Abstract
Background
Ethanol is a frequently abused, addictive drug that impairs cognitive function. Ethanol may disrupt cognitive processes by altering attention, short-term memory, and/ or long-term memory. Interestingly, some research suggests that ethanol may enhance cognitive processes at lower doses. The current research examined the dose-dependent effects of ethanol on contextual and cued fear conditioning. In addition, the present studies assessed the importance of stimulus salience in the effects of ethanol and directly compared the effects of ethanol on short-term and long-term memory.
Methods
This study employed both foreground and background fear conditioning, which differ in the salience of contextual stimuli, and tested conditioning at 4 hours, 24 hours, and 1 week in order to assess the effects of ethanol on short-term and long-term memory. Foreground conditioning consisted of 2 presentations of a foot shock unconditioned stimulus (US) (2 seconds, 0.57 mA). Background conditioning consisted of 2 auditory conditioned stimulus (30 seconds, 85 dB white noise)–foot shock (US; 2 seconds, 0.57 mA) pairings.
Results
For both foreground and background conditioning, ethanol enhanced short-term and long-term memory for contextual and cued conditioning at a low dose (0.25 g/kg) and impaired short-term and long-term memory for contextual and cued conditioning at a high dose (1.0 g/kg).
Conclusions
These results suggest that ethanol has long-lasting, biphasic effects on short-term and long-term memory for contextual and cued conditioning. Furthermore, the effects of ethanol on contextual fear conditioning are independent of the salience of the context.
Keywords: Alcohol, Learning, Mice, NMDA Receptors, Addiction, Hippocampus
Despite its low potency, approximately 17.6 million adult Americans consume enough alcohol on a regular basis to be considered abusers (National Institute on Alcohol Abuse and Alcoholism, 2004). Symptoms of alcohol abuse include disruptions of both physiological and psychological functioning that can lead to long-term changes in behavior. One function altered by ethanol is learning. Acute ethanol produces dose-dependent impairments in learning (for review, Ryback, 1971). These impairments have been demonstrated in many tasks, including passive avoidance, delay matching-to-position, free recall, radial maze, signal detection, and fear conditioning (Bammer and Chesher, 1982; Escher and Mittleman, 2004; Gibson, 1985; Gould, 2003; Higgins et al., 1992; Melia and Ehlers, 1989). Fear conditioning is useful for investigating the effects of ethanol on different types of learning that differentially involve the hippocampus because it tests both hippocampus-dependent contextual learning, in which contextual features are associated with an unconditioned stimulus (US) foot shock, and hippocampus-independent cued associations, in which a conditioned stimulus (CS) auditory cue is associated with the US (Logue et al., 1997; Phillips and LeDoux, 1992). Previous work has demonstrated that ethanol severely disrupts contextual learning and impairs cued learning to a lesser extent (Celerier et al., 2000; Gould, 2003; Melia et al., 1996). Although ethanol impairs both forms of fear conditioning, it should still be noted that the effects of ethanol are specific to the type of memory being tested; a dose of ethanol that impairs fear conditioning (Gould, 2003) enhances cue-based reward learning (Matthews et al., 1999). Thus, the behavioral requirements and/or underlying neural substrates of a learning task may determine the susceptibility of the task to the effects of ethanol.
A number of studies have demonstrated ethanol-induced impairments in fear conditioning (Celerier et al., 2000; Gould, 2003; Melia et al., 1996) and diverse explanations for these deficits can be proposed. Ethanol could have direct effects on learning, attention, consolidation, or sensitivity to the shock stimulus. Although ethanol may decrease shock sensitivity, prior research in a passive avoidance paradigm suggests that 2.0 g/kg ethanol does not alter shock sensitivity within a range of shock intensities that include the intensity used in the current study (Bammer and Chesher, 1982). Furthermore, if ethanol was mediating learning by altering sensitivity to the shock, contextual and cued conditioning should show the same level of impairment in response to ethanol treatment. However, other studies have demonstrated that ethanol impairs contextual conditioning to a greater degree than cued conditioning (Gould, 2003; Gould and Lommock, 2003; Melia et al., 1996). Ethanol could also be altering processes involved in consolidation but prior studies have demonstrated that post-training ethanol does not affect fear conditioning (Gould and Lommock, 2003); this suggests that ethanol is not altering processes involved in consolidation but may alter processes involved in acquisition.
Ethanol can impair attentional processes (Givens and McMahon, 1997; Lyon et al., 1975; Rezvani and Levin, 2003), and selective attention is necessary for acquisition of associative conditioning (Steele-Russell et al., 2006). However, it is unclear whether the effects of ethanol on attention are related to ethanol-induced impairments in contextual fear conditioning. Previous studies that examined the effects of ethanol on contextual fear conditioning have used background fear conditioning (i.e., pairing an auditory CS with the shock US), relegating the context to a background stimulus (Rescorla and Wagner, 1972). Following background fear conditioning, the tone is a more robust predictor of the shock because the tone and the shock have a consistent temporal relationship. In contrast, contextual cues are present throughout the training session and may, therefore, be a less robust predictor of the shock because greater attention may be paid to the auditory CS than to the context during background fear conditioning (Odling-Smee, 1978), although this overshadowing has not been seen in all studies (Lolordo et al., 2001). It is unclear whether the differential effects of ethanol on contextual and cued conditioning are due to an ethanol-induced decrease in attention to the background contextual cues. Conducting contextual fear conditioning in the absence of an auditory CS renders the context a foreground stimulus (Odling-Smee, 1975, 1978), which is more salient than a background stimulus. Changes in salience may alter the level of contextual fear conditioning, possibly due to the activation of divergent substrates during the encoding of background versus foreground contextual fear conditioning (Majchrzak et al., 2006; Phillips and LeDoux, 1994; Stiedl et al., 1999). Thus, examining the effects of ethanol on background and foreground contextual fear conditioning will further our understanding of the behavioral effects of ethanol by determining the importance of stimulus salience in the effects of ethanol on learning.
If ethanol is affecting the acquisition of learning, as suggested by other research (Bammer and Chesher, 1982; Birnbaum et al., 1978), then the effects of ethanol may extend to both short-term and long-term memory. Studies of working memory tasks have demonstrated strong ethanol-induced deficits (Escher and Mittleman, 2004; Givens, 1995; Givens and McMahon, 1997). In addition, Sircar and Sircar (2005) have demonstrated that ethanol-treated animals perform poorly at testing sessions immediately following training, as well as at 4, 7, and 25 days post-training in a Morris Water Maze task. However, ethanol has differential effects on learning depending on the type of memory being tested, and it is unclear if ethanol differentially affects short-term and long-term memory for fear conditioning. In fear conditioning, 1.0 g/kg ethanol impairs contextual fear conditioning at 1 and 7 days post-training (Gould and Lommock, 2003), suggesting that the effects of ethanol are long-lasting. As the neural substrates underlying working memory, short-term memory, and long-term memory differ across tasks (for review, Squire and Kandel, 1999), it is important to independently determine the effects of ethanol on different forms of memory. A direct comparison of the effects of ethanol on short-term and long-term memory for fear conditioning will help to determine whether there are differences in the degree to which ethanol affects memory at diverse time points.
Finally, most studies of the effects of ethanol on cognition have focused on the impairing effects that occur at high doses of the drug. These studies have demonstrated that acute ethanol impairs learning in many paradigms, usually at doses of 1.0 g/kg and greater (Bammer and Chesher, 1982; Birnbaum et al., 1978; Gould, 2003; Markwiese et al., 1998; Matthews et al., 1995; Popke et al., 2000). Far fewer studies have examined the effects of low doses of ethanol on cognition. The studies that have been conducted suggest that lower doses of ethanol may enhance learning in paradigms such as the 8-arm radial maze, serial learning during stress, and eye blink conditioning (Devenport et al., 1983; Hernandez and Powell, 1986; Hernandez and Valentine, 1990; Korman et al., 1960). To date, no studies have examined the effects of ethanol on fear conditioning using both doses that have been shown to enhance learning in other studies and doses associated with impairments.
The goals of this study were to investigate the dose-dependent effects of ethanol on fear conditioning and to determine whether these effects varied with changes in the interval between training and testing and whether they depended on the salience of the context. In addition, the present study tested an extended dose range for the effects of ethanol on fear conditioning to examine if the effects of ethanol were biphasic. Based on prior ethanol studies, we predicted that ethanol would enhance contextual fear conditioning at lower doses while impairing it at higher doses, regardless of the salience of the context or the time of testing.
MATERIALS AND METHODS
Subjects
Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were tested at 8 to 12 weeks of age (20 to 30 g). Mice were housed in groups of 4 mice per cage and had ad libitum access to food and water. A 12-hr light–dark cycle (lights on at 0700) was maintained, with all testing carried out between 9:00 am and 5:00 pm. Procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Apparatus
Training took place in an identical conditioning chambers housed in sound-attenuating boxes (MED Associates, St Albans, VT). Each 17.78 × 19.05 × 38.10 cm chamber consisted of Plexiglas panels in the front, back, and ceiling and 2 stainless-steel walls on the sides. The metal grid floor of each chamber, through which the foot-shock US (0.57 mA) was delivered, was connected to a shock generator and scrambler. Background noise and air exchange (69 dB) were provided by ventilation fans mounted on the right wall of each sound-attenuating box, and speakers that were used to deliver a white noise CS (85 dB) were mounted on the right wall of each chamber. An IBM PC-compatible computer running MED Associates software (MED Associates, St Albans, VT) controlled presentation of training and testing stimuli. Chambers were cleaned with 70% ethanol before each session.
Testing for freezing to the CS occurred in a separate room in altered context chambers that were housed in sound-attenuating boxes. Speakers that delivered the white noise CS used at training were mounted on the left wall of each chamber. The 20.32 × 22.86 × 17.78 cm chambers were constructed of 4 Plexiglas walls, a Plexiglas ceiling, and a metal grid floor covered with opaque white plastic. In addition to the differences in location, visual cues (e.g., the inside of the sound-attenuating boxes in the training context were white, and the inside of the sound-attenuating boxes in the altered context room were black), chamber dimensions, floor construction, and a vanilla extract olfactory cue (no olfactory cue was present in the training chambers) further distinguished the altered context chambers from the original training chambers.
Procedure
We examined the effects of an extended dose range of ethanol on acquisition of foreground and background contextual and cued fear conditioning at 4 and 24 hours, as well as at 1 week post-training. Unlike long-term memory, short-term memory is independent of protein synthesis (Davis and Squire, 1984), and the protein synthesis inhibitor anisomycin, which disrupts long-term memory, fails to disrupt learning at 1 and 6 hours post-training (Bourtchouladze et al., 1998). The 4-hour time point was chosen to represent short-term memory as it falls squarely into this time frame but well after the 2-hour time point by which 1.0 g/kg ethanol is eliminated by mice (Haseba et al., 2006). We also examined the effects of ethanol on consolidation of background fear conditioning. Blood alcohol concentrations (BACs) were measured for the doses used in the current study. Groups in all conditions except the blood alcohol analysis consisted of 8 to 12 animals. Saline and ethanol (20% v/v in saline) were administered by intraperitoneal (i.p.) injection in all experiments.
Foreground Contextual Fear Conditioning
We first examined the effect of ethanol administered at training on short- and long-term foreground contextual fear conditioning. Saline or ethanol (0.25, 0.5, and 1.0 g/kg; 20% v/v in saline) was administered 15 minutes prior to training. Mice were placed in conditioning chambers and the US (0.57 mA foot shock) was presented at 148 and 298 seconds. They remained in the chambers for a total of 5.5 minutes. Baseline freezing, i.e., freezing before the US presentation, was recorded during the first 120 seconds and immediate freezing was measured after the first CS–US presentation (methods based on Davis et al., 2006).
Mice were tested at either 4 or 24 hours post-training. Freezing to the context was assessed and then freezing to the CS was assessed 1 hour later. To evaluate freezing to the context, mice were placed in the training chamber for 5 minutes during which freezing behavior was recorded. To evaluate freezing to the CS, mice were placed in an altered context consisting of a modified conditioning chamber located in a different room. During the first 3 minutes of the altered context test, the CS was not presented (pre-CS test). During the last 3 minutes, the CS was continuously presented (CS test). Freezing was assessed during the entire 6 minute period. It was expected that the low levels of freezing to the CS would be seen because mice were trained without a CS.
Freezing to the context and the CS were again assessed 7 days after training for mice originally tested at 4 or 24 hours. To evaluate freezing to the context, mice were placed in the training chamber for 5 minutes during which freezing behavior was recorded. To evaluate freezing to the CS, mice were placed in the altered context and tested as before.
Background Contextual Fear Conditioning
The next experiment examined the effect of ethanol administered at training on short- and long-term background contextual fear conditioning. Saline or ethanol (0.25, 0.5, and 1.0 g/kg; 20% v/v in saline) was administered 15 minutes prior to training. On training day, mice were placed in conditioning chambers. After a 120 second baseline period, they received 2 co-terminating CS (30-seconds, 85 dB white noise)–US (2-seconds, 0.57 mA foot shock) pairings. At 120 and 270 seconds, the CS sounded for 30 seconds; the US occurred during the last 2 seconds of the CS. The mice remained in the chamber for 30 seconds after the second CS–US presentation, for a total of 5.5 minutes. Baseline freezing behavior was recorded during the first 120 seconds and immediate freezing was measured after the first CS–US presentation (methods based on Gould and Higgins, 2003). Mice were tested at either 4 or 24 hours post-training. Freezing to the context and the CS were again assessed 7 days after training for mice originally tested at 4 or 24 hours. Furthermore, we ran an additional analysis comparing the saline-treated groups in foreground versus background contextual conditioning at both the initial time points and at the 1 week retest to examine differences between foreground and background conditioning.
In a follow-up study, we used the same background fear conditioning paradigm to examine the effects of a lower dose range of ethanol on background contextual and cued fear conditioning. Saline or ethanol (0.0625, 0.125, and 0.25 g/kg; 20% v/v in saline) was administered 15 minutes prior to training. There were no tests at 4 hours or 1 week in this experiment, as the initial studies demonstrated similar dose–response curves for the effects of ethanol at 4 hours, 24 hours, and 1 week.
Single CS–US Pairing
In the initial studies, there was no significant difference in cued fear conditioning between saline and the 0.25 g/kg ethanol groups, but the high levels of learning in the saline groups for cued fear conditioning indicate that a ceiling effect may be responsible for the lack of differences. A single CS–US pairing during training leads to lower conditioning than the 2 CS–US pairings used in the previous studies (Gould et al., 2004); lower levels of freezing across all conditions could reveal any enhancements previously masked by a ceiling effect. Saline or ethanol (0.25 g/kg; 20% v/v in saline) was administered 15 minutes before training, and testing occurred 24 hours post-training.
Mice were placed in conditioning chambers in which they received a single co-terminating CS (15-seconds, 85 dB white noise)–US (2-seconds, 0.57 mA foot shock) pairing. After the 135 second baseline period, the CS sounded for 15 seconds. The mice remained in the chamber for 30 seconds after the end of the CS–US presentation, for a total of 3 minutes. Baseline freezing behavior was recorded during the first 120 seconds (methods based on Gould et al., 2004). Mice were tested at 24 hours post-training. Freezing to the context was assessed and then freezing to the CS was assessed 1 hour later.
Post-training Ethanol Administration
Ethanol elimination from the blood requires approximately 2 hours for a 1.0 g/kg dose administered to mice (Haseba et al., 2006), creating the possibility that ethanol may alter learning through processes that occur after acquisition, such as consolidation. The administration of ethanol occurred 15 minutes before training in the initial studies; as a result, ethanol was present in the animals during both the acquisition and consolidation phases of learning. In order to rule out the possibility that the effects of 0.25 g/kg ethanol on learning are due to modulation of consolidation rather than acquisition, saline or ethanol (0.25 g/kg; 20% v/v in saline) was administered 2 minutes after training, creating a situation in which ethanol was present only during consolidation.
On training day, mice were placed in conditioning chambers. After a 120 second baseline period, they received 2 co-terminating CS (30-seconds, 85 dB white noise)–US (2 seconds, 0.57 mA foot shock) pairings. At 120 and 270 seconds, the CS sounded for 30 seconds; the US occurred during the last 2 seconds of the CS. The mice remained in the chamber for 30 seconds after the second CS–US presentation, for a total of 5.5 minutes. Baseline freezing behavior was recorded during the first 120 seconds and immediate freezing was measured after the first CS–US presentation (methods based on Gould and Higgins, 2003). Mice were tested at 24 hours post-training. Freezing to the context was assessed and then freezing to the CS was assessed 1 hour later.
Scoring
Freezing behavior was observed using a time-sampling procedure for pairs of mice during training and testing sessions. At 10-second intervals, mice were assessed for freezing during a 1-second period. Freezing was defined as the absence of visible movement with the exception of respiration (methods based on Gould and Wehner, 1999).
Blood Alcohol Concentration Analysis
Mice received injections of ethanol (0.0625, 0.125, and 0.25 g/kg; 20% v/v in saline). Groups consisted of 4 to 5 animals. Blood was collected from each group at 20 minutes postinjection to approximate the blood alcohol levels during training. Blood was collected in a capillary tube (Analox Instruments, Lunenberg, MA) and spun down in a centrifuge for 2 minutes at 14,000 rev/min. The plasma was then analyzed by an AM1 Analyzer (Analox Instruments) to obtain BAC (mg/dl). To assure the accuracy of the reading, each sample was analyzed twice and a mean from the 2 readings was used for data analysis (methods based on Gould and Lommock, 2003).
Statistical Testing
Data were analyzed using a two-way (time of testing vs. drug dose) analysis of variance (ANOVA) for the initial foreground and background study. t-Tests were used for the post-training ethanol and single CS–US experiments, as well as the foreground–background comparisons, and a one-way ANOVA was used for the BAC study. Tukey’s post hoc analysis was used to detect significant pair-wise differences at the p < 0.05 level. Statistics were run on SPSS (Version 13; SPSS, Chicago, IL).
RESULTS
Effects of Ethanol on Foreground Fear Conditioning
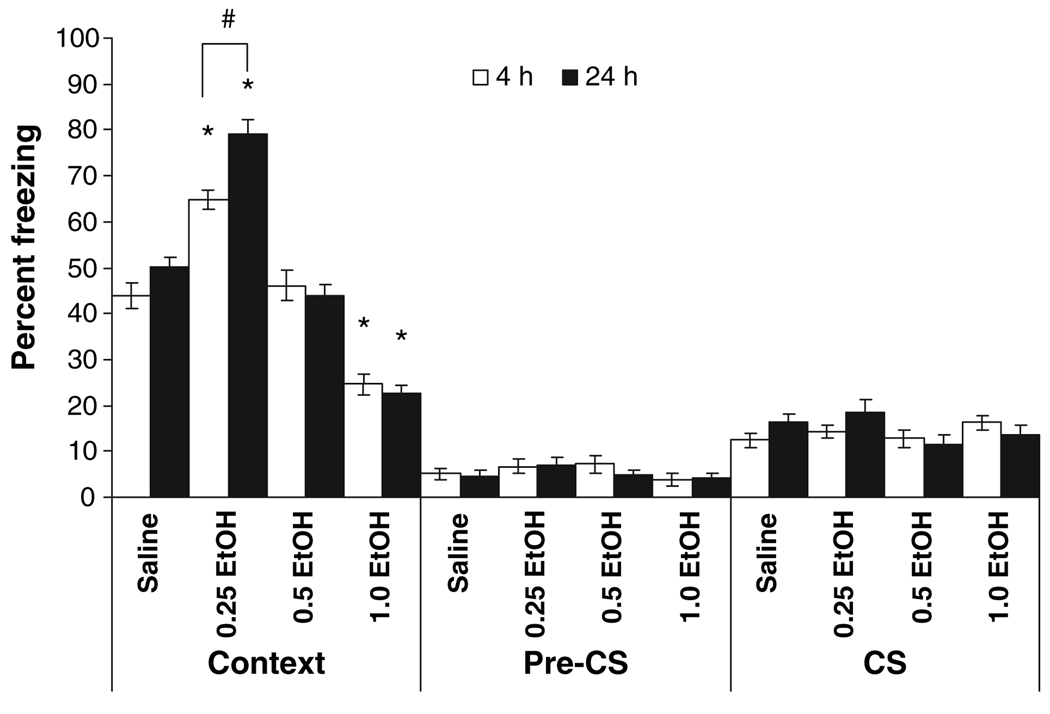
The effects of ethanol on foreground fear conditioning were assessed at either 4 or 24 hours. The levels of baseline and immediate freezing measured before and after the first US presentation, respectively, were similar across all groups. There was a significant main effect of drug condition on contextual fear conditioning, F(3,101) = 107.18, p < 0.001, and a significant interaction between drug condition and time of testing, F(3, 101) = 6.41, p < 0.01. Post hoc analysis revealed that during the context testing at both 4 and 24 hours, the 0.25 g/kg ethanol groups froze significantly more than the saline group while the 1.0 g/kg ethanol groups froze significantly less than the saline groups (p < 0.001), regardless of the time of testing. Additionally, in the groups administered 0.25 g/kg ethanol, the group tested at 24 hours froze significantly more than the group tested at 4 hours (p < 0.05). There was no effect of 0.5 g/kg ethanol at either 4 hours (p > 0.05) or 24 hours (p > 0.05). Thus, for foreground conditioning, 0.25 g/kg ethanol enhanced, and 1.0 g/kg ethanol impaired both short-term and long-term memory, while 0.5 g/kg ethanol had no effect (Fig. 1).
Fig. 1.
Effects of ethanol on foreground contextual fear conditioning at 4 and 24 hours post-training. Ethanol or saline was administered 15 minutes before training. Groups administered 0.25 g/kg ethanol froze to the context significantly more than controls, while groups administered 1.0 g/kg ethanol froze significantly less than controls. In addition, the enhancement by the 0.25 g/kg dose of ethanol was significantly greater at 24 hours compared with 4 hours. The groups administered 0.5 g/kg ethanol were not significantly different from controls. The absence of significant differences in freezing to an altered context (pre-conditioned stimulus; pre-CS) or to a tone (CS) was expected because mice were not trained to an auditory CS (mean ± SEM; *indicates significant difference from controls, p < 0.05; #indicates significant difference, p < 0.05).
There were no significant main effects for freezing to the altered context or the CS. There was no training with a CS in foreground contextual fear conditioning, so the absence of higher levels of freezing in cued testing suggests that the effects of ethanol were not due to general changes in locomotion or generalized fear.
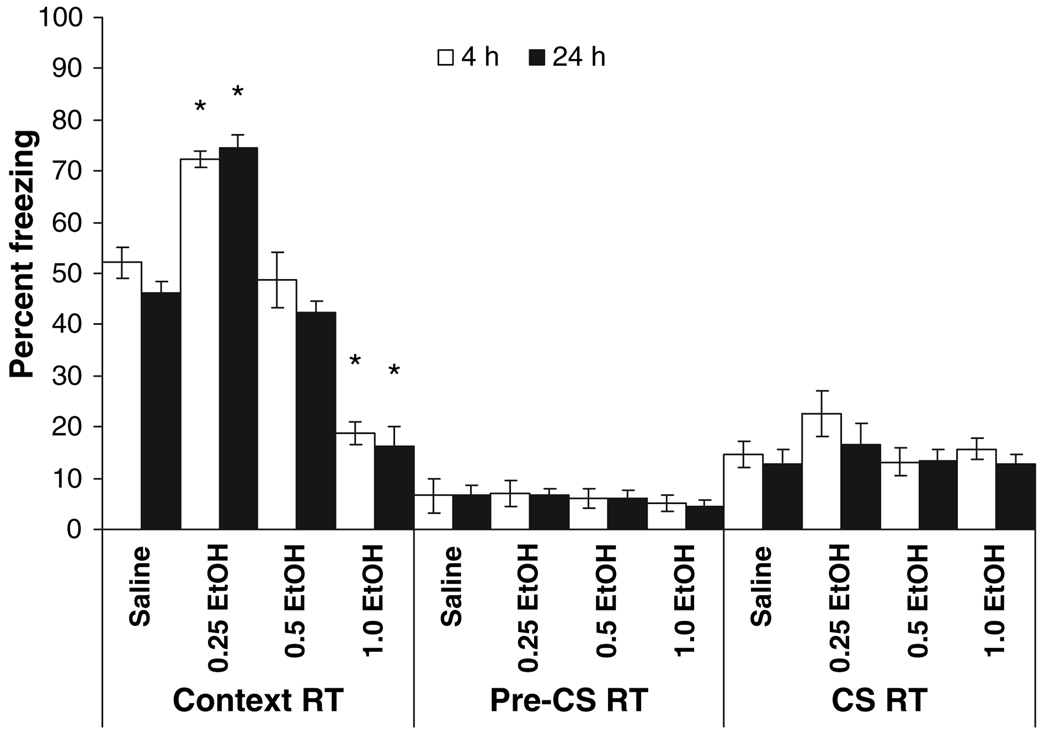
In order to examine the long-term effects of ethanol on foreground contextual fear conditioning, all animals initially tested at 4 or 24 hours were retested at 1 week post-training. There was a significant main effect of drug condition for freezing to the context, F(3,101) = 124.27, p < 0.001. Post hoc analysis revealed that at testing 1 week post-training, groups administered 0.25 g/kg ethanol at training froze significantly more in the context test than all other groups (p < 0.001), whereas groups administered 1.0 g/kg ethanol at training froze significantly less than all other groups (p < 0.001). The groups administered 0.5 g/kg froze at levels similar to the saline groups (p > 0.05) (Fig. 2). These results suggest that the effects of ethanol on fear conditioning are long-lasting and that the initial time of testing does not alter the responses measured 1 week post-training.
Fig. 2.
Effects of ethanol on foreground contextual fear conditioning at testing 1 week after training. Animals were initially tested at 4 or 24 hours post-training. RT indicates the 1 week retest; no drug was administered at retest. Groups administered 0.25 g/kg ethanol before training froze to the context (context RT) significantly more than controls, whereas groups administered 1.0 g/kg ethanol froze significantly less than controls. The groups administered 0.5 g/kg ethanol were not significantly different from controls. Post hoc tests revealed no significant differences in freezing to an altered context (pre-RT) or to a tone [conditioned stimulus (CS RT)] (mean ± SEM; *indicates significant difference from controls, p < 0.05).
For the CS period, there was a significant main effect of drug condition, F(3,101) = 2.23, p < 0.05 at the 1 week retest, but post hoc analysis revealed that the differences between saline and all other groups were not significant (p > 0.05). The absence of significant differences between groups again suggests that the freezing to the context was not due to generalized fear.
Effects of Ethanol on Background Fear Conditioning
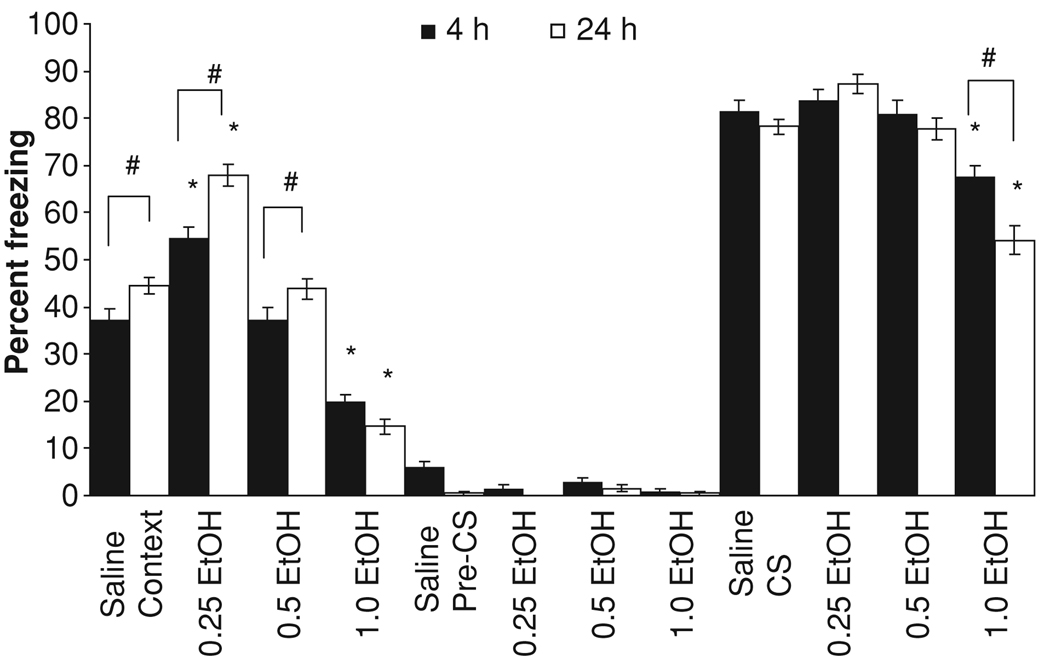
The effects of ethanol on background fear conditioning were assessed at either 4 or 24 hours post-training and again 1 week later. The levels of baseline and immediate freezing were similar across all groups. For freezing to the context, there were significant main effects of drug condition, F(3,101) = 3.92, p < 0.05, and of time of testing, F(1,101) = 12.43, p < 0.05. Post hoc analysis revealed that during the context testing, the 0.25 g/kg ethanol groups froze significantly more than the saline group whereas the 1.0 g/kg ethanol groups froze significantly less than the saline groups (p < 0.001), regardless of the initial time of testing. There was also a significant interaction of drug condition and time of testing, F(3,101) = 6.45, p < 0.001. Post hoc analysis revealed higher levels of freezing at the 24 hour time point for saline (p < 0.05), 0.25 g/kg ethanol (p < 0.001), and 0.5 g/kg ethanol (p < 0.05) compared with groups tested at 4 hours, but there was no difference in freezing between 4 and 24 hours for the groups administered 1.0 g/kg ethanol (p > 0.05). The groups administered 0.5 g/kg ethanol were not significantly different from the saline groups at either 4 hours (p > 0.05) or 24 hours (p > 0.05). There were no significant differences for freezing to the altered context (Fig. 3).
Fig. 3.
Effects of ethanol on background contextual and cued fear conditioning at 4 and 24 hours post-training. Ethanol or saline was administered 15 minutes before training. Groups administered 0.25 g/kg ethanol froze to the context significantly more than controls, while groups administered 1.0 g/kg ethanol froze significantly less than controls. Groups administered 1.0 g/kg ethanol were also significantly different from controls in freezing to a cue (conditioned stimulus; CS). Freezing to the context in the saline, 0.25, and 0.5 g/kg ethanol groups was greater at 24 hours than at 4 hours, while freezing to the cue in the 1.0 g/kg ethanol group was more impaired at 24 hours than at 4 hours. The groups administered 0.5 g/kg ethanol were not significantly different from controls. There were no significant differences in freezing to an altered context (pre-CS) (mean ± SEM; *indicates significant difference from controls, p < 0.05; #indicates significant difference, p < 0.05).
During the CS period, there was a significant main effect of drug condition F(3,101) = 34.32, p < 0.001, and a significant interaction of drug condition and time of testing, F(3,101) = 4.25, p < 0.01. Post hoc analysis revealed that the groups administered 1.0 g/kg ethanol were significantly different from all other groups due to their decreased freezing (p < 0.05) and that the group tested at 24 hours froze significantly less than the group tested at 4 hours (p < 0.05). There was no significant enhancement of freezing to the cue by the 0.25 g/kg dose of ethanol.
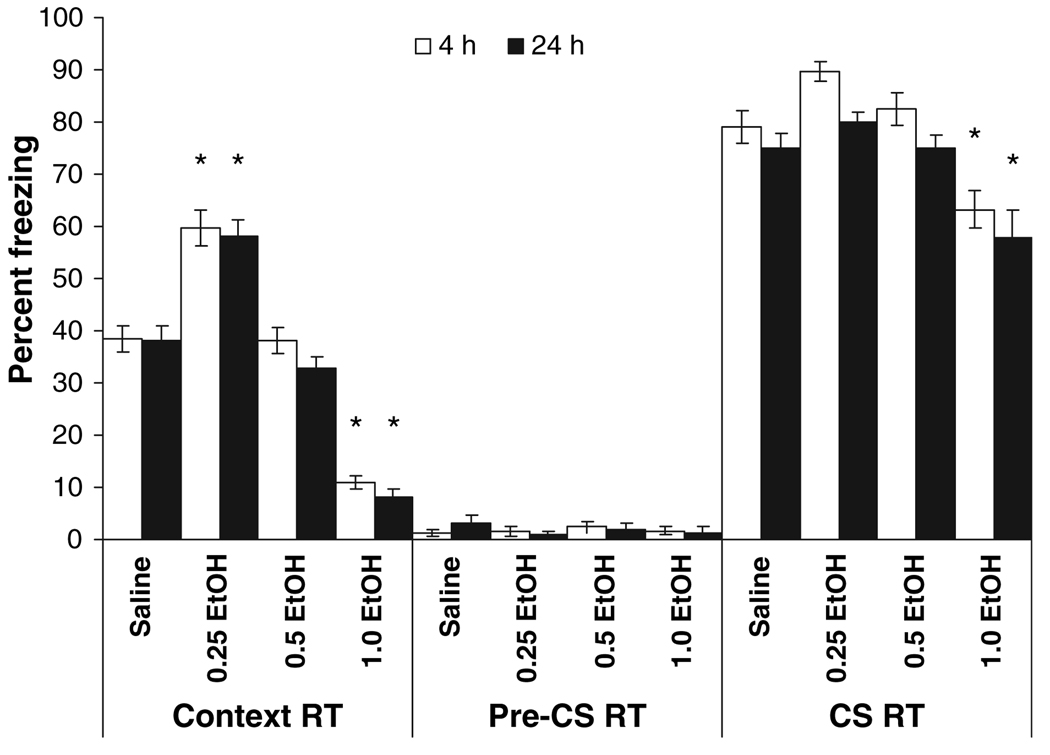
In order to examine the long-term effects of ethanol on background contextual fear conditioning, animals were retested at 1 week post-training. For freezing to the context, there was a significant main effect of drug condition, F(3,101) = 134.95, p < 0.001. Post hoc analysis revealed that groups administered 0.25 g/kg ethanol at training froze significantly more to the context than all other groups (p < 0.001), while groups administered 1.0 g/kg ethanol at training froze significantly less than all other groups (p < 0.001). The groups administered 0.5 g/kg froze at levels similar to the saline groups (p > 0.05) (Fig. 4). Thus, similar effects of ethanol were seen on short-term and long-term contextual memory in both foreground and background conditioning.
Fig. 4.
Effects of ethanol on background contextual and cued fear at testing 1 week after training. Animals were initially tested at either 4 or 24 hours post-training. Groups administered 0.25 g/kg ethanol before training froze to the context significantly more than controls, whereas groups administered 1.0 g/kg ethanol froze significantly less than controls. Groups administered 1.0 g/kg ethanol were also significantly different from controls in freezing to a cue (conditioned stimulus; pre-CS RT). There were no significant differences in freezing to an altered context (pre-RT) (mean ± SEM; *indicates significant difference from controls, p < 0.05).
For freezing to the CS, there was a significant main effect of drug condition, F(3,101) = 22.68, p < 0.001 at the 1 week retest. Post hoc analysis revealed a decrease in freezing in the 1.0 g/kg ethanol groups (p < 0.05). There was no significant enhancement of freezing to the cue by the 0.25 g/kg dose of ethanol.
Comparison of Foreground and Background Conditioning in Saline-Treated Animals
In order to determine whether there were significant differences in conditioning to foreground and background contextual stimuli, we also compared the levels of contextual conditioning in the saline-treated groups. There was no significant difference between groups at the initial time point, t(22) = 1.96, p > 0.05 for the groups tested at 4 hours (foreground: Mean (M) = 44.55, Standard Error of the Mean (SEM) = 3.24; background: M = 37.18, SEM = 2.35; reported for percent freezing), and t(21) = 1.94, p > 0.05 for groups tested at 24 hours (foreground: M = 49.39, SEM = 2.10; background: M = 44.44; SEM = 1.68; reported for percent freezing). However, there were significant differences between foreground and background conditioning at the 1 week retest, t(22) = 3.69, p < 0.05 for the groups tested at 4 hours (foreground: M = 52.5, SEM = 2.81; background: M = 38.46; SEM = 2.44; reported for percent freezing), and t(21) = 3.38, p < 0.05 for the groups tested at 24 hours (foreground: M = 47.58, SEM = 2.11; background: M = 37.22; SEM = 2.38; reported for percent freezing). Comparisons of group means revealed that animals trained in foreground conditioning froze more to the context than animals trained in background conditioning at the 1 week time point.
Effects of Low Ethanol Doses on Background Fear Conditioning
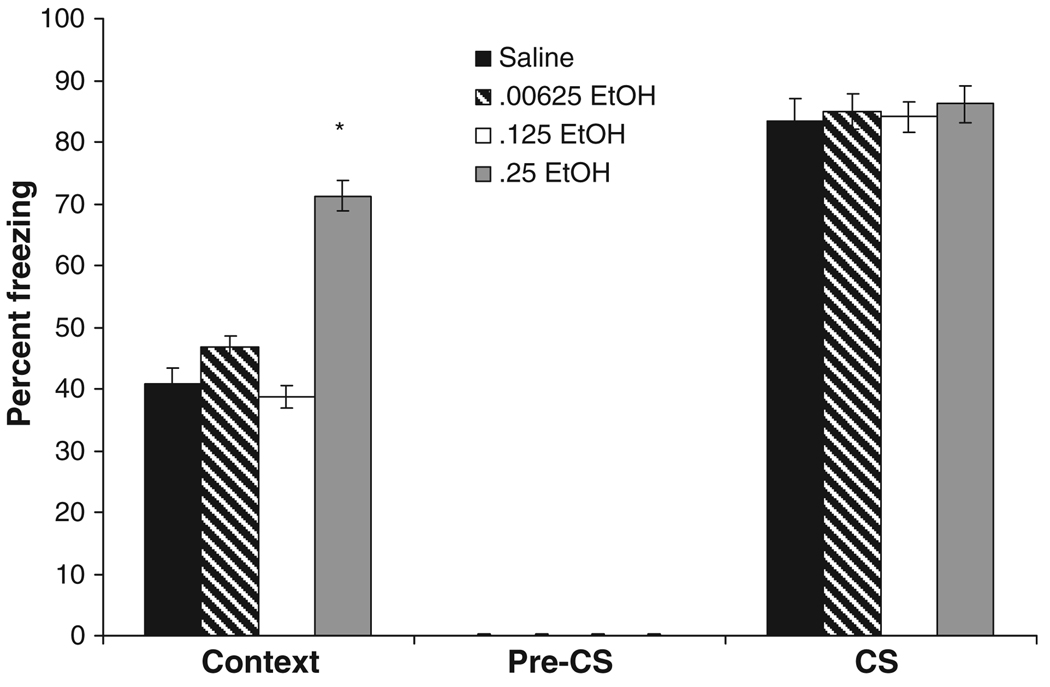
We next examined the effects of a lower dose range of ethanol administered at training on background fear conditioning, with testing 24 hours later. The levels of baseline and immediate freezing were similar across all groups. There was a significant main effect for freezing to the context, F(3,28) = 45.94, p < 0.001, but no significant effect for freezing during the pre-CS or CS periods. Post hoc analysis of contextual conditioning revealed that the 0.25 g/kg ethanol group froze significantly more than all other groups (p < 0.001). The saline, 0.0625 g/kg, and 0.125 g/kg ethanol groups were not significantly different (Fig. 5).
Fig. 5.
Effects of lower doses of ethanol on background contextual and cued fear tested 24 hours after training. Ethanol or saline was administered 15 minutes before training. The group administered 0.25 g/kg ethanol froze to the context significantly more than all other groups; there were no significant differences between groups administered saline, 0.00625, and 0.125 g/kg ethanol. There were no significant differences in freezing to an altered context (pre-conditioned stimulus; CS) or to a cue (CS) (mean ± SEM; *indicates significant difference from controls, p < 0.05).
Effects of a Single CS–US Pairing on Background Fear Conditioning
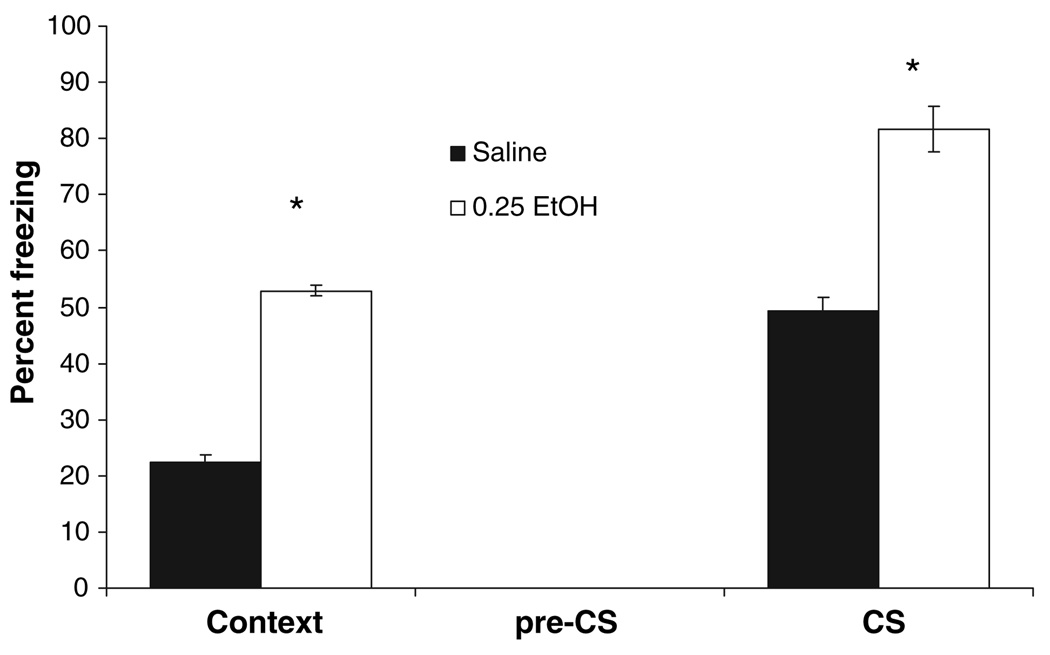
To examine the possibility that ethanol could enhance cued conditioning when a more difficult training procedure was used, animals were trained with a single CS–US pairing. Saline or 0.25 g/kg ethanol was administered before training, and animals were tested for both contextual and cued fear conditioning 24 hours later. Similar levels of baseline freezing were found across groups. There were significant differences in both freezing to the context, t(13) = 20.27, p < 0.001, and to the cue, t(13) = 7.6, p < 0.001 but no significant difference in freezing during the pre-CS period. The group administered 0.25 g/kg ethanol froze significantly more to both the context and the cue than did the group administered saline (Fig. 6).
Fig. 6.
Effects of ethanol on conditioning to a single conditioned stimulus–conditioned stimulus (CS–US) pairing in background contextual fear conditioning. Saline or 0.25 g/kg ethanol was administered 15 minutes before training. The 0.25 g/kg ethanol group froze significantly more than the saline group to both the context and the cue (CS). There were no significant differences in freezing to an altered context (pre-CS) (mean ± SEM; *indicates significant difference from controls, p < 0.05).
Effects of Post-Training Ethanol on Background Fear Conditioning
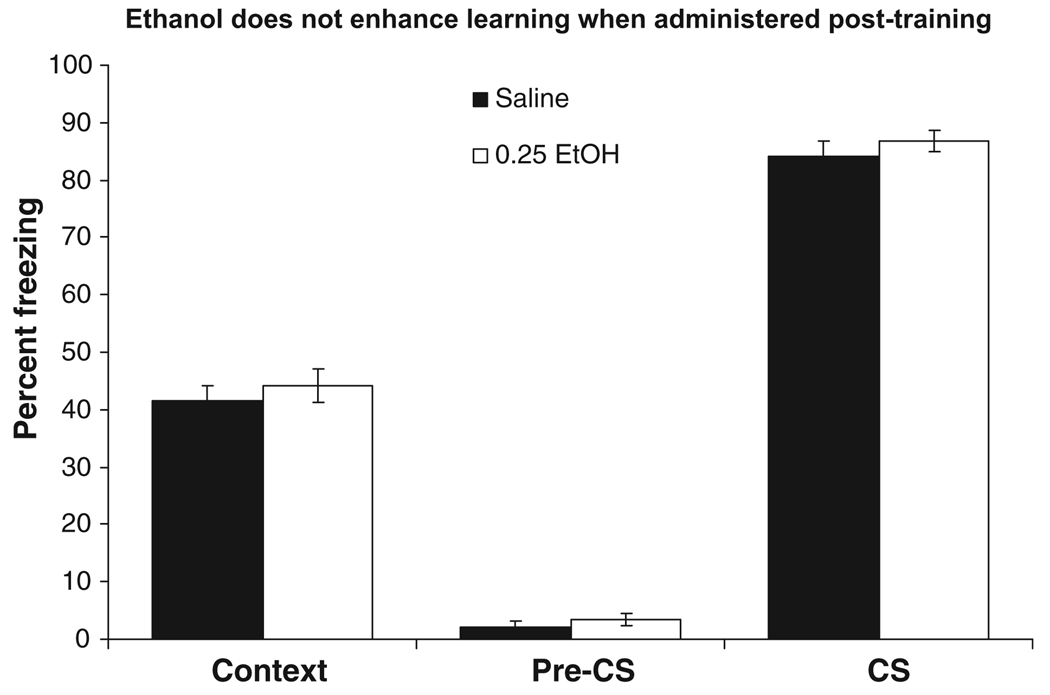
To determine whether the enhancement of contextual and cued fear conditioning by ethanol was due to effects on acquisition or consolidation, saline or 0.25 g/kg ethanol was administered immediately after background conditioning. Similar levels of baseline and immediate freezing were found across groups. There were no significant differences in freezing during the pre-CS period. In addition, there were no significant differences detected during testing for freezing to the context or to the cue (Fig. 7).
Fig. 7.
Effects of post-training ethanol on background contextual and cued fear conditioning. Saline or 0.25 g/kg ethanol was administered immediately after training. There were no significant differences in freezing to the training context to an altered context (pre-conditioned stimulus; pre-CS) or to a cue (CS) 24 hours later (mean ± SEM).
Blood Alcohol Concentration at Training
Blood alcohol levels at 20 minutes after injection were determined for ethanol (0.0625, 0.125, 0.25, 0.5, and 1.0 g/kg) treated mice. There were significant differences in the mean blood alcohol levels between groups, F(4,20) = 144.8, p < 0.001. Tukey post hoc analyses revealed a significant difference between the 1.0 g/kg ethanol group and all other groups (p < 0.001) and between the 0.5 g/kg ethanol group and all other groups (p < 0.001). There were no significant differences between the 0.0625, 0.125, and 0.25 g/kg ethanol groups (p > 0.05); however, these doses produced blood alcohol levels at which detection of minute differences in BAC may become difficult, and even small changes in BAC may have behavioral consequences (Lima-Landman and Albuquerque, 1989).
DISCUSSION
This study demonstrates that ethanol dose-dependently alters acquisition of both short-term and long-term memories for contextual and cued fear conditioning. Perhaps the most interesting finding to emerge from this study was that ethanol altered fear conditioning in both a positive and negative direction. Regardless of whether the context was a foreground or a background stimulus, ethanol impaired conditioning at a high dose but enhanced conditioning at a low dose. In addition, the enhancement of fear conditioning by ethanol was selective for the acquisition, but not consolidation, of learning. By altering acquisition, ethanol may have similar effects on both short-term and long-term memory, in agreement with other studies showing that high doses of ethanol impair both short-term and long-term memory systems (Givens, 1996; Grattan-Miscio and Vogel-Sprott, 2005; Schweizer et al., 2006). This result is similar to prior findings demonstrating that higher doses of ethanol that impaired learning when administered before conditioning did not impair learning when administered after conditioning (Alkana and Parker, 1979; Bammer and Chesher, 1982; Gould and Lommock, 2003). Together, these findings suggest that ethanol alters learning by mediating processes involved in acquisition of short-term and long-term memories rather than consolidation. However, these processes have not yet been fully characterized, and it is unclear whether the effects of ethanol on acquisition are due to changes in attention or other cognitive processes that contribute to acquisition.
Prior studies have demonstrated that hippocampus-dependent learning, such as contextual conditioning, is more sensitive to the effects of ethanol than hippocampus-independent learning, such as cued conditioning (Celerier et al., 2000; Gould, 2003; Melia et al., 1996); while the current study does show greater percent deficits due to ethanol in contextual conditioning (a 57% decrease in freezing to the context compared with a 24% decrease in freezing to the cue), both contextual and cued conditioning were significantly affected. There are at least 2 possible explanations for the differential effects of ethanol on contextual and cued conditioning. Ethanol may alter learning-related processes in both the hippocampus and amygdala, leading to greater deficits in contextual conditioning, which is dependent on both areas (Phillips and LeDoux, 1992). Alternatively, ethanol may act solely on learning-related processes in the amygdala; in this case, it may be that cued conditioning is less susceptible to the effects of ethanol because it is more salient, and thus stronger, than contextual conditioning. Identifying the mechanism by which ethanol disrupts fear conditioning will aid in determining whether ethanol affects a process common to both contextual and cued fear conditioning or parallel processes.
One process that ethanol may alter, resulting in the disruption of learning, is N-methyl-d-aspartate (NMDA) receptor-mediated activity. NMDA receptors are involved in learning and, more specifically, fear conditioning (Bast et al., 2003; Maren et al., 1996). In the current study, the highest dose of ethanol produced blood alcohol levels (80 to 90 mg/dl) in the range that produces intoxication in humans (50 to 100 mg/dl) (Beers et al., 2005). Interestingly, at intoxicating doses, ethanol inhibits NMDA receptors by altering channel kinetics to decrease the efficacy of agonist binding (Peoples and Weight, 1995; Snell et al., 1993) and decrease NMDA receptor-mediated currents (Wright et al., 1996). Furthermore, ethanol decreases NMDA receptor-mediated activity in both the hippocampus and amygdala (Lovinger et al., 1989; Roberto et al., 2004), which are involved in fear conditioning (Phillips and LeDoux, 1992). It is also of interest that ethanol disrupts acquisition but not consolidation of learning (Alkana and Parker, 1979; Bammer and Chesher, 1982) because NMDA receptors appear to be involved in the acquisition but not consolidation of fear conditioning (Bast et al., 2003; Gould et al., 2002; Maren et al., 1996). Thus, disruption of NMDA receptor function may be 1 mechanism responsible for ethanol disruption of fear conditioning.
Ethanol-induced changes in NMDA receptor function could also explain the enhancement of fear conditioning by ethanol. Similar to the present results for fear conditioning, prior research demonstrates that ethanol enhances other forms of learning including eye blink conditioning (Devenport et al., 1983; Hernandez and Powell, 1986; Hernandez and Valentine, 1990; Korman et al., 1960). What is interesting is that eye blink conditioning involves plasticity in the cerebellum (Gould and Steinmetz, 1996; Kim and Thompson, 1997; McCormick and Thompson, 1984), while fear conditioning involves plasticity in the hippocampus and amygdala (Kim et al., 1993; Le Doux, 1995; Logue et al., 1997; Phillips and LeDoux, 1992); yet although the underlying neural areas involved in eye blink conditioning differ from those involved in fear conditioning, the dose of ethanol that enhanced fear conditioning was within the range of doses that enhanced eye blink conditioning. This suggests that common cellular processes across these tasks may be affected by low doses of ethanol. One possible mechanism is the potentiation of NMDA receptor-mediated activity by low doses of ethanol. In the hippocampus, low doses of ethanol potentiate NMDA receptor-mediated currents (Lima-Landman and Albuquerque, 1989). Like fear conditioning, eye blink conditioning is dependent on NMDA receptor-mediated activity (Chen and Steinmetz, 2000), and it may be that the potentiation of NMDA receptor function by low doses of ethanol underlies the enhancing effects of ethanol on learning. The lower doses of ethanol used in the present study produced blood alcohol levels (less than 50 mg/dl) below the range associated with intoxication. This finding suggests that the enhancing effects of ethanol on learning occur at blood alcohol levels less than those at which the physical symptoms of intoxication develop.
Previous research on the effects of ethanol on contextual fear conditioning has used the context as a background stimulus. Although some research suggests that the neural substrates underlying foreground and background conditioning may differ (Bourtchouladze et al., 1998; Phillips and LeDoux, 1994; Trifilieff et al., 2006) and that the salience of the context may differ between foreground and background conditioning (Odling-Smee, 1975, 1978), we found that ethanol had similar effects on contextual learning in both background and foreground fear conditioning. Other research in our laboratory has demonstrated that acute nicotine enhances both foreground and background fear conditioning (Davis et al., 2006), supporting the current finding that background and foreground fear conditioning may share common modulatory pathways. Further evidence for this comes from studies demonstrating that mice with altered hippocampal function have deficits in both foreground and background contextual fear conditioning (Paylor et al., 1994) and that both forms of contextual conditioning are dependent on NMDA receptor-mediated activity in the dorsal hippocampus (Bast et al., 2003). Taken together, our results suggest that the effects of ethanol on foreground and background conditioning are similar and that these effects are not related to the salience of the context.
Finally, it should be noted that there was a significant difference between foreground and background contextual conditioning. At the 1 week retest, foreground conditioning was greater than background conditioning. In contrast, no difference between foreground and background contextual conditioning was seen at the initial time points, 4 and 24 hours. This suggests that, in the present study, differences between background and foreground conditioning may affect long-term consolidation processes rather than processes activated during initial acquisition and consolidation.
ACKNOWLEDGMENTS
This work was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grant AA015515 (TJG).
REFERENCES
- Alkana RL, Parker ES. Memory facilitation by post-training injection of ethanol. Psychopharmacology. 1979;66:117–119. doi: 10.1007/BF00427617. [DOI] [PubMed] [Google Scholar]
- Bammer G, Chesher GB. An analysis of some effects of ethanol on performance in a passive avoidance task. Psychopharmacology. 1982;77:66–73. doi: 10.1007/BF00436101. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: effects of local NMDA-receptor blockade and stimulation. Hippocampus. 2003;13:657–675. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Beers MH, Porter RS, Jones TV, Kaplan JL, Berkwits M. Merck Manual of Diagnosis and Therapy [online] Whitehouse, NJ: Merck Publishing; 2005. [Accessed November 15, 2006]. Psychiatric disorders: drug use and dependence, Chapter 195. November 2005: Section 15, Available from: [Google Scholar]
- Birnbaum IM, Parker ES, Hartley JT, Noble EP. Alcohol and memory: retrieval processes. J Verb Learn Verb Behav. 1978;17:325–335. [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel E. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Celerier A, Ognard R, Decorte L, Beracochea D. Deficits of spatial and non-spatial memory and of auditory fear conditioning following anterior thalamic lesions in mice: comparison with chronic alcohol consumption. Eur J Neurosci. 2000;12:2575–2584. doi: 10.1046/j.1460-9568.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Steinmetz JE. Intra-cerebellar infusion of NMDA receptor antagonist AP5 disrupts classical eyeblink conditioning in rabbits. Brain Res. 2000;887:144–156. doi: 10.1016/s0006-8993(00)03005-5. [DOI] [PubMed] [Google Scholar]
- Davis JA, Porter J, Gould TJ. Nicotine enhances both foreground and background contextual fear conditioning. Neurosci Lett. 2006;394:202–205. doi: 10.1016/j.neulet.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Devenport LD, Merriman VJ, Devenport JA. Effects of ethanol on enforced spatial variability in the 8-arm radial maze. Pharmacol Biochem Behav. 1983;18:55–59. doi: 10.1016/0091-3057(83)90251-4. [DOI] [PubMed] [Google Scholar]
- Escher T, Mittleman G. Effects of ethanol and GABAb drugs on working memory. Psychopharmacology. 2004;176:166–174. doi: 10.1007/s00213-004-1875-x. [DOI] [PubMed] [Google Scholar]
- Gibson WE. Effects of alcohol on radial maze performance in rats. Physiol Behav. 1985;35:1003–1005. doi: 10.1016/0031-9384(85)90273-2. [DOI] [PubMed] [Google Scholar]
- Givens B. Low doses of ethanol impair spatial working memory and reduce hippocampal theta activity. Alcohol Clin Exp Res. 1995;19:763–767. doi: 10.1111/j.1530-0277.1995.tb01580.x. [DOI] [PubMed] [Google Scholar]
- Givens B. Behavioral correlates of single units in the medial septal area: the effect of ethanol. Neuroscience. 1996;71:417–427. doi: 10.1016/0306-4522(95)00443-2. [DOI] [PubMed] [Google Scholar]
- Givens B, McMahon K. Effects of ethanol on nonspatial working memory and attention in rats. Behav Neurosci. 1997;111:275–282. doi: 10.1037//0735-7044.111.2.275. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Ethanol disrupts fear conditioning in C57BL/6J mice. J Psychopharmacol. 2003;17:77–81. doi: 10.1177/0269881103017001702. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Feiro O, Moore D. Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning. Behav Brain Res. 2004;155:167–173. doi: 10.1016/j.bbr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav Neurosci. 2003;117:1276–1282. doi: 10.1037/0735-7044.117.6.1276. [DOI] [PubMed] [Google Scholar]
- Gould TJ, McCarthy MM, Keith RA. MK-801 disrupts acquisition of contextual fear conditioning but enhances memory consolidation of cued fear conditioning. Behav Pharmacol. 2002;13:287–294. doi: 10.1097/00008877-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Steinmetz JE. Changes in rabbit cerebellar cortical and interpositus nucleus activity during acquisition, extinction, and backward classical eyelid conditioning. Neurobiol Learn Mem. 1996;65:17–34. doi: 10.1006/nlme.1996.0003. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Grattan-Miscio KE, Vogel-Sprott M. Effects of alcohol and performance incentives on immediate working memory. Psychopharmacology. 2005;181:188–196. doi: 10.1007/s00213-005-2226-2. [DOI] [PubMed] [Google Scholar]
- Haseba T, Tomito Y, Kurosu M, Ohno Y. Dose and time changes in liver alcohol dehydrogenase (ADH) activity during acute alcohol intoxication involve not only class I but also class III ADH and govern elimination rate of blood ethanol. Leg Med. 2006;5:202–211. doi: 10.1016/s1344-6223(03)00080-4. [DOI] [PubMed] [Google Scholar]
- Hernandez LL, Powell DA. Ethanol enhancement of Pavlovian conditioning: comparison with instrumental conditioning. Psychopharmacology. 1986;88:75–81. doi: 10.1007/BF00310516. [DOI] [PubMed] [Google Scholar]
- Hernandez LL, Valentine JD. Mild ethanol intoxication may enhance Pavlovian conditioning. Drug Dev Res. 1990;20:155–167. [Google Scholar]
- Higgins ST, Rush CR, Hughes JR, Bickel WK, Lynn M, Capeless MA. Effects of cocaine and alcohol, alone and in combination, on human learning and performance. J Exp Anal Behav. 1992;58:87–105. doi: 10.1901/jeab.1992.58-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short-term and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- Korman M, Knopf IJ, Austin RB. Effects of alcohol on serial learning under stress conditions. Psychol Rep. 1960;7:217–220. [Google Scholar]
- LeDoux JE. Emotion: clues from the brain. Annu Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- Lima-Landman MT, Albuquerque EX. Ethanol potentiates and blocks NMDA-activated single-channel currents in rat hippocampal pyramidal cells. FEBS Lett. 1989;247:61–67. doi: 10.1016/0014-5793(89)81241-4. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111:104–133. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Lolordo VM, Williams DA, McPhee JE. Overshadowing of situational cues with variable but not fixed intertrial intervals. Anim Learn Behav. 2001;29:143–152. [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lyon RJ, Tong JE, Leigh G, Clare G. The influence of alcohol and tobacco on the components of choice reaction time. J Stud Alcohol. 1975;36:587–596. doi: 10.15288/jsa.1975.36.587. [DOI] [PubMed] [Google Scholar]
- Majchrzak M, Ferry B, Marchand AR, Herbeaux K, Seillier A, Barbelivien A. Entorhinal cortex lesions disrupt fear conditioning to background context but spare fear conditioning to a tone in rat. Hippocampus. 2006;16:114–124. doi: 10.1002/hipo.20138. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav Neurosci. 1996;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory I adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Matthews DB, Ilgen M, White AM, Best PJ. Acute ethanol administration impairs spatial performance while facilitating nonspatial performance in rats. Neurobiol Learn Mem. 1999;72:169–179. doi: 10.1006/nlme.1998.3900. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Simson PE, Best PJ. Acute ethanol impairs spatial memory but not stimulus/response memory in the rat. Alcohol Clin Exp Res. 1995;19:902–909. doi: 10.1111/j.1530-0277.1995.tb00965.x. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ehlers CL. Signal detection analysis of ethanol effects on a complex conditional discrimination. Pharmacol Biochem Behav. 1989;33:581–584. doi: 10.1016/0091-3057(89)90391-2. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Corodimas KP, Wilson MC, LeDoux JE. Hippocampal-dependent learning and experience-dependent activation of the hippocampus are preferentially disrupted by ethanol. Neuroscience. 1996;74:313–322. doi: 10.1016/0306-4522(96)00138-8. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. [Accessed October 12, 2006]; Available at: http://pubs.niaaa.nih.gov/publications/GettheFacts_HTML/Facts.pdf.
- Odling-Smee FJ. The role of background stimuli during Pavlovian conditioning. Q J Exp Psychol. 1975;27:201–209. doi: 10.1080/14640747508400480. [DOI] [PubMed] [Google Scholar]
- Odling-Smee FJ. The overshadowing of background stimuli: some effects of varying amounts of training and UCS intensity. Q J Exp Psychol. 1978;30:737–746. doi: 10.1080/14640747808400698. [DOI] [PubMed] [Google Scholar]
- Paylor R, Tracy R, Wehner JM, Rudy JW. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci. 1994;108:810–817. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- Peoples RW, Weight FF. Cutoff in potency implicates alcohol inhibition of NMDA receptors in alcohol intoxication. PNAS. 1995;92:2825–2829. doi: 10.1073/pnas.92.7.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem. 1994;1:34–44. [PubMed] [Google Scholar]
- Popke EJ, Allen SR, Paule MG. Effects of acute ethanol on indices of cognitive-behavioral performance in rats. Alcohol. 2000;20:187–192. doi: 10.1016/s0741-8329(99)00081-6. [DOI] [PubMed] [Google Scholar]
- Rescorla A, Wagner AR. A theory of Pavlovian conditioning: variation 278 in the effectiveness of reinforcement and non reinforcement. In: Black P, editor. Classical Conditioning II: Current Research and Theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Rezvani AH, Levin ED. Nicotine-alcohol interactions and attentional performance on an operant visual signal detection task in female rats. Pharmacol Biochem Behav. 2003;76:75–83. doi: 10.1016/s0091-3057(03)00193-x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryback RS. The continuum and specificity of the effects of alcohol on memory. Q J Stud Alcohol. 1971;32:996–1016. [PubMed] [Google Scholar]
- Schweizer TA, Vogel-Sprott M, Danckert J, Roy EA, Skakum A, Broderick CE. Neuropsychological profile of acute alcohol intoxication during ascending and descending blood alcohol concentrations. Neuropsycho-pharmcology. 2006;31:1301–1309. doi: 10.1038/sj.npp.1300941. [DOI] [PubMed] [Google Scholar]
- Sircar R, Sircar D. Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcohol Clin Exp Res. 2005;29:1402–1410. doi: 10.1097/01.alc.0000175012.77756.d9. [DOI] [PubMed] [Google Scholar]
- Snell LD, Tabakoff B, Hoffman PL. Radioligand binding to the NMDA receptor/ionophore complex: alterations by ethanol in vitro and by chronic in vivo ethanol ingestion. Brain Res. 1993;602:91–98. doi: 10.1016/0006-8993(93)90246-j. [DOI] [PubMed] [Google Scholar]
- Squire LR, Kandel ER. Memory: From Mind to Molecules. New York: Scientific American Library; 1999. [Google Scholar]
- Steele-Russell I, Russell MI, Castiglioni JA, Reuter JA, van Hof MW. Selective attention and Pavlovian conditioning. Exp Brain Res. 2006;173:587–602. doi: 10.1007/s00221-006-0404-z. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Palve M, Radulovic J, Birkenfeld K, Spiess J. Differential impairment of auditory and contextual fear conditioning by protein synthesis inhibition in C57BL/6N mice. Behav Neurosci. 1999;113:496–506. doi: 10.1037//0735-7044.113.3.496. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, Riedel G, Mons N, Micheau J. Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn Mem. 2006;13:349–358. doi: 10.1101/lm.80206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JM, Peoples RW, Weight FF. Single-channel and whole-cell analysis of ethanol inhibition of NMDA-activated currents in cultured mouse cortical ad hippocampal neurons. Brain Res. 1996;738:249–256. doi: 10.1016/s0006-8993(96)00780-9. [DOI] [PubMed] [Google Scholar]