Abstract
Remote ischemic postconditioning (RIP) refers to an ischemia conducted in a distant organ that protects against a prior ischemia in another organ. We tested whether RIP protects against focal ischemia in the rat brain. Stroke was generated by a permanent occlusion of the left distal middle cerebral artery combined with a 30 min occlusion of the bilateral common carotid arteries (CCA) in male rats. After CCA release, RIP was generated by 3 cycles of 15 min occlusion/15 min release of the left hind femoral artery. The results showed that rapid RIP performed immediately after CCA release reduced infarction by 67% measured at 2 d after stroke. In addition, delayed RIP initiated as late as 3 h, but not 6 h, still robustly reduced infarction by 43% 2 d after stroke. RIP's protective effect was abolished by injecting the protein synthesis inhibitor, cycloheximide, and the afferent nerve blocker, capsaicin, suggesting that RIP blocks ischemic injury by modulating protein synthesis and nerve activity. Nevertheless, rapid RIP did not reduce infarction size 2 months after stroke while it ameliorated the outcome of the behavioral test. In conclusion, RIP attenuates brain injury after focal ischemia.
Keywords: stroke, cerebral ischemia, preconditioning, remote postconditioning
1. Introduction
Ischemic preconditioning refers to a short period of sublethal ischemia conducted in one organ protecting against a subsequent prolonged ischemia in the same organ [8,14,17]. Numerous reports have confirmed that it is the strongest endogenous neuroprotectant against brain injury after stroke [8,14,17]. However, the clinical translation of ischemic preconditioning is only feasible when stroke occurrence is predictable. Ideally, the clinical application of one treatment targeting stroke requires that the treatment be applied after stroke onset, such as ischemic postconditioning [30]. We have recently demonstrated that ischemic postconditioning protects against stroke [6,7,22,31], in which ischemic postconditioning is conducted by a series of brief occlusion and release of the bilateral common carotid arteries (CCA) after reperfusion.
The above concepts of both conventional preconditioning and postconditioning have been extended to that of remote pre- [23] and postconditioning [1,11,13], in which pre- or postconditioning is induced in a distant non-vital organ. Previous studies have demonstrated that both remote pre- [16] and postconditioning [13] performed in the limbs reduces ischemic injury in the heart. For cerebral ischemia, a few groups have demonstrated that remote preconditioning performed in the hind limbs attenuates hippocampal injury after transient global ischemia [9,10,26,33,34]. Most recently, we have shown that remote preconditioning performed in the ipsilateral hind limb reduces brain injury after focal ischemia [23]. However, whether remote postconditioning inhibits brain injury after cerebral ischemia is unknown. In this study, we tested the hypothesis that limb remote postconditioning protects the brain from stroke and the potential protective mechanisms related with protein synthesis and afferent nerve pathways.
2. Results
Remote postconditioning reduced infarct size
To study the acute protective effect of remote postconditioning, infarct sizes were measured at 2d after stroke. The results show that control ischemia caused infarct size of 44.4±1.0% (Fig.2). Treatment with isoflurane alone did not affect infarct size. However, rapid remote postconditioning conducted immediately at CCA release significantly reduced infarct size to 14.5±2.8% (P<0.001) (Fig.2). In addition, delayed remote postconditioning performed 3h after CCA release also significantly reduced infarct size to 25.4±6.3% (P<0.01). However, delayed remote postconditioning initiated at 6h did not significantly reduce infarct size (Fig.2).
Fig. 2.
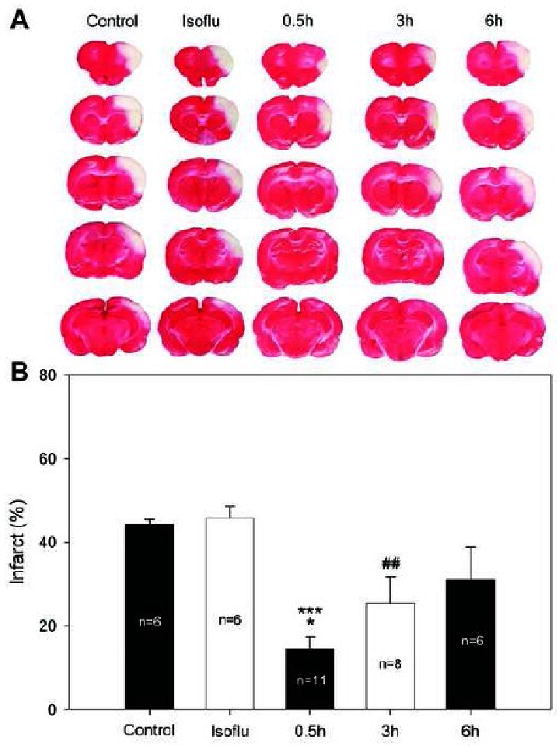
Remote limb postconditioning reduced infarct size measured at 2d after stroke. A: Representative infarcts stained by TTC from each group. Rat brains were cut into 5 slices and stained by TTC. Infarct cortex of each slice was measured and normalized to non-ischemic cortex, and expressed as percentage. B: Bar graphs show the average infarct size of the 5 slices. Control, control ischemia; Isoflu, isoflurane of 90 min after reperfusion. * vs 6h, P=0.014; *** vs control, isoflu, P<0.001; ## vs control, isoflu, P<0.01.
To further test the reproducibility of remote postconditioning's protection, the protective effect of rapid remote postconditioning was repeated by a different individual in our laboratory. The result demonstrated that rapid remote postconditioning significantly reduced infarct size from 58.4±2.3% to 44.5±3.6 (P=0.008).
Both protein synthesis inhibitor and nerve blocker abolish the protective effect of rapid remote postconditioning
We then studied the potential mechanisms of remote postconditioning. Treatment of the protein synthesis inhibitor, cycloheximide, increased infarct size from 18.5±4.2% in rats receiving vehicle to 35.3±4.7% (P<0.05), while capsaicin injection increased infarct size to 47.5±2.9% from 18.9±4.5% in rats receiving vehicle (P<0.001) (Fig.3). Note that treatment with either cycloheximide or capsaicin alone did not affect infarct size in rats receiving control ischemia.
Fig.3.
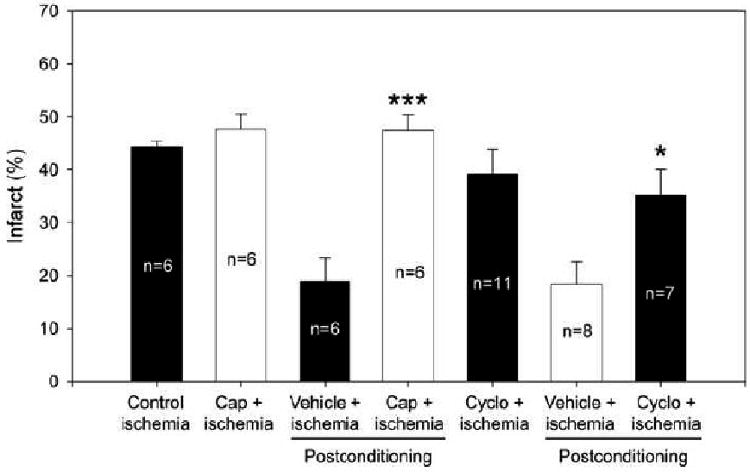
The effects of capsaicin and cycloheximide on rapid remote postconditioning's protection. The infarct size of control group from Fig. 2 was re-portrayed with the rest of the 6 groups receiving drugs or vehicles plus rapid remote postconditioning after focal ischemia. Cap, capsaicin; cyclo, cycloheximide. * vs vehicle for cycloheximide, p=0.019; *** vs vehicle for capsaicin, P<0.001.
Long-term protective effect and behavioral test of rapid remote postconditioning
At last, we studied whether rapid remote postconditioning offers long-term protection. The results showed that it did not affect the infarct size measured 2 months after stroke; however, the neurological functions, which were stimated by the behavioral test of vibrissae method, were markedly improved by rapid remote postconditioning (Fig.4).
Fig. 4.
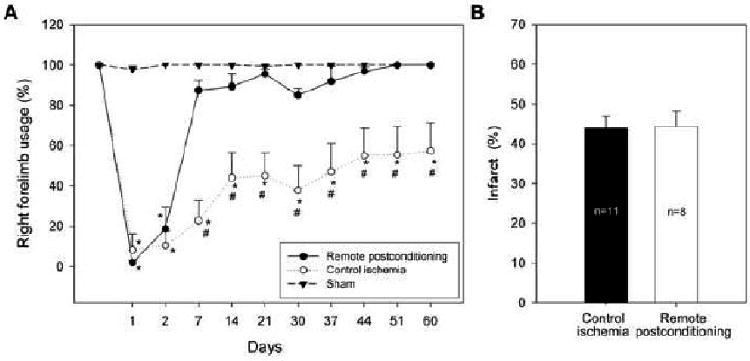
Long-term protective effect of rapid remote postconditioning. A: Rapid remote postconditioning attenuated neurological deficits after stroke. * vs sham P<0.001, # vs control ischemia, P<0.001. B: Rapid remote postconditioning did not block ischemic injury measured at two months after stroke. The bar graph represents the average.
3. Discussion
We found that both rapid and delayed remote postconditioning, which were conducted immediately or 3h after reperfusion in the ipsilateral hind limb, protected against focal cerebral ischemia in rats. It is plausible that remote postconditioning protects the ischemic brain through protein synthesis and afferent nerve activity, since inhibition of protein synthesis and nerve activity abolished its protection. However, the effect of rapid remote postconditioning on infarct size was lost at two months after stroke while it improved the neurological outcomes.
Currently, there are at least two types of ischemic postconditioning, conventional and remote postconditioning. The conventional postconditioning refers to a series of brief, repetitive mechanical occlusion/reperfusion of the occluded blood vessels after ischemia/reperfusion [31,35]. We demonstrated in 2006 that conventional postconditioning, which was performed by 3 cycles of 30 sec occlusion/10 sec release of the bilateral CCA after reperfusion, robustly reduced infarct size in the same focal ischemia model used in this current study [31]. This protective concept of ischemic postconditioning has been confirmed by a number of groups using in vivo global and focal ischemia models [6,12,19,28], and in vitro ischemic models [19].
In our current study, we further tested our hypothesis that remote postconditioning, which is conducted in the ipsilateral hind limb, protects against focal ischemia. This idea was derived from our most recent study showing that remote limb preconditioning reduces infarct size [23]. In our previous study, remote preconditioning performed immediately, 12h and 48h before the onset of cerebral ischemia robustly reduced infarct size; remote preconditioning was conducted in the ipsilateral hind limb by 3 cycles of 15 min occlusion/15min release of the femoral artery [23]. In addition, other previous studies have shown that remote postconditioning reduces heart injury after ischemia [1]. We therefore speculated that limb ischemia could be extrapolated to remote postconditioning. Indeed, as expected, we have demonstrated that remote postconditioning conducted immediately after reperfusion markedly reduced infarct size. To our surprise, the therapeutic time windows of remote postconditioning can be as late as 3h after reperfusion, which provides a wide time window for clinical translation.
To further examine the reproducibility of the protective effect of rapid remote postconditioning, another individual in our laboratory independently repeated the experiment, and the result shows significant protection at 2d after stroke. Although it seems less protective in the latter experiment (24% reduction of infarction) compared with that in the former experiment (67% reduction in Fig.2), this difference may be caused by the experimental variability from different operators. Although in both experiments the CCA occlusion time was the same (30 min), the actual ischemic severity can be different due to the exact occlusion sites of the MCA; the more proximal the occlusion site, the worse the injury. The infarct size of the control ischemia is 58.4% for the confirming experiment, which is larger than the infarction of 45.8% in the former experiment (Fig. 2). Therefore, the protective effect of remote postconditioning may be dependent on ischemic severity.
The underlying protective mechanisms of remote postconditioning are unknown. Nevertheless, research from remote preconditioning against ischemia in other organs may shed light on our understanding of the protective mechanisms of remote postconditioning. In the heart, accumulating evidence suggests that neural pathways serve as a connection between the remote preconditioned organ and the heart. Wolfrum et al. reported that remote preconditioning with brief mesenteric artery occlusion/reperfusion reduced heart infarction by activating εPKC in rats [29]. However, pretreatment with the ganglion blocker, hexamethonium, inhibited εPKC activation thus blocking remote preconditioning's protection [29]. In addition, Schoemaker and colleagues showed that bradykinin released during remote preconditioning stimulates sensory nerves and offers protection, which is blocked by the ganglion blocker, hexamethonium [25]. Moreover, inhibition of afferent nerves with capsaicin also abolishes remote preconditioning's protection against gastric ischemia, in which remote preconditioning was conducted in the heart or liver by two-5 min ischemic occlusions of the coronal or hepatic arteries [4]. Accordingly, we have found that both hexamethonium and capsaicin abolished the protective effect of remote preconditioning against stroke (unpublished results). As expected, we currently demonstrated that capsaicin treatment reversed remote postconditioning's protection, suggesting that the afferent nerve pathways may sever a connection between the remote organ, limb, and the ischemic brain.
Moreover, we demonstrated that the protein synthesis inhibitor, cycloheximide, also robustly attenuated the protective effect of remote postconditioning. Cycloheximide is usually used to test the hypothesis that preconditioning protects against ischemic injury via protein synthesis [2]. In those studies, preconditioning was carried out a few hours to days before ischemia onset [2,15,27]. Thus, preconditioning may stimulate the organ to adapt to a future ischemic event, including protein synthesis. It is thus not surprising that a protein synthesis inhibitor blocks the protective effect of preconditioning. Nevertheless, remote postconditioning is performed immediately after stroke onset; it seems that there is no time for the brain to synthesize the new protein for neuroprotection. Therefore, the result that protein synthesis inhibition abolished remote postconditioning's protection is beyond of our expectation. Our result implies that protein synthesis may play critical roles in brain recovery after remote postconditioning.
Remote postconditioning no longer reduced infarct size at 2 months after stroke, but still improved neurological functions, suggesting its transient protection on infarct size with long-term protection on neurological function. Therefore, infarct size alone does not reflect the protective effect of a neuroprotectant. Such transient protection for infarct size is not new for neuroprotectants. A previous report has demonstrated that post-ischemic hypothermia (30 °C) attenuated hippocampal CA1 neuronal loss measured at 3 days but not at 2 months after transient global ischemia [5]. Similar transient protection was reported for the protective effect of rapid ischemic preconditioning [18]. Nevertheless, future studies need to address why remote postconditioning did not reduce infarction but still improved neurological functions at 2 months after stroke.
In conclusion, limb remote postconditioning reduced infarct size of focal ischemia in rats in a prolonged time window as late as 3h after reperfusion. It appears that remote postconditioning protects against ischemia via the nerve pathway and via modulating protein synthesis.
4. Experimental Procedure
Focal Cerebral Ischemia
Focal cerebral ischemia was generated in male Sprague–Dawley rats (270 to 330 g) as previously described [7,31,32]. Experimental protocols were approved by the Stanford University Administrative Panel on Laboratory Animal Care. Anesthesia was induced by 5% isoflurane and maintained with 1-2% isoflurane in 20% O2 plus 80% air during surgery and early reperfusion. Core body temperatures were monitored with a rectal probe and maintained at 36.5-37.5°C using a heating pad and lamp during the whole experiment. Focal ischemia was induced by occluding the bilateral CCAs for 30 min combined with permanent occlusion of the left distal middle cerebral artery (MCA) above the rhinal fissure. The right femoral artery was cannulated for blood collecting for blood gas analysis. Arterial pO2, pCO2 and pH, were controlled in normal ranges.
Limb remote postconditioning
For the initial experiments testing the protective effect of remote postconditioning, male Sprague–Dawley rats were randomly assigned into 5 groups (Fig. 1). Group 1 rats were subjected to control ischemia (n=6); rats were returned to the home cage 30 min after stroke. For rats in groups 2 (n=6), 3 (n=11), 4 (n=8), and 5 (n=6), the left femoral artery was separated below the left groin ligament for later induction of femoral artery occlusion (group 3, 4 and 5) or for sham limb surgery (group 2). Rats in group 2 were exposed to an extra 90 min of 1-2% isoflurane starting from 30 min after stroke; this group serves as a control for group 3, in which rats were anesthetized with 1-2% isoflurane for 90 min during remote postconditioning. Remote postconditioning was conducted in the left limb by occluding and releasing the femoral artery for 3 cycles; each occlusion or release lasted for 15 min. Remote postconditioning was initiated immediately, 3 h and 6 h after bilateral CCA release in groups 3, 4 and 5, respectively, which was performed in a blind manner. At 30 min after stroke onset, a second person who did not perform surgery randomly assigned the rats into different groups. The rat brains were harvested 2 d after stroke for TTC staining, as described, by a person who was blind to the experimental conditions.
Fig. 1.
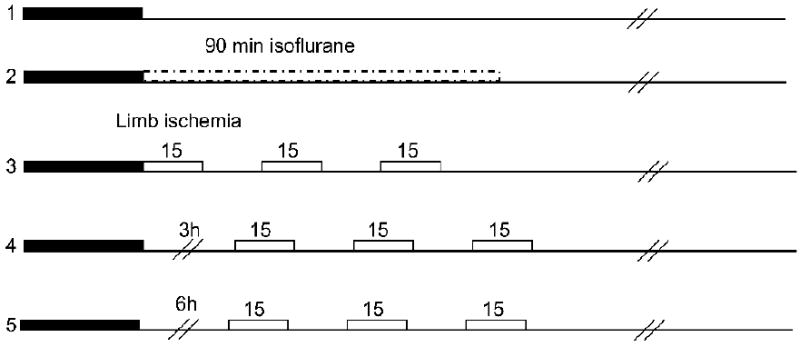
Surgery protocols. Rats were divided into 5 groups. Group 1: control ischemia was induced by 30 min occlusion of the bilateral CCA combined with a permanent occlusion of the left MCA. Group 2: a corresponding isoflurane group was used as a control for group 3, for which rapid limb postconditioning was conducted in the left hind limb by 3 cycles of 15 min occlusion/reperfusion. For group 4 and 5, limb postconditioning was induced 3h and 6h after CCA release, respectively.
To further confirm the reproducibility of the acute protective effect of remote postconditioning, experiments of group 2 (n=8) and 3 (n=10) were repeated by a different individual in our laboratory. The rats were sacrificed 2d after stroke, perfused with 4% PFA, stained with cresyl/violet staining for infarction measurement, as described [32].
Behavioral testing
The vibrissae-elicited forelimb placement test was used to quantify motor asymmetry [7,24,32] caused by a unilateral cortical stroke. In brief, forelimb placing was induced by gently brushing the rats' vibrissas on each side against the edge of a table; rats respond reflexively by placing their forelimb on that side onto the countertop. Reflexive placing is disrupted contralateral to the cortical injury. The reflex was tested 10 times on each side per trial, and two trials occurred per test session. The percentage of vibrissa stimulations in which a paw placement occurred was calculated. Rats were divided into three groups: 1) sham surgery without ischemia; 2) ischemia for 30 min of CCA occlusion plus permanent distal MCA occlusion (control ischemia), 3) ischemia plus remote postconditioning conducted immediately after CCAs release. Rats were gently handled for 3 d before stroke, and behavior baseline was tested on the day before surgery. Two to three persons who were blind to the experimental conditions performed all behavior tests, on days 1, 2, 7, 14, 21, 30, 37, 44, 51, and 60 d post-stroke. At the end of the test, the rats were sacrificed, perfused with 4% PFA, stained with cresyl/violet staining for infarction measurement.
Drug injection: Two drugs, the afferent nerve blocker, capsaicin, and the protein synthesis inhibitor, cycloheximide, were used to study the potential protective mechanisms of remote postconditioning.
The method for capsaicin injection was modified from previous studies [3,20]. Capsaicin was dissolved in 10% ethanol, 10% Tween-80 and 80% saline to a final concentration of 4 mg/ml. Under isoflurane anesthesia, rats were subcutaneously injected with capsaicin or vehicle for 4 consecutive days (n=6): day 1, 12.5 mg/kg; day 2, 12.5 mg/kg, twice at 12 h intervals; day 3, 25 mg/kg at 12 h intervals; day 4, 25 mg/kg. To check the effectiveness of the capsaicin denervation, a drop of 0.1 mg/ml solution of capsaicin was instilled into the eye of each rat and their protective movements were observed. All animals pretreated with capsaicin showed no wiping movements, thus confirming functional denervation of the capsaicin-sensitive nerves. Focal ischemia and rapid remote postconditioning was performed 2 weeks after the last capsaicin injection. The operator performing the surgery was blind to the rats' conditions.
Cycloheximide was dissolved in saline. The dosage was adopted from previous studies [21]. After focal ischemia, rats were intraperitoneally (i.p) injected with 1 mg/kg cycloheximide or vehicle before postconditioning induction (n=7).
All rats receiving drug and vehicle treatments were sacrificed 2 days after stroke for infarction measurement.
Infarct size measurement
The rats were reanesthetized with an overdose of isoflurane at desired time after stroke, perfused intracardially with 100 ml of cold 10 mM sodium phosphate buffered saline (PBS; pH 7.4). The rats were then decapitated and the brains rapidly removed and sectioned coronally at 2 mm intervals, generating a total of 5 sections. As defined above, slices were incubated for 20 min in a 2% solution of 2,3,7-triphenyltetrazolium chloride (TTC) at room temperature and fixed by immersion in 2% paraformaldehyde solution, or cut on a cryostat and stained with cresyl/violet. Using a computerized image analysis system (NIH image, version 1.61), the area of infarction on both sides of each section was measured. Infarct size of the ischemic cortex was normalized to the non-ischemic cortex and expressed as a percentage, and an average value from the 5 slices was presented, as described [7,31,32]. For rats surviving 2 months, brain slices from 5 blocks of each brain were stained with cresyl/violet. The normal cortices in both the ipsilateral and contralateral hemispheres were measured, from which the infarct was calculated according to the formula: [(area of the cortex in the non-ischemic hemisphere – area of the normal cortex in the ischemic hemisphere)/area of the non-ischemic cortex] × 100%.
Statistical Analyses
One-way analysis of variance (ANOVA) was used to compare each group on infarct size followed by Fisher's least square difference post hoc test when there were multiple groups; t-test was used to compare infarct size when only two groups were studied. For behavioral tests, one-way repeated measures anova was used to compare a test at different time points in the same group, and two-way anova was used to compare between remote postconditioning and control ischemia, followed by Student–Newman–Keuls test. Tests were considered statistically significant at P-values < 0.05. Data were presented as means±s.e.m.
Acknowledgments
We wish to thank Elizabeth Hoyte for figure preparation, Felicia Beppu for manuscript editing. This study was supported in part by AHA grant SDG 0730113N (HZ) and NIH grant 1R21NS057750-01A2 (HZ).
Abbreviations
- CCA
common carotid arteries
- MCA
middle cerebral artery
- PBS
sodium phosphate buffered saline
- TTC
2,3,7-triphenyltetrazolium chloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literatures
- 1.Andreka G, Vertesaljai M, Szantho G, Font G, Piroth Z, Fontos G, Juhasz ED, Szekely L, Szelid Z, Turner MS, Ashrafian H, Frenneaux MP, Andreka P. Remote ischaemic postconditioning protects the heart during acute myocardial infarction in pigs. Heart. 2007;93:749–752. doi: 10.1136/hrt.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barone FC, White RF, Spera PA, Ellison J, Currie RW, Wang X, Feuerstein GZ. Ischemic preconditioning and brain tolerance: temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29:1937–1950. doi: 10.1161/01.str.29.9.1937. discussion 1950-1931. [DOI] [PubMed] [Google Scholar]
- 3.Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Kwiecien S, Pawlik M, Drozdowicz D, Sliwowski Z, Pawlik WW. Ischemic preconditioning of remote organs attenuates gastric ischemia-reperfusion injury through involvement of prostaglandins and sensory nerves. Eur J Pharmacol. 2004;499:201–213. doi: 10.1016/j.ejphar.2004.07.072. [DOI] [PubMed] [Google Scholar]
- 4.Brzozowski T, Konturek PC, Pajdo R, Kwiecien S, Sliwowski Z, Drozdowicz D, Ptak-Belowska A, Pawlik M, Konturek SJ, Pawlik WW, Hahn GG. Importance of brain-gut axis in the gastroprotection induced by gastric and remote preconditioning. J Physiol Pharmacol. 2004;55:165–177. [PubMed] [Google Scholar]
- 5.Dietrich WD, Busto R, Alonso O, Globus MY, Ginsberg MD. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1993;13:541–549. doi: 10.1038/jcbfm.1993.71. [DOI] [PubMed] [Google Scholar]
- 6.Gao X, Ren C, Zhao H. Protective effects of ischemic postconditioning compared with gradual reperfusion or preconditioning. J Neurosci Res. 2008 doi: 10.1002/jnr.21703. [DOI] [PubMed] [Google Scholar]
- 7.Gao X, Zhang H, Takahashi T, Hsieh J, Liao J, Steinberg GK, Zhao H. The Akt signaling pathway contributes to postconditioning's protection against stroke; the protection is associated with the MAPK and PKC pathways. J Neurochem. 2008;105:943–955. doi: 10.1111/j.1471-4159.2008.05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 9.Jin RL, Li WB, Li QJ, Zhang M, Xian XH, Sun XC, Zhao HG, Qi J. The role of extracellular signal-regulated kinases in the neuroprotection of limb ischemic preconditioning. Neurosci Res. 2006;55:65–73. doi: 10.1016/j.neures.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Kakimoto M, Kawaguchi M, Sakamoto T, Inoue S, Furuya H, Nakamura M, Konishi N. Evaluation of rapid ischemic preconditioning in a rabbit model of spinal cord ischemia. Anesthesiology. 2003;99:1112–1117. doi: 10.1097/00000542-200311000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Kerendi F, Kin H, Halkos ME, Jiang R, Zatta AJ, Zhao ZQ, Guyton RA, Vinten-Johansen J. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100:404–412. doi: 10.1007/s00395-005-0539-2. [DOI] [PubMed] [Google Scholar]
- 12.Lee JJ, Li L, Jung HH, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–1062. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li CM, Zhang XH, Ma XJ, Luo M. Limb ischemic postconditioning protects myocardium from ischemia-reperfusion injury. Scand Cardiovasc J. 2006;40:312–317. doi: 10.1080/14017430600925292. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Kato H, Nakata N, Kogure K. Protection of rat hippocampus against ischemic neuronal damage by pretreatment with sublethal ischemia. Brain Res. 1992;586:121–124. doi: 10.1016/0006-8993(92)91380-w. [DOI] [PubMed] [Google Scholar]
- 15.Meldrum DR, Cleveland JC, Jr, Rowland RT, Banerjee A, Harken AH, Meng X. Early and delayed preconditioning: differential mechanisms and additive protection. Am J Physiol. 1997;273:H725–733. doi: 10.1152/ajpheart.1997.273.2.H725. [DOI] [PubMed] [Google Scholar]
- 16.Oxman T, Arad M, Klein R, Avazov N, Rabinowitz B. Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. Am J Physiol. 1997;273:H1707–1712. doi: 10.1152/ajpheart.1997.273.4.H1707. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Pinzon MA. Mechanisms of neuroprotection during ischemic preconditioning: lessons from anoxic tolerance. Comp Biochem Physiol A Mol Integr Physiol. 2007;147:291–299. doi: 10.1016/j.cbpa.2006.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Pinzon MA. Neuroprotective effects of ischemic preconditioning in brain mitochondria following cerebral ischemia. J Bioenerg Biomembr. 2004;36:323–327. doi: 10.1023/B:JOBB.0000041762.47544.ff. [DOI] [PubMed] [Google Scholar]
- 19.Pignataro G, Meller R, Inoue K, Ordonez AN, Ashley MD, Xiong Z, Gala R, Simon RP. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab. 2008;28:232–241. doi: 10.1038/sj.jcbfm.9600559. [DOI] [PubMed] [Google Scholar]
- 20.Polidori C, Massi M, Guerrini R, Grandi D, Lupo D, Morini G. Peripheral mechanisms involved in gastric mucosal protection by intracerebroventricular and intraperitoneal nociceptin in rats. Endocrinology. 2005;146:3861–3867. doi: 10.1210/en.2005-0397. [DOI] [PubMed] [Google Scholar]
- 21.Puisieux F, Deplanque D, Bulckaen H, Maboudou P, Gele P, Lhermitte M, Lebuffe G, Bordet R. Brain ischemic preconditioning is abolished by antioxidant drugs but does not up-regulate superoxide dismutase and glutathion peroxidase. Brain Res. 2004;1027:30–37. doi: 10.1016/j.brainres.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 22.Ren C, Gao X, Niu G, Yan Z, Chen X, Zhao H. Delayed postconditioning protects against focal ischemic brain injury in rats. PLoS ONE. 2008;3:e3851. doi: 10.1371/journal.pone.0003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience. 2008;151:1099–1103. doi: 10.1016/j.neuroscience.2007.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 25.Schoemaker RG, van Heijningen CL. Bradykinin mediates cardiac preconditioning at a distance. Am J Physiol Heart Circ Physiol. 2000;278:H1571–1576. doi: 10.1152/ajpheart.2000.278.5.H1571. [DOI] [PubMed] [Google Scholar]
- 26.Steiger HJ, Hanggi D. Ischaemic preconditioning of the brain, mechanisms and applications. Acta Neurochir (Wien) 2007;149:1–10. doi: 10.1007/s00701-006-1057-1. [DOI] [PubMed] [Google Scholar]
- 27.Thornton J, Striplin S, Liu GS, Swafford A, Stanley AW, Van Winkle DM, Downey JM. Inhibition of protein synthesis does not block myocardial protection afforded by preconditioning. Am J Physiol. 1990;259:H1822–1825. doi: 10.1152/ajpheart.1990.259.6.H1822. [DOI] [PubMed] [Google Scholar]
- 28.Wang JY, Shen J, Gao Q, Ye ZG, Yang SY, Liang HW, Bruce IC, Luo BY, Xia Q. Ischemic postconditioning protects against global cerebral ischemia/reperfusion-induced injury in rats. Stroke. 2008;39:983–990. doi: 10.1161/STROKEAHA.107.499079. [DOI] [PubMed] [Google Scholar]
- 29.Wolfrum S, Schneider K, Heidbreder M, Nienstedt J, Dominiak P, Dendorfer A. Remote preconditioning protects the heart by activating myocardial PKCepsilon-isoform. Cardiovasc Res. 2002;55:583–589. doi: 10.1016/s0008-6363(02)00408-x. [DOI] [PubMed] [Google Scholar]
- 30.Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1114–1121. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- 32.Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, Steinberg GK. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao HG, Li WB, Li QJ, Chen XL, Liu HQ, Feng RF, Ai J. Limb ischemic preconditioning attenuates apoptosis of pyramidal neurons in the CA1 hippocampus induced by cerebral ischemia-reperfusion in rats. Sheng Li Xue Bao. 2004;56:407–412. [PubMed] [Google Scholar]
- 34.Zhao HG, Sun XC, Xian XH, Li WB, Zhang M, Li QJ. The role of nitric oxide in the neuroprotection of limb ischemic preconditioning in rats. Neurochem Res. 2007;32:1919–1926. doi: 10.1007/s11064-007-9381-2. [DOI] [PubMed] [Google Scholar]
- 35.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]


