducible endometriosis would allow investigators to characterize factors involved at the early onset of disease and throughout disease progression allowing for the evaluation of molecular changes that cause infertility and chronic pelvic pain. The experimentally induced model also allows for the investigation of the efficacy of therapeutic intervention strategies.
Animal models of endometriosis
Immunocompromised rodents have been used for the development of an animal model of endometriosis by surgically implanting human endometrial tissue into the peritoneal wall (Zamah et al., 1984; Bruner et al., 1997; Awwad et al., 1999). Another version of the rodent model transplants endometrial tissue from genetically similar mice to induce disease (Rossi et al., 2000). Although the use of rodents is a cost-effective animal model that does allow for the generation of specific knock-outs, the disadvantages are numerous. Ectopic lesions are very small and are not physiologically similar to those found in advanced stages of human disease. Also the use of immunocompromised rodents eliminates the investigation of any immunoregulatory component involved in the pathogenesis of disease. Additionally, these animals do not develop spontaneous disease since they are a non-menstruating species. In contrast though, the rodent model would be cost-effective for preliminary efficacy trials of medical therapies.
Several non-human primates (NHP) have been used for the development of experimental endometriosis: Japanese macaque, pigtailed macaque, rhesus monkeys and baboons (Story and Kennedy, 2004). The use of NHP is advantageous for the study of endometriosis because it provides a phyogenetically similar animal model to the human, and this use of NHP allows for the evaluation of disease pathogenesis as well as therapeutic targets. However, the use of NHP is very costly and supply is very limited. Of all the NHP models developed, the baboon (Papio anubis) is the most favorable because of its size and similar reproductive anatomy. Unlike rodents and other primate models of disease, baboons have a menstrual cycle similar to humans in both duration and endometrial remodeling (Hendrickx, 1971). Baboons also have similar changes in the eutopic endometrium during the window of uterine receptivity and a similar type of placentation as that seen in humans (Hendrickx, 1971). In addition, baboons also develop spontaneous endometriosis in which ectopic lesions resemble those of humans (Merrill, 1968; Folse and Stout, 1978). Endometriosis can also be induced in baboons by injecting autologous menstrual effluent into the pelvic cavity (D'Hooghe, 1997; Fazleabas et al., 2002). The intraperitoneal injection of menstrual tissue mimics the normal physiological process of retrograde menstruation and allows investigators to study disease progression from the initial onset of disease. In baboons, we are able to perform evaluative laparoscopies and collect biological samples prior to the induction of disease and then compare these control samples to post-inoculation samples of the same animal (Fazleabas et al., 2002). The employment of each animal to serve as its own control reduces inter-animal variability in experimental designs and reduces the animal numbers required per study. The body size of baboons allows for multiple and complex surgical procedures and repeated collection of measurable biological samples such as blood, tissue and peritoneal fluid. Because laparoscopic evaluation can also cause inflammation of the peritoneal cavity (D'Hooghe et al., 1999), it is important that animals induced with disease be compared with healthy control animals which have also undergone the same number of laparoscopic procedures at the same intervals for each study.
Therefore, the baboon provides an excellent model to study the pathogenesis of endometriosis, the identification of molecules in multiple physiological systems that are affected by the presence of ectopic peritoneal lesions and the effectiveness of novel therapeutic strategies. This review will summarize the current findings and ongoing studies, utilizing the experimentally induced baboon model of endometriosis.
Pathogenesis of ectopic endometrium
Although endometriosis was first described nearly a century ago, the understanding of the pathogenesis of endometriosis remains unclear. The key to understanding the pathogenesis of endometriosis is to evaluate molecular changes associated with the peritoneal lining and the sites of ectopic lesion invasion during the initial stages of development. These studies are impossible to perform in humans because disease is always established prior to clinical symptoms. Using an induced model of disease, we are able to test Sampson's theory of endometriosis by investigating the early molecular events that are necessary for the establishment of ectopic endometrial lesions. The initial step for the development of ectopic lesions is the attachment of menstrual endometrium to the peritoneal lining followed by the invasion of the menstrual tissue through the peritoneal surface and finally the establishment of a blood supply.
Attachment
The process of retrograde menstruation has been shown to increase several inflammatory mediators in the peritoneal fluid of baboons. It has been reported that in peritoneal fluid of baboons, peritoneal cells expressed higher levels of tumor necrosis factor (TNF-α), transforming growth factor (TGF-β1) and intracellular adhesion molecule ICAM-1, and the peritoneal fluid contained higher levels of TGF-β1 and interleukin (IL-6) (D'Hooghe et al., 2001). These inflammatory mediators have the capacity to enhance the binding of menstrual tissue to the peritoneal lining of abdominal structures.
In vitro studies have challenged the previous concept that ectopic lesions invade at locations only where the peritoneal surface has been damaged (Koks et al., 1999). Multiple studies have shown that human menstrual tissue fragments cannot only attach but also invade peritoneal cell monolayers (Witz et al., 1999, 2003; Debrock et al., 2002). However, these studies found that the invasion of menstrual tissue through peritoneal monolayers was not dependent on the presence of endometriosis, indicating that the peritoneal environment is important for normal clearance of menstrual tissue.
If retrograde menstruation occurs in the majority of human females and NHP, then why is not there a higher prevalence of endometrosis? The efficient immunological clearance of menstrual tissue or a decreased immuno-tolerance may explain why the prevalence of endometriosis is not greater than that observed. It has been reported that the peritoneal fluid from women with endometriosis have decreased natural killer and macrophage activity (Oosterlynck et al., 1991), but elevated levels of IL-1, IL-6, IL-10, p40, TNF-α and TGF-β have also been reported (Punnonen et al., 1996; Mazzeo et al., 1998; Harada et al., 1999; Gazvani and Templeton, 2002). In baboons, TNF-α, TGF-β, CD3 and HLA-DR were expressed on a larger number of cells within the peritoneal fluid following the induction of endometriosis compared with pre-inoculatory controls (D'Hooghe et al., 2001).
Invasion
The increased levels of cytokines in the peritoneal fluid of both women and NHP with endometriosis may aid the invasion of ectopic lesions by activating tissue remodeling enzymes known as matrix metalloproteinases (MMPs). The expression profile for MMPs in the human endometrium throughout the menstrual cycle and their regulation by ovarian steroid hormones has been well documented (Rodgers et al., 1993, 1994; Singer et al., 1999; Curry and Osteen, 2001). Increases in these mediators of inflammation may support the remodeling of the peritoneal mesothelium which would allow for the invasion and subsequent growth of endometrial fragments. Our laboratory has reported that MMP-7 levels are increased in baboon ectopic endometrium (Fazleabas et al., 2002), and these data are supported by human studies (Bruner-Tran et al., 2002). The inhibition of MMP action may be a beneficial therapeutic target for the reduction of ectopic lesion development.
Angiogenesis and maintenance of disease
The development of ectopic endometrium is heavily dependent on the establishment of a sufficient blood supply at the site of invasion and its maintenance by steroid hormones. The establishment of angiogenesis can be seen macroscopically at sites of ectopic endometrial invasion. In both humans and baboons, vascular endothelial cell growth factor (VEGF) levels are elevated in eutopic and ectopic endometrium (Donnez et al., 1998; Fujishita et al., 1999; Tan et al., 2002; Takehara et al., 2004; Gashaw et al., 2006). Additionally, CYR61 (an angiogenic factor important for cell proliferation, development and tumorigenesis) has been shown to be up-regulated in both the eutopic and ectopic endometrium (Absenger et al., 2004; Gashaw et al., 2006). These data indicate that the enhanced expression of angiogenic factors in the eutopic endometrium may attribute to the development of ectopic lesions in the peritoneal cavity.
Ectopic endometrial lesions are steroid hormone responsive. In human and baboon endometriotic tissues, there is an up-regulation of aromatase, estrogen receptor-2 (ESR2) expression and downregulation of progesterone receptor beta (PGR-β) (Noble et al., 1996, 1997; Brandenberger et al., 1999; Attia et al., 2000; Fazleabas et al., 2003; Jackson et al., 2007). The up-regulation of estrogen biosynthesis in ectopic endometrium has been shown to occur through alterations in the methylation patterns of ESR2 and steroidigenic factor 1 (Xue et al., 2007a, b). It has also been shown that ectopic endometrial stromal cells fail to convert estradiol (biologically active estrogen) to estrone because of reduced expression of 17-β-hydroxysteroid dehydrogenase type 2 in ectopic endometrial epithelial cells (Zeitoun et al., 1998). This enzyme is regulated by PGR, and the reduction of PGR in ectopic lesions may in turn cause the dysregulation of 17-β-hydroxysteroid dehydrogenase type 2 expression (Cheng et al., 2007). The dysregulation of steroid synthesis enzymes and steroid receptors allows for ectopic lesion development by maintenance of cell proliferation through the use of exogenous steroids and endogenous synthesis of estrogen. The ability of these lesions to regulate their own steroid hormone production within their local environment renders them hormonally independent from the eutopic endometrium (Attar et al., 2009; Bulun et al., 2009).
Infertility
There are many factors that contribute to endometriosis-associated subfertility: immune dysfunction, distortion of the pelvic anatomy, poor oocyte quality and fertilization rates, and the dysregulation of the genetic profile of the eutopic endometrium, during the window of receptivity, resulting in decreased implantation success (Ayers et al., 1987; Halme et al., 1987; Mills et al., 1992; Burney et al., 2007). The identification of dysregulated genes in the eutopic endometrium could reveal potential precursors for the development of endometriosis and also therapeutic targets for infertility treatment.
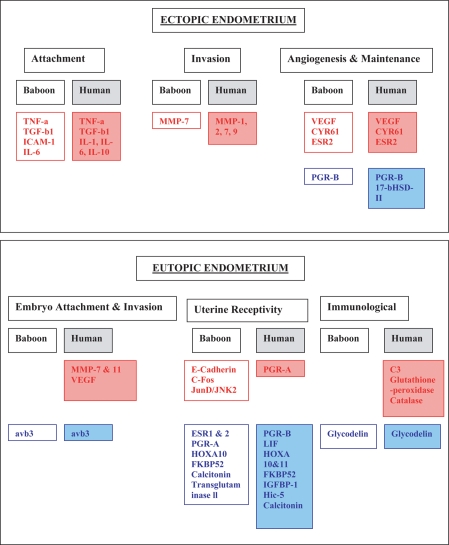
The power of microarray technology allows the identification of genes that are dysregulated in endometriotic tissue among several physiological pathways. Several genes have been found to be dysregulated in the eutopic endometrium of women with endometriosis, and these studies have been validated in both the rodent and baboon models of induced endometriosis (Eyster et al., 2002; Giudice et al., 2002; Giudice, 2004; Matsuzaki et al., 2005; Flores et al., 2007; Mettler et al., 2007; Pan et al., 2007; Wren et al., 2007; Gaetje et al., 2008). In general, these studies have consistently found that the following genes are dysregulated in eutopic endometriotic tissue: aromatase, endometrial bleeding factor, hepatocyte growth factor, 17-β-hydroxysteroid dehydrogenase, HOXA10, HOXA11, leukemia inhibitory factor, MMPs 3, 7 and 11, tissue inhibitors of metalloproteinases, progesterone-receptor isoforms, complement 3, glutathione peroxidase, catalase, thrombospondin 1, VEGF, integrin αvβ3 and glycodelin. These genes have been associated with regulating processes such as embryonic attachment and invasion, angiogenesis, endometrial tissue remodeling, fetal immunological tolerance and uterine steroid hormone responsiveness, which are all physiological functions vital for successful embryonic implantation (summarized in Fig. 1).
Figure 1.
Gene dysregulation in ectopic and eutopic endometrium in baboons and women with endometriosis. All references can be found within the manuscript. Red denotes upregulated genes, blue denotes downregulated genes. Abbreviations: TNF, tumour necrosis factor; TGF, transforming growth factor; ICAM, intracellular adhesion molecule; IL, interleukin; MMP, matrix metalloproteinase; VEGF, vascular endothelial growth factor; ESR, oestrogen receptor; PGR, progesterone receptor; HSD, hydroxysteroid dehydrogenase; LIF, leukaemia inhibitory factor; FKBP, regulator of progesterone responsiveness; IGFBP, insulin-like binding protein; CYR, cysteine-rich angiogenic inducer; Hic-5, progesterone responsive gene; avb3, integrin avb3.
Immunological genes
It is known that endometriosis induces an inflammatory environment in the peritoneal cavity of women (Oosterlynck et al., 1991, 1994; D'Hooghe et al., 2001; Gazvani and Templeton, 2002; Umezawa et al., 2009), but does the presence of ectopic lesions also induce an inflammatory environment in the eutopic endometrium? Microarray analysis of endometrium from women with or without endometriosis has revealed several genes that are dysregulated during the secretory stage are important for successful implantation (Burney et al., 2007). This study reported that MUC-1 and osteopontin, facilitators of embryo adhesion, and glycodelin, immune regulator of maternal fetal tolerance (Bolton et al., 1987), were down-regulated during the secretory phase of the menstrual cycle. Studies from our laboratory have corroborated these findings using a simulated model of pregnancy in the baboon. We have found that in normal endometrium, glycodelin levels increase in response to the embryonic signal CG (chorionic gonadotrophin); however, glycodelin induction by CG failed in endometriotic eutopic tissue (Fazleabas et al., 2003).
This study prompted our laboratory to investigate other immunological genes that may be dysregulated in the eutopic endometrium during the window of receptivity. Using a focused microarray, we analyzed eutopic endometrium from control and diseased animals and found that there was no significant change in several immunological factors during the window of receptivity in eutopic endometriotic tissue (Hastings et al., 2006). These data indicate that although endometriosis does not alter the expression of immune factors, it impairs the function of immunoregulatory proteins to adequately suppress the activity of endometrial T cells to sustain proper embryo implantation.
Steroid hormone responsiveness
The coordinated changes that occur in the endometrium throughout the menstrual cycle are regulated by the ovarian steroid hormones estrogen (E2) and progesterone. Hormonal action is mediated through their interaction with their respective receptors on both endometrial stromal and epithelial cells. Using the baboon model of induced endometriosis, our laboratory was able to identify the dysregulation of estrogen and progesterone responsive genes as well as ESR1 and ESR2 and PGR-A and PGR-B during disease progression.
In healthy baboons without endometriosis, eutopic endometrial stromal expression of ESR1 was primarily evident during the window of receptivity, but in endometriotic animals, this increase was not observed (Jackson et al., 2007). The decrease in expression was seen primarily in the stromal cells 6 months after the induction of disease. No changes were observed in the glandular epithelial layer through disease progression. The expression pattern of ESR2 was also altered in the presence of disease. During the window of receptivity, ESR2 was decreased in the eutopic endometrium of diseased animals compared with controls, and this decrease was evident in both the glandular and stromal compartments at 6 months of disease. The decrease in ESR1 and ESR2 expression, in diseased animals, renders the endometrium unresponsive to estrogen stimulation and as a consequence PGR induction, which is necessary during the window of receptivity. These changes are not apparent at the onset of disease (<6 months) but rather after adequate establishment of disease (>6 months), indicating that ectopic lesions altered the expression of ESR in the eutopic endometrium.
Following the early onset of disease, c-FOS, an estrogen-responsive gene, was highly up-regulated, in addition to junD and JNK2 (Hastings et al., 2006). These genes are important for controlling cell cycle and cellular fate by repressing gene expression or silencing. One of the targets of AP-1 transcription factors is PGR isoform A (Shemshedini et al., 1991). The early expression of this family of transcription factors may affect the responsiveness of the endometrium to ovarian steroids during the window of receptivity throughout disease progression.
The expression of the PGR-A and PGR-B is important during the window of receptivity for successful implantation. During the progression of endometriosis, it has been reported that the eutopic endometrium becomes resistant to progesterone action by the dysregulation of a number of progesterone-responsive genes (Hic-5, glycodelin, IGFBP-1,HOXA10, calcitonin) and progesterone chaperone genes (FKBP52) (Kao et al., 2003; Burney et al., 2007; Jackson et al., 2007, 2009; Kim et al., 2007; Aghajanova et al., 2009). Using our baboon model of endometriosis, we examined the eutopic endometrial expression of PGR-A and PGR-B and downstream targets of progesterone action. We found a decrease in PGR-A immunolocalization in glandular epithelial cells, although there was no change in the immunolocalization of PGR-B. In stromal cells, there was no difference in the immunolocalization of PGR-A or PGR-B, but interestingly, the ability of these cells to respond to PR stimulation was decreased (Kim et al., 2007). This decrease was attributed to the finding that FKBP52, a regulator of progesterone responsiveness, was diminished at later time points (>6 months) of disease (Jackson et al., 2007), a finding that has been also confirmed in women with endometriosis (Hirota et al., 2008).
In addition to the characterization of the steroid receptor profiles in diseased eutopic endometrium, it has also been reported that HOXA10, a downstream target of PR which is also down-regulated in FKBP52 null mice (Tranguch et al., 2005, 2007), was decreased in endometriotic tissue from women and baboons (Taylor et al., 1999; Kim et al., 2007). In baboons, the decrease of HOXA10 expression resulted in a decrease of target gene integrin B3 and an increase of empty spiracles homolog 2 (Kim et al., 2007). The dysregulation of HOXA10 in endometriosis may be the result of the methylation state of the gene. In baboons with disease, the F1 region of HOXA10 was hypermethylated compared with disease-free animals. In women with severe endometriosis, HOXA10 expression was also associated with hypermethylation of all three regions of the HOXA10 gene (Gui et al., 1999). These studies indicate that endometriosis may affect post-transcriptional modifications as a method of gene silencing or activation.
HOXA10 has also been shown to be important in endometrial stromal cell decidualization, in that null mice are infertile due to a decidualization defect (Benson et al., 1996). The role of HOXA10 during the process of stromal cell decidualization was examined by investigating the potential HOXA10 regulation of insulin-like growth factor-binding protein-1 (IGFBP-1), a major marker for decidualization in the baboon. Endometrial stromal cells isolated from baboons with endometriosis have shown increased expression of IGFBP-1 following decidualization treatment (Kim et al., 2007), indicating a hyper response to decidualization. In contrast, human endometrial stromal cells from diseased women have shown reduced IGFBP-1 secretion (Klemmt et al., 2006). Both of these studies indicate that there is an altered decidualization response in eutopic endometrial stromal cells from diseased endometrium.
The importance of FKBP52 for embryonic implantation was proven by the use of FKBP52 null mice. When FKBP52 was deleted, the levels of HOXA10, Indian hedgehog (Ihh) and calcitonin (CALC) were also reduced during the window of receptivity (Tranguch et al. 2005, 2007; Yang et al., 2006). Our laboratory evaluated the localization of calcitonin and calcitonin-modulated proteins, E-cadherin and transglutaminase II in the eutopic endometrium during the window of receptivity in baboons with induced endometriosis to determine whether these proteins were downregulated as a result of decreased FKBP52 expression. We found that in diseased animals, calcitonin staining was decreased in both endometrial epithelial and stromal cells, whereas E-cadherin staining was increased in endometrial epithelial cells, and transglutaminase-II staining was decreased in endometrial stromal cells compared with disease-free healthy controls (Jackson et al., 2009). These data support the theory that, during disease progression, the eutopic endometrium has the ability to become resistant to progesterone actions probably as a consequence of impaired PGR function directly or indirectly by the attenuated expression of critical chaperone proteins such as FKBP52 and Hic-5 (Aghajanova et al., 2009).
The aberrant dysregulation of the steroid receptors and their downstream targets, during the window of receptivity, affects not only the ability of the eutopic endometrium to respond to ovarian hormonal priming but also the response of the endometrium to the implanting embryo, resulting in implantation failure. This is clearly evident in our baboon model of simulated pregnancy. Following the induction of endometriosis, the eutopic endometrium is unresponsive to chorionic gonadotrophin (CG) stimulation, and the expression of a number of genes thought to be critical for implantation and decidualization are markedly altered (Fazleabas et al., 2003; Hastings et al., 2008). The examination of steroid hormone responsiveness can elucidate the effectiveness of hormonal treatment of endometriosis and corroborate the finding of progesterone therapy resistance in some cases of endometriosis (Bulun et al., 2006; Burney et al., 2007).
Ultrastructural abnormalities
The endometrium is one of the most dynamic tissues in the entire body. At the beginning of each menstrual cycle, this unique tissue regenerates following the shedding of endometrial tissue known as menstruation. Because the endometrium goes through histological changes each menstrual cycle, pathologists have created a dating system to classify the different phases of the menstrual cycle (Noyes et al., 1975). Recently, we have reported changes in the ultrastructure of the endometrium in each phase of the menstrual cycle (Jones et al., 2006). Control eutopic endometrial tissues from the late proliferative, mid-secretory and late secretory phases of the menstrual cycle were analyzed using electron microscopy. During the window of uterine receptivity, the endometrium was characterized by columnar cells containing glycogen-filled vesicles which were associated with Golgi bodies, small and narrow mitochondria, lateral membranes with few interdigitations and apical junctional complexes (Jones et al., 2006). Following the induction of endometriosis, eutopic endometrium isolated during the window of receptivity (mid-secretory) did not resemble that of control mid-secretory endometrium. Instead, we observed a shift in the ultrastructure of the endometrium to that of late proliferative control endometrium at early stages of disease. At later stages of disease, the ultrastructure of the eutopic endometrium resembled that of control late secretory endometrium (Jones et al., 2006). These findings have since been validated in the eutopic and ectopic endometrium of women (Jones et al., 2009a, b). Thus, in addition to the changes in gene expression, changes in the ultrastructural morphology of the eutopic endometrium during the window of uterine receptivity are also clearly documented. Thus, it is evident that the presence of peritoneal disease is associated with an inherent endometrial effect that is manifested in multiple etiologies, all of which may be detrimental to successful embryo implantation.
The studies summarized here indicate that the sub-infertility that is associated with endometriosis is the result of several factors. The use of the baboon model of inducible endometriosis has clearly established that ectopic endometrial lesions distinctly influence the eutopic endometrium, and these changes sequentially result in an overall resistance to progesterone. These types of studies are not feasible when evaluating the consequences of spontaneous disease. Thus, on the basis of these studies, the effectiveness of treatment modalities can be evaluated to determine whether these therapies can re-establish normal fecundity rates in women with endometriosis.
Translational research
The baboon model of endometriosis, either inducible or spontaneous, provides an excellent tool to evaluate the effectiveness of therapeutic targets on reducing disease development and also on improving pregnancy success rates. The inflammatory cytokine TNF-α is a potent stimulator of the inflammatory process and has been shown to be elevated in the peritoneal fluid of women with endometriosis (Halme, 1989; Arici et al., 1998). For this reason, it has been the primary target for therapeutic design and development.
Anti-TNF therapy has shown to be a potent, non-hormonal therapeutic option for the treatment of endometriosis. Pretreatment of the pelvic cavity with recombinant human TNF-binding protein-1 (r-hTBP-1) prior to disease induction resulted in a reduction in the lesion surface area and revised American Fertility Society (rAFS) scoring of disease (D'Hooghe et al., 2006). When r-hTBP-1 was given after disease induction, they found that r-hTBP-1 treatment could reduce the surface area of lesions, improve rAFS scoring and eliminate the formation of adhesions involving the reproductive organs and cul-de-sac. However, neither pre- nor post-treatment with r-hTBP-1 affected inflammatory cytokine mRNA levels for TNF-α, ICAM-1, MMP-1, IL-8, IL-6 or RANTES( regulated upon activation, normal T-cell expressed, and secreted) in either the ectopic or the eutopic endometrium (Kyama et al., 2008). The authors did report that post-disease induction treatment with r-hTBP-1 did reduce TGF-β mRNA levels in the ectopic endometrium but not in the eutopic endometrium.
Another compound, etanercept, currently used for rheumatoid arthritis, juvenile rheumatoid arthritis and psoriatic arthritis has been investigated for its effectiveness as therapy for endometriosis (Barrier et al., 2004). Etanercept, a fusion protein consisting of human recombinant soluble TNF receptor 2 which neutralizes TNF activity, reduced the formation of red lesions in baboons with established endometriosis. This treatment did not have an effect on blue or white lesions.
Because anti-TNF-α therapy has had beneficial results for the reduction of endometriosis development, it has recently been used to examine the potential benefits on pregnancy rate and outcome in endometriotic animals. Treatment of the anti-TNF-α agent c5N following disease induction has been shown to reduce the formation of ectopic lesions (Falconer et al., 2006). Subsequent to this study, these same animals were then evaluated for pregnancy success and outcome (Falconer et al., 2008). The treatment of animals with c5N was not able to increase pregnancy success or pregnancy outcome. The authors believe that the failure of c5N to improve fertility was due to the short time of disease induction, 2 months, and this short time period of disease was not adequate for the induction of subfertility. The treatment of animals with spontaneous proven infertility with c5N would be indicative of the effectiveness of c5N for the improvement of pregnancy parameters.
Another family of immunomodulators that have been investigated as a potential therapeutic treatment for endometriosis are peroxisome proliferator-activated receptors (PPARs). The ligand for PPAR-γ induces the regression of endometriotic explants in the rat model and also in the induced baboon model (Lebovic et al., 2004, 2007). The reduction of lesion surface area was not due to a decrease in serum E2 or P4 levels, but instead they believe that PPAR-γ actions may be through its inhibition of nuclear factor-κB (NF-κB) in macrophages (Chinetti et al., 1998; Jiang et al., 1998; Wang et al., 2001; Chen et al., 2002; Liu et al., 2005). The constitutive expression of NF-κB in endometriotic lesions may play a role in the development of endometriosis (Guo, 2007), and targeting this transcription factor is a potential therapy for reducing the pathogenesis of this disease.
Summary
The use of the NHP (baboon) model of endometriosis allows for the investigation of molecules involved not only in the pathogenesis of disease but also in the genetic dysregulation of the eutopic endometrium and immune system. It is heavily debated whether defects of the endometrium cause ectopic lesion development or whether the presence of ectopic lesions causes defects of the eutopic endometrium. Because the baboon can develop endometriosis spontaneously, as well as in response to induction with menstrual tissue, investigators can begin to elucidate factors that are dysregulated due to the presence of ectopic lesions and compare these factors to the spontaneous model. This information could identify markers early in disease development that could be used as diagnostic tools eliminating the need for surgical diagnosis. Additionally, the baboon is a powerful model for evaluating and testing therapeutic targets for the treatment of disease. The use of the baboon allows for repetitive surgical evaluations of disease, which provides a well-controlled model for the study of the effectiveness of drug therapies and reduces the ethical and medical concerns that arise in human studies requiring surgical evaluation and intervention. The baboon model of endometriosis is critical for the discovery of a future treatment or possible prevention of endometriosis.
Funding
Supported by the Eunice Kennedy Shriver NICHD/National Institutes of Health through cooperative agreement U54 HD 40093 (to A.T.F.) as part of the Specialized Cooperative Centers Program in Reproductive Research and T32 HL 007692 (to A.G.B).
References
- Abbott JA, Hawe J, Clayton RD, Garry R. The effects and effectiveness of laparoscopic excision of endometriosis: a prospective study with 2–5 year follow-up. Hum Reprod. 2003;18:1922–1927. doi: 10.1093/humrep/deg275. [DOI] [PubMed] [Google Scholar]
- Absenger Y, Hess-Stumpp H, Kreft B, Kratzschmar J, Haendler B, Schutze N, Regidor PA, Winterhager E. Cyr61, a deregulated gene in endometriosis. Mol Hum Reprod. 2004;10:399–407. doi: 10.1093/molehr/gah053. [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Velarde M, Giudice LC. The progesterone receptor co-activator Hic-5 is involved in the pathophysiology of endometriosis. Endocrinology. 2009;150:3863–3870. doi: 10.1210/en.2009-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arici A, Seli E, Zeyneloglu HB, Senturk LM, Oral E, Olive DL. Interleukin-8 induces proliferation of endometrial stromal cells: a potential autocrine growth factor. J Clin Endocrinol Metab. 1998;83:1201–1205. doi: 10.1210/jcem.83.4.4743. [DOI] [PubMed] [Google Scholar]
- Attar E, Tokunaga H, Imir G, Yilmaz MB, Redwine D, Putman M, Gurates B, Attar R, Yaegashi N, Hales DB, et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab. 2009;94:623–631. doi: 10.1210/jc.2008-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85:2897–2902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- Awwad JT, Sayegh RA, Tao XJ, Hassan T, Awwad ST, Isaacson K. The SCID mouse: an experimental model for endometriosis. Hum Reprod. 1999;14:3107–3111. doi: 10.1093/humrep/14.12.3107. [DOI] [PubMed] [Google Scholar]
- Ayers JW, Birenbaum DL, Menon KM. Luteal phase dysfunction in endometriosis: elevated progesterone levels in peripheral and ovarian veins during the follicular phase. Fertil Steril. 1987;47:925–929. doi: 10.1016/s0015-0282(16)59224-3. [DOI] [PubMed] [Google Scholar]
- Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740–745. doi: 10.1016/0002-9378(92)91706-g. [DOI] [PubMed] [Google Scholar]
- Barrier BF, Bates GW, Leland MM, Leach DA, Robinson RD, Propst AM. Efficacy of anti-tumor necrosis factor therapy in the treatment of spontaneous endometriosis in baboons. Fertil Steril. 2004;81(Suppl. 1):775–779. doi: 10.1016/j.fertnstert.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Batt RE, Smith RA. Embryologic theory of histogenesis of endometriosis in peritoneal pockets. Obstet Gynecol Clin North Am. 1989;16:15–28. [PubMed] [Google Scholar]
- Batt RE, Smith RA, Buck GM, Severino MF, Naples JD. Mullerianosis. Prog Clin Biol Res. 1990;323:413–426. [PubMed] [Google Scholar]
- Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz MJ, Gallagher N, Bashore RA, Tenckhoff H. Retrograde menstruation in women undergoing chronic peritoneal dialysis. Obstet Gynecol. 1981;57:667–670. [PubMed] [Google Scholar]
- Bolton AE, Pockley AG, Clough KJ, Mowles EA, Stoker RJ, Westwood OM, Chapman MG. Identification of placental protein 14 as an immunosuppressive factor in human reproduction. Lancet. 1987;1:593–595. doi: 10.1016/s0140-6736(87)90235-2. [DOI] [PubMed] [Google Scholar]
- Brandenberger AW, Lebovic DI, Tee MK, Ryan IP, Tseng JF, Jaffe RB, Taylor RN. Oestrogen receptor (ER)-alpha and ER-beta isoforms in normal endometrial and endometriosis-derived stromal cells. Mol Hum Reprod. 1999;5:651–655. doi: 10.1093/molehr/5.7.651. [DOI] [PubMed] [Google Scholar]
- Bruner KL, Matrisian LM, Rodgers WH, Gorstein F, Osteen KG. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest. 1997;99:2851–2857. doi: 10.1172/JCI119478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab. 2002;87:4782–4791. doi: 10.1210/jc.2002-020418. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248:94–103. doi: 10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Utsunomiya H, Lin Z, Yin P, Cheng YH, Pavone ME, Tokunaga H, Trukhacheva E, Attar E, Gurates B, et al. Steroidogenic factor-1 and endometriosis. Mol Cell Endocrinol. 2009;300:104–108. doi: 10.1016/j.mce.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–3826. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- Chen GG, Lee JF, Wang SH, Chan UP, Ip PC, Lau WY. Apoptosis induced by activation of peroxisome-proliferator activated receptor-gamma is associated with Bcl-2 and NF-kappaB in human colon cancer. Life Sci. 2002;70:2631–2646. doi: 10.1016/s0024-3205(02)01510-2. [DOI] [PubMed] [Google Scholar]
- Cheng YH, Imir A, Fenkci V, Yilmaz MB, Bulun SE. Stromal cells of endometriosis fail to produce paracrine factors that induce epithelial 17beta-hydroxysteroid dehydrogenase type 2 gene and its transcriptional regulator Sp1: a mechanism for defective estradiol metabolism. Am J Obstet Gynecol. 2007;196:391.e1–391.e7. doi: 10.1016/j.ajog.2006.12.014. [Discussion 391.e7–391.e8] [DOI] [PubMed] [Google Scholar]
- Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J, Staels B. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Garg R, Brenner RM, Schubert G, Elger W. Selective progesterone receptor modulators (SPRMs): a novel therapeutic concept in endometriosis. Ann NY Acad Sci. 2002;955:373–388. doi: 10.1111/j.1749-6632.2002.tb02798.x. [Discussion 389–393, 396–406] [DOI] [PubMed] [Google Scholar]
- Clement PB. The pathology of endometriosis: a survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects. Adv Anat Pathol. 2007;14:241–260. doi: 10.1097/PAP.0b013e3180ca7d7b. [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Osteen KG. Cyclic changes in the matrix metalloproteinase system in the ovary and uterus. Biol Reprod. 2001;64:1285–1296. doi: 10.1095/biolreprod64.5.1285. [DOI] [PubMed] [Google Scholar]
- Debrock S, Perre S, Meuleman C, Moerman P, Hill J, D'Hooghe T. In-vitro adhesion of endometrium to autologus peritoneal membranes: effect of the cycle phase and the stage of endometriosis. Hum Reprod. 2002;17:2523–2528. doi: 10.1093/humrep/17.10.2523. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM. Clinical relevance of the baboon as a model for the study of endometriosis. Fertil Steril. 1997;68:613–625. doi: 10.1016/s0015-0282(97)00277-x. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM. Immunomodulators and aromatase inhibitors: are they the next generation of treatment for endometriosis? Curr Opin Obstet Gynecol. 2003;15:243–249. doi: 10.1097/01.gco.0000072859.73466.e3. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Bambra CS, Raevmaekers BM, Hill JA. Pelvic inflammation induced by diagnostic laparoscopy in baboons. Fertil Steril. 1999;72:1134–1141. doi: 10.1016/s0015-0282(99)00406-9. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Bambra CS, Xiao L, Peixe K, Hill JA. Effect of menstruation and intrapelvic injection of endometrium on inflammatory parameters of peritoneal fluid in the baboon (Papio anubis and Papio cynocephalus) Am J Obstet Gynecol. 2001;184:917–925. doi: 10.1067/mob.2001.111715. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Nugent NP, Cuneo S, Chai DC, Deer F, Debrock S, Kyama CM, Mihalyi A, Mwenda JM. Recombinant human TNFRSF1A (r-hTBP1) inhibits the development of endometriosis in baboons: a prospective, randomized, placebo- and drug-controlled study. Biol Reprod. 2006;74:131–136. doi: 10.1095/biolreprod.105.043349. [DOI] [PubMed] [Google Scholar]
- Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod. 1998;13:1686–1690. doi: 10.1093/humrep/13.6.1686. [DOI] [PubMed] [Google Scholar]
- Donnez J, Pirard C, Smets M, Jadoul P, Squifflet J. Pre- and post-surgical management of endometriosis. Semin Reprod Med. 2003;21:235–242. doi: 10.1055/s-2003-41329. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- Eyster KM, Boles AL, Brannian JD, Hansen KA. DNA microarray analysis of gene expression markers of endometriosis. Fertil Steril. 2002;77:38–42. doi: 10.1016/s0015-0282(01)02955-7. [DOI] [PubMed] [Google Scholar]
- Falconer H, Mwenda JM, Chai DC, Wagner C, Song XY, Mihalyi A, Simsa P, Kyama C, Cornillie FJ, Bergqvist A, et al. Treatment with anti-TNF monoclonal antibody (c5N) reduces the extent of induced endometriosis in the baboon. Hum Reprod. 2006;21:1856–1862. doi: 10.1093/humrep/del044. [DOI] [PubMed] [Google Scholar]
- Falconer H, Mwenda JM, Chai DC, Song XY, Cornillie FJ, Bergqvist A, Fried G, D'Hooghe TM. Effects of anti-TNF-mAb treatment on pregnancy in baboons with induced endometriosis. Fertil Steril. 2008;89(Suppl. 5):1537–1545. doi: 10.1016/j.fertnstert.2007.05.062. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Gurates B, Chai D, Bulun S. A modified baboon model for endometriosis. Ann NY Acad Sci. 2002;955:308–317. doi: 10.1111/j.1749-6632.2002.tb02791.x. [Discussion 340–302, 396–406] [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Chai D, Langoi D, Bulun SE. Steroid receptor and aromatase expression in baboon endometriotic lesions. Fertil Steril. 2003;80(Suppl. 2):820–827. doi: 10.1016/s0015-0282(03)00982-8. [DOI] [PubMed] [Google Scholar]
- Flores I, Rivera E, Ruiz LA, Santiago OI, Vernon MW, Appleyard CB. Molecular profiling of experimental endometriosis identified gene expression patterns in common with human disease. Fertil Steril. 2007;87:1180–1199. doi: 10.1016/j.fertnstert.2006.07.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folse D, Stout L. Endometriosis in the baboon. Lab Anim Sci. 1978;28:217–219. [PubMed] [Google Scholar]
- Fujishita A, Hasuo A, Khan KN, Masuzaki H, Nakashima H, Ishimaru T. Immunohistochemical study of angiogenic factors in endometrium and endometriosis. Gynecol Obstet Invest. 1999;48(Suppl. 1):36–44. doi: 10.1159/000052867. [DOI] [PubMed] [Google Scholar]
- Gaetje R, Holtrich U, Engels K, Kissler S, Rody A, Karn T, Kaufmann M. Differential expression of claudins in human endometrium and endometriosis. Gynecol Endocrinol. 2008;24:442–449. doi: 10.1080/09513590802242694. [DOI] [PubMed] [Google Scholar]
- Garry R. The effectiveness of laparoscopic excision of endometriosis. Curr Opin Obstet Gynecol. 2004;16:299–303. doi: 10.1097/01.gco.0000136496.95075.79. [DOI] [PubMed] [Google Scholar]
- Gashaw I, Hastings JM, Jackson KS, Winterhager E, Fazleabas AT. Induced endometriosis in the baboon (Papio anubis) increases the expression of the proangiogenic factor CYR61 (CCN1) in eutopic and ectopic endometria. Biol Reprod. 2006;74:1060–1066. doi: 10.1095/biolreprod.105.049320. [DOI] [PubMed] [Google Scholar]
- Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction. 2002;123:217–226. doi: 10.1530/rep.0.1230217. [DOI] [PubMed] [Google Scholar]
- Giudice LC. Microarray expression profiling reveals candidate genes for human uterine receptivity. Am J Pharmacogenomics. 2004;4:299–312. doi: 10.2165/00129785-200404050-00003. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Telles TL, Lobo S, Kao L. The molecular basis for implantation failure in endometriosis: on the road to discovery. Ann NY Acad Sci. 2002;955:252–264. doi: 10.1111/j.1749-6632.2002.tb02786.x. [Discussion 293–255, 396–406] [DOI] [PubMed] [Google Scholar]
- Gui Y, Zhang J, Yuan L, Lessey BA. Regulation of HOXA-10 and its expression in normal and abnormal endometrium. Mol Hum Reprod. 1999;5:866–873. doi: 10.1093/molehr/5.9.866. [DOI] [PubMed] [Google Scholar]
- Guo SW. Nuclear factor-kappab (NF-kappaB): an unsuspected major culprit in the pathogenesis of endometriosis that is still at large? Gynecol Obstet Invest. 2007;63:71–97. doi: 10.1159/000096047. [DOI] [PubMed] [Google Scholar]
- Halme J. Release of tumor necrosis factor-alpha by human peritoneal macrophages in vivo and in vitro. Am J Obstet Gynecol. 1989;161:1718–1725. doi: 10.1016/0002-9378(89)90957-5. [DOI] [PubMed] [Google Scholar]
- Halme J, Becker S, Haskill S. Altered maturation and function of peritoneal macrophages: possible role in pathogenesis of endometriosis. Am J Obstet Gynecol. 1987;156:783–789. doi: 10.1016/0002-9378(87)90333-4. [DOI] [PubMed] [Google Scholar]
- Harada T, Enatsu A, Mitsunari M, Nagano Y, Ito M, Tsudo T, Taniguchi F, Iwabe T, Tanikawa M, Terakawa N. Role of cytokines in progression of endometriosis. Gynecol Obstet Invest. 1999;47(Suppl. 1):34–39. doi: 10.1159/000052857. [Discussion 39–40] [DOI] [PubMed] [Google Scholar]
- Harrison RF, Barry-Kinsella C. Efficacy of medroxyprogesterone treatment in infertile women with endometriosis: a prospective, randomized, placebo-controlled study. Fertil Steril. 2000;74:24–30. doi: 10.1016/s0015-0282(00)00577-x. [DOI] [PubMed] [Google Scholar]
- Hastings JM, Jackson KS, Mavrogianis PA, Fazleabas AT. The estrogen early response gene FOS is altered in a baboon model of endometriosis. Biol Reprod. 2006;75:176–182. doi: 10.1095/biolreprod.106.052852. [DOI] [PubMed] [Google Scholar]
- Hastings JM, Sherwin RA, Mavrogianis PA, Jackson KS, Sharkey AM, Fazleabas AT. The eutopic endometrial response to chorionic gonadotrophin is abnormal in a baboon model of endometriosis: a possible explanation for endometriosis associated implantation failure. Fertil Steril. 2008;90:S2. [Google Scholar]
- Hendrickx A. Reproduction: methods. In: Hendrickx A, editor. Embryology of the Baboon. Chicago: University of Chicago Press; 1971. pp. 1–44. [Google Scholar]
- Hirota Y, Tranguch S, Daikoku T, Hasegawa A, Osuga Y, Taketani Y, Dey SK. Deficiency of immunophilin FKBP52 promotes endometriosis. Am J Pathol. 2008;173:1747–1757. doi: 10.2353/ajpath.2008.080527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard FM. The role of laparoscopy in chronic pelvic pain: promise and pitfalls. Obstet Gynecol Surv. 1993;48:357–387. doi: 10.1097/00006254-199306000-00001. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Fedorkow DM, Collins JA. A quantitative overview of controlled trials in endometriosis-associated infertility. Fertil Steril. 1993;59:963–970. [PubMed] [Google Scholar]
- Jackson KS, Brudney A, Hastings JM, Mavrogianis PA, Kim JJ, Fazleabas AT. The altered distribution of the steroid hormone receptors and the chaperone immunophilin FKBP52 in a baboon model of endometriosis is associated with progesterone resistance during the window of uterine receptivity. Reprod Sci. 2007;14:137–150. doi: 10.1177/1933719106298409. [DOI] [PubMed] [Google Scholar]
- Jackson K, Hastings J, Mavrogianis P, Bagchi I, Fazleabas A. Alterations in the calcitonin and calcitonin modulated proteins, E-cadherin and the enzyme tissue transglutaminase II during the window of implantation in a baboon model of endometriosis. J Endometriosis. 2009;1:57–67. [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Denton J, Fazleabas AT. Morphological and glycosylation changes associated with the endometrium and ectopic lesions in a baboon model of endometriosis. Hum Reprod. 2006;21:3068–3080. doi: 10.1093/humrep/del310. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Inuwa IM, Nardo LG, Litta P, Fazleabas AT. Eutopic endometrium from women with endometriosis shows altered ultrastructure and glycosylation compared to that from healthy controls—a pilot observational study. Reprod Sci. 2009;a 16:559–572. doi: 10.1177/1933719109332825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CJ, Nardo LG, Litta P, Fazleabas AT. Peritoneal ectopic lesions from women with endometriosis show abnormalities in progesterone-dependent glycan expression. Fertil Steril. 2009;b 91(Suppl. 4):1608–1610. doi: 10.1016/j.fertnstert.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- Kettel LM, Murphy AA. Combination medical and surgical therapy for infertile patients with endometriosis. Obstet Gynecol Clin North Am. 1989;16:167–177. [PubMed] [Google Scholar]
- Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, Wu Y, Guo SW, Fazleabas AT. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod. 2007;13:323–332. doi: 10.1093/molehr/gam005. [DOI] [PubMed] [Google Scholar]
- Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85:564–572. doi: 10.1016/j.fertnstert.2005.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koks C, Groothuis P, Dunselman G, de Goeij AF, Evers J. Adhesion of shed menstrual tissue in an in-vitro model using amnion and peritoneum: a light and electron microscopic study. Hum Reprod. 1999;14:816–822. doi: 10.1093/humrep/14.3.816. [DOI] [PubMed] [Google Scholar]
- Kyama C, Overbergh L, Mihalyi A, Cuneo S, Chai D, Debrock S, Mwenda J, Mathieu C, Nugent NP, D'Hooghe T. Effect of recombinant human TNF-binding protein-1 and GnRH antagonist on mRNA expression of inflammatory cytokines and adhesion and growth factors in endometrium and endometriosis tissues in baboons. Fertil Steril. 2008;89:1306–1313. doi: 10.1016/j.fertnstert.2006.11.205. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Kir M, Casey C. Peroxisome proliferator-activated receptor gamma induces regression of endometrial explants in a rat model of endometriosis. Fertil Steril. 2004;82:1008–1013. doi: 10.1016/j.fertnstert.2004.02.148. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Mwenda JM, Chai DC, Mueller MD, Santi A, Fisseha S, D'Hooghe T. PPAR-gamma receptor ligand induces regression of endometrial explants in baboons: a prospective, randomized, placebo- and drug-controlled study. Fertil Steril. 2007;88(Suppl. 4):1108–1119. doi: 10.1016/j.fertnstert.2006.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA. Medical management of endometriosis and infertility. Fertil Steril. 2000;73:1089–1096. doi: 10.1016/s0015-0282(00)00519-7. [DOI] [PubMed] [Google Scholar]
- Liu D, Zeng BX, Zhang SH, Yao SL. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, reduces pulmonary inflammatory response in a rat model of endotoxemia. Inflamm Res. 2005;54:464–470. doi: 10.1007/s00011-005-1379-0. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Canis M, Vaurs-Barriere C, Boespflug-Tanguy O, Dastugue B, Mage G. DNA microarray analysis of gene expression in eutopic endometrium from patients with deep endometriosis using laser capture microdissection. Fertil Steril. 2005;84(Suppl. 2):1180–1190. doi: 10.1016/j.fertnstert.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Mazzeo D, Vigano P, Di Blasio AM, Sinigaglia F, Vignali M, Panina-Bordignon P. Interleukin-12 and its free p40 subunit regulate immune recognition of endometrial cells: potential role in endometriosis. J Clin Endocrinol Metab. 1998;83:911–916. doi: 10.1210/jcem.83.3.4612. [DOI] [PubMed] [Google Scholar]
- Merrill J. Spontaneous endometriosis in the Kenya baboon. Am J Obstet Gynecol. 1968;101:569–570. doi: 10.1016/0002-9378(68)90572-3. [DOI] [PubMed] [Google Scholar]
- Mettler L, Salmassi A, Schollmeyer T, Schmutzler AG, Pungel F, Jonat W. Comparison of c-DNA microarray analysis of gene expression between eutopic endometrium and ectopic endometrium (endometriosis) J Assist Reprod Genet. 2007;24:249–258. doi: 10.1007/s10815-007-9116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. uber den Stand der Frage der Ademomyositis and Ademomyome serosepithelialis und Adenomyometritis sarcomatosa. Zentrakbl Gynako. 1919;43:745–750. [Google Scholar]
- Mills MS, Eddowes HA, Cahill DJ, Fahy UM, Abuzeid MI, McDermott A, Hull MG. A prospective controlled study of in-vitro fertilization, gamete intra-fallopian transfer and intrauterine insemination combined with superovulation. Hum Reprod. 1992;7:490–494. doi: 10.1093/oxfordjournals.humrep.a137677. [DOI] [PubMed] [Google Scholar]
- Moghissi KS. Medical treatment of endometriosis. Clin Obstet Gynecol. 1999;42:620–632. doi: 10.1097/00003081-199909000-00016. [DOI] [PubMed] [Google Scholar]
- Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. J Clin Endocrinol Metab. 1996;81:174–179. doi: 10.1210/jcem.81.1.8550748. [DOI] [PubMed] [Google Scholar]
- Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, Agarwal VR, Zhao Y, Carr BR, Bulun SE. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600–606. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- Oosterlynck DJ, Cornillie FJ, Waer M, Vandeputte M, Koninckx PR. Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertil Steril. 1991;56:45–51. doi: 10.1016/s0015-0282(16)54414-8. [DOI] [PubMed] [Google Scholar]
- Oosterlynck D, Meuleman C, Waer M, Koninckx PR. Transforming growth factor-b activity is increased in peritoneal fluid from women with endometriosis. Obstet Gynecol. 1994;83:287–292. [PubMed] [Google Scholar]
- Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod. 2007;13:797–806. doi: 10.1093/molehr/gam063. [DOI] [PubMed] [Google Scholar]
- Punnonen J, Teisala K, Ranta H, Bennett B, Punnonen R. Increased levels of interleukin-6 and interleukin-10 in the peritoneal fluid of patients with endometriosis. Am J Obstet Gynecol. 1996;174:1522–1526. doi: 10.1016/s0002-9378(96)70600-2. [DOI] [PubMed] [Google Scholar]
- Rodgers WH, Osteen KG, Matrisian LM, Navre M, Giudice LC, Gorstein F. Expression and localization of matrilysin, a matrix metalloproteinase, in human endometrium during the reproductive cycle. Am J Obstet Gynecol. 1993;168:253–260. doi: 10.1016/s0002-9378(12)90922-9. [DOI] [PubMed] [Google Scholar]
- Rodgers WH, Matrisian LM, Giudice LC, Dsupin B, Cannon P, Svitek C, Gorstein F, Osteen KG. Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J Clin Invest. 1994;94:946–953. doi: 10.1172/JCI117461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G, Somigliana E, Moschetta M, Santorsola R, Cozzolino S, Filardo P, Salmaso A, Zingrillo B. Dynamic aspects of endometriosis in a mouse model through analysis of implantation and progression. Arch Gynecol Obstet. 2000;263:102–107. doi: 10.1007/s004040050005. [DOI] [PubMed] [Google Scholar]
- Sampson J. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the pelvic cavity. Am J Obstet Gynecol. 1927;14:422–469. [Google Scholar]
- Shaw RW. The role of GnRH analogues in the treatment of endometriosis. Br J Obstet Gynaecol. 1992;99(Suppl. 7):9–12. doi: 10.1111/j.1471-0528.1992.tb13532.x. [DOI] [PubMed] [Google Scholar]
- Shemshedini L, Knauthe R, Sassone-Corsi P, Pornon A, Gronemeyer H. Cell-specific inhibitory and stimulatory effects of Fos and Jun on transcription activation by nuclear receptors. EMBO J. 1991;10:3839–3849. doi: 10.1002/j.1460-2075.1991.tb04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715–2724. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- Singer CF, Marbaix E, Lemoine P, Courtoy PJ, Eeckhout Y. Local cytokines induce differential expression of matrix metalloproteinases but not their tissue inhibitors in human endometrial fibroblasts. Eur J Biochem. 1999;259:40–45. doi: 10.1046/j.1432-1327.1999.00001.x. [DOI] [PubMed] [Google Scholar]
- Story L, Kennedy S. Animal studies in endometriosis: a review. ILAR J. 2004;45:132–138. doi: 10.1093/ilar.45.2.132. [DOI] [PubMed] [Google Scholar]
- Takehara M, Ueda M, Yamashita Y, Terai Y, Hung YC, Ueki M. Vascular endothelial growth factor A and C gene expression in endometriosis. Hum Pathol. 2004;35:1369–1375. doi: 10.1016/j.humpath.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Tan XJ, Lang JH, Liu DY, Shen K, Leng JH, Zhu L. Expression of vascular endothelial growth factor and thrombospondin-1 mRNA in patients with endometriosis. Fertil Steril. 2002;78:148–153. doi: 10.1016/s0015-0282(02)03187-4. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14:1328–1331. doi: 10.1093/humrep/14.5.1328. [DOI] [PubMed] [Google Scholar]
- Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF, Dey SK. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci USA. 2005;102:14326–14331. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranguch S, Wang H, Daikoku T, Xie H, Smith DF, Dey SK. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest. 2007;117:1824–1834. doi: 10.1172/JCI31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa M, Tanaka N, Tainaka H, Takeda K, Ihara T, Sugamata M. Microarray analysis provides insight into the early steps of pathophysiology of mouse endometriosis model induced by autotransplantation of endometrium. Life Sci. 2009;84:832–837. doi: 10.1016/j.lfs.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Valle RF. Endometriosis: current concepts and therapy. Int J Gynaecol Obstet. 2002;78:107–119. doi: 10.1016/s0020-7292(02)00132-7. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Cortesi I, Crosignani PG. Progestins for symptomatic endometriosis: a critical analysis of the evidence. Fertil Steril. 1997;68:393–401. doi: 10.1016/s0015-0282(97)00193-3. [DOI] [PubMed] [Google Scholar]
- Verkauf BS. Incidence, symptoms, and signs of endometriosis in fertile and infertile women. J Fla Med Assoc. 1987;74:671–675. [PubMed] [Google Scholar]
- Wang P, Anderson PO, Chen S, Paulsson KM, Sjogren HO, Li S. Inhibition of the transcription factors AP-1 and NF-kappaB in CD4 T cells by peroxisome proliferator-activated receptor gamma ligands. Int Immunopharmacol. 2001;1:803–812. doi: 10.1016/s1567-5769(01)00015-7. [DOI] [PubMed] [Google Scholar]
- Wilson ML, Farquhar CM, Sinclair OJ, Johnson NP. Surgical interruption of pelvic nerve pathways for primary and secondary dysmenorrhoea. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD001896. CD001896. [DOI] [PubMed] [Google Scholar]
- Witz CA. Current concepts in the pathogenesis of endometriosis. Clin Obstet Gynecol. 1999;42:566–585. doi: 10.1097/00003081-199909000-00013. [DOI] [PubMed] [Google Scholar]
- Witz CA, Monotoya-Rodriguez IA, Schenken RS. Whole explants of peritoneum and endometrium: a novel model of the early endometriosis lesion. Fertil Steril. 1999;71:56–60. doi: 10.1016/s0015-0282(98)00400-2. [DOI] [PubMed] [Google Scholar]
- Witz CA, Cho S, Centonze V, Monotoya-Rodriguez IA, Schenken RS. Time series analysis of transmesothelial invasion by endometrial stromal and epithelial cells using three-dimensional confocal microscopy. Fertil Steril. 2003;79:770–778. doi: 10.1016/s0015-0282(02)04834-3. [DOI] [PubMed] [Google Scholar]
- Wren JD, Wu Y, Guo SW. A system-wide analysis of differentially expressed genes in ectopic and eutopic endometrium. Hum Reprod. 2007;22:2093–2102. doi: 10.1093/humrep/dem129. [DOI] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, et al. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;a 77:681–687. doi: 10.1095/biolreprod.107.061804. [DOI] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, Reierstad S, Bulun SE. Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab. 2007;b 92:3261–3267. doi: 10.1210/jc.2007-0494. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, Yong W, Shi S, Zhao W, Xu J, Srivastava A, et al. FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform. Mol Endocrinol. 2006;20:2682–2694. doi: 10.1210/me.2006-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamah NM, Dodson MG, Stephens LC, Buttram VC, Jr, Besch PK, Kaufman RH. Transplantation of normal and ectopic human endometrial tissue into athymic nude mice. Am J Obstet Gynecol. 1984;149:591–597. doi: 10.1016/0002-9378(84)90240-0. [DOI] [PubMed] [Google Scholar]
- Zeitoun K, Takayama K, Sasano H, Suzuki T, Moghrabi N, Andersson S, Johns A, Meng L, Putman M, Carr B, et al. Deficient 17beta-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17beta-estradiol. J Clin Endocrinol Metab. 1998;83:4474–4480. doi: 10.1210/jcem.83.12.5301. [DOI] [PubMed] [Google Scholar]