Abstract
Mutations in the X-linked inhibitor of apoptosis (XIAP) have recently been identified in patients with the rare genetic disease, X-linked lymphoproliferative syndrome (XLP), which was previously thought to be solely attributable to mutations in a distinct gene, SAP. To further understand the roles of these two factors in the pathogenesis of XLP, we have compared mice deficient in Xiap with known phenotypes of Sap-null mice. We show here that in contrast to Sap-deficient mice, animals lacking Xiap have apparently normal NKT cell development and no apparent defect in humoral responses to T cell-dependent antigens. However, Xiap-deficient cells were more susceptible to death upon infection with the murine herpesvirus MHV-68 and gave rise to more infectious virus. These differences could be rescued by restoration of XIAP. These data provide insight into the differing roles of XIAP and SAP in the pathogenesis of XLP.
Keywords: immunodeficiency, viral, apoptosis, signal transduction
1. INTRODUCTION
X-linked lymphoproliferative syndrome (XLP) is a rare primary immunodeficiency, with manifestations ranging from fatal infectious mononucleosis to B cell lymphomas and hypogammaglobulinemia (reviewed in [1]). Primary disease is often associated with Epstein-Barr virus infection, which patients are unable to control, and which frequently results in death. XLP affects approximately 1–3 in 1,000,000 males, though it may be under-diagnosed. Age of onset varies by primary feature of the disease, but 70% of patients die by the age of ten years. Bone marrow transplant has emerged as a promising treatment [2].
XLP was first described in the Duncan family in the early 1970s [3] but it was not until the late 1990s that the gene responsible for the majority of diagnosed cases was identified [4–6]. The defect was mapped to a single locus within the Xq25 band of the X chromosome, which encodes SLAM-associated protein (SAP/DSP1/SH2D1A), an adaptor molecule for the SLAM family of receptors, a subset of the CD2 subfamily of Ig receptors [7]. The majority of patients have a variety of described mutations in the SAP coding region leading to loss of protein expression. A minority, who do not exhibit loss of protein mutations, have been explained primarily by misdiagnosis of XLP or the existence of mutations outside the coding sequence which affect SAP expression levels [8], although the genetic basis of others remained unclear.
Mice lacking Sh2d1a, the murine SAP homolog, have been generated and studied in depth [9, 10]. Sap-null mice are defective in the development of natural killer T (NKT) cells, which has been reported to recapitulate the phenotype of XLP patients [11–13]. In addition, T cell help for humoral immunity is severely impaired, which is observed in the mice as an inability to develop germinal centers [14, 15]. Defects in the ability to produce Th2 cytokines in response to TCR stimulation are also observed [16]. Natural killer (NK) cells stimulated via the SLAM family receptor 2B4 also have a reduced ability to kill target cells in the absence of SAP [17, 18]. Possibly related to one or more of the above defects, Sap-null mice display aberrant immune responses to MHV-68 [19], a murine γ-herpesvirus similar to Epstein-Barr virus (EBV). These defects have been instrumental in describing the function of SAP as well as the functions of the SLAM family of receptors.
Recent studies have described a number of XLP kindreds that express normal SAP and shared the majority of their symptoms with patients having mutations in SAP [20, 21]. Interestingly, genetic analysis of these patients has revealed loss of function mutations mapping to the same locus, but in XIAP, which encodes the X-linked inhibitor of apoptosis. At the immunologic level, this cohort of XLP patients was originally reported to exhibit a loss of NKT cells, similar to patients lacking SAP.
XIAP, a well-characterized member of the IAP family of proteins, has been described to function as an inhibitor of the apoptotic cell death pathway. XIAP directly binds and inhibits the activity of caspases: aspartate-specific cysteine proteases that carry out the process of cell death [22]. Interestingly, the original report describing the murine knockout of XIAP indicated no obvious apoptotic defect [23], which raises the possibility that XIAP may have different functions in mice. XIAP has additionally been shown to be involved in several signaling pathways, including TGF-β-mediated signaling and activation of NF-κB and JNK [24], as well as in copper trafficking [25]. Through its carboxy-terminal RING domain, XIAP can also catalyze the ubiquitination of target proteins [26]. Remarkably, though both demonstrate a wide array of functions, SAP and XIAP show no obvious structural or functional similarity beyond a common chromosomal locus and their involvement in XLP. Therefore, analysis of Xiap-deficient mice for known phenotypes of Sap-deficient mice, as well as exploration of potential interactions between the two molecules, could provide valuable insight into both the function of XIAP and the pathogenesis of XLP.
In this study, we compare the functions and interactions of SAP and XIAP in the context of known phenotypes of Sap-deficient mice. We do not find evidence that XIAP and SAP interact, nor are they observed to act similarly in the pathways controlling NKT development and humoral immune responses. However, we find that Xiap-null cells differ from control cells in response to the murine herpesvirus MHV-68, raising the possibility of an alternate mechanism by which patients lacking XIAP may demonstrate an XLP-like disease when infected with a γ-herpesvirus.
2. MATERIALS AND METHODS
2.1. Immunoprecipitations
Cells were cultured in DMEM (Cellgro; Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS) (Cellgro; Manassas, VA, USA), 2mM glutamine (Invitrogen; Carlsbad, CA, USA; Carlsbad, CA, USA) and 1% penicillin/streptomycin (Invitrogen; Carlsbad, CA, USA; Carlsbad, CA, USA) at 37°C, 5% CO2. HEK293 cells were transfected using a standard calcium phosphate procedure with plasmids that have been previously described [7]. Whole cell lysates were prepared using RIPA lysis buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1mM DTT, 1mM PMSF in 1x PBS) or Triton X-100 lysis buffer (1% Triton X-100, 10% glycerol, 25mM Hepes, 100mM NaCl, 1mM EDTA, 1mM DTT, 1mM PMSF, 1mM NaF, and 1mM NaOV4) supplemented with complete protease inhibitor tablets (Roche Applied Science; Indianapolis, IN, USA). For immunoprecipitations, lysates were incubated with glutathione sepharose beads or antibody to FLAG followed by protein A agarose beads. Centrifugation was performed to recover agarose beads, followed by washing in the indicated lysis buffer. Precipitated proteins were eluted by adding LDS sample buffer (Invitrogen; Carlsbad, CA, USA) and heating the samples for 5 min at 95°C. Recovered proteins were subsequently separated by electrophoresis, and immunoblot analysis was performed as described below.
2.2. Immunoblotting
Samples were resolved on 4–12% gradient SDS-PAGE gels (Invitrogen; Carlsbad, CA, USA), transferred onto nitrocellulose (Invitrogen; Carlsbad, CA, USA) and blocked in 5% milk in Tris-buffered saline containing 0.1% Tween (Bio-Rad; Hercules, CA, USA). Membranes were incubated at room temperature for 1h or overnight at 4°C with the following antibodies: GST (Santa Cruz Biotechnology; Santa Cruz, CA, USA), XIAP (BD Biosciences; San Jose, CA, USA), HA-HRP (Sigma-Aldrich; St. Louis, MO, USA), FLAG-HRP (Sigma-Aldrich; St. Louis, MO, USA), SAP [9], and β-actin (Sigma-Aldrich; St. Louis, MO, USA). Secondary horseradish peroxidase-conjugated anti-mouse or anti-rabbit (GE Healthcare; Piscataway, NJ, USA) were used for 1h at room temperature. Enhanced chemiluminescence (GE Healthcare; Piscataway, NJ, USA) and Kodak XAR film were used for visualization purposes.
2.3. Mice
XIAP KO mice [23] were backcrossed in the C57BL/6 strain for at least 12 generations. All mice were housed under specific pathogen-free conditions within the animal care facility at the University of Michigan. All animal experiments were approvied by the University of Michigan Committee on the Use and Care of Animals.
2.4. NKT cell quantification
Single-cell suspensions were generated from XIAP wildtype and knockout thymi and spleens and immediately stained with the following antibodies purchased from BD Biosciences (San Jose, CA, USA): CD24-FITC (M1/69); NK1.1-PE-CY7 (PK136); TCR-beta-PE-Cy5 (H57–597). Thymocytes were also stained with PE-conjugated, αGC-loaded CD1d tetramer. Expression data was collected by flow cytometry on a Cytomics FC500 from Beckman Coulter and analyzed using FlowJo (TreeStar Inc; Ashland, OR, USA).
2.5. SRBC treatment
Mice were injected intraperitoneally with sheep red blood cells (SRBC; Colorado Serum Co.; Denver, CO, USA) diluted 1:10 in PBS, 200ul per mouse. Control mice were injected similarly with PBS alone. Six days later, spleens were harvested and split for two procedures. Frozen sections were made and stained according to standard procedures with peanut agglutinin (PNA)-biotin (Vector Labs; Burlingame, CA, USA) and B220 (BD Biosciences; San Jose, CA, USA), with streptavidin-Alexa488 (Invitrogen; Carlsbad, CA, USA) and anti-rat-594 (Invitrogen; Carlsbad, CA, USA), and imaged on an Olympus BX-51 microscope with an Olympus DP-70 high resolution digital camera. The remainder of the spleen was made into a single-cell suspension and stained with the following antibodies: B220-PE-Cy7, IgD-biotin, Fas-PE, GL7-FITC, and CD38-FITC (BD Biosciences; San Jose, CA, USA) and PNA-FITC (Biomeda; Plovdiv, Bulgaria) with a secondary of streptavidin-PE-alexa610 (Invitrogen; Carlsbad, CA, USA). Data was collected by flow cytometry as above.
2.6. MHV-68 infection of MEFs
XIAP WT and littermate KO MEFs were generated by standard procedures and reconstituted with either full length murine XIAP or a D148A/W310A double mutant generated by site-directed mutagenesis, by infection with lentivirus as described [27]. MEFs were infected with MHV-68 (ATCC, WUMS strain) at 0.1 pfu/cell and washed once with PBS 24 hours later. 72 hours after infection, cells were visualized with a Nikon Eclipse TS100 microscope with A CoolSnap-Pro cf camera (Media Cybernetics; Bethesda, MD, USA). Floating cells were then collected and combined with adherent cells lifted with trypsin-EDTA (Cellgro; Manassas, VA, USA), and all were resuspended in propidium iodide (PI) buffer (2μg/ml PI [Sigma-Aldrich; St. Louis, MO, USA], 1% bovine serum albumin [Sigma-Aldrich; St. Louis, MO, USA] in 1x PBS) for flow cytometry, performed as above. Supernatant was saved, filtered through 0.45 um PVDF (Millipore; Billerica, MA, USA) and serially diluted 1:2 in media, starting with 1:1000. 3T12 cells were washed in the viral supernatant for 1 hour at 37°C, and carboxymethylcellulose (CMC, Sigma-Aldrich; St. Louis, MO, USA) mixture (CMC, culture media, 2x MEM [Lonza; Basel, Switzerland], FCS, penicillin/streptomycin, glutamine, Hepes, NEAA [HyClone; Waltham, MA, USA], fungizone [Invitrogen; Carlsbad, CA, USA; Carlsbad, CA, USA]) was added for 1 week. Plaques were visualized by fixing and staining with 70% methanol plus 0.35% methylene blue (Fisher Scientific; Pittsburgh PA, USA).
3. RESULTS AND DISCUSSION
3.1. No detectable interactions between XIAP and SAP
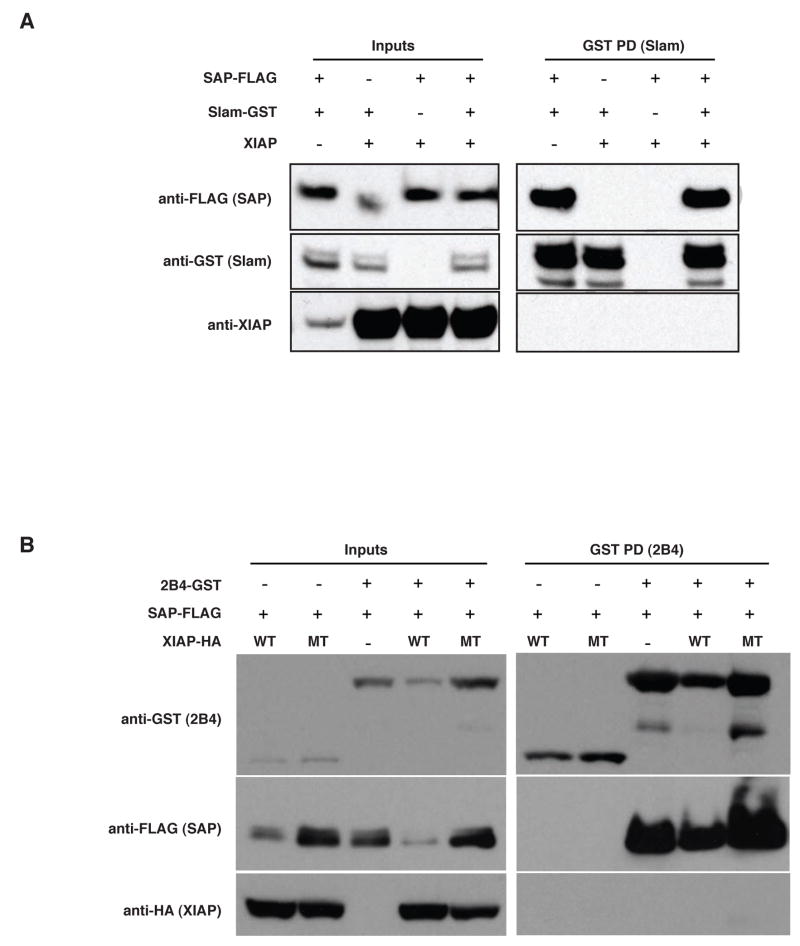
The discovery that human X-linked lymphoproliferative syndrome can be caused by mutations in the genes encoding either SAP and XIAP led us to determine whether the two proteins might interact. We have previously described a system in which the association of SAP with the cytoplasmic tails of several members of the CD2 family, including SLAM and 2B4, can be readily evaluated [7]. Using this system, the cytoplasmic signaling domain of SLAM fused in-frame with glutathione-S-transferase (SLAM-GST) was expressed with FLAG-epitope-tagged SAP (SAP-FLAG) and XIAP. Upon precipitation with glutathione sepharose beads, SLAM was observed to interact with SAP, but not with XIAP (Figure 1A). In co-immunoprecipitation using FLAG antibody, SAP-FLAG was expressed with SLAM-GST and XIAP (data not shown). While an association between SAP and SLAM was observed, validating this experimental approach, no XIAP was detectable in the complex. XIAP was not found to coprecipitate with either SLAM or SAP, and notably, it was also not observed to disrupt the association between these two proteins.
Fig. 1. No detectable interaction between XIAP and SAP.
(A) XIAP and FLAG-tagged SAP were coexpressed with the cytoplasmic tail of SLAM-GST or GST alone in HEK293 cells. Glutathione-sepharose beads were added to lysates and bead-associated proteins were separated by SDS-PAGE and immunoblotted for FLAG, GST and XIAP. Additionally, the last panel shows immunoprecipitation using an anti-FLAG monoclonal antibody and IgA beads to assess binding of SLAM and XIAP to SAP. (B) HA-XIAP (both wildtype and a H467A point mutant) and FLAG-SAP were expressed in HEK293 cells in the presence of the tyrosine kinase Lck and either a GST-tagged cytoplasmic tail construct of the 2B4 receptor or GST alone. As in A, GST coprecipitations and immunoblots were performed assessing the ability of wildtype (WT) or D148A/W310A double mutant (MT) XIAP to bind SAP or 2B4. All samples include Lck.
While interactions between SAP and SLAM are phosphorylation-independent, another CD2 family member, the 2B4 receptor, requires phosphorylation to associate with SAP [7]. We examined the possibility of a phosphorylation-dependent interaction of XIAP with 2B4 by expression of a GST-2B4 chimera along with the tyrosine kinase Lck, SAP-FLAG, and XIAP. As demonstrated previously, 2B4 was capable of precipitating SAP in the presence of Lck, but XIAP was not detected (Figure 1B). Additionally, a point mutant of XIAP, H467A, was utilized, which is incapable of ubiquitinating target proteins [26], and which may increase the stability of otherwise transient interactions. Similar to the wildtype protein, this point mutant also not found to coprecipitate with SAP and 2B4. Thus, we found no evidence of a physical interaction between XIAP and SAP.
3.2. Similar expression of murine proteins
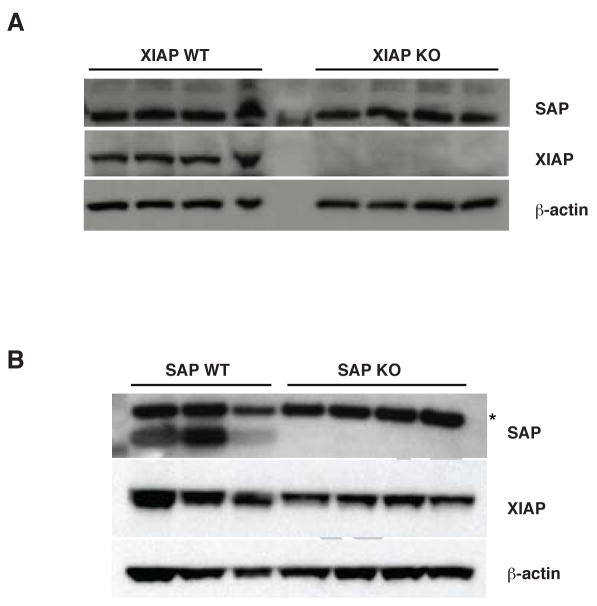
Although no evidence of a direct interaction between XIAP and SAP was observed, the possibility remained that expression of XIAP or SAP might be coordinately regulated, for example through mechanisms such as epigenetic silencing or posttranslational modifications such as ubiquitination. To explore this possibility, SAP expression was examined by immunoblot in thymocytes from several Xiap-null mice and littermate controls. As shown in Figure 2A, no differences in SAP protein levels were detected in lysates from Xiap-deficient mice and control littermates. Similarly, lysates from thymocytes from Sap-null mice were separated by electrophoresis and immunoblotted with an antibody to XIAP (Figure 2B). XIAP levels were indistinguishable between Sap-null mice and littermate controls. These findings suggest that XIAP and SAP do not physically interact, and that the expression of these two factors are independently regulated. Therefore, the loss of XIAP does not appear to contribute to XLP by altering SAP expression.
Fig. 2. Murine expression of XIAP and SAP.
Thymocytes were harvested from XIAP (A) and SAP (B) WT and KO mice, lysed and immunoblotted for SAP, XIAP and β-actin. Asterisk (*) indicates a non-specific band.
3.3. Murine NKT cells are not affected by loss of XIAP
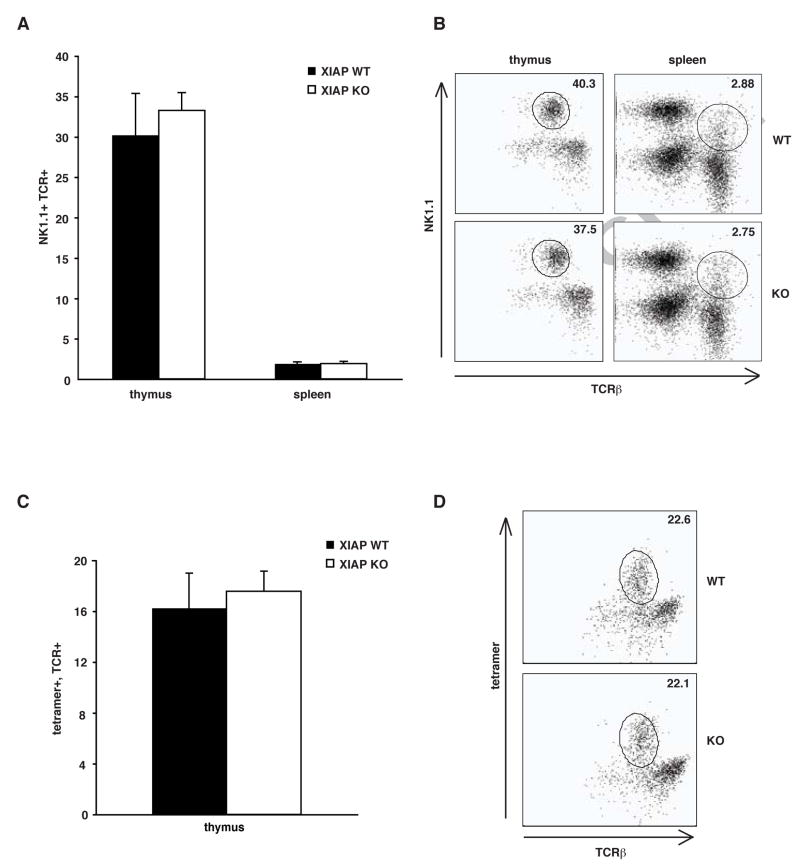
Since the findings described above suggest that XIAP does not interact with SAP or affect SAP protein, the mechanism by which the loss of XIAP leads to the pathogenesis of XLP remains unclear. One potential explanation is that XIAP might be involved in similar molecular pathways as SAP, but at different levels, a possibility that could be addressed by examining Xiap-deficient mice for phenotypes previously reported in SAP-deficient mice. A primary hallmark of SAP deficiency is impaired development of NKT cells, resulting in a severe lack of NKT cells in SAP−/Y mice [12, 13]. NKT cells have diverse immunomodulatory functions, and so their loss has been proposed to contribute directly to the pathogenesis of XLP. Therefore, NKT cell populations were analyzed by flow cytometry in Xiap-deficient mice and littermate controls, as shown in Figure 3. Surprisingly, both cohorts contained similar populations of classical NKT cells, defined by expression of NK1.1 and the T cell receptor (TCR) when gated on the CD24low population. In the thymus, Xiap-deficient littermates contained 33±2% NK1.1+, TCRβ+ cells, and 30±5% of cells were NK1.1+, TCRβ+ in controls. In the spleen, these cells comprised 1.9±0.3% and 1.9±0.2% of the Xiap-null and control CD24low populations, respectively (Figure 3A and B). We verified these results using a CD1d tetramer that recognizes NKT cells, which confirmed that control thymocytes (16±3%) were comprised of similar numbers of NKT cells as Xiap-deficient mice (18±1%) (Figure 3C and D). The lack of a difference between NKT cell populations in mice with or without XIAP suggests that XIAP is not involved in NKT cell development in mice.
Fig. 3. NKT cells normal in XIAP KO mice.
Splenocytes and thymocytes were isolated from three XIAP WT and three XIAP KO mice, and stained with anti-CD24, NK1.1+ and TCR-β+ (A and B), as well as PE-conjugated α-galactosylceramide-loaded CD1d tetramer, specific for NKT cells (C and D). NKT cells are defined as CD24low, NK1.1+, TCR-β+, and tetramer+. A and C show total results of at least 3 individual mice, error bars shown are standard error of the mean. B and D are representative FACS plots of the CD24low subpopulation.
3.4. Normal humoral responses in the absence of XIAP
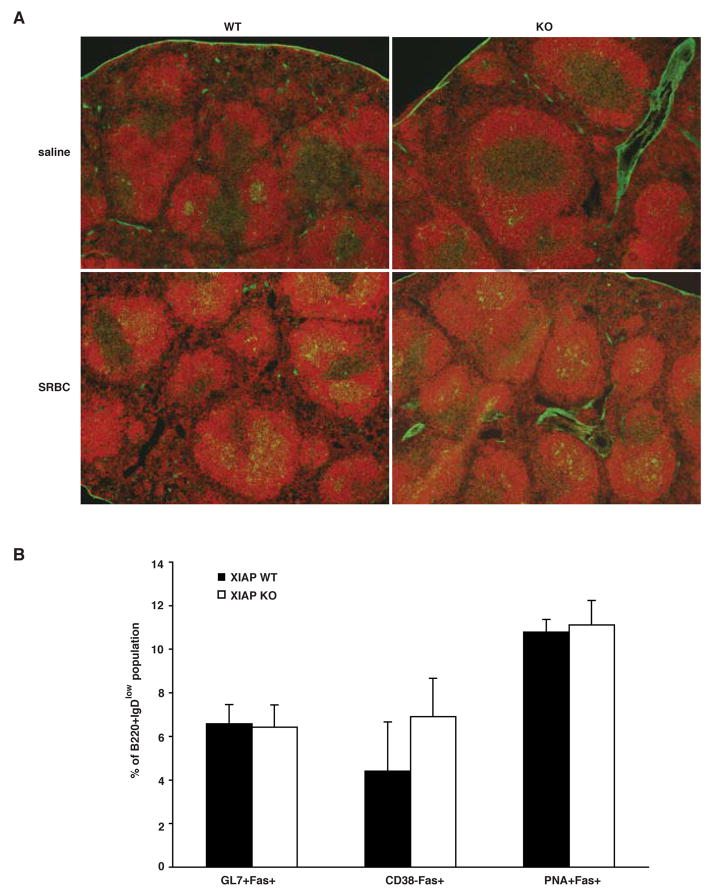
Several reports have shown that SAP-null mice are defective in their ability to form humoral responses to helper T cell-dependent antigens, which reflects the dysgammaglobulinemia found in human XLP patients [14, 15]. Since germinal center formation is a primary indicator of an intact humoral response, the ability of Xiap-deficient animals to form germinal centers was tested. Xiap-null mice and controls were injected intraperitoneally with sheep red blood cells (SRBC), and splenic germinal center formation was analyzed six days later. Splenic sections were stained with antibody to B220 to detect B cells, and with peanut agglutinin (PNA), a lectin that binds germinal center lymphoid cells. While germinal centers (B220+, PNA+) were rare in saline-injected mice, they were clearly visible in both Xiap-null and -replete SRBC-injected mice (Figure 4A), indicating that mice lacking XIAP have normal responses to T cell-dependent antigens.
Fig. 4. Normal germinal center formation in XIAP KO mice.
(A) XIAP WT and KO mice were injected with either SRBC or saline and spleens were harvested 6 days later. Frozen sections were stained with PNA-FITC and anti-B220 and viewed on an Olympus microscope. (B) Splenocytes were harvested from mice treated in A and stained with antibodies to B220, IgD and Fas. Subsets were also stained with PNA or antibodies to GL7 or CD38 to specifically identify germinal center B cells. Data are representative of at least three individual mice, and error bars shown are standard error of the mean.
Additionally, germinal center formation was analyzed by flow cytometry. Whole splenocytes were gated for B220+, IgDlow cells and tested for Fas expression in conjunction with other germinal center markers (GL7+, PNA+ and CD38low). All three markers indicated no difference in germinal center formation between Xiap-null and littermate spleens (Figure 4B). These data indicate that, unlike SAP [14, 15], XIAP is not involved in signaling for CD4+ T cell-mediated B cell help.
3.5. Different response to murine γ-herpesvirus-68 in Xiap-null mice
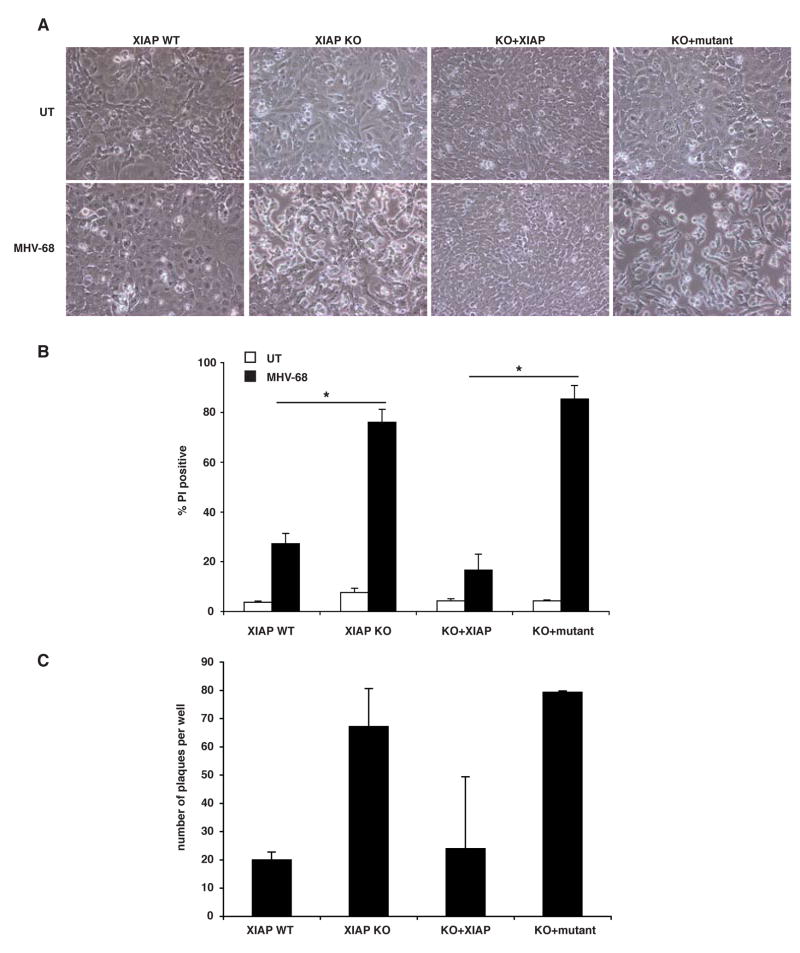
Symptoms of XLP are commonly first triggered or exacerbated by EBV infection, which often results in fulminant infectious mononucleosis [1]. Since EBV cannot infect mouse cells, a related virus, murine γ-herpesvirus-68 (MHV-68), is frequently used as a mouse model for human EBV infection (reviewed in [28]). To evaluate how XIAP affects responses to MHV-68, cells isolated from Xiap-null mice were infected with MHV-68 in culture. Upon MHV-68 infection, murine embryonic fibroblasts (MEFs) deficient for XIAP appeared by contrast microscopy to be dying after 72 hours, while control cells were not (Figure 5A). To evaluate this quantitatively, we performed propidium iodide exclusion assays on infected cells after 72 hours of infection. As shown in Figure 5B, this method demonstrated that cells lacking XIAP (XIAP KO) were significantly more sensitive to death than their wildtype counterparts (XIAP WT), and reconstitution with wildtype XIAP (KO+XIAP) restored the wildtype, resistant, phenotype. Reintroduction of XIAP with both point mutations, D148A and W310A (KO+mutant) affecting the ability to bind caspases [29], was unable to render Xiap-null cells resistant to death. Thus, the sensitivity to death during viral infection was a specific result of the loss of XIAP and further, its caspase-binding activity, implying that the cell death was apoptotic.
Fig. 5. Xiap-null cells are sensitive to virus-induced death.
(A) The indicated MEF cell lines were infected with 0.1 pfu/cell MHV-68 and cultured for 72 hours, after which they were visualized with light microscope. (B) Cells were treated as in A, then floating and adherent cells were harvested and PI stained for viability by flow cytometry. Data represent at least three experiments, with error bars illustrating standard error, and significance (<0.001 indicated with an asterisk [*]) was calculated using a one-way ANOVA. (C) Supernatants from cells treated as in A were serially diluted and plated on 3T12 cells for quantitation by plaque assay. Three wells were counted from the 1:32,000 dilution of supernatant in each of two experiments.
Finally, we examined how the loss of XIAP might affect the production of intact virus particles in embryonic fibroblasts, assessing viral titer in the supernatant by plaque assay. XIAP-null cells were found to produce more virus than wildtype counterparts, which was reversed by reintroduction of XIAP (Figure 5C). Interestingly, viral titers were markedly elevated in cells expressing altered version of XIAP that are incapable of inhibiting caspases, suggesting a role for caspase activity in the production or release of MHV-68. Taken together, these results suggest that enhanced viral replication in the Xiap-null cells is associated with increased apoptotic cell death.
4. DISCUSSION
Despite the involvement of both proteins in XLP, the findings described above indicate that XIAP and SAP do not directly interact, and are neither coordinately nor reciprocally regulated. The data led us to pursue the contribution of XIAP to biological processes in which SAP is known to be involved. We show here that unlike SAP, in mice XIAP does not appear to play a role in NKT cell development or in generating T cell help for humoral immunity. Thus XIAP does not appear to be involved in the same physiological processes as SAP, even though paradoxically it is implicated in the same disease. The results presented here suggest that the pathogenesis of the disease in patients lacking XIAP is likely different from SAP-null patients, even if the outcomes are similar.
Since a major hallmark of XLP disease is EBV-associated fulminant infectious mononucleosis, examination of the immune response to γ-herpesviruses has been crucial to our understanding of the pathogenesis of the disease. It has been proposed that the lack of NKT cells, whose numbers are greatly impaired in the absence of SAP, contributes to the phenotypes of XLP [12, 13]. Xiap-null mice, however, have normal development of NKT cells, even though humans lacking XIAP have few NKT cells. This discrepancy suggests that there may be a difference between mice and humans with respect to the function of XIAP, and illustrates a caveat to the direct application of conclusions from mouse models to human disease.
Despite the apparent difference in NKT cell development between mice and humans, cells from Xiap-null mice exhibit greater death than controls in response to MHV-68, which suggests that XIAP may yet play a role in the response to γ-herpesviruses such as EBV. The data suggest that the defect in individuals lacking XIAP is cell-intrinsic, at the level of the infected cell itself, rather than the immune response to it, as is seen in SAP-deficient patients. Cells lacking XIAP are more sensitive to death, presumably apoptotic, than cells with intact XIAP, and release more virus into the surrounding milieu.. If XIAP affects the infected cells in a cell-autonomous manner, leading to increased cell death and increased viral release, this may provide an explanation by which increased immune activation may occur. If the immune system is overwhelmed by this excess of viral particles, this would potentially lead to a similarly uncontrolled infection as in the absence of SAP.
Despite the close genomic proximity of the genes encoding the two proteins, XIAP and SAP appear to contribute to the pathogenesis of XLP through very different mechanisms. The function of XIAP in this context may be related to its primary role as a modulator of apoptosis, Cells lacking XIAP appear more susceptible to death, release more virus, and thus may disseminate the infection more quickly. It also remains a formal possibility that XIAP limits viral replication and that in its absence, viral replication is enhanced, leading to increased lytic viral production. These findings highlight the fact that SAP and XIAP play distinct physiological roles in mammalian responses to virus infections, even though loss of function mutations in either gene contribute to the pathogenesis of XLP.
Acknowledgments
We thank Dr. J. Silke for providing murine XIAP plasmids, and to María Soengas for providing E1A and Ras plasmids. We are grateful to Josh Stoolman for help in the construction of plasmids for reconstitution of Xiap-null MEFs, to Carol Wilke for assistance with MHV-68 studies and to members of the Duckett lab for critical reading of the manuscript. This work was supported in part by award W81XWH-06-1-0429 from the Department of Defense to K.A.O., an American Heart Association and National Institutes of Health Grants CA86867 to P.L.S., AI065543 and HL087846 to B.B.M., and GM067827 and an award from the Sandler Program for Asthma Research to C.S.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nichols KE, Ma CS, Cannons JL, Schwartzberg PL, Tangye SG. Molecular and cellular pathogenesis of X-linked lymphoproliferative disease. Immunol Rev. 2005;203:180–199. doi: 10.1111/j.0105-2896.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann T, Heilmann C, Madsen HO, Vindelov L, Schmiegelow K. Matched unrelated allogeneic bone marrow transplantation for recurrent malignant lymphoma in a patient with X-linked lymphoproliferative disease (XLP) Bone Marrow Transplant. 1998;22:603–604. doi: 10.1038/sj.bmt.1701389. [DOI] [PubMed] [Google Scholar]
- 3.Purtilo DT, Cassel CK, Yang JP, Harper R. X-linked recessive progressive combined variable immunodeficiency (Duncan’s disease) Lancet. 1975;1:935–940. doi: 10.1016/s0140-6736(75)92004-8. [DOI] [PubMed] [Google Scholar]
- 4.Nichols KE, Harkin DP, Levitz S, Krainer M, Kolquist KA, Genovese C, Bernard A, Ferguson M, Zuo L, Snyder E, Buckler AJ, Wise C, Ashley J, Lovett M, Valentine MB, Look AT, Gerald W, Housman DE, Haber DA. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 1998;95:13765–13770. doi: 10.1073/pnas.95.23.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffey AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, Cahn AP, Durham J, Heath P, Wray P, Pavitt R, Wilkinson J, Leversha M, Huckle E, Shaw-Smith CJ, Dunham A, Rhodes S, Schuster V, Porta G, Yin L, Serafini P, Sylla B, Zollo M, Franco B, Bolino A, Seri M, Lanyi A, Davis JR, Webster D, Harris A, Lenoir G, de St Basile G, Jones A, Behloradsky BH, Achatz H, Murken J, Fassler R, Sumegi J, Romeo G, Vaudin M, Ross MT, Meindl A, Bentley DR. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nature Genetics. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 6.Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, van Schaik S, Notarangelo L, Geha R, Roncarolo MG, Oettgen H, De Vries JE, Aversa G, Terhorst C. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 7.Lewis J, Eiben LJ, Nelson DL, Cohen JI, Nichols KE, Ochs HD, Notarangelo LD, Duckett CS. Distinct interactions of the X-linked lymphoproliferative syndrome gene product SAP with cytoplasmic domains of members of the CD2 receptor family. Clin Immunol. 2001;100:15–23. doi: 10.1006/clim.2001.5035. [DOI] [PubMed] [Google Scholar]
- 8.Sumegi J, Huang D, Lanyi A, Davis JD, Seemayer TA, Maeda A, Klein G, Seri M, Wakiguchi H, Purtilo DT, Gross TG. Correlation of mutations of the SH2D1A gene and epstein-barr virus infection with clinical phenotype and outcome in X-linked lymphoproliferative disease. Blood. 2000;96:3118–3125. [PubMed] [Google Scholar]
- 9.Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, Chen A, Sher A, Duckett CS, Ahmed R, Schwartzberg PL. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci U S A. 2001;98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C, Nguyen KB, Pien GC, Wang N, Gullo C, Howie D, Sosa MR, Edwards MJ, Borrow P, Satoskar AR, Sharpe AH, Biron CA, Terhorst C. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat Immunol. 2001;2:410–414. doi: 10.1038/87713. [DOI] [PubMed] [Google Scholar]
- 11.Pasquier B, Yin L, Fondaneche MC, Relouzat F, Bloch-Queyrat C, Lambert N, Fischer A, de Saint-Basile G, Latour S. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174:3153–3157. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- 13.Nichols KE, Hom J, Gong SY, Ganguly A, Ma CS, Cannons JL, Tangye SG, Schwartzberg PL, Koretzky GA, Stein PL. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, Anderson S, Cheever AW, Sher A, Schwartzberg PL. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCausland MM, Yusuf I, Tran H, Ono N, Yanagi Y, Crotty S. SAP regulation of follicular helper CD4 T cell development and humoral immunity is independent of SLAM and Fyn kinase. J Immunol. 2007;178:817–828. doi: 10.4049/jimmunol.178.2.817. [DOI] [PubMed] [Google Scholar]
- 16.Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D, Nichols KE, Antonellis A, Koretzky GA, Gardner K, Schwartzberg PL. SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-kappaB1. Immunity. 2004;21:693–706. doi: 10.1016/j.immuni.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Benoit L, Wang X, Pabst HF, Dutz J, Tan R. Defective NK cell activation in X-linked lymphoproliferative disease. J Immunol. 2000;165:3549–3553. doi: 10.4049/jimmunol.165.7.3549. [DOI] [PubMed] [Google Scholar]
- 18.Gao N, Schwartzberg P, Wilder JA, Blazar BR, Yuan D. B cell induction of IL-13 expression in NK cells: role of CD244 and SLAM-associated protein. J Immunol. 2006;176:2758–2764. doi: 10.4049/jimmunol.176.5.2758. [DOI] [PubMed] [Google Scholar]
- 19.Chen G, Tai AK, Lin M, Chang F, Terhorst C, Huber BT. Signaling lymphocyte activation molecule-associated protein is a negative regulator of the CD8 T cell response in mice. J Immunol. 2005;175:2212–2218. doi: 10.4049/jimmunol.175.4.2212. [DOI] [PubMed] [Google Scholar]
- 20.Rigaud S, Fondaneche MC, Lambert N, Pasquier B, Mateo V, Soulas P, Galicier L, Le Deist F, Rieux-Laucat F, Revy P, Fischer A, de Saint Basile G, Latour S. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–114. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- 21.Marsh RA, Villanueva J, Zhang K, Snow AL, Su HC, Madden L, Mody R, Kitchen B, Marmer D, Jordan MB, Risma KA, Filipovich AH, Bleesing JJ. A rapid flow cytometric screening test for X-linked lymphoproliferative disease due to XIAP deficiency. Cytometry B Clin Cytom. 2009 in press. [Google Scholar]
- 22.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 23.Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB. Characterization of XIAP-deficient mice. Mol Cell Biol. 2001;21:3604–3608. doi: 10.1128/MCB.21.10.3604-3608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis J, Burstein E, Birkey Reffey S, Bratton SB, Roberts AB, Duckett CS. Uncoupling of the signaling and caspase-inhibitory properties of XIAP. J Biol Chem. 2004;279:9023–9029. doi: 10.1074/jbc.M312891200. [DOI] [PubMed] [Google Scholar]
- 25.Mufti AR, Burstein E, Csomos RA, Graf PC, Wilkinson JC, Dick RD, Challa M, Son JK, Bratton SB, Su GL, Brewer GJ, Jakob U, Duckett CS. XIAP Is a copper binding protein deregulated in Wilson’s disease and other copper toxicosis disorders. Mol Cell. 2006;21:775–785. doi: 10.1016/j.molcel.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science. 2000;288:874–877. doi: 10.1126/science.288.5467.874. [DOI] [PubMed] [Google Scholar]
- 27.Rumble JM, Bertrand MJ, Csomos RA, Wright CW, Albert L, Mak TW, Barker PA, Duckett CS. Apoptotic sensitivity of murine IAP-deficient cells. Biochem J. 2008;415:21–25. doi: 10.1042/BJ20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevenson PG, Efstathiou S. Immune mechanisms in murine gammaherpesvirus-68 infection. Viral Immunol. 2005;18:445–456. doi: 10.1089/vim.2005.18.445. [DOI] [PubMed] [Google Scholar]
- 29.Bratton SB, Lewis J, Butterworth M, Duckett CS, Cohen GM. XIAP inhibition of caspase-3 preserves its association with the Apaf-1 apoptosome and prevents CD95- and Bax-induced apoptosis. Cell Death Differ. 2002;9:881–892. doi: 10.1038/sj.cdd.4401069. [DOI] [PubMed] [Google Scholar]