Abstract
Virus like particles (VLPs) derived from enteric pathogens like Norwalk virus (NV) are well suited to study oral immunization. We previously described stable transgenic plants that accumulate recombinant NV-like particles (rNV) that were orally immunogenic in mice and humans. The transgenic approach suffers from long generation time and modest level of antigen accumulation. We now overcome these constraints with an efficient tobacco mosaic virus (TMV)-derived transient expression system using leaves of Nicotiana benthamiana. We produced properly assembled rNV at 0.8 mg/g leaf 12 days post infection. Oral immunization of CD1 mice with 100 or 250 μg/dose of partially purified rNV elicited systemic and mucosal immune responses. We conclude that the plant viral transient expression system provides a robust research tool to generate abundant quantities of rNV as enriched, concentrated VLP preparations that are orally immunogenic.
Keywords: Virus-like particles, Plant viral vector, Mucosal vaccination
1. Introduction
Norwalk virus (NV) is the prototype of human noroviruses, the major cause of non-bacterial gastroenteritis in developed and developing countries. In the United States alone, recent estimates indicated that noroviruses are responsible for more than 95% of viral gastroenteritis [1,2]. The NV genome is a 7.7 kb single stranded positive sense RNA containing a 5′ untranslated region (UTR), three open reading frames (ORF), a 3′ UTR and a poly(A) tail [3]. The viral morphology is generated by the icosahedral arrangement of the 58 kDa NV capsid protein (NVCP) that assembles into a non-enveloped capsid. Heterologous expression of recombinant NVCP in insect cells, using the baculovirus expression system, and in stably transformed transgenic plants showed that the structural protein alone can self-assemble into virus like particles (VLPs), antigenically and morphologically similar to the native NV particles [4-7]. The empty 38-nm capsids are composed of 90 dimers of NVCP and exhibit a T=3 icosahedral symmetry [8].
VLPs are highly immunogenic due to their particulate, ordered and repetitive structures, which trigger B cell and T cell response [9,10] and hence, are excellent subunit vaccine candidates. Studies of mucosal immunogenicity in mice with purified recombinant Norwalk virus-like particles (rNV) produced in insect cells showed both humoral and mucosal responses, including serum IgG and intestinal IgA [11]. Nasal administration of rNV achieved the same responses at lower antigen levels [12]. Moreover, the oral administration of insect cell derived rNVs to human subjects proved safe and immunogenic [13].
Plants have been extensively investigated as an economical expression and delivery system for antigens; several plant-produced antigens showed immunogenicity and protection against different infectious diseases [14-16]. Properly assembled rNV produced in potatoes elicited serum IgG and intestinal IgA in mice when fed as raw material, either with or without the use of cholera toxin as mucosal adjuvant [6]. The ingestion of lyophilized powder of transgenic tomato fruits expressing rNV induced IgG and IgA responses in mice [17]. Oral immunization using uncooked transgenic potatoes expressing rNV showed that purification of the antigen was not required to achieve an immune response, and that plant-derived rNV is safe and immunogenic in human volunteers [18]. The principle limitation to further development of tomato or potato-derived rNV toward development of a commercial vaccine has been modest antigen expression, usually <1% of total soluble protein (TSP).
Advancements in plant biotechnology in recent years offer new approaches to achieve high levels of protein expression. In particular, the manipulation of plant viruses for transient expression in plant leaves has shown that many proteins can be rapidly expressed at levels that rival more traditional fermentation-based protein expression. Such systems offer the possibility to express a large number of different genetic constructs to determine the best candidate(s) for product development [19]. A tobacco mosaic virus (TMV)-based “deconstructed” viral system (magnICON) has been extensively engineered to achieve very high levels of recombinant protein accumulation in leaves [20-22]. It was proven highly effective in the production of protective antigens from Yersinia pestis and also for immunogenic hepatitis B core VLPs [16,23]. In this paper, we describe the rapid and high level expression of rNV in Nicotiana benthamiana leaves using the magnICON system, a VLP preparation method used to process the plant material, and the systemic and mucosal antibody responses generated in mice following gavage with rNV. A concerted effort to evaluate the composition of an effective NV vaccine using plant-derived antigen is now feasible, using a plant-made vaccine strategy.
2. Materials and methods
2.1 Plasmid construction
A synthetic plant optimized gene (sNVCP, GenBank accession number: AY360474) encoding NV ORF2 (without ORF3) [3], was moved from plasmid psNV210 [17] to pICH10990 in order to generate pICHsNVCP (Fig. 1). The subcloning was performed by the ligation of three fragments. The 5′ fragment of the gene was obtained by PCR, using primers NV Fwd1 (5′-TTTTGGTCTCAAGGTATGATGATGGCTTCTAAGGATGC) and NVRev1 (5′-CAAAACATCACCTGGGGTGTTG), which introduced a BsaI site at the 5′ of ORF2. The PCR product was digested with BsaI/NcoI and ligated together with the 3′ fragment of the gene, obtained by digestion from psNV210 with NcoI/SacI, and the plasmid vector pICH10990 (Icon Genetics) prepared BsaI/SacI. The PCR generated fragment was subsequently sequenced to assure fidelity to the template. The module containing the integrase, pICH14011, and the 5′ modules pICH15879, pICH10530, pICH8420, pICH18750 are described in [20] and represented in [16]. Vector pICH15879 mediates cytosolic accumulation, vectors pICH10530 and pICH8420 mediate apoplastic accumulation while pICH18750 provides chloroplastic targeting, for simplicity here they will be named, CYTO, APO1, APO2 and CHLOR respectively. For comparative studies, pICH-GFP was used in some experiments; this 5′ module contains green florescence protein reporter gene [20]. The plasmids were mobilized into Agrobacterium tumefaciens GV3101 [20] and confirmed by restriction enzyme digestion after plasmid preparation from transformed bacterial clones.
Fig. 1.

3′ ICON modules used in the study. LB and RB, left and right borders of the T-DNA region; AttB, specific recombination site from phage PhiC31; I, intron; NTR, 3′ non translated region; Nos-T, nopaline synthase terminator.
2.2 Agro infiltration and plant infection
Two hundred μl of Agrobacterium suspensions, OD600 ranging from 1.6 to 2.5, were sedimented at 6,000 × g for 3 min. The pellet was resuspended in 1 ml of a solution containing 10 mM Mes (pH 5.5) and 10 mM MgSO4. Leaves of greenhouse-grown N. benthamiana plants, 6 to 8 weeks old, were infiltrated with the bacterial suspension by applying a syringe without needle to the underside of the tobacco leaves, and slowly injecting the solution into the leaf interior space.
2.3 Plant extraction and partial purification of rNV
Tobacco leaves were harvested at 12 days post-infection (dpi) and homogenized by blending in acid extraction buffer (25mM sodium phosphate buffer, 100mM NaCl, 50mM sodium ascorbate, 10 μg/ml leupeptin, pH 5.3) at a proportion of 1 ml for each g of leaf fresh weight (FW). Homogenates were immediately filtered through cheesecloth and then incubated at room temperature for 1h. The plant extract was then centrifuged at 5,000 × g for 5 min, and the supernatant passed through a 0.22 μm filter (Millipore Corporation, Billerica, Massachusetts). The samples were incubated at room temperature for an hour and then at 4°C overnight and centrifuged for 20 min at 5,000 × g. The supernatant was removed and applied to a Centricon Plus-70 100 kDa size exclusion column (Millipore) and centrifuged for 10 min at 3,000 × g, until almost all the solution flowed through. The excluded protein material was suspended in acid extraction buffer and subjected to an additional centrifugation through the 100 kDa filtration column as described above. The second retained pellet was suspended in acid extraction buffer for further analysis.
2.4 NVCP ELISA
NVCP in plant extracts was quantified by ELISA as described [6]. Rabbit anti-(rNV) serum diluted 1:10,000 in 0.01 M phosphate-buffered saline (PBS; 50 μl per well) was bound to 96-well polyvinylchloride microtiter plates for 4 h at 23°C, the plates were blocked with 5% non fat dry milk in PBS (DMyPBS) for 1 h at 37°C. After washing the wells three times with PBS containing 0.05% Tween 20 (PBST), samples (50 μl per well) serially diluted in PBS were added and incubated 16 h at 4°C. A standard curve was generated with two-fold dilutions of insect cell-derived rNV [3] at concentrations ranging from 50 to 0.8 ng/ml. Several dilutions of the plant samples, depending on the expression levels, were tested in order to reach the linear range of the reference standard absorbance levels. The wells were washed and incubated in succession with guinea pig anti-(rNV) serum and goat anti-guinea pig IgG–horseradish peroxidase conjugate, each diluted 1:5000 in 2% DMyPBS, for 2 h at 37°C. Plates were developed with TMB substrate (Pierce) for 5 min at 23°C, the reaction was ended by addition of an equal volume of 0.5 M H2SO4, and the absorbance was read at 450 nm.
2.5 SDS-PAGE, dot blot and Western blot
Plant protein crude extracts and insect cells purified rNV were denatured by boiling in SDS–PAGE sample buffer and separated on 4–20% gradient polyacrylamide gels. Proteins were either visualized by Coomassie blue staining or electrophoretically transferred to polyvinlidene difluoride (PVDF) membrane (Amersham Pharmacia, Piscataway, NJ), and probed in succession with rabbit anti-rNV serum [6] at dilution of 1:10,000 in 1% DMyPBS and goat anti-rabbit-IgG-horseradish peroxidase conjugate (Sigma-Aldrich, St. Louis, MO) diluted in 1:10,000 in 1% DMyPBS. The membranes were developed by chemiluminescence using the ECL plus detection reagent (Amersham Pharmacia). For dot blot analysis, 2 μl from the fractions collected from sucrose gradient were spotted onto nitrocellulose membrane and dried. The membranes were probed with the same antibodies combinations and developing system previously described for western blot analysis.
2.6 Sucrose gradient
The sucrose gradient was performed as described in [6,18] with modifications. Briefly, the gradient was generated by layering 833 μl each of 60%, 50%, 40%, 30%, 20% and 10% sucrose dissolved in modified phosphate buffer (25mM Na-phosphate pH 6.2, 100mM NaCl), into Beckman SW55 Ti tubes (Beckman Coulter, Fullerton, CA), and incubated 2 hours at 4°C to allow diffusion into a continuous gradient; 100 μl of crude or partially purified extract were layered on the top of the gradient, and centrifuged at 90,000 × g for 3h at 4°C. Fifteen fractions were generated by carefully removing 333 μl from the top to the bottom of the tube and analyzed by ELISA, dot blot and western blot.
2.7 Electron microscopy (EM)
Sucrose gradient purified suspensions of tobacco-derived and insect cell-derived rNV were subjected to negative staining and examination by transmission EM. Ten μl of each suspension were positioned on 200-mesh copper grids and allowed to incubate at room temperature for 15 minutes in order for the particles to adhere; remaining aqueous solution was then removed from the grids with filter paper. The grids were transferred to 10 μl of 2% uranyl acetate for 30 s and examined with a Philips CM-12 TEM.
2.8 Mice, immunization and sample collection
Adult (24 to 28 g) female CD1 mice (Charles River Laboratories, Portage, Mich.) were immunized by gavage of partially purified rNV from tobacco leaves (Section 2.3). Five groups (7 mice per group) were used in the study, with two different doses of plant-derived rNV (100 or 250 μg) gavaged with or without 10 μg CT (Sigma Chemical Co., St. Louis, Mo.). Doses were delivered by gavaging 250 μl samples on days 1, 21 and 42. Mice of the control group were given partially purified extracts from tobacco leaves inoculated with Agrobacterium not harboring the sNVCP construct. Individual preimmune serum, fecal samples and vaginal lavages were collected before the first immunization, day 0. Blood samples were obtained by tail bleed 2 weeks after each immunization (days 14, 35 and 56), and additional samples were taken at days 77 and 105. Vaginal lavage samples were collected at days 35, 56, 77, and 105. Samples of vaginal lavage were obtained using a 20G × 1½ inch animal feeding needle with plastic tip (Popper and Sons, Inc, New Hyde Park, N.Y.) attached to a 1 ml syringe; 50 μl of PBS buffer was used to wash 5 times the mice vaginal cavity. Fecal material was collected from single caged individual mice at days 56 and 77.
2.9 Antibody measurement
The specific serum IgG, fecal and vaginal IgA responses were determined by endpoint titer ELISA as described [11,12]. Fecal extracts were prepared as described [17]. Polyvinyl chloride 96 well ELISA plates were coated with 50 μl of insect cell-derived rNV (1 ng/μl) in PBS and incubated 4h at 37°C. Plates were blocked with 5% skim milk in PBST over night at 4°C. Subsequently, the plates were incubated with the serum, vaginal lavage, and fecal extracts diluted with 2.5% skim milk in PBST for 2h at 37°C and then with HRP–conjugated goat anti-mouse IgG or IgA (Sigma, St. Louis, MO) for 1h at 37°C. For the IgG subtype evaluation serum samples from the same mice group at the same time point were pooled and processed as described above using specific HRP–conjugated goat anti-mouse IgG1 or IgG2a (Sigma, St. Louis, MO). After color development, absorbance was detected at 450nm in a 96-well plate reader. Endpoint titer was reported as the reciprocal of the highest serum dilution that had an absorbance ≥ 0.1 OD unit above the absorbance of the preimmune samples. Geometric mean titer (GMT) was calculated for every group of mice. All non-responders were included in the computation of the GMTs. For serum IgG, the lowest dilution tested (1:20) was divided by 2 and used as the titer for the negative control samples; for example the preimmune samples and negative control group mice were assigned a titer of 10. For vaginal and fecal IgA antibodies, the lowest dilution tested was 1:2 and negative samples were assigned a titer of 1. Differences in immunological response were compared for significance using analysis of variance followed by a t-test. P-values less than 0.05 were considered significant. Statistical analysis was performed using SPSS software (SPSS Inc.).
3. Results
3.1. Rapid and efficient viral system-based expression of NVCP in N. benthamiana leaves
The deconstructed TMV-based expression system [20,21] is divided into two major cDNA modules, the 5′ of which contains the viral replicase gene and the 3′ module harbors the gene of interest. Different 5′ modules may carry different organelle targeting signals, which fuse in frame with the gene of interest after recombination and nuclear processing. In this study we used the same 5′ and integrase modules described in [16]. Each 5′ module was coupled pair-wise with pICH-sNVCP, the 3′ module carrying a plant optimized NVCP gene (Fig.1). Non-infiltrated leaf samples and GFP expressing samples (pICH-GFP) were used as negative controls. Different 5′ vector modules allowed us to examine subcellular targeting of the antigen to cytosolic, endomembrane/apoplast and chloroplast sites. Samples were harvested at 8, 10, and 12 dpi, and expression levels were calculated by analyzing a minimum of three independent infection sites on three different plants. In every case antigenic NVCP was detected (Table1) and no phytotoxic effects were noticeable even when the infections were carried out up to 21 dpi (data not shown). The maximal expression level of 0.86 mg NVCP per g leaf FW was achieved with cytosolic targeting at 12 dpi; these conditions were selected for subsequent studies. Western blot of leaf extracts (Fig. 2) showed that the vector expressing NVCP (lane 4) produced a band of the expected 58 kDa, co-migrating with insect cell-derived rNVCP (lane 1). The 58 kDa band was absent from extracts of non-infected or GFP-expressing leaves (Fig. 2, lanes 2 and 3).
Table 1. NVCP accumulated (mg/g leaf FW) for different subcellular targeting vectors.
| Localization | 8 dpi | 10 dpi | 12 dpi |
|---|---|---|---|
| APO1 | 0.03 ± 0.007 | 0.08 ± 0.008 | 0.17 ± 0.015 |
| APO2 | 0.05 ± 0.009 | 0.07 ± 0.01 | 0.15 ± 0.02 |
| CHLOR | 0.10 ± 0.02 | 0.10 ± 0.025 | 0.11 ± 0.01 |
| CYTO | 0.70 ± 0.08 | 0.80 ± 0.04 | 0.86 ± 0.03 |
N. benthamiana leaves were infiltrated with the 3′ module pICHsNVCP and the integrase module pICH14011 coupled with each one of the 5′ localization modules: pICH10530 and pICH8420 mediate apoplast/endomembrane targeting (APO1 and APO2), pICH18750 provides chloroplast targeting (CHLOR) and pICH15879 gives cytosolic localization (CYTO). Data were obtained by ELISA as described in the Material and Methods section and are mean ± SD from a minimum of three independently infected leaves of three different plants; dpi, days post infection; FW, fresh weight.
Fig. 2.
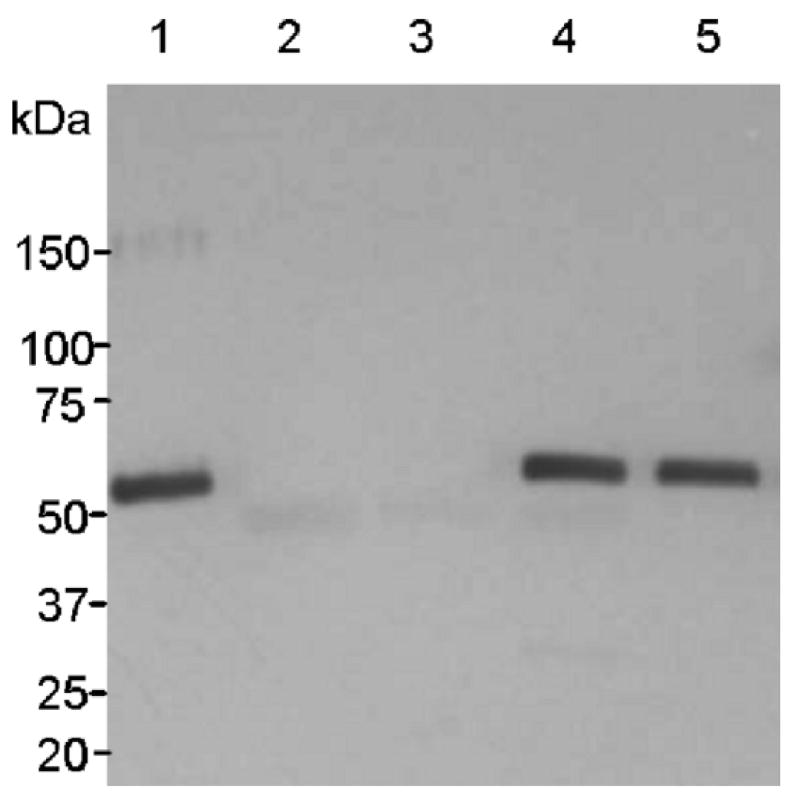
Western blot analysis of the NVCP produced in N. benthamiana leaves harvested at 12 dpi. The membrane was probed with rabbit anti-NVCP serum. Lane 1, insect cell-derived NVCP reference standard; lane 2, uninfected leaf extract; lane 3, extract from pICH-GFP infiltrated leaves; 4, crude extract from pICH-sNVCP infiltrated leaves, lane 5, rNV partially purified from pICH-sNVCP infiltrated leaves.
3.2 VLPs assembly of plant-derived NVCP
NVCP expressed in insect cells or plant systems forms empty (devoid of viral RNA) VLPs [4-7]. In the current study, we asked if proper assembly of VLPs occurred in N. benthamiana leaves using the magnICON system using sucrose gradient sedimentation. The crude leaf extract shows two distinct NVCP peaks (by ELISA) of approximately equal proportion (Fig. 3), also confirmed by western blot and dot blot (data not shown). Examination of gradient fractions by EM revealed VLPs in the rapidly sedimenting peak in sucrose gradients of plant-derived rNV and insect cell-derived rNV (Fig. 4, fractions 9-11). In both samples, particles of 38 nm in diameter were clearly detected. We conclude that the faster sedimenting peak in plant-derived samples represents 38nm VLPs. Although we did not examine the slower sedimenting peak by EM, we surmise that it represents partial assembly products and/or small 23nm VLP that were observed in insect cells [5] and stably transformed tomato fruit [17].
Fig. 3.

Evaluation of rNV particle formation by sucrose gradient sedimentation. Insect cell-derived rNV (i-rNV) and crude extracts from N. benthamiana infiltrated with pICH-sNVCP (cp-rNV) were sedimented on a 10-60% sucrose gradient; 15 fractions were collected. The distribution of NVCP across gradient fractions was determined by ELISA for NVCP. The top of the gradient is at left.
Fig. 4.

Visualization by negative staining and electron microscopy of rNV. (A) insect cell-derived rNV; (B) rNV partially purified by sucrose gradient from pICH-sNVCP infected N. benthamiana leaves.
3.3 Partial purification of plant derived rNV
We developed a method for partial purification of rNV in order to enrich the faster-sedimenting VLPs fraction with respect to the endogenous plant proteins and slower-sedimenting NVCP. Chloroplast–associated ribulose bisphosphate carboxylase (Rubisco, the major protein component of leaves), was removed by sedimentation following leaf extraction and incubation in acidic buffer [24], while the NVCP remained in the supernatant fraction. The subsequent filtration of the supernatant with a 100 kDa-cutoff membrane allowed concentration and preferential enhancement of the rNV (Fig. 5A, lane 5). No change in the size of the NVCP was observed during these manipulations (Figure 2, lane 5). Samples of the concentrated rNV co-sedimented on sucrose gradient with insect cell rNV and were largely devoid of the slower-sedimenting peak seen in crude extracts (Fig. 5B, C compare with Fig. 3). These data indicate that filtration allowed selective removal of slower-sedimenting NVCP forms and other small proteins, and preferentially enriched the remaining, concentrated rNV in the form of VLPs.
Fig. 5.
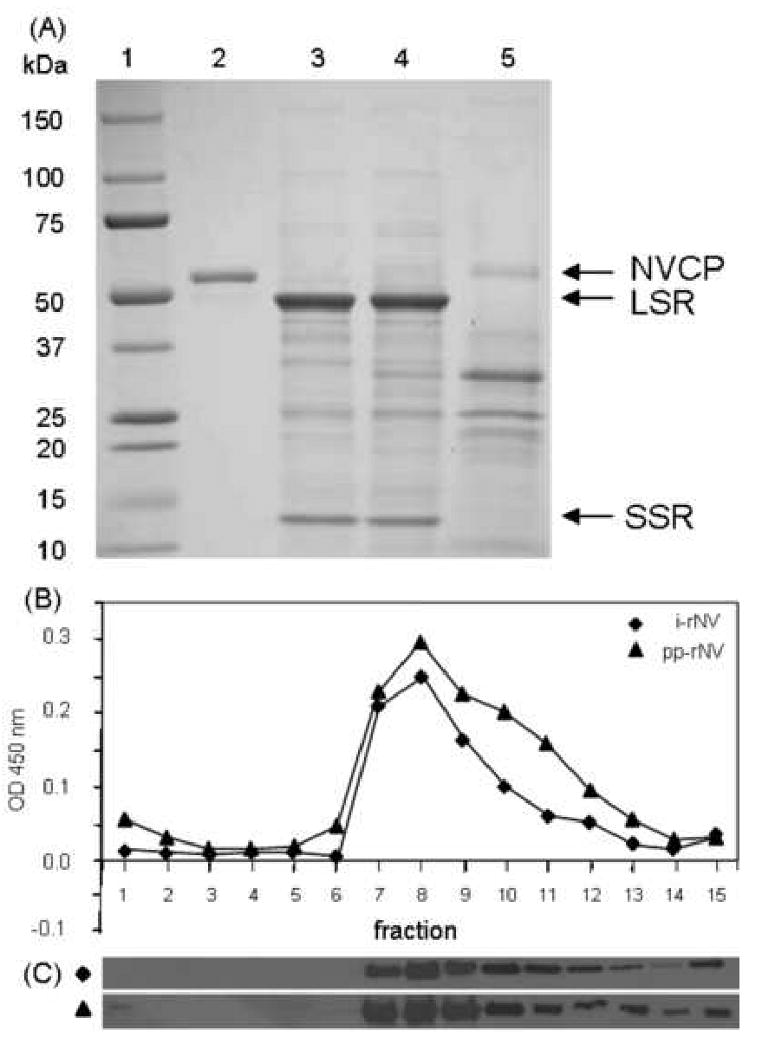
Partial purification of plant derived rNV. (A) Protein samples were analyzed on a 4-20% gradient polyacrylamide gel and visualized by Coomassie staining. Lane 1, Molecular weight marker; lane 2, insect cell-derived rNV reference standard (2 μg); lane 3, crude protein extract from uninfected leaves (20 μg); lane 4, crude protein extract from pICH-sNVCP infiltrated leaves (20 μg); lane 5, rNV partially purified from pICH-sNVCP infected N. benthamiana leaves (20 μg). NVCP, Norwalk virus capsid protein; LSR, large subunit of Rubisco; SSR, small subunit of Rubisco. (B) ELISA profile of sucrose gradient fractions of rNV. i-rNV, insect cell derived rNV; pp-rNV, partially purified extracts from N. benthamiana leaves infiltrated with pICH-sNVCP. The top of the gradient is at left. (C) Western blot analysis of sucrose gradient fractions probed with rabbit polyclonal anti-NVCP.
3.4 Serum antibody response to orally delivered plant-derived rNV
CD1 mice were gavaged with three doses of rNV delivered over 6 weeks (days 0, 21, and 42). Two different dosages were tested, 100 μg and 250 μg, with or without 10 μg of CT (4 groups, n=7). The fifth group (n=7) was sham immunized with 10 μg of CT combined with leaf extracts inoculated with Agrobacterium that did not contain an expression plasmid for NVCP. Individual serum antibody responses were evaluated by ELISA and the GMT was calculated for each group at different time points (Fig. 6). Serum samples from sham-immunized mice taken throughout the course of the experiment and preimmune sera were negative (titer <20) for the presence of anti-rNV specific IgG (data not shown). All mice responded after the first administration of rNV at either dosage, with or without CT (Fig. 6A). Titers increased after each immunization and reached a maximal level at week 8, two weeks after the third immunization, and remained high through week 15, the last data point. The amplitude of the immune response was dose dependent, with and without CT, and the highest titers were produced with 250 μg rNV + CT. The rNV clearly was immunogenic even without the use of CT adjuvant, although the groups dosed with equal amounts of antigen with CT showed significantly stronger responses than those lacking CT.
Fig. 6.

Time course of NVCP specific serum IgG (A) and vaginal IgA (B) responses in mice after oral delivery of rNV produced in N. benthamiana leaves. CD1 mice (n=7 per group) were gavaged on weeks 0, 3 and 6, as indicated by arrows (↑). Group 1 received 100 μg rNV; group 2, 250 μg rNV; group 3, 100 μg rNV + 10 μg CT; and group 4, 250 μg rNV + 10 μg CT. Serum IgG and vaginal IgA were measured by ELISA. The y-axis shows the geometric means titers (GMT) and the error bars show the standard error of the mean.
IgG subtypes IgG1 and IgG2a specific to rNV were analyzed by ELISA, in order to evaluate the Th type response. Pooled serum samples obtained at week 11 (approximate midpoint of the maximal response plateau) were analyzed. IgG1 and IgG2a subtypes were detected in all groups, and IgG1/IgG2a ratios were 0.95 and 0.92 for the groups 1 and 2, suggesting that oral immunization with plant-derived rNV alone stimulated a balanced NV-specific immune response. Co-delivery of 100 μg dose rNV + CT did not shift the ratio (0.93), while the higher dose (250 μg) with CT caused a slightly lower proportion of IgG1 (0.79).
3.5 Mucosal antibody response to orally delivered plant-derived rNV
Oral immunization with rNV in previous studies stimulated specific mucosal antibody responses in the gut as well as at distant mucosal surfaces [11,13]. We asked if the plant-derived rNV retained similar immunogenic properties. Vaginal lavage samples from the negative control group taken throughout the experiment and preimmune samples were negative (titer <2) for the presence of anti-rNV specific IgA (data not shown). Specific vaginal IgA responses were induced in all of the mice of all immunized groups. There was no significant difference (P> 0.05) between the groups that received 100 μg or 250 μg doses in absence of adjuvant, while the groups dosed with CT generated a greater response. In particular, 250 μg of rNV + CT induced a significantly higher and longer lasting response than all the other groups.
Specific fecal IgA response was assayed at 5 and 8 weeks post immunization, one week before and two weeks after the second boosting immunization, respectively (Fig. 7). Fecal IgAs were detected in all of the animals that received rNV, although titers were moderate. Every animal had significantly higher GMT values when compared to control group animals which, as previously reported [12], showed a low background titer. Intra-group variability was apparent, but for groups 1 and 2 the third dose generated a statistically significant increase in IgA titer. For groups 3 and 4, which received CT, differences between weeks 5 and 8 were not significant (P >0.05), suggesting that CT accelerated rate of the antibody response as well as the amplitude. Overall, the magnitude of the responses, both at 5 and at 8 weeks post immunization, was significantly higher for the 250 μg dose + CT (group 4) than for other groups.
Fig. 7.

Intestinal IgA responses in CD1 mice to different doses of partially purified plant-derived rNV administered by gavage in presence or absence of CT. The fecal rNV-specific IgA titer was evaluated at 5 and 8 weeks post oral immunization and expressed as GMT. The error bars show the standard error of the mean.
4. Discussion
Many studies conducted over the last two decades have demonstrated that transgenic plants are a convenient antigen expression system, and that plant tissue can be used directly as a delivery system for orally administered subunit vaccines [25]. Prior studies focused on oral vaccine delivery of unprocessed (raw potato [6]) and minimally processed (freeze-dried [7, 17]) plant material. As was previously discussed [26], the use of unpurified plant-delivered antigens as vaccines may meet regulatory approval in animal health markets, but will likely face daunting challenges for commercialization as human vaccines, due to regulatory factors including quality assurance for antigen quantity and uniformity in perishable or freeze-dried plant tissues. The current study was designed to explore the feasibility of developing cost-effective antigen purification from plant tissue as a step toward formulation of a standardized subunit vaccine. We focused our efforts on: 1) finding a plant-based expression system that drives high level antigen accumulation to increase cost-effectiveness of the purification process, and 2) validating the oral immunogenic properties of processed plant-derived antigen.
We previously showed that rNV accumulates in transgenic tobacco, tomato and potato [6,7,17,18], and that the VLPs resemble those produced in insect cells driven by a baculovirus expression system [4,5,8]. Furthermore, the oral immunogenicity of plant made rNV were similar to that obtained from insect cells in both preclinical and human clinical trials [6,7,11,13,17,18]. However, we noted that a major limitation of the system was a modest level of antigen accumulation in the transgenic plant tissue, which required that volunteers eat a very large bolus of plant sample [18]. Subsequent studies by our group (unpublished) have failed to identify a strategy to overcome constraints in NVCP accumulation in stably nuclear transgenic plants. We therefore evaluated transient approaches to NVCP expression, and we here describe our success in adopting a plant viral vector system to achieve rNV accumulation at a level that is suitable for commercial development of a subunit vaccine.
In 2004 a novel new strategy was introduced in which the viral genome was “deconstructed” and modified to utilize the bacterium Agrobacterium tumefaciens for delivery of DNA vectors into fully expanded plant leaves [20]. Vectors delivered by Agrobacterium into leaf cells trigger replication and transient expression of RNA. For small scale production, the bacteria can be infiltrated into leaves using a syringe; while for large scale production, whole plants can be vacuum infiltrated [22,27]. This “magnICON” system produced very high levels of protein expression [16,20] compared with stably transgenic lines and other viral transient systems that are often hampered by post-transcriptional RNAi-mediated gene silencing. It is likely that the robust performance of magnICON vectors is at least in part due to the gene silencing suppressor function of one of the TMV replicase subunits [28].
In plant tissues, the dominant soluble protein is Rubisco [29]; it comprises up to 50% of the total leaf protein and must, therefore, be removed if a target protein is to be utilized for demanding vaccine applications. We employed protocols that can be utilized in cost-effective plant extract manipulation, asking how wine and food industry technology can be applied to antigen purification. Our protocol is a low pH extraction of leaves followed by centrifugation/filtration, similar to cell disruption in a wine press followed by filtration. As shown in Fig.5, we removed all easily detectable levels of Rubisco from our sample, and enriched the NVCP to produce an “antigen concentrate”.
There is extensive prior evidence that VLPs can assemble in plant cells [30]. We therefore asked if rNV were formed when the antigen was expressed using the magnICON vector and purified; by both EM and sucrose gradient sedimentation we gained affirmative results (Figs. 3, 4, and 5). The results discussed above allow us to reach a conclusion – very “low technology” procedures (and therefore cost-effective) can be used to concentrate and partially purify rNV from leaves after use of the magnICON expression system. The expression level (0.86 mg antigen/g leaf FW) is ∼30-fold greater than we have previously achieved in nuclear stably transformed N. tabacum [6], ∼35-fold higher than transient expression in N. benthamiana leaf with a nonreplicating vector (Mason et al., unpublished data), and ∼60-fold greater than in stably transformed tomato fruit [17].
It was essential to validate the mucosal immunogenicity of the partially purified rNV. We observed both humoral and mucosal responses, which is consistent with previous data using both insect cell-derived and plant-derived rNV [6,7,11,12]. All animals responded to antigen presentation with mucosal antibody responses (enteric and vaginal IgA), and CT adjuvant enhanced these responses. We conclude that the magnICON expression system coupled with a “low technology” purification protocol will yield immunogenically active rNV.
There is no commercial vaccine to prevent norovirus infections. Introduction of a vaccine must take into account the variability of capsid protein composition in different viral serotypes [31-35]. Additional research is needed: 1) to determine the extent of cross-protection among VLPs from different serotypes, 2) to derive a correlate of protection to define a successful immunization regime, and 3) to establish the most effective formulation and delivery strategy (including adjuvants). The data in this paper have validated a robust plant-based expression technology that is highly cost-effective and rapid (2-3 weeks after vector construction) for the production of candidate subunit vaccines for noroviruses. Ongoing research by our group is establishing protocols for Good Manufacturing Practices for production of rNV for human testing to meet stringent federal guidelines. We anticipate that plant-based antigen production technology will speed research on norovirus vaccine candidates and provide the capacity to allow cost efficiencies that will make the manufacture of a multivalent subunit vaccine a feasible venture.
Acknowledgments
The authors thank Huafang Lai and Chandana Uppalapati for their technical assistance, Dr. Yuri Gleba (Icon Genetics, Halle Germany) for the magnICON expression system, and Dr. Michelle Kilcoyne for critical reading of the manuscript. This research was supported by the US National Institutes of Health grant number U19-AI062150
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fankhauser RL, Noel JS, Monroe SS, Ando T, Glass RI. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J Infect Dis. 1998;178:1571–1578. doi: 10.1086/314525. [DOI] [PubMed] [Google Scholar]
- 2.Billgren M, Christenson B, Hedlund KO, Vinje J. Epidemiology of Norwalk-like human caliciviruses in hospital outbreaks of acute gastroenteritis in the Stockholm area in 1996. J Infect. 2002;44:26–32. doi: 10.1053/jinf.2001.0946. [DOI] [PubMed] [Google Scholar]
- 3.Jiang X, Wang M, Wang K, Estes MK. Sequence and genomic organization of Norwalk virus. Virology. 1993;195:51–61. doi: 10.1006/viro.1993.1345. [DOI] [PubMed] [Google Scholar]
- 4.Jiang X, Wang M, Graham DY, Estes MK. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992;66:6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White L, Hardy M, Estes M. Biochemical characterization of a smaller form of recombinant Norwalk virus capsids assembled in insect cells. J Virol. 1997;71:8066–8072. doi: 10.1128/jvi.71.10.8066-8072.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mason HS, Ball JM, Shi JJ, Jiang X, Estes MK, Arntzen CJ. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc Natl Acad Sci U S A. 1996;93:5335–5340. doi: 10.1073/pnas.93.11.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Z, Elkin G, Maloney BJ, Beuhner N, Arntzen CJ, Thanavala Y, et al. Virus-like particle expression and assembly in plants: hepatitis B and Norwalk viruses. Vaccine. 2005;23:1851–1858. doi: 10.1016/j.vaccine.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Prasad BV, Rothnagel R, Jiang X, Estes MK. Three-dimensional structure of baculovirus-expressed Norwalk virus capsids. J Virol. 1994;68:5117–5125. doi: 10.1128/jvi.68.8.5117-5125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 10.Bachmann MF, Zinkernagel RM, Oxenius A. Journal Of Immunology. Vol. 161. Baltimore, Md.: 1950. 1998. Immune responses in the absence of costimulation: viruses know the trick; pp. 5791–5794. [PubMed] [Google Scholar]
- 11.Ball JM, Hardy ME, Atmar RL, Conner ME, Estes MK. Oral Immunization with Recombinant Norwalk Virus-Like Particles Induces a Systemic and Mucosal Immune Response in Mice. J Virol. 1998;72:1345–1353. doi: 10.1128/jvi.72.2.1345-1353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerrero RA, Ball JM, Krater SS, Pacheco SE, Clements JD, Estes MK. Recombinant Norwalk Virus-Like Particles Administered Intranasally to Mice Induce Systemic and Mucosal (Fecal and Vaginal) Immune Responses. J Virol. 2001;75:9713–9722. doi: 10.1128/JVI.75.20.9713-9722.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clinical Immunology. 2003;108:241–247. doi: 10.1016/s1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 14.Dong JL, Liang BG, Jin YS, Zhang WJ, Wang T. Oral immunization with pBsVP6-transgenic alfalfa protects mice against rotavirus infection. Virology. 2005;339:153–163. doi: 10.1016/j.virol.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Koya V, Moayeri M, Leppla SH, Daniell H. Plant-Based Vaccine: Mice Immunized with Chloroplast-Derived Anthrax Protective Antigen Survive Anthrax Lethal Toxin Challenge Infect. Immun. 2005;73:8266–8274. doi: 10.1128/IAI.73.12.8266-8274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santi L, Giritch A, Roy CJ, Marillonnet S, Klimyuk V, Gleba Y, et al. Protection conferred by recombinant Yersinia pestis antigens produced by a rapid and highly scalable plant expression system. Proc Natl Acad Sci U S A. 2006;103:861–866. doi: 10.1073/pnas.0510014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Buehner NA, Hutson AM, Estes MK, Mason HS. Tomato is a highly effective vehicle for expression and oral immunization with Norwalk virus capsid protein. Plant Biotechnol J. 2006;4:419–432. doi: 10.1111/j.1467-7652.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 18.Tacket CO, Mason HS, Losonsky G, Estes MK, Levine MM, Arntzen CJ. Human immune responses to a novel Norwalk virus vaccine delivered in transgenic potatoes. J Infect Dis. 2000;182:302–305. doi: 10.1086/315653. [DOI] [PubMed] [Google Scholar]
- 19.Canizares MC, Nicholson L, Lomonossoff GP. Use of viral vectors for vaccine production in plants. Immunol Cell Biol. 2005;83:263–270. doi: 10.1111/j.1440-1711.2005.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marillonnet S, Giritch A, Gils M, Kandzia R, Klimyuk V, Gleba Y. In planta engineering of viral RNA replicons: Efficient assembly by recombination of DNA modules delivered by Agrobacterium. Proc Natl Acad Sci U S A. 2004 doi: 10.1073/pnas.0400149101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marillonnet S, Thoeringer C, Kandzia R, Klimyuk V, Gleba Y. Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat Biotechnol. 2005;23:718–723. doi: 10.1038/nbt1094. [DOI] [PubMed] [Google Scholar]
- 22.Gleba Y, Klimyuk V, Marillonnet S. Magnifection--a new platform for expressing recombinant vaccines in plants. Vaccine. 2005;23:2042–2048. doi: 10.1016/j.vaccine.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Huang Z, Santi L, LePore K, Kilbourne J, Arntzen CJ, Mason HS. Rapid, high-level production of hepatitis B core antigen in plant leaf and its immunogenicity in mice. Vaccine. 2006;24:2506–2513. doi: 10.1016/j.vaccine.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Staehelin LA, Arntzen CJ. Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol. 1983;97:1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason HS, Warzecha H, Mor T, Arntzen CJ. Edible plant vaccines: applications for prophylactic and therapeutic molecular medicine. Trends in Molecular Medicine. 2002;8:324–329. doi: 10.1016/s1471-4914(02)02360-2. [DOI] [PubMed] [Google Scholar]
- 26.Arntzen C, Plotkin S, Dodet B. Plant-derived vaccines and antibodies: potential and limitations. Vaccine. 2005;23:1753–1756. doi: 10.1016/j.vaccine.2005.01.090. [DOI] [PubMed] [Google Scholar]
- 27.Gleba Y, Klimyuk V, Marillonnet S. Viral vectors for the expression of proteins in plants. Curr Opin Biotechnol. 2007;18:134–141. doi: 10.1016/j.copbio.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Csorba T, Bovi A, Dalmay T, Burgyan J. The p122 subunit of Tobacco Mosaic Virus replicase is a potent silencing suppressor and compromises both small interfering RNA- and microRNA-mediated pathways. J Virol. 2007;81:11768–11780. doi: 10.1128/JVI.01230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis RJ. The most abundant protein in the world. Trends in Biochemical Sciences. 1979;4:241–244. [Google Scholar]
- 30.Santi L, Huang Z, Mason H. Virus-like particles production in green plants. Methods. 2006;40:66–76. doi: 10.1016/j.ymeth.2006.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardy ME, Tanaka TN, Kitamoto N, White LJ, Ball JM, Jiang X, et al. Antigenic mapping of the recombinant Norwalk virus capsid protein using monoclonal antibodies. Virology. 1996;217:252–261. doi: 10.1006/viro.1996.0112. [DOI] [PubMed] [Google Scholar]
- 32.Kitamoto N, Tanaka T, Natori K, Takeda N, Nakata S, Jiang X, et al. Cross-reactivity among several recombinant calicivirus virus-like particles (VLPs) with monoclonal antibodies obtained from mice immunized orally with one type of VLP. J Clin Microbiol. 2002;40:2459–2465. doi: 10.1128/JCM.40.7.2459-2465.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang X, Matson DO, Cubitt WD, Estes MK. Genetic and antigenic diversity of human caliciviruses (HuCVs) using RT-PCR and new EIAs. Arch Virol Suppl. 1996;12:251–262. doi: 10.1007/978-3-7091-6553-9_27. [DOI] [PubMed] [Google Scholar]
- 34.Green J, Vinje J, Gallimore CI, Koopmans M, Hale A, Brown DW, et al. Capsid protein diversity among Norwalk-like viruses. Virus Genes. 2000;20:227–236. doi: 10.1023/a:1008140611929. [DOI] [PubMed] [Google Scholar]
- 35.Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus classification and proposed strain nomenclature. Virology. 2006;346:312–323. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]


