Abstract
Dietary nitrate, found in abundance in green vegetables, can be converted to the cytoprotective molecule nitrite by oral bacteria, suggesting that nitrate and nitrite may represent active cardioprotective constituents of the Mediterranean diet.
We therefore tested the hypothesis that dietary nitrate and nitrite levels modulate tissue damage and ischemic gene expression in a mouse liver ischemia-reperfusion model. We found that stomach content, plasma, heart and liver nitrite levels were significantly reduced after dietary nitrate and nitrite depletion, and could be restored to normal levels with nitrite supplementation in water. Remarkably, we confirmed that basal nitrite levels significantly reduced liver injury after ischemia-reperfusion. Consistent with an effect of nitrite on the post-translational modification of complex I of the mitochondrial electron transport chain, the severity of liver infarction was inversely proportional to complex I activity after nitrite repletion in the diet. The transcriptional response of dietary nitrite after ischemia was more robust than after normoxia, suggesting a hypoxic potentiation of nitrite-dependent transcriptional signaling.
Our studies indicate that normal dietary nitrate and nitrite levels modulate ischemic stress responses and hypoxic gene expression programs, supporting the hypothesis that dietary nitrate and nitrite are cytoprotective components of the diet.
Introduction
The anion nitrite functions as a blood and tissue reservoir of nitric oxide (NO) that is activated by deoxygenated hemoglobin, myoglobin, xanthine oxidoreductase and other heme-based enzymes during physiological and pathological hypoxia [1]. NO-meditated cytoprotection after ischemia and reperfusion (I/R) has been well documented [2-4] and it has recently been discovered that the reduction of nitrite to NO in ischemic tissues is associated with potent cytoprotection. Nitrite has been shown to limit tissue apoptosis and infarction in animal models of ischemia-reperfusion injury of the heart, liver, brain and kidney [5-8] and to be dependent on the generation of NO. An important mechanism explaining the cytoprotective effect of nitrite is its ability to nitrosate mitochondrial complex I, thereby limiting the generation of reactive oxygen species ROS formation and cell injury[9].
More specifically, Duranski and colleagues [10] showed in a mouse model of ischemia reperfusion that intraperitoneal injection of doses of sodium nitrite (NaNO2) doses as low as 2.4 nmol reduced liver and heart infarction volumes by approximately 50%. These lowest doses studied increased plasma levels of nitrite by 400 nM or 50% of baseline, similar to the levels of nitrite that form in plasma after the consumption of a leafy green salad [11].
Nitrite in the plasma and organ tissues originates from several sources. First, nitric oxide formed by nitric oxide synthase in the endothelium (eNOS) can be oxidized to nitrite by ceruloplasmin [12]. Kleinbongard et al. reported that in mice about 70% of plasma nitrite is derived from eNOS [13]. Second, nitrite is also present in food such as green vegetables, however concentrations are about 500-fold lower than its oxidation product nitrate [14]. Processed food sources, like cured meat, in which nitrite is added as a preservative to inhibit bacterial growth are therefore the main source of direct food nitrite intake. A third source of nitrite is derived from dietary nitrate. Commensal bacteria in the posterior crypts of the tongue and the digestive tract are able to convert approximately 20% of salivary nitrate into nitrite. The salivary glands of various mammals, including humans and mice, are able to concentrate and secrete plasma nitrate in micromolar levels into saliva [15]. This mechanism allows nitrate to come into repetitive contact with mouth bacteria realizing a more efficient conversion of nitrate to nitrite over time. Indeed, infusion or ingestion of nitrate is associated with significant increases in plasma nitrite. A report by Dich et al. [16] provides an overview of 15 studies conducted in the Europe and the US between 1975 and 1996. The ingested nitrate levels in these studies ranged between 50 and 120 mg nitrate (mean 77 mg) per day with vegetables contributing from 72% up to 94%. A study by Meah et al. [17] showed that the amount of vegetables consumed in the UK was about 160 g per day. According to a study by Trichopoulos et al. [18] the amount of vegetables consumed in the so called Mediterranean diet is more than 3 fold higher (550 g per day). Table 2 displays the nitrate levels of the vegetables most commonly consumed in the Mediterranean diet with a mean of 800 mg nitrate/kg. Based on these numbers the amount of nitrate ingested by the Mediterranean diet would be around 400 mg, or more than 4 fold the level of the average European or US diet. Furthermore, the so-called Western diet of uncured meats, carbohydrates and fats contains minimal nitrate and nitrite, and the levels of nitrate in drinking water are strictly controlled by regulatory agencies to limit such intake.
Table 2. Nitrate amount in vegetables typical for the Mediterranean diet.
| Typical vegetables in the Mediterranean diet | Nitrate content (mg/kg) |
|---|---|
| Aubergine | 460 |
| Beans | 400 |
| Beets | 1500 |
| Carrots | 170 |
| Fennel | 2000 |
| Potatoes | 120 |
| Zucchini | 810 |
| Mean | 780 |
Values obtained from Meah et al.[17]
We and others have considered the fact that the Mediterranean diet and a diet rich in green vegetables is associated with lower blood pressures and a reduced risk of coronary artery disease [11, 19-24]. Indeed a recent study by Webb and colleagues reports that the ingestion of beet root juice, high in nitrate content (2.8 g/L), results in decreased blood pressure, inhibition of platelet activity and increased endothelial resilience to hypoxic stress. These effects were related to the conversion of nitrate to bioactive nitrite [25].
Considering the relationship between dietary nitrate levels and plasma nitrite levels and the recent understanding that nitrite participates in a protective cellular response to ischemia, we hypothesized that dietary levels of nitrate and nitrite modulate cellular resistance to ischemia-reperfusion injury and more globally modulate the signaling transcriptome during both normoxia and following ischemic stress. Consistent with the first thesis, Bryan and colleagues recently reported that the depletion of dietary nitrite results in greater susceptibility to ischemia-reperfusion injury in a mouse myocardial infarction model [26]. In an earlier paper they described that nitrite besides conversion into NO, may directly affect cytoprotection by nitrosation and that nitrite can act as a signaling molecule and regulator of gene expression [27]. However, due to the huge difference in composition between the standard chow and the low nitrite chow, effects of other dietary components on gene expression cannot be excluded.
We therefore used in a mouse model of liver ischemia-reperfusion the same low nitrite and nitrate chow and supplemented with two different nitrite concentrations in the drinking water. One to obtain a similar plasma nitrite level as found with our standard chow NIH-31 and the other to increase plasma nitrite levels with approximately 50 %. Stomach, plasma, heart and liver nitrite levels were measured and the effects of nitrite depletion and supplementation on liver gene expression were determined using DNA microarrays during basal conditions and liver ischemia-reperfusion. Since the nitrite amounts in the NIH-31 chow and the low nitrite and nitrate chow are comparable, it effectively was the depletion of its precursor nitrate that reduced plasma and tissue nitrite in the mice. We choose to supplement with nitrite instead of nitrate to avoid the indirect processes of systemic recirculation, salivary secretion and bacterial conversion that are required for bioconversion of nitrate into nitrite.
If dietary nitrate and nitrite are able to modulate cytoprotection and gene expression they could play a role as “active” ingredients in the Mediterranean diet and as necessary dietary supplements. Furthermore, the exploration of this hypothesis in mouse models of disease could have broad implications for control experiments if the animals are intrinsically protected by substances in diet and water that are not monitored or controlled.
Material and Methods
Materials
All materials were purchased from Sigma (St. Louis, MO), unless otherwise specified.
Animals
This study was reviewed and approved by the Animal Care and Use Committee of the National Heart, Lung and Blood Institute, Bethesda, MD. All mice used in this study were 8–10 weeks old C57BL6/J (The Jackson Laboratory, Bar Harbor, ME). All mice were allowed to acclimatize for at least one week and were fed ad libitum on the standard NIH-31 chow (Zeigler Bros Inc., Gardners, PA) and tap water.
Study setup
This diet study was comprised of 4 groups and was carried out with 4 mice in a cage for 1 week. The first group was kept on the standard NIH-31diet and tap water. The 2nd group was put on a purified low NOx chow TD99366 (Harlan Teklad, Madison, WI) and sterilized de-ionized water and labeled as 0 mg/L NaNO2 in the rest of the article. The 3rd and 4th group were placed on the low NOx chow and the de-ionized water was supplemented with either 300 or 1500 mg/L sodium nitrite (NaNO2), labeled as 300 mg/L NaNO2 and 1500 mg/L NaNO2 in the rest of the article, respectively. These repletion doses of nitrite were determined based on their ability to normalize or increase plasma nitrite levels by 50% in the mice on the low NOx diet, respectively. Baseline plasma and tissue nitrite and nitrate levels were determined in the 4 diet groups on 3 different experimental days using 4 mice per group. Plasma and tissue nitrite and nitrate levels from the liver ischemia-reperfusion experiments were determined in the 4 dietary groups on 6 different days using 2 mice per group.
Liver ischemia and reperfusion
Animals on the previously described four dietary regimens were subjected to 45 min. of liver ischemia and 5 hours of reperfusion as described previously [10]. After reperfusion, liver damage was determined by plasma levels of alanine transaminase (ALT) and aspartate transaminase (AST). Liver mitochondrial complex I activity was determined as described previously [9]. Further details are described in the on-line data supplement.
Measurement of plasma and whole blood nitrite and nitrate levels
Nitrite levels in chow, plasma and organs were measured using tri-iodide–based reductive chemiluminescence and nitrate levels were measured using vanadium(III) chloride as described and validated previously [28, 29]. Further details are described in the on-line data supplement.
DNA micro-array analysis
RNA isolation, microarray data processing and analysis
To determine normoxic gene expression, total liver RNA was isolated in two separate experiments from 4 or 5 mice per group that were in the same cage for one week on the 4 diets described above. For the ischemic gene expression total RNA was isolated from the livers from mice that had undergone 45 min ischemia and reperfusion for 4 hours. The experiments were carried out on two different days with 4 mice in two groups that were either on the low NOx chow or that same chow supplemented with 1500 mg/L sodium nitrite. Total RNA extraction from mouse liver, microarray data processing were performed as described before [30]. Further details are described in the on-line data supplement.
Effect of nitrite on basal gene expression and after ischemia-reperfusion injury
Nitrite levels in plasma and tissue were measured for basal gene expression studies and the expression profiles from animals with low levels of plasma nitrite were compared to profiles from animals on the nitrite water repletion and the NIH-31 group. For this analysis, the 0 mg/L NaNO2 diet and 300 mg/L NaNO2 diet groups were combined into a single 0 +300 mg/L NaNO2 group, since plasma nitrite levels were low in both of these two groups in these experimental batches (see Figure 1 on-line data supplement). Because animal-to-animal variability was high within treatment groups, only the mean values for each group were utilized, yielding two independent measurements of gene expression level per group. To determine which genes were transcriptionally modulated by nitrite, independent of diet effects, only the genes that increased with both nitrite repletion in water and in NIH-31 were considered. To identify these genes, a consistency test [31] was applied to the comparisons between the 1500 mg/L NaNO2 diet versus the 0 + 300 mg/L NaNO2 diet and the standard chow NIH-31 versus the 0 +300 mg/L NOx diet to determine genes with significant differential expression for that comparison in two independent runs. Significant genes were defined as having a p-value corresponding to a false discovery rate less than 10% and a 1.6 fold change. For the calculation of the fold-change values the individual fold-change from the two batches were averaged. A gene set was then obtained from both consistency tests that was modulated with both nitrite repletion in water and with nitrate repletion in NIH-31 (overlapping ven diagram for both analyses); this gene set was specific to nitrite repletion and independent of background diet. Pathway analysis was performed using Ingenuity Pathway Analysis (Ingenuity Systems Inc., Redwood City, CA).
The RNA samples from the liver that underwent ischemia-reperfusion were chipped all in one batch. Nitrite levels in plasma (162 ± 13 vs 677 ± 106 nM) and liver (35 ± 4 vs 70 ± 6 pmoles/mg protein) were measured for the 0 mg/l NaNO2 and 1500 mg/L NaNO2 diet, respectively. Because the effect of nitrite on transcription was much more robust after ischemia and reperfusion, a more simple analysis of nitrite induced changes in gene expression was performed for these experiments. Affymetrix GCOS version 1.4 was used to calculate the signal intensity and the absent and present calls on the probe set level as described before [30] and in more detail in the on-line data supplement.
Statistics
Values are presented as mean ± SEM, unless indicated otherwise. Differences between groups were analyzed using a t-test, with p < 0.05 considered to be significant. The software used for statistical analysis was Graphpad Prism 3.0 (GraphPad Software Inc., San Diego, CA) and JMP 7.0 (SAS Institute Inc., Cary, NC) for calculation of the Spearman correlation.
Results
Determination of nitrate and nitrite levels in different rodent chows and tap water
Several rodent chows were tested for their levels of nitrate and nitrite. We compared the standard wheat based chow NIH-31 and TD 2018, both used at the NIH with three purified amino acid based chows TD99366, TD94045 and TD 94048 (Harlan Teklad, Madison, WI). The nitrate (NO3-) level in the NIH-31 chow was 8-fold higher compared to the other standard chow TD2018 and 55-fold higher than the purified diet TD99366 which had the lowest level of nitrate. The nitrite (NO2-) level in NIH-31 was about a third of the levels found in TD 2018 while the NO2- levels in the purified diets were comparable to NIH-31 (Table 1). The nitrate and nitrite levels were also evaluated in the drinking water provided to the mice. We found that the tap water contained 183 ± 47 μM (12 ± 2.9 mg/L) of nitrate and 220 ± 20 nM (10.3 ± 1.0 ug/l) of nitrite, while levels in the de-ionized water were negligible (Table 1).
Table 1. Nitrite and nitrate content of chow and water.
| Nitrate content | Nitrite content | |||
|---|---|---|---|---|
| Diet | (nmol/g) | (mg/kg) | (nmol/g) | (mg/kg) |
| NIH-31 | 1480.4 ± 87.3 | 91.8 ± 5.4 | 2.8 ± 0.1 | 0.13 ± 0.01 |
| TD 2018 | 178.0 ± 9.0 | 11.0 ± 0.6 | 8.0 ± 2.8 | 0.37 ± 0.13 |
| TD 99366 | 26.6 ± 2.5 | 1.7 ± 0.2 | 2.6 ± 0.2 | 0.12 ± 0.01 |
| TD 94045 | 66.7 ± 10.7 | 4.1 ± 0.7 | 4.2 ± 0.5 | 0.19 ± 0.03 |
| TD 94048 | 115.8 ± 22.5 | 7.2 ± 1.4 | 3.0 ± 0.4 | 0.14 ± 0.02 |
| (μM) | (mg/L) | (μM) | (μg/l) | |
| Tap water | 183.0 ± 47.4 | 11.3 ± 2.9 | 0.22 ± 0.02 | 10.3 ± 1.0 |
Values are expressed as mean ± SEM and n = 3 for each group
Stomach content nitrate and nitrite levels
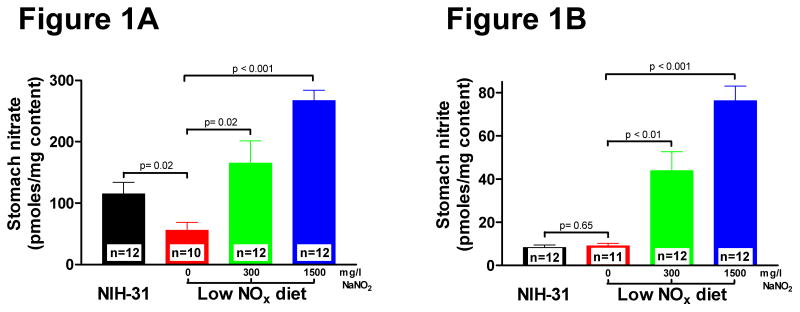
Mice on the standard NIH-31 diet had a stomach content nitrate level of 115 ± 20 pmoles/mg (n = 11) (Figure 1A). Stomach content nitrate levels dropped more than half (56 ± 14 pmoles/mg, n = 10) in mice on the low NOx diet (Figure 1A). Supplementation with 300 mg/L sodium nitrite increased stomach content nitrate levels by almost 1.5-fold (165 ± 38 pmoles/mg, n = 12) compared to NIH-31. A 2.3-fold (267 ± 18 pmoles/mg, n = 12) increase in stomach nitrate levels occurred after supplementation with 1500 mg/L sodium nitrite (Figure 1A). The nitrite level in the stomach contents of mice on the standard NIH-31 diet was 8 ± 1 pmoles/mg total stomach contents (n = 12) (Figure 1B). Similar levels (9 ± 1 pmoles/mg contents, n= 11) were found for the mice in the 0 mg/L NaNO2 diet group (Figure 1B). Supplementation with 300 or 1500 mg/L NaNO2 resulted in a 5-fold (44 ± 9 pmoles/mg content, n = 12) and 9-fold (76 ± 7 pmoles/mg contents, n= 12) increase in stomach content nitrite, respectively (Figure 1B).
Figure 1. Dietary effect on stomach content nitrate and nitrite levels.
Mice were fed for 7 days with indicated diets. A. Stomach content nitrate levels B. Stomach content nitrite levels. Values are expressed as mean ± SEM. Differences were determined by t-test.
Plasma nitrate and nitrite levels in mice on low NOx chow supplemented with nitrite
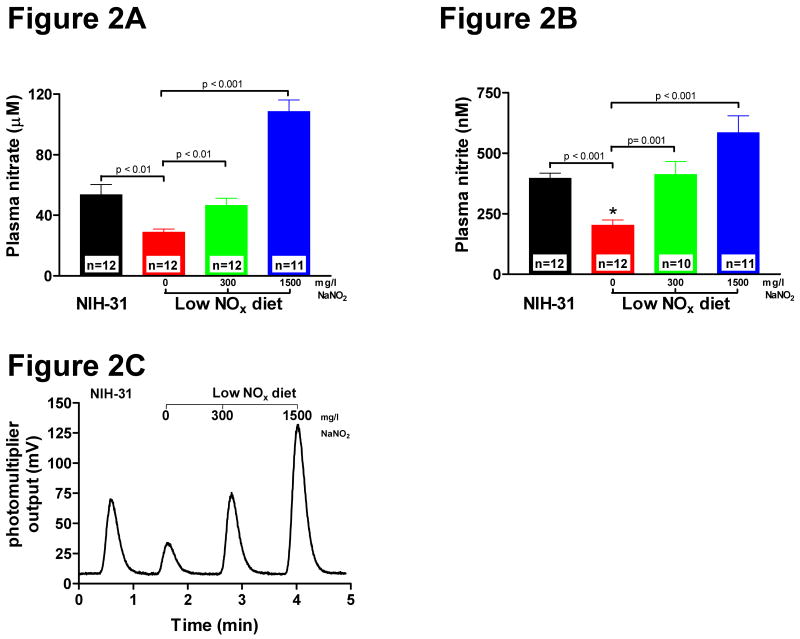
As displayed in Figure. 2A, plasma levels of nitrate in mice fed on the standard NIH-31 chow and tap water were 54 ± 7 μM (n= 12). We observed about a 50% decrease in plasma nitrate levels (29 ± 2 μM, n=12) in mice fed the low NOx chow TD99366 and de-ionized water and this group is referred to as 0 mg/L NaNO2(Figure 2A). Supplementation with 300 mg/L sodium nitrite in the drinking water restored plasma nitrate levels (47 ± 5 μM, n=12) towards those found in mice on the NIH-31 chow (Figure 2A) and this group will be referred to as 300 mg/L NaNO2 group. A 5-fold higher supplementation of 1500 mg/L sodium nitrite resulted only in a two-fold increase in plasma nitrate levels (109 ± 8 μM, n= 11) compared to the NIH-31 chow (Figure 2A) and this group is referred to as 1500 mg/L NaNO2 group.
Figure 2. Dietary effect on plasma nitrate and nitrite levels.
Mice were fed for 7 days with indicated diets. A. Plasma nitrate levels. B. Plasma nitrite levels C. Typical raw experimental chemilluminescence data of plasma nitrite levels as obtained with a NO analyzer. Values are expressed as mean ± SEM. Differences were determined by t-test.
Plasma nitrite in NIH-31 group was 397 ± 22 nM (n= 12), and similar to plasma nitrate levels there was an almost 50% reduction in plasma nitrite levels in mice fed with the low NOx chow (203 ± 23 nM, n= 12) (Figure 2B). Supplementation with 300 mg/L sodium nitrite restored the plasma levels of nitrite (412 ± 57 nM, n= 10), while supplementation with 1500 mg/L sodium nitrite increased plasma nitrate levels 1.5-fold (586 ± 73 nM, n= 11) (Figure 2B).
Liver and heart nitrite levels
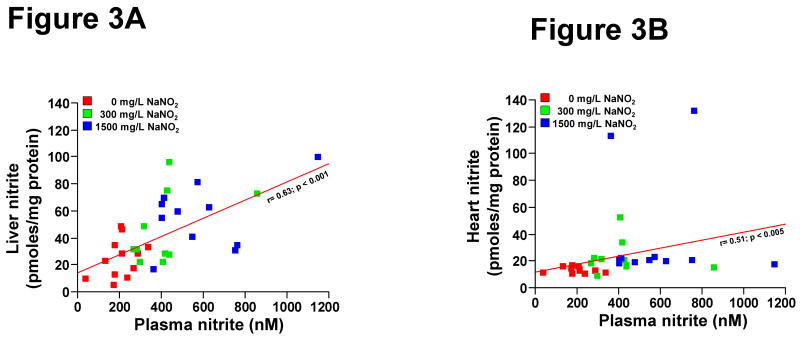
Dietary modulation of nitrate and nitrite significantly altered liver and heart nitrite levels. While there was sufficient variability between animals, likely reflecting different intake or processing rates between mice, a non-parametrical regression of the nitrite levels in plasma compared to liver and heart revealed a significant correlation on the different diets (Figure 3A and 3B, Spearman's rank correlation, r=0.63 and 0.51 for plasma vs. liver or heart nitrite, p<0.001 and p<0.005, respectively).
Figure 3. Dietary effect on liver and heart nitrite levels.
Mice were fed for 7 days with indicated diets. A. Correlation of liver nitrite levels with plasma nitrite levels. A Spearman's rank correlation was performed resulting in a r of 0.63 for plasma vs liver, p<.0001). B. Correlation of heart nitrite levels with plasma nitrite levels. A Spearman's rank correlation was performed resulting in a r of 0.51 for plasma vs. heart nitrite, p>0.0025).
Dietary nitrite levels modulate the cellular resilience to ischemia-reperfusion injury
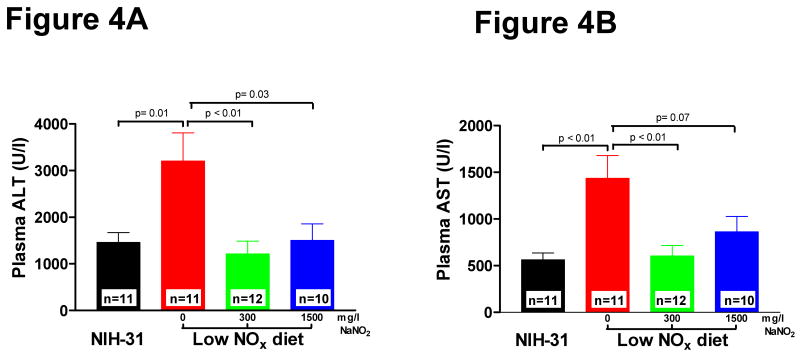
To explore whether basal levels of dietary nitrate or nitrite contribute to the cellular resilience to ischemic stress, mice were subjected to ischemia-reperfusion injury to the liver. The extent of hepatocellular damage was assessed by measuring the plasma levels of enzymes alanine transaminase (ALT) and aspartate transaminase (AST) 5 hours after liver reperfusion as previously described 9. ALT and AST levels in mice in the NIH-31 diet group were 1367 ± 262 U/l and 502 ± 78 U/l, respectively (Figure 4A and 4B). ALT and AST levels in the 0 mg/L NaNO2 group were about 2.5-fold higher (3308 ± 780 U/l and 1323 ± 303 U/l, respectively) than in mice on NIH-31 (Figure 4A and 4B), indicating more severe cellular injury. Supplementation of the low NOx chow with 300 or 1500 mg/L NaNO2 resulted in a comparable reduction in ALT and AST levels for both groups towards the ALT and AST levels observed in the standard NIH-31 (Figure 4A and 4B).
Figure 4. Dietary effects on liver ischemia-reperfusion injury.
Mice were fed for 7 days with indicated diets and subjected to liver IR injury. Plasma transaminases were measured 5 hours after reperfusion. A. Plasma alanine transaminase (ALT) levels B. Plasma aspartate transaminase (AST) levels. Values are expressed as mean ± SEM. Differences were determined by t-test.
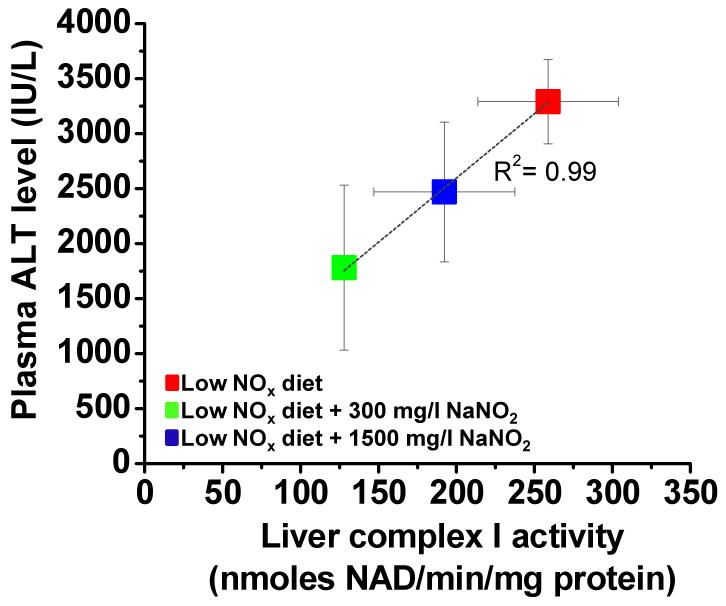
Shiva et al. [9] have shown that cytoprotection by nitrite is due to nitrosation and subsequent inhibition of mitochondrial complex I which decreases ROS formation and reduces tissue damage. We therefore determined complex I activity after ischemia-reperfusion in two experiments and correlated them with plasma ALT levels. Complex I activity was lowest in the low NOx chow with 300 mg/L NaNO2 and highest in the low NOx diet and there was a clear inverse correlation with plasma ALT values (Figure 5). This data confirms the earlier data from Shiva et al. [9] that complex I activity was the lowest in the most protected tissue.
Figure 5. Correlation of mitochondrial complex I activity with plasma liver enzymes after liver ischemia-reperfusion.
Mice were fed for 7 days with indicated diets and subjected to liver IR injury. Plasma transaminases and liver mitochondrial complex I activity were measured 5 hours after reperfusion. Values are expressed as mean ± SEM. N=2-4 per group.
Dietary nitrite levels modulate the liver transcriptional program during ischemia followed by reperfusion
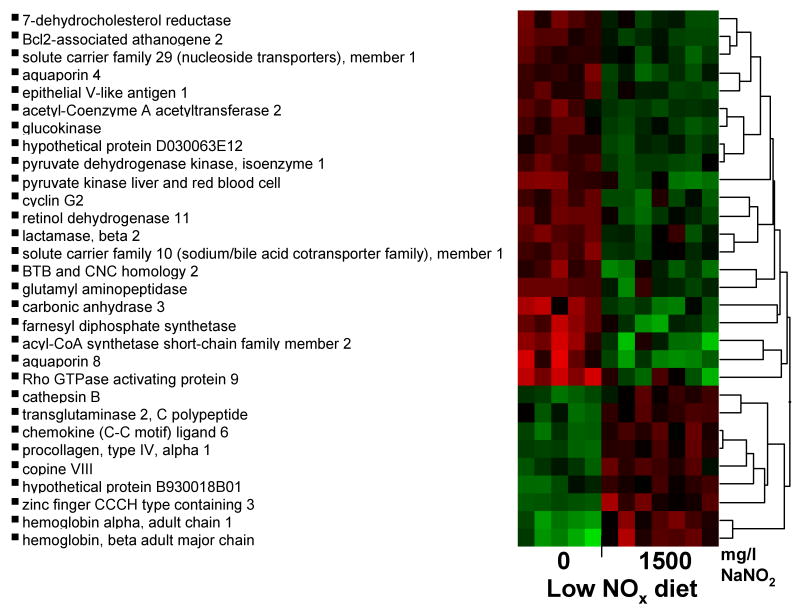
The results above suggest that standard levels of nitrate in food or nitrite supplementation can potently modulate cellular response to ischemic stress. Prior studies have revealed an effect of nitrite on gene expression for HO-1, Hsp70 and P450 under normoxia [27]. Because nitrite conversion to NO is potentiated during hypoxia, we hypothesized that the effects of nitrite on gene expression might be even larger after ischemia reperfusion in the presence of nitrite. We tested the early transcriptional response in mice fed the low NOx chow and mice on the same diet but repleted with 1500 mg/L NaNO2 after 45-minutes of ischemia followed by four hours of reperfusion. Differential gene expression in livers from mice on the low and high nitrite diet intake was found in 144 probe-sets, of which 83 probe-sets were up-regulated and 61 were down-regulated, indicating a marked effect of hypoxia on nitrite signaling. Hierarchical cluster analysis for the gene expression during ischemia is displayed in Figure 6 with an additional filter cut-off in fold change of 1.5-fold. We used pathway analysis to determine if these genes fell into common biological pathways. Genes involved in lipid and carbohydrate metabolism, molecular transport, and genes related to protein synthesis, turnover, and repair were the gene pathways to be predominantly up-regulated (see table 1A, on-line data supplement).
Figure 6. Hierarchical cluster analysis of the effects of dietary nitrite on mouse liver gene expression during ischemia and reperfusion.
Mice were fed for 7 days with indicated diets. 0 mg/L NaNO2 or 1500 mg/L NaNO2 diet (ischemia) or NIH-31, and 0, 300, 1500 mg/L NaNO2 diet (normoxia). RNA from normoxic and ischemic mouse liver was incubated with 45,101 probe sets on Mouse 430 2.0 gene chips and was analyzed. Differential gene expression in ischemic mouse liver 4 hours after reperfusion. Student t-test was applied to normalized and transformed gene expression data and significant genes were defined as having a p-value corresponding to a false discovery rate less than 20% and a change of at least 1.5 fold corresponding to 19 up-regulated and 9 down-regulated genes. Hierarchical clustering using Ward method was then applied on selected genes for expression pattern. Black in the figure indicates the mean of transformed intensities, and red and green are above and below mean, respectively.
Dietary nitrite levels have limited effect on the basal “normoxic” hepatocyte transcriptional program
During normoxia, no difference in liver gene expression was detected using the standard analysis of differential gene expression, as used for the ischemia-reperfusion experiments. This result is consistent with our hypothesis that more genes would be induced during hypoxia than during normoxia. We therefore applied a more statistically powerful analytic approach (consistency test, see material and methods) to identify genes displaying differential expression based on nitrite levels.
Illustrating the major effect of the higher carbohydrate and lipid levels in the 0 mg/L NaNO2 group, which may have nothing to do with nitrite levels, 787 probe-sets were found to be differentially expressed in the comparison between the NIH-31 diet group and the 0 + 300 mg/L NaNO2 diet group. In contrast, only 162 probe-sets were found to be differentially expressed for the comparison of the 1500 mg/L NaNO2 diet group and the 0 + 300 mg/L NaNO2 group. This large difference in gene expression suggests that the effect of switching from the standard NIH-31 chow to the low NOx diet had a much more pronounced effect on gene expression then the levels of liver nitrite per se. Further analysis of normoxic gene expression induced by nitrite under normoxia can be found in the result section of the on-line data supplement.
Discussion
We show in this study that mice fed a low nitrate and nitrite (NOx) chow (TD 99366) and de-ionized water drop their plasma and liver nitrite levels by 50% compared to the mice fed a standard NIH-31 chow, with similar reductions occurring in the heart. This reduction in plasma and liver nitrite is associated with a marked increase in liver injury after ischemic stress. In addition, we find that a diet rich in either natural nitrate (NIH-31) and low NOx diet (TD99366) supplemented with nitrite reduces liver enzyme release to the same extend. This finding provides clear evidence that the amount of nitrate and nitrite is the critical cytoprotective element in the diet that besides cardiac protection from ischemia reperfusion injury [26], also protect the liver after a period of ischemia. Our study also indicates that chronic administration of dietary nitrate or nitrite protects the liver after ischemia and reperfusion. The fact that nitrite after 7 days of continuous supplementation remained protective is an important finding suggesting that nitrite, in contrast to organic nitrates (i.e. nitroglycerin), doesn't induce direct tolerance.
Our results are consistent with a growing number of studies that indicate that nitrite is a potent cytoprotective signaling molecule in animal models of myocardial infarction, stroke, liver infarction and renal ischemia-reperfusion injury [5-7, 10, 32]. Doses of nitrite delivered intraperitoneally or intravenously in mice significantly reduce both cardiac and liver infarction volumes by approximately 50% at doses as low as 2.4 nmoles [10]. This amount of nitrite increases plasma levels acutely by 50%, leading to the hypothesis that even the low levels in the diet may modulate this response. The mechanism of cytoprotection by nitrite is likely to be mediated by reversible post-translational S-nitrosation of complex I of the mitochondrial electron transfer chain [9]. Complex I inhibition by nitrite and a variety of other agents such as S-nitrosoglutathione, amobarbitol and rotenone limit electron flow in the electron transport chain during pathological reperfusion, limiting superoxide formation at complex I and limiting mitochondrial and cellar oxidative damage [33-35]. Unlike other complex I inhibitors, like rotenone or S-nitrosothiols [36], nitrite only inhibits complex I after ischemia reperfusion injury and not during normal physiology. This hypoxic bioactivation of nitrite by nitrosation likely explains both the protective effect even after 7 days administration with minimal basal transcriptional effects during normoxia and marked transcriptional activation after ischemia.
A diet rich in green leafy vegetables has been reported to correlate with a reduced risk for coronary heart disease and reduced blood pressures [21, 22]. Also the Mediterranean diet, which is rich in green vegetables as well (Table 2), has been correlated with reducing cardiovascular risk [37]. Both antioxidants as well as unsaturated fatty acids have been considered as responsible for its cardioprotective effect. We and others have speculated that based on the potent effect of plasma nitrite on limiting cardiac infarction, nitrate and nitrite may represent one of the active molecules in the Mediterranean diet [1, 20, 24]. Recently Webb et al. [25] showed that ingestion of beetroot juice, which is high in nitrate, restored flow mediated dilatation induced by an acute ischemic insult in the forearm. In addition, blood pressure was decreased by 10 mm Hg which correlated with peak increases in plasma nitrite concentration and platelet aggregation was attenuated. Importantly, plasma nitrite levels did not rise and no drop in blood pressure occurred when the test subjects were not allowed to swallow their saliva. This finding supports the idea that nitrate conversion to nitrite could play a critical role in cardiovascular changes observed with the Mediterranean Diet due to its blood pressure lowering and cardioprotective effect.
We found a robust effect of nitrite on differential gene expression after ischemia-reperfusion while with the same analysis no transcriptional changes were detected during normoxia. This finding confirms our hypothesis that nitrite preferentially would activate gene expression during hypoxia. It also indicates that nitrite by itself has little effect on gene expression in our model, but it is only during hypoxia, when nitrite is converted to NO or can nitrosate proteins directly, that gene expression is increased.
To evaluate effects of dietary nitrite on normoxic gene expression we used a more powerful gene expression analysis where we first determined the differential gene expression of the standard NIH-31 chow versus the low NOx diet and that of the low nitrite NOx versus that diet supplemented with 1500 mg/L NaNO2 separately using consistency testing (see material and methods). We found almost 5 times more probe-sets in the first group suggesting that just switching from NIH-31 to the low NOx diet had more pronounced effects on gene expression than the effect of nitrite itself on the low NOx diet background. The genes for HSP-70 and HO-1 of which Bryan et al. [27] have shown a dose dependent modulating effect of nitrite on protein expression during normoxia were not in our list of genes that were modulated by nitrite under normoxia. A difference in animal species (rat vs mouse), period of nitrite administration (1-2 days vs 7 days) or organ that was measured (heart and aorta vs liver) could easily explain this discrepancy.
In contrast to the minimal, but measurable, effect on gene expression during normoxia, there is a robust effect of nitrite on the cellular response to ischemic stress. There was an upregulation of genes involved in protein synthesis (Rpl7, Rpl35, Rps15a, Mrpl30), but also protein degradation (Psmd6) corresponding to a necessity of these functions for damaged tissue. Procollagen (type4, alpha1) was 1.6-fold upregulated, also compatible with the need for renewed extracellular matrix synthesis after tissue injury. Transglutaminase was 1.5-fold upregulated and is a multi-functional enzyme with GTP binding, disulphide isomerease and kinase properties that was shown to protect cardiomyocytes against ischemia-reperfusion injury by regulating ATP synthesis [38]. Upregulation of transglutaminase in neurons during hypoxia and glucose deprivation recently was shown to protect against cell death by attenuation of the HIF1 hypoxic response pathway through its interaction with HIF1β [39].
Future studies will have to further explore whether the cytoprotecive effect of nitrite is mainly by direct nitrosation of proteins involved in cytoprotection (e.g. mitrochiondrial complex I) or that nitrosation of proteins that modulate cytoprotective gene expression is also involved. Most likely there is a place for both mechanisms depending whether the acute or chronic effects of nitrite administration are studied.
The acute dose of sodium nitrite Duranski et al. [10] needed for optimal protection both in liver and cardiac ischemia-reperfusion injury was 48 nmol sodium nitrite (=3.3 ug). Chronic supplementation of sodium nitrite (50 mg-300 mg/L, 0.2-1.2 mg per mouse based on drinking of 4 ml of water per day) as performed by our study and the study of Bryan et al. [26] asks for considerable higher amounts to observe tissue protection by nitrite. A possible reason for this discrepancy could be that direct injection of nitrite in the abdomen as performed by Duranski et al. [10] avoids acidic nitrite conversion in the stomach as well as reaction with stomach content that will occur when nitrite is administered in the drinking water. However, Shiva et al. [9] showed that nitrite administration by oral gavage (20 ug) in mice was enough to reduce cardiac I.R injury even 24 hrs after administration by nitrosation of mitochondrial complex I. This result suggests that administering nitrite as a bolus instead of a chronic dose is more important than loss of nitrite in the stomach. It is conceivable that plasma nitrite has to reach a certain threshold at which antioxidant systems are saturated and above which mitochondrial protein nitrosation can occur.
We haven't observed any gross pathology with these dose administrations for one week. Although we haven't measured methemoglobin levels and blood pressure in our mice supplemented with nitrite, a previous study by Hunter et al. [40] using nebulized nitrite (300mg in 20 min) in newborn sheep (BW 6-7 kg) showed that methemoglobin levels marginally increased from 2.1 ± 0.1 % to 2.8 ± 0.2 % while no measureable effect on blood pressure was reported.
In conclusion, the importance of our study is that we extended an earlier finding of Bryan et al. [26] that dietary nitrite protects against cardiac ischemia- reperfusion injury to liver ischemia reperfusion injury. We show for the first time directly that nitrite can modulate gene expression after hypoxia. These findings support the hypothesis that dietary nitrate and nitrite are active components of the cardio-protective Mediterranean diet. Besides, our study shows the importance for monitoring and controlling dietary nitrate and nitrite levels in laboratories studying NO bioavailability and ischemia-reperfusion injury.
Supplementary Material
Plasma nitrite levels for the two batches of mice on the four diets used for gene chipping at normoxia.
Differential gene expression in normoxic mouse liver. Cluster analysis was applied to the gene expression data and significant genes were defined as having a p-value corresponding to a false discovery rate less than 10% and a change of at least 1.6 fold. A total of 48 probe-sets and 36 unique genes were found to have changed in expression in the comparison of the combination of the 0 + 300 mg/L NaNO2 diet and the 1500 mg/L NaNO2 diet, corresponding to 12 up-regulated and 24 down-regulated genes.
Plasma and liver nitrite levels for batch of mice on the low NOX diet and Low NOX diet supplemented with 1500 mg NaNO2/l used for gene chipping of ischemic and reperfused liver tissue.
Gene categories differential gene expression by nitrite after ischemia-reperfusion
Gene categories differential gene expression by nitrite during normoxia. The mean fold change ± standard deviation is expressed for the two individual animal batches
Acknowledgments
Funding: This research was supported by the National Heart Lung and Blood Institute, the Critical Care Medicine Department at the Clinical Center and the Intramural Research Program at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Dr. Gladwin is listed as a co-inventor on a US government patent for the use of nitrite for cardiovascular diseases. Dr. Gladwin is the PI on a US government Collaborative Research and Development Agreement (CRADA) with INO-Ikaria.
References
- 1.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 2.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. Journal of Molecular and Cellular Cardiology. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 4.Bell RM, Maddock HL, Yellon DM. The cardioprotective and mitochondrial depolarising properties of exogenous nitric oxide in mouse heart. Cardiovasc Res. 2003;57:405–415. doi: 10.1016/s0008-6363(02)00675-2. [DOI] [PubMed] [Google Scholar]
- 5.Tripatara P, Patel NS, Webb A, Rathod K, Lecomte FM, Mazzon E, Cuzzocrea S, Yaqoob MM, Ahluwalia A, Thiemermann C. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. J Am Soc Nephrol. 2007;18:570–580. doi: 10.1681/ASN.2006050450. [DOI] [PubMed] [Google Scholar]
- 6.Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, Park DK, Kim JM, Song EC, Kim M, Roh JK. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37:2744–2750. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- 7.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu P, Liu F, Wang CY, Chen DD, Yao Z, Tian Y, Zhang JH, Wu YH. Gender differences in hepatic ischemic reperfusion injury in rats are associated with endothelial cell nitric oxide synthase-derived nitric oxide. World J Gastroenterol. 2005;11:3441–3445. doi: 10.3748/wjg.v11.i22.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, Macarthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol. 2006;2:486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 13.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 14.Walker R. Nitrates, nitrites and N-nitrosocompounds: a review of the occurrence in food and diet and the toxicological implications. Food Addit Contam. 1990;7:717–768. doi: 10.1080/02652039009373938. [DOI] [PubMed] [Google Scholar]
- 15.Spiegelhalder B, Eisenbrand G, Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet Toxicol. 1976;14:545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 16.Dich J, Jarvinen R, Knekt P, Penttila PL. Dietary intakes of nitrate, nitrite and NDMA in the Finnish Mobile Clinic Health Examination Survey. Food Addit Contam. 1996;13:541–552. doi: 10.1080/02652039609374439. [DOI] [PubMed] [Google Scholar]
- 17.Meah MN, Harrison N, Davies A. Nitrate and nitrite in foods and the diet. Food Addit Contam. 1994;11:519–532. doi: 10.1080/02652039409374250. [DOI] [PubMed] [Google Scholar]
- 18.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 19.Dejam A, Hunter CJ, Gladwin MT. Effects of dietary nitrate on blood pressure. N Engl J Med. 2007;356:1590. doi: 10.1056/NEJMc070163. [DOI] [PubMed] [Google Scholar]
- 20.Lundberg JO, Feelisch M, Bjorne H, Jansson EA, Weitzberg E. Cardioprotective effects of vegetables: is nitrate the answer? Nitric Oxide. 2006;15:359–362. doi: 10.1016/j.niox.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 22.Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Colditz G, Ascherio A, Rosner B, Spiegelman D, Willett WC. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 23.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 24.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO, III, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster JR, Jr, Feelisch M, Lundberg JO. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1:308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 25.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. 2005;1:290–297. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- 28.Yang BK, Vivas EX, Reiter CD, Gladwin MT. Methodologies for the sensitive and specific measurement of S-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radic Res. 2003;37:1–10. doi: 10.1080/1071576021000033112. [DOI] [PubMed] [Google Scholar]
- 29.Macarthur PH, Shiva S, Gladwin MT. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:93–105. doi: 10.1016/j.jchromb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Jison ML, Munson PJ, Barb JJ, Suffredini AF, Talwar S, Logun C, Raghavachari N, Beigel JH, Shelhamer JH, Danner RL, Gladwin MT. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104:270–280. doi: 10.1182/blood-2003-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munson PJA. GeneLogic Workshop of Low Level Analysis of Affymetrix GeneChip Data. Bethesda, MD: A consistency test for determining the significance of gene expression changes on replicate samples and two convenient variance-stabilizing transformations. Link: http://stat-www.berkeley.edu/users/terry/zarray/Affy/GL_Workshop/genelogic2001.html. [Google Scholar]
- 32.Lu P, Liu F, Yao Z, Wang CY, Chen DD, Tian Y, Zhang JH, Wu YH. Nitrite-derived nitric oxide by xanthine oxidoreductase protects the liver against ischemia-reperfusion injury. Hepatobiliary Pancreat Dis Int. 2005;4:350–355. [PubMed] [Google Scholar]
- 33.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther. 2006;319:1405–1412. doi: 10.1124/jpet.106.110262. [DOI] [PubMed] [Google Scholar]
- 34.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during ischemia protects cardiac mitochondria. J Biol Chem. 2004;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- 36.Dahm CC, Moore K, Murphy MP. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: implications for the interaction of nitric oxide with mitochondria. J Biol Chem. 2006;281:10056–10065. doi: 10.1074/jbc.M512203200. [DOI] [PubMed] [Google Scholar]
- 37.Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, Fiol M, Gomez-Gracia E, Lopez-Sabater MC, Vinyoles E, Aros F, Conde M, Lahoz C, Lapetra J, Saez G, Ros E. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 38.Szondy Z, Mastroberardino PG, Varadi J, Farrace MG, Nagy N, Bak I, Viti I, Wieckowski MR, Melino G, Rizzuto R, Tosaki A, Fesus L, Piacentini M. Tissue transglutaminase (TG2) protects cardiomyocytes against ischemia/reperfusion injury by regulating ATP synthesis. Cell Death Differ. 2006;13:1827–1829. doi: 10.1038/sj.cdd.4401889. [DOI] [PubMed] [Google Scholar]
- 39.Filiano AJ, Bailey CD, Tucholski J, Gundemir S, Johnson GV. Transglutaminase 2 protects against ischemic insult, interacts with HIF1beta, and attenuates HIF1 signaling. FASEB J. 2008;22:2662–2675. doi: 10.1096/fj.07-097709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasma nitrite levels for the two batches of mice on the four diets used for gene chipping at normoxia.
Differential gene expression in normoxic mouse liver. Cluster analysis was applied to the gene expression data and significant genes were defined as having a p-value corresponding to a false discovery rate less than 10% and a change of at least 1.6 fold. A total of 48 probe-sets and 36 unique genes were found to have changed in expression in the comparison of the combination of the 0 + 300 mg/L NaNO2 diet and the 1500 mg/L NaNO2 diet, corresponding to 12 up-regulated and 24 down-regulated genes.
Plasma and liver nitrite levels for batch of mice on the low NOX diet and Low NOX diet supplemented with 1500 mg NaNO2/l used for gene chipping of ischemic and reperfused liver tissue.
Gene categories differential gene expression by nitrite after ischemia-reperfusion
Gene categories differential gene expression by nitrite during normoxia. The mean fold change ± standard deviation is expressed for the two individual animal batches