Abstract
The human parainfluenza viruses (hPIVs) and respiratory syncytial viruses (RSV) are the leading causes of hospitalizations due to respiratory viral disease in infants and young children, but no vaccines are yet available. Here we describe the use of recombinant Sendai viruses (rSeVs) as candidate vaccine vectors for these respiratory viruses in a cotton rat model. Two new SeV-based hPIV-2 vaccine constructs were generated by inserting the fusion (F) gene or the hemagglutinin-neuraminidase (HN) gene from hPIV-2 into the rSeV genome. The inoculation of either vaccine into cotton rats elicited neutralizing antibodies toward both homologous and heterologous hPIV-2 virus isolates. The vaccines elicited robust and durable antibodies toward hPIV-2, and cotton rats immunized with individual or mixed vaccines were fully protected against hPIV-2 infections of the lower respiratory tract. The immune responses toward a single inoculation with rSeV vaccines were long-lasting and cotton rats were protected against viral challenge for as long as 11 months after vaccination. One inoculation with a mixture of the hPIV2-HN-expressing construct and two additional rSeVs (expressing the F protein of RSV and the HN protein of hPIV-3) resulted in protection against challenge viruses hPIV-1, hPIV-2, hPIV-3, and RSV. Results identify SeV vectors as promising vaccine candidates for four different paramyxoviruses, each responsible for serious respiratory infections in children.
Keywords: respiratory syncytial virus, parainfluenza virus, protective immunity
1. INTRODUCTION
Respiratory syncytial virus (RSV) and the human parainfluenza viruses (hPIV) are negative-strand RNA viruses (Paramyxoviridae) that replicate in epithelial cells of the human respiratory tract. RSV is the leading cause of hospitalizations for infants in the USA. [1–3]. Most patients recover from RSV in the developed world, but in developing countries there are close to 1 million RSV-related deaths per year [4]. The human parainfluenza viruses (hPIVs) are also responsible for serious respiratory infections in the pediatric population. These viruses are categorized into four serotypes (hPIV-1,-2,-3 and –4). Human PIV-1 and hPIV-2 infections are often associated with severe croup involving the larynx and upper trachea, while RSV and hPIV-3 are more often associated with bronchiolitis, bronchitis and pneumonia. hPIV-4-disease is less frequent and less severe, but hPIV-4 can mediate significant morbidity/mortality in immunodeficient patients [5–13].
Since the 1960s, vaccine strategies have included the use of killed viruses [14], attenuated viruses [15–17], virus subunits [18;19] virus recombinants [20–23], and Jennerian vaccines [24]. The latter strategy refers to the use of a vaccine virus that is derived from a non-human species, but that has some similarity with the human virus target and that grows poorly or not at all in humans. Clinical trials have been initiated with several different vaccine candidates [16;25;26], each demonstrating some promise, but no vaccines have yet been clinically proven.
Sendai virus (SeV) is now being considered as a novel candidate vaccine for the pediatric respiratory viruses. SeV was originally discovered in Sendai Japan by Kuroya et. al. during an epidemic of fatal pneumonitis in newborns [27]. At that time, lung tissue was taken from sick newborns and transferred to mice for expansion and isolation of the pathogen. When SeV was subsequently isolated, researchers believed that it derived from humans, but later realized that the virus originated in laboratory mice and was not responsible for the infant disease [28;29]. More than fifty years have since passed, revealing no association between SeV and human infection or illness. Therefore, leaders in the field now describe SeV as a pathogen of mice and not of humans [10].
Since its first discovery, SeV has provided laboratories with a rich research model for the study of hPIV-1 infections in mice [30;31]. Investigators then found that SeV elicited robust immunity in mice and established a permanent serum antibody response due to a long-sustained population of antibody-secreting cells in the bone marrow [32]. In addition, the virus elicited robust virus-specific T-helper cell and cytotoxic T lymphocyte function [33–37]. When individual T-cell clones were transferred to naïve animals, they conferred protection against subsequent virus challenge. The humoral and cellular responses were apparently complementary, as the antibodies neutralized virus particles while T-cells cleared infected cells [36;37].
During laboratory studies of SeV, the profound sequence similarities between SeV (murine PIV-1) and hPIV-1 were recognized [38;39]. Antigenic similarities were also revealed when virus- specific B-cells and T-cells were shown to cross -react between hPIV-1 and SeV [40;41]. In fact, when hPIV-1 was administered intranasally to infant or adult mice, recipients were protected against a challenge with Sendai virus [42] and when African green monkeys were immunized with SeV, they were completely protected against hPIV-1 (protection was slightly superior to that conferred by hPIV-1 itself [43]). Each of these results highlighted SeV as a potential new Jennerian (xenotropic) vaccine for the protection of humans from hPIV-1, and prompted the initiation of clinical trials which have thus far demonstrated safety in adults and children ([44] and unpublished results).
As clinical trials were designed and implemented with SeV, further basic research indicated that the virus might also serve as a backbone for vaccines against pathogens other than hPIV-1. Specifically, reverse genetics was considered as a means to recombine foreign genes with the rSeV genome [45–49]. The current report describes the design of two new SeV recombinant constructs that express the hemagglutinin-neuraminidase (HN) and fusion (F) genes of hPIV-2. We show that each rSeV elicits immune activity and protection against hPIV-2 challenge. A single intranasal inoculation with rSeV is sufficient to sustain protection for as long as 11 months. Additionally, we show that an hPIV-2 recombinant SeV can be mixed with rSeVs expressing hPIV-3 and RSV antigens [46;47] to protect cotton rats from challenges with four different respiratory viruses: hPIV-1, hPIV-2, hPIV-3 and RSV.
2. MATERIALS AND METHODS
2.1 Construct design
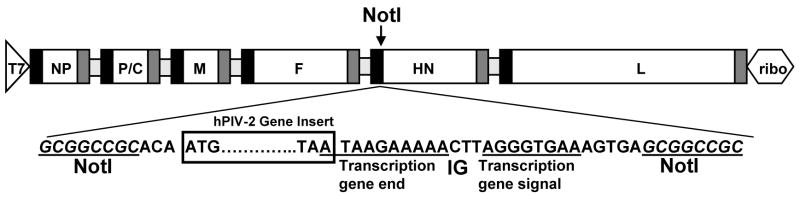
Replication-competent recombinant SeVs were rescued using a reverse genetics system, described previously [45–47;50]. For cloning of the hPIV-2 F and HN genes into the plasmid containing full length cDNA of Sendai virus (Enders strain) pSV(E) [48], LLC-MK2 (ATCC, Rockland, MD) cells were infected with the VR92 Greer strain of hPIV-2 (VR92, American Type Culture Collection [ATCC], Rockland, MD) and viral RNA was extracted. The hPIV-2 F and HN genes were amplified by RT-PCR (Titan One Tube System; Roche). The PCR forward primer included a NotI site and the reverse primer included an SeV transcription termination signal, an intergenic (IG) sequence CTT, a transcription initiation signal, and a second NotI site. The hPIV-2 F and HN cDNAs were digested with NotI and ligated into the NotI site of pSV(E)(Figure 1).
Figure 1. Design of rSeVs expressing hPIV-2 F or hPIV-2 HN.
A schematic representation is shown of the constructs used to produce the recombinant virus. A unique NotI restriction enzyme site in the non-coding region of the HN gene was used for insertion of the hPIV-2 F or hPIV-2 HN gene. These genes were obtained by RT-PCR, digested with NotI and cloned into the NotI site of pSeV(E). T7 = T7 promoter; ribo = hepatitis delta virus ribozyme sequence. The SeV proteins are indicated: NP, nucleoprotein; P, polymerase; M, matrix protein; F, fusion protein; HN, hemagglutinin-neuraminidase; L, large protein. IG = intergenic sequence.
2.2 Virus Rescue
To rescue the recombinant viruses, we 293T cells were infected with a UV-inactivated, T7 RNA polymerase-expressing recombinant vaccinia virus (vTF7.3 [51;52] for 1 h at 37 °C at an MOI of 3. Cells were then co-transfected with cDNA plasmids containing either the hPIV-2 F or HN gene (1 μg) and three supporting T7-driven plasmids expressing the NP, P, or L gene of SeV (1 μg pTF1SVNP, 1 μg pTF1SVP, and 0.1 μg pTF1SVL, respectively) using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen, Carlsbag, CA, USA). Supernatants were replaced with fresh growth media containing 10% FBS at 24 h after transfection. Cells were then incubated for an additional 24 h. Cell lysates were prepared and inoculated into 10-day-old embryonated chicken eggs. Allantoic fluids were harvested after 72 h and viruses were plaque purified on LLC-MK2 cells. Recombinant SeV clones were amplified once more in embryonated eggs to prepare vaccine stocks. Recovered viruses were designated rSeV-hPIV2-F (SeV expressing hPIV-2 F protein) or rSeV-hPIV2-HN (SeV expressing hPIV-2 HN protein). The viruses were each sequenced by RT-PCR to confirm maintenance of passenger gene sequences.
2.3 Animals, Inoculations and challenges
Groups of 2–5 adult female cotton rats (Sigmodon hispidus) purchased from Ace Animals (Boyertown, PA) were intranasally inoculated with rSeV-hPIV2-F (2×106 plaque-forming units [PFU]), rSeV-hPIV2-HN (2×106 PFU), a mixture of 1×106 rSeV-hPIV2-F and 1×106 rSeV-hPIV2-HN, or a mixture of 2×106 rSeV-hPIV2-HN, 2×106 rSeV-hPIV3-HN, and 2×106 rSeV-RSV-F (previously described rSeVs expressing the full-length RSV F or hPIV-3 HN proteins [47]). Additional animals received wild-type SeV (2 × 106 PFU/cotton rat). Controls received PBS or no inoculation. Sera were taken at regular intervals after vaccination. Challenges were performed at 1 month (4–5 weeks), 9 months or 11 months post-vaccination. Animals were challenged intranasally with hPIV-1 (strain C35 from ATCC, 2×106 PFU/cotton rat), hPIV-2 (strain VR92 Greer from ATCC, 2×106 PFU/cotton rat), hPIV-3 (strain C243 from ATCC, 2×106 PFU/cotton rat) or RSV (strain A2 from ATCC, 1.5×106 PFU/cotton rat). Lungs were harvested 3–5 days post-challenge for virus measurements.
2.4 Enzyme-linked immunosorbent assay (ELISA)
For the detection of anti-hPIV-2 F- or HN-specific antibodies, hPIV-2 stock virus was prepared from culture supernatants by concentration with a Millipore Amicon filter unit. Concentrates were lysed in disruption buffer (0.5% TritonX-100, 0.6M KCl, 0.05M Tris pH7.8), diluted with PBS (1:3000) and coated on 96-well ELISA plates. After overnight incubation, plates were blocked with PBS containing 1–3% bovine serum albumin (BSA, Sigma, St Louis, MO). Serum samples from vaccinated and control animals were diluted and incubated on plates for 2 h at 37°C or overnight at 4°C. Plates were then washed and incubated with rabbit anti–cotton rat antibody (kindly provided by Dr. Greg Prince, Virion Systems, Rockville, MD) for 2–3 h at room temperature. After further washing, plates were incubated with goat anti-rabbit IgG-horseradish peroxidase conjugate (diluted 1:3,000 in PBS/1% BSA, Bio-Rad, Hercules, CA) for 30–60 min at room temperature. Plates were washed again, and incubated with a 50% mixture of a 3,3′,5,5′-tetramethylbenzidine solution and a 0.02% solution of hydrogen peroxide in citric acid buffer (TMB Microwell Peroxidase Substrate System, KPL, Gaithersburg, MD). Reactions were stopped (after approximately 5–10 min) with an equal volume of 1M phosphoric acid. Absorbance was read at 450 nm.
2.5 Neutralization assays
To conduct neutralization assays, we mixed diluted sera with approximately 10 TCID50 hPIV-2 per well in DMEM (Cambrex Bio Science Walkersville, Inc, Walkersville, MD), in some cases with supplements of 0.1% BSA, 0.5 mg/ml gentamicin, 2 mM L-glutamine and 5μg/ml acetylated trypsin for 1 h at 37°C. Viruses in this assay were either homologous (VR92 Greer, the virus from which vaccine was derived) or heterologous (viruses of distinct origin, named by their date of isolation at St. Jude Children’s Research Hospital). The virus-serum mixtures were then added to wells of LLC-MK2 cell monolayers, which were incubated for 1 h (37°C, 5% CO2) and then fed with DMEM supplemented with glutamine, antibiotics and 5% fetal calf serum (FCS). After 4–5 days of culture (37°C, 5% CO2), supernatants from test wells (50–100 μl) were mixed with an equal volume of 0.5% fresh guinea pig red blood cells in multi-well plates and incubated at 4°C or room temperature for 30–60 min. Hemagglutination was scored as positive or negative for each well and the percent neutralization was calculated as the reduction in frequency of positive wells.
2.6 Virus challenge assay
Three-five days after intranasal viral challenge, cotton rats were sacrificed and lungs were harvested for measurement of virus titer. Briefly, lungs were homogenized on ice with a mechanical Dounce homogenizer (PowerGen125 PCR Tissue Homogenizing kit; Fisher Scientific) to yield 2–5 ml of homogenate in PBS. Homogenates were centrifuged to remove debris and supernatants were collected.
For hPIV-1 and hPIV-3 detection, 100–200 μl of serially diluted lung supernatants in DMEM (in some cases with supplements of 0.1% BSA, 0.5 mg/ml gentamicin, 2mM L-glutamine and 5 μg/ml acetylated trypsin, the latter required for PIV-1 growth) were added to wells of LLC-MK2 cell monolayers. Cultures were incubated for 1 hr (37°C, 5% CO2) and then fed with DMEM supplemented with glutamine, antibiotics and 5% FCS. After 4–5 days incubation, 50–100 μl supernatants from wells were used for standard hemagglutination (HA) assays with an equal volume of turkey red blood cells (for hPIV-3) and chicken red blood cells (for hPIV-1) for 30–60 min. TCID50 were calculated using the Reed-Muench formula.
For hPIV-2 detection, TCID50 scores were calculated as above except that guinea pig red blood cells were used in the HA assays. hPIV-2 was also quantified by plating serial dilutions of supernatants from homogenized lungs onto confluent LLC-MK2 cell monolayers in 0.15% BSA in PBS/calcium/magnesium and gentamicin. After 1 hr at 33°C, 5% CO2, the cells were overlaid with medium supplemented with 0.15% BSA, vitamins, amino acids and 0.9% agarose (electrophoresis grade, BRL, Gaithersberg, MD). After the agarose was set, plates were inverted and incubated at 33°C in a 5% CO2 incubator. 5 days later, plates received a second overlay similar to the first, but with 5% FCS instead of BSA and 0.0035% neutral red. Plates were incubated for one more day and plaques were counted.
For RSV measurements, serially diluted supernatants from lung homogenates were inoculated on Hep-2 cell monolayers in 12-well plates; after 1 h at 37 °C and 5% CO2, the wells were overlaid with EMEM medium supplemented with glutamine, antibiotics, 10% fetal calf serum and 0.75% methylcellulose. After incubation for 5 to 6 days at 37°C and 5% CO2, the methylcellulose was removed, cells were fixed with formalin phosphate, and the plates were stained with hematoxylin and eosin for enumeration of plaques. For each virus, the pulmonary burden per homogenized cotton rat lung was scored.
3. RESULTS
3.1 Sendai virus vaccines expressing hPIV-2 F or HN induce hPIV-2-specific binding and neutralizing antibodies in a cotton rat model
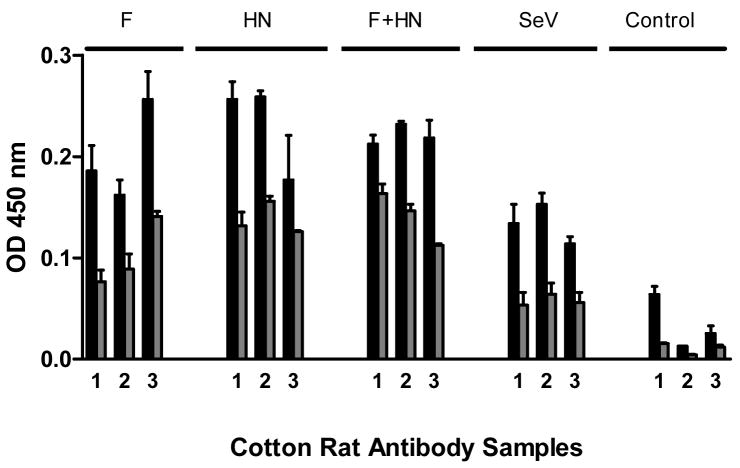
To study the capacity of SeV to serve as an hPIV-2 vaccine backbone, we inserted either hPIV-2 F or HN genes into the full-length SeV cDNA (Enders strain) and rescued two new viruses, rSeV-hPIV2-F and rSeV-hPIV2-HN (see Figure 1 and Materials and Methods for details). The immunogenicity of each new SeV-based vaccine was then examined by the inoculation of groups of 3–5 cotton rats intranasally. Animals received 2 × 106 PFU rSeV-hPIV-2-F (abbreviated ‘F’), rSeV-hPIV-2-HN (abbreviated ‘HN’), or a mixture of 1 × 106 PFU of each of the two vaccines (‘F+HN’). Unmodified SeV and PBS were used as controls. Blood was collected 1 month later for measurement of hPIV-2-specific antibodies by ELISA (using 1:100 and 1:500 serum dilutions). As shown in Figure 2, all cotton rats immunized with the single or mixed recombinant vaccines generated antibodies with specificity for hPIV-2. Repeat experiments showed no consistent difference between the antibody titers from animals that received rSeV-hPIV2-F, rSeV-hPIV-2 HN or a mixture of the two vaccines, and in some experiments, titers scored as high as 1:10,000 (see below). Animals that were immunized with unmanipulated SeV also generated antibody responses above background, demonstrating a weak antigenic relationship between SeV and hPIV-2 viruses.
Figure 2. Recombinant SeV immunizations elicit hPIV-2-specific antibodies.
Groups of cotton rats were inoculated with rSeV-hPIV2-F (denoted ‘F’, 2 × 106 PFU/cotton rat), rSeV-hPIV2-HN (denoted ‘HN’, 2 × 106 PFU), a combination of the two vaccines (denoted ‘F + HN’, each at 1 × 106 PFU), unmodified SeV (2 × 106 PFU), or PBS (Control). Sera were collected 1 month later, diluted, and tested by ELISA for hPIV-2-specific antibody activity. Sera were diluted 1:100 (black bars) and 1:500 (gray bars) and tested in replicate at each dilution. Each bar represents the mean absorbance value and standard error from an individual cotton rat. Unpaired T-tests showed that antibody responses elicited by each of the vaccines, including unmanipulated SeV, were statistically significant at both dilutions (p<.05).
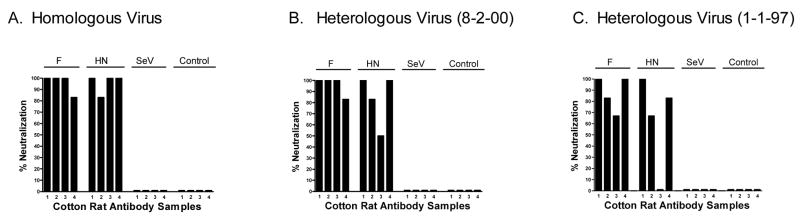
Having demonstrated antibody responses toward hPIV-2, we next examined neutralizing antibody activity. Serum samples taken 1 month after vaccination were tested against homologous virus (from which vaccine was derived) and heterologous isolates (viruses derived from a distinct origin). Heterologous viruses were from different yearly outbreaks of hPIV-2 (viruses 8-2-00 and 1-1-97 were obtained from the Clinical Pathology Department of St. Jude Children’s Research Hospital and named by their data of isolation). As shown in Figure 3, both homologous and heterologous viruses were neutralized by sera (diluted 1:256) from vaccinated animals, demonstrating the potent cross-neutralizing capability of antibodies elicited by recombinant SeV hPIV-2 vaccines (neutralization was scored as the reduction in frequency of positive viral cultures; see Materials and Methods). When sera were diluted 1:1024, neutralizing activity generally fell below the >50% cut-off on all three viruses, demonstrating a titer of ≥ 1:256, but less than 1:1024. The mixed vaccine (HN + F) was also tested on homologous and heterologous viruses, but elicited responses that did not exceed those induced by the individual vaccine vectors alone (data not shown).
Figure 3. rSeV immunizations induce neutralizing antibodies against homologous and heterologous hPIV-2 virus isolates.
Serum samples were from cotton rats inoculated with rSeV-hPIV2-F (2 × 106 PFU), rSeV-hPIV2-HN (2 × 106 PFU), unmodified SeV (2 × 106 PFU) or PBS (Control). Tested viruses were the homologous VR92 (ATCC) and the heterologous 8-2-00 and 1-1-97 (kindly provided by Dr. Randy Hayden and named for the date of isolation). Sera (1:256 dilution) were mixed with virus for one hour at 37°C and then inoculated onto LLC-MK2 monolayer cell cultures. Neutralization was scored 4 days later as the percent reduction of wells with hemagglutination activity on guinea pig RBC. Most samples from rSeV-vaccinated animals scored positively on all three viruses (>50% neutralization). Sera were also tested at a dilution of 1:1024, but neutralization fell below 50% at this dilution. Statistical analyses were performed with Fisher’s Exact Tests to evaluate the presence of neutralizing antibodies (scores of >50% were considered positive) in test groups compared to the control. Results were significant for all three viruses with sera from rSeV-hPIV2-F-primed animals, and for two viruses (panels A and B) for rSeV-hPIV2-HN-primed animals (p<.05; rSeV-hPIV2-HN-primed animal sera neutralized all three viruses at a 1:64 dilution, data not shown). The unmanipulated SeV did not induce significant neutralizing antibody responses toward hPIV-2.
3.2 rSeV-hPIV2-F and rSeV-hPIV2-HN vaccines confer complete protection against homologous hPIV-2 challenges in the cotton rat model
We next assessed vaccine-induced protection from hPIV-2 challenge. One month after inoculations with rSeV-hPIV2-F, rSeV-hPIV2-HN, a mixture of the two constructs, wild-type SeV or PBS, cotton rats were challenged intranasally with the hPIV-2 VR92 Greer strain at a dose of 2×106 PFU/animal. Four days after challenge, animals were sacrificed and lungs were collected for determination of hPIV-2 burden. As shown in Figure 4, animals vaccinated with individual or mixed rSeV vaccines were fully protected from hPIV-2 challenge. Wild-type SeV did not confer significant protection (defined as a 1 log reduction of challenge virus relative to the mean control value). The results clearly demonstrated the efficacy of the rSeV-hPIV2-F and rSeV-hPIV2-HN constructs as vaccines against hPIV-2, and that full protection against virus challenge could be gained without mixing the two rSeVs.
Figure 4. Recombinant SeV vaccines confer protection against hPIV-2 challenge.
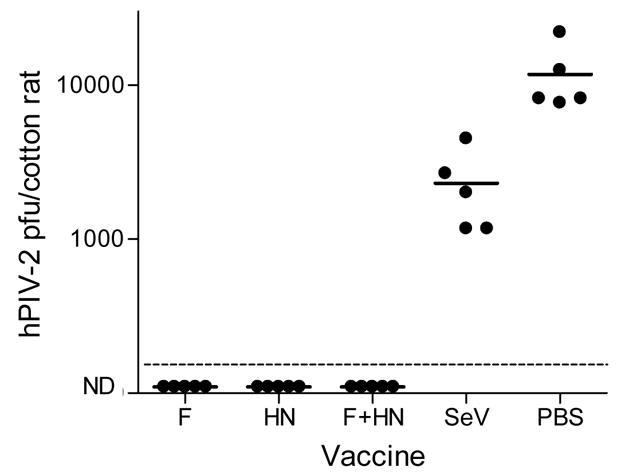
One month after immunizations with rSeV-hPIV2-F or rSeV-hPIV2-HN (2×106 PFU/cotton rat), animals were challenged with the homologous VR92 hPIV-2 (2×106 PFU/cotton rat). Pulmonary virus loads were measured on day 4 post-challenge. Each symbol represents the pulmonary titer (PFU) in an individual animal. Control animals received wild-type SeV (2 × 106 PFU/cotton rat) or PBS. ND=No Virus Detected. The dotted horizontal line indicates the lower limit of detection (LLOD) for virus in test animals (167 pfu). The Fishers Exact Test was conducted to evaluate achievement of a 1 log virus reduction compared to mean control values. Results with recombinant vaccines, but not wildtype SeV, were significant (p<.05).
3.5 Durability of immune responses elicited by rSeV-hPIV2-F and rSeV-hPIV2-HN vaccines
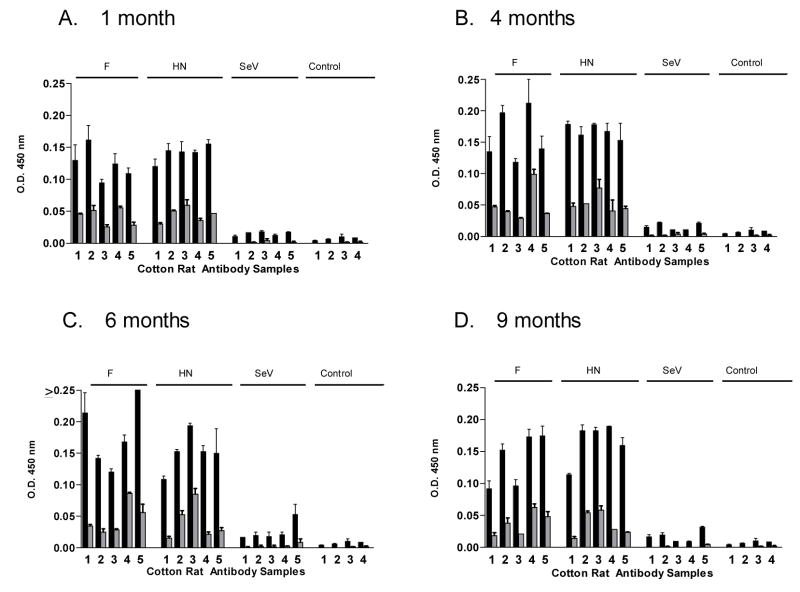
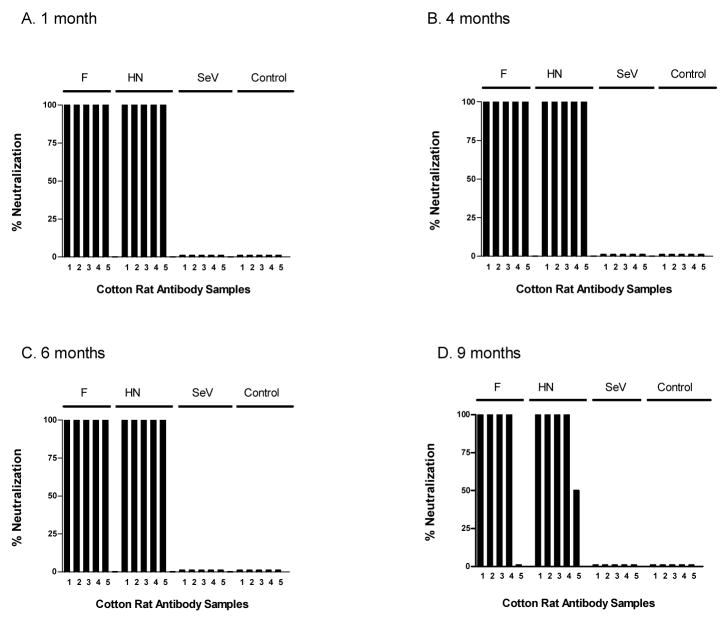
Given that live virus vaccines have in some cases elicited potent and durable immunity [32;53], we asked if the immune responses elicited by our new recombinant vaccines were long-lasting. We therefore immunized animals with either the rSeV-hPIV2-HN or rSeV-hPIV2-F vaccines. Cotton rats were then sampled for antibody activities after 1, 4, 6 and 9 months. As shown in Figure 5, the antibody binding responses were sustained in rSeV-hPIV2-HN- and rSeV-hPIV2-F-primed animals throughout the 9 month period without apparent loss of titer (responses were scored at serum dilutions of 1: 1,000 and 1:10,000). As shown in Figure 6, neutralization activity was also sustained in these test animals (≥ 1:256 serum titer). This result demonstrates the extraordinary activity of responses elicited by the rSeV live virus vaccines.
Figure 5. Persistent antibody binding responses toward recombinant SeV.
Cotton rats were immunized intranasally with rSeV-hPIV2-F, rSeV-hPIV2-HN or unmanipulated SeV (2×106 PFU/cotton rat) and sera were sampled after 1, 4, 6 and 9 months. Serum samples were tested for hPIV-2-specific antibodies by ELISA on virus-coated plates. Results from experiments with 1:1,000 (black bars) and 1:10,000 (grey bars) serum dilutions (tested in replicate) are shown. Sera from unprimed animals were used as controls. Panels A, B, C and D represent results from months 1, 4, 6 and 9, respectively. Unpaired T-tests showed that antibody responses elicited by rSeV-hPIV2-F and rSeV-hPIV2-HN vaccines were statistically significant at both dilutions; antibody responses elicited by the unmanipulated SeV vaccine were significant only at the 1:1000 dilution when sampled on months 1 and 4 (p<.05).
Figure 6. Persistent antibody neutralizing responses elicited by rSeVs.
Cotton rats were immunized intranasally with rSeV-hPIV2-F, rSeV-hPIV2-HN or unmanipulated SeV (2×106 PFU/cotton rat) and sera were sampled after 1, 4, 6 and 9 months. Neutralizing activity was tested by mixing homologous virus (VR92 Greer, ATCC, approximately 10 TCID50/well) with sera at a 1:256 dilution for 1 h at 37°C. Mixed samples were then inoculated onto LLC-MK2 cells for 5 days. Neutralization was scored as the percent reduction of infected wells as determined by hemagglutination activity on guinea pig RBC. Most rSeV-primed animals were positive for neutralizing activity, while sera from unmanipulated SeV-primed animals were not. Statistical analyses were performed with Fisher’s Exact Tests to evaluate the presence of neutralizing antibodies (scores of >50% were considered positive) in test groups compared to the control. Results were significant at all time points for rSeV-hPIV2-F- and hPIV2-HN-primed animals (p<.05). Results from SeV-primed animals were not significant.
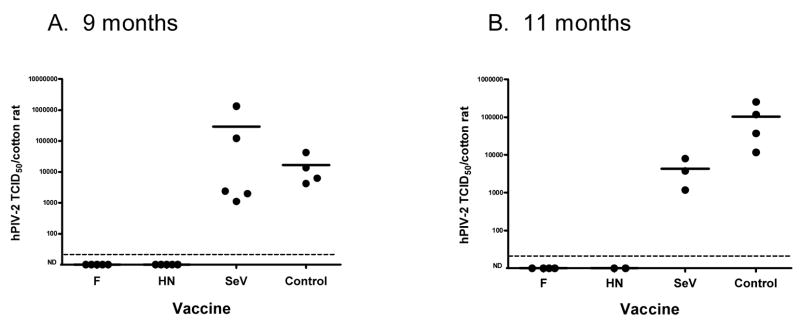
A most critical question was whether the protective responses were retained months after vaccination. Animals were therefore challenged with hPIV-2 after 9 and 11 months. As shown in Figure 7, animals were fully protected at both time-points after the single intranasal inoculations. Control animals were not protected.
Figure 7. Persistent protection conferred by recombinant SeV.
Cotton rats were immunized with rSeV-hPIV2-HN, rSeV-hPIV2-F, or unmodified SeV (2×106 PFU/cotton rat) by the intranasal route. After 9 (Panel A) or 11 (Panel B) months post-inoculation, animals were challenged with VR92 hPIV-2 (2×106 PFU/cotton rat). Pulmonary virus loads were measured on day 4 post-challenge. Each symbol represents the pulmonary titer in an individual animal. Control animals were unvaccinated. ND=No virus detected. The dotted horizontal lines indicate the LLODs (21 TCID50). The Fishers Exact Test was conducted with combined data from 9- month and 11-month challenge experiments to evaluate achievement of a 1 log virus reduction compared to mean control values. Results with recombinant vaccines, but not unmanipulated SeV, were significant (p<.05).
3.6 A mixed rSeV-RSV-F, rSeV-hPIV3-HN, and rSeV-hPIV2-HN vaccine confers protection against RSV, hPIV-1, hPIV-2 and hPIV-3 challenges in the cotton rat model
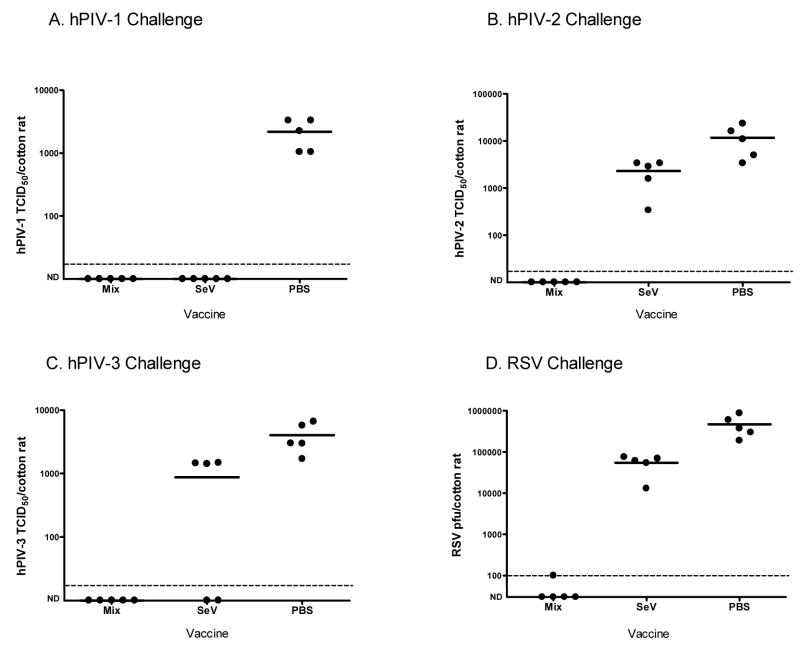
Once the potency of the hPIV-2 vaccines was revealed, a mixture of rSeV vaccines was tested to determine if protection could be gained against more than one virus pathogen. To this end, the rSeV-hPIV2-HN construct was mixed with two other recombinants, rSeV-RSV-F and rSeV-hPIV3-HN; these viruses have been previously described [47;48]. The final dose for each of the three viruses in the trimeric vaccine was 2×106 PFU. Groups of cotton rats then received the mixed vaccine, the wild-type SeV or PBS. One month after vaccination, the groups were challenged with RSV, hPIV-1, hPIV-2 or hPIV-3. The animals were sacrificed for lung harvest and virus titration on days 4 (for hPIV-1, hPIV-3 and RSV) and day 5 (for hPIV-2). As shown in Figure 8 (panels A-D), cotton rats inoculated with the mixed vaccine were protected against all four pathogens. The wild-type SeV also conferred protection against hPIV-1, minimal protection against hPIV-3 (in this experiment, only 2 of 5 animals were protected), and poor activity toward hPIV-2 and RSV. Together, these findings demonstrated that a single nasal inoculation with a mixture of three different recombinant SeVs was sufficient to prevent measurable pulmonary infection with four different viruses in the cotton rat model.
Figure 8. Mixed rSeV-hPIV3-HN and rSeV-RSV-F rSeV vaccines confer protection against challenges with hPIV-1, hPIV-2, hPIV-3 and RSV.
One month after immunizations with a vaccine mixture (2×106PFU rSeV-RSV-F, 2×106PFU rSeV-hPIV2-HN and 2×106PFU rSeV-hPIV3-HN per cotton rat), groups of 5 animals were challenged with hPIV-1 (C35, 2×106 PFU/cotton rat), hPIV-2 (VR92, 2×106 PFU/cotton rat), hPIV-3 (C243, 2×106 PFU/cotton rat) or RSV (A2, 1.5×106 PFU/cotton rat). Groups of cotton rats were also immunized with wild-type SeV (2×106 PFU/cotton rat) or PBS. Viral loads were measured in animal lungs on days 4 (hPIV-1, hPIV-3, RSV) and 5 (hPIV-2) post-challenge. hPIVs were measured as TCID50/cotton rat (on LLC-MK2 cells) while RSV viral loads were measured as PFU/cotton rat (on Hep2 cells). ND=No virus detected. The dotted horizontal lines indicate the LLODs (17 TCID50 for hPIV-1, hPIV-2 and hPIV-3, and 100 pfu for RSV). The Fishers Exact Test was conducted to evaluate achievement of a 1 log virus reduction compared to mean control values. Results with the mixed recombinant vaccines and SeV were significant for the hPIV-1 challenge; results with the mixed recombinant vaccines, but not wildtype SeV, were significant for challenges with hPIV-2, hPIV-3 and RSV (p<.05).
4. DISCUSSION
This report describes the construction of two new rSeV vaccines that express the hPIV-2 F (rSeV-hPIV2-F) and HN (rSeV-hPIV2-HN) proteins. In a cotton rat model, each vaccine was shown to elicit binding and neutralizing antibodies and to protect animals against hPIV-2 challenge. The antibodies elicited by the new hPIV-2 vaccines recognized both homologous and heterologous viruses. Antibodies were durable and protection was sustained for many months after vaccination. We also tested a mixture of three SeV recombinants expressing RSV, hPIV-2, and hPIV-3 proteins (rSeV-RSV-F, rSeV-hPIV2-HN and rSeV-hPIV3-HN constructs) in cotton rats and compared these to unmanipulated SeV. Unmanipulated SeV protected against hPIV-1, reflecting the close homology between mouse and human PIV-1 viruses [43]. There was also minimal protection conferred by unmanipulated SeV against hPIV-3. This proved statistically significant in some experiments but not others, reflecting the partial homology between respiroviruses PIV-1 and PIV-3 (a comparison of HN and F genes between hPIV-1 and hPIV-3 revealed a respective amino acid sharing of 47% and 42% between viruses; cross-reactivity between hPIV-1 and hPIV-3 was also described in previous B- and T-cell studies [11;48;54–56]). The mixed recombinant vaccine was far superior to unmanipulated SeV in the cotton rat model system, as there was virtually complete lower respiratory tract protection against challenges with four different pathogens: hPIV-1, hPIV-2, hPIV-3 and RSV. These results highlight the enormous capacity of mixed rSeVs to protect against four serious respiratory pathogens of children [44–47;57].
A major mediator of viral protection is the virus-specific antibody, as is best demonstrated by the capacity of licensed anti-RSV-F monoclonal and polyclonal antibodies to control lower respiratory tract viral disease in clinical pediatrics [58–65]. Antibodies provide a first line of defense against infection by preventing virus entry into target host cells [32;66;67]. The current report demonstrates that antibody responses elicited by rSeV passenger proteins are both potent and long-lasting. The durability of serum antibody responses likely reflects the capacity of SeV to induce lifelong plasma cell activity in the bone marrow of immunized animals [31;32;66]. This remarkable durability typifies a number of live virus vaccines, yet the mechanism underlying sustained responsiveness remains a topic of debate [68]. Infectious SeV is cleared from sero-negative experimental mice [69], cotton rats (our unpublished data) and primates [43] within 1–2 weeks post-infection, but antigens deposited following live virus infections and other forms of immunizations survive considerably longer (weeks or months [68;70–72]). Some investigators argue that antigen persistence is responsible for continuous low-level B-cell proliferative activity, while others argue that long-sustained B-cell memory has no requirement for persistent antigen [68]. In support of the latter argument, it has been demonstrated that memory B-cells persist in vivo even after a cre-lox induced alteration of antibody specificity [73]. Perhaps there is merit to both schools of thought; memory B-cells may be sustained for long periods in the absence of antigen, but benefit by re-stimulation when antigen persists. These concepts will be topics of future research, as will be the study of T-cell responses toward the hPIV-2 rSeV vaccines.
Results in this and previous reports encourage further testing of both unmodified and recombinant SeV vaccines [57]. Clinical trial results thus far have confirmed the safety of unmodified SeV in adults as no serious vaccine-related adverse events were observed in any study participants [44]. Clinical trials in a younger volunteer population are ongoing. The absence of serious vaccine-related adverse events was predicted in humans, because SeV is a mouse pathogen and is host-range restricted. This is in part due to the sensitivity of SeV to the innate immune activities elicited by human interferon [74]. As further evidence of SeV safety and efficacy in primates, it has been demonstrated that the wild-type virus causes no adverse events in African Green Monkeys or chimpanzees, yet confers complete protection against hPIV-1 [43;75]. It was also shown that the peak growth of SeV in the lower respiratory tract (LRT) of chimpanzees was less than that of bovine parainfluenza virus-type 3 (bPIV-3), a vaccine that has been tested in human infants and reported to be safe [24;75;76]. Histopathology tests have also been conducted to examine cotton rat lungs after vaccinations with rSeV-RSV-G or rSeV-RSV-F and challenges with RSV, demonstrating no enhanced immunopathology relative to unvaccinated animals[45–47]. Additional analyses of recombinant SeV in a non-human primate study are currently planned.
While clinical trials are the only true tests of vaccine safety and efficacy in humans, the results from the current cotton rat study are encouraging in light of correlations made during studies of the monoclonal anti-RSV antibody Palivizumab (and polyclonal RSV-specific antibodies) in pre-clinical and clinical trials [60–65;77]. The licensed Palivizumab product is known to confer significant protection against disease in infants (in a placebo-controlled trial, Palivizumab prophylaxis yielded a 55% reduction in RSV-associated hospitalizations [62]). In humans, the target serum trough level for Palivizumab is 25 μg/ml [64;65] which correlates with a micro-neutralization titer of 1:250 and a 2-log reduction of RSV in cotton rat challenge studies [77]. In comparison, the results of our studies with the rSeV-RSV-F construct are promising in that neutralization titers exceed 1:250 and challenge virus loads are reduced by more than 2 logs when vaccinated animals are compared to controls [47].
The SeV recombinants add to a variety of vaccine candidates that have been tested in the respiratory virus vaccine field. One prominent candidate is the bovine parainfluenza virus type 3 (bPIV-3) vaccine which was originally advanced as a Jennerian (xenotrophic) vaccine for human PIV-3 (hPIV-3). Intranasal inoculations of infants and children with live bPIV-3 at doses of up to 106 TCID50 were tolerated with no apparent ill effects. Antibodies toward virus were recognized [78], but the cross-reactivity of these antibodies with hPIV-3 was weak, encouraging a change in vaccine strategy [11;24]. Researchers then employed reverse genetics strategies to replace the bovine PIV-3 genes F and HN with human PIV-3 genes [79;80] and to add RSV G and F passenger genes to the construct [81]. Vaccines of this type elicited RSV-specific neutralizing antibodies in both hamsters and non-human primates [82]. A bPIV-3-based hPIV-3/RSV vaccine (MEDI-534) was recently tested in adult, sero-positive humans [25]. Initial results showed no medically significant vaccine-related adverse events and no boost to RSV or hPIV-3 antibody titers in the study population.
Reverse genetics has also been used to exchange genes between the human PIVs. For example, in an hPIV-3/hPIV-1 chimeric virus designated rPIV3-1, the HN and F genes of hPIV-3 were replaced with genes from hPIV-1. Further manipulations yielded a vector named rPIV3-1.2HN, which additionally expressed the HN protein of hPIV-2 [83;84]. rPIV3-1.2HN proved to be temperature sensitive and was restricted in replication when tested in hamsters. It elicited serum antibodies to both hPIV-1 and hPIV-2 and induced resistance to challenge by both viruses. Reverse genetics has also been used to exchange genes between RSV A and B subgroups, or bovine and human RSV isolates [85–88].
An additional long-standing strategy for the development of respiratory virus vaccines has been to generate temperature sensitive mutants by cold-passage of human viruses. This strategy was used to generate cp45, an hPIV-3 mutant. cp45 was shown to be of attenuated phenotype in chimpanzees and to induce a degree of resistance to hPIV-3 challenge [89]. When tested in the clinic, the vaccine retained its attenuated phenotype and elicited a moderate immune response in children [90–92]. A recombinant version of cp45 is now being studied in clinical trials [11].
The vaccine strategies described above have each shown some promise in the clinic, but despite decades of work, none has yet emerged as a clearly efficacious vaccine [17;93]. The development of rSeV now provides an alternative candidate vaccine for protection against the pediatric respiratory virus diseases. Recombinant SeV products are particularly attractive, primarily because of natural host-range restriction and because, as shown in the present report, a single-dose 3-component vaccine can prevent four different respiratory virus infections in the cotton rat model: hPIV-1, hPIV-2, hPIV-3 and RSV.
In conclusion, reverse genetics has permitted the development of two new rSeV constructs (expressing the F and HN genes of hPIV-2), each capable of eliciting durable protective immune responses against hPIV-2. A 3-component vaccine also conferred complete protection against hPIV-1, hPIV-2, hPIV-3 and RSV infections in the lower respiratory tracts of cotton rats. Perhaps an rSeV vaccine cocktail will eventually protect human infants from serious respiratory pathogens, an outcome that would offer great benefit to the pediatric population.
Acknowledgments
We thank Dr. Greg Prince and Jorge Blanco (Virion Systems) for providing cotton rat antibody reagents. We thank Dr. Randy Hayden (SJCRH) and the American Type Culture Collection (ATCC, Rockville, MD) for providing virus isolates. We thank Robert Sealy and Ruth Ann Scroggs for expert technical assistance. This work was supported by NIH NIAID grant P01 AI054955, NIH NCI grant P30-CA21765 and the American-Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collins PL, Chanock RM, Murphy BR, Knipe DM, Howley PM. Fields Virology. Lippincott Williams and Wilkins; Philadelphia, PA: 2001. Respiratory Syncytial Virus; pp. 1443–1485. [Google Scholar]
- 2.Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect DIs J. 2002;21:629–632. doi: 10.1097/00006454-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143:S127–S132. doi: 10.1067/s0022-3476(03)00510-9. [DOI] [PubMed] [Google Scholar]
- 4.State of the World’s Vaccines and Immunization, 1996. Vol. 161. Geneva: World Health Organization and United Nations Children’s Fund; 1996. Publication WHO/GPV 96.04. [Google Scholar]
- 5.Vachon ML, Dionne N, Leblanc E, Moisan D, Bergeron MG, Boivin G. Human parainfluenza type 4 infections, Canada. Emerg Infect Dis. 2006;12:1755–1758. doi: 10.3201/eid1211.060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garbino J, Inoubli S, Mossdorf E, Weber R, Tamm M, Soccal P, Aubert JD, Bridevaux PO, Tapparel C, Kaiser L. Respiratory viruses in HIV-infected patients with suspected respiratory opportunistic infection. AIDS. 2008;22:701–705. doi: 10.1097/QAD.0b013e3282f470ac. [DOI] [PubMed] [Google Scholar]
- 7.Glezen WP, Frank AL, Taber LH, Kasel JA. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis. 1984;150:851–857. doi: 10.1093/infdis/150.6.851. [DOI] [PubMed] [Google Scholar]
- 8.Glezen WP. Morbidity associated with the major respiratory viruses. Pediatr Ann. 1990;19:535–540. doi: 10.3928/0090-4481-19900901-09. [DOI] [PubMed] [Google Scholar]
- 9.Henrickson KJ, Kuhn SM, Savatski LL. Epidemiology and cost of infection with human parainfluenza virus types 1 and 2 in young children. Clin Inf Dis. 1994;18:770–779. doi: 10.1093/clinids/18.5.770. [DOI] [PubMed] [Google Scholar]
- 10.Chanock RM, Murphy BR, Collins PL. Parainfluenza viruses. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams and Wilkins; Philadelphia PA: 2001. pp. 1341–1379. [Google Scholar]
- 11.Karron RA, Collins PL. Parainfluenza Viruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. Lippincott Williams and Wilkins; 2007. pp. 1497–1526. [Google Scholar]
- 12.Downham MA, McQuillin J, Gardner PS. Diagnosis and clinical significance of parainfluenza virus infections in children. Arch Dis Child. 1974;49:8–15. doi: 10.1136/adc.49.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lujan-Zilbermann J, Benaim E, Tong X, Srivastava DK, Patrick CC, DeVincenzo JP. Respiratory virus infections in pediatric hematopoietic stem cell transplantation. Clin Infect Dis. 2001;33:962–968. doi: 10.1086/322628. [DOI] [PubMed] [Google Scholar]
- 14.Heilman CA. From the National Institute of Allergy and Infectious Diseases and the World Health Organization. Respiratory syncytial and parainfluenza viruses. J Infect Dis. 1990;161:402–406. doi: 10.1093/infdis/161.3.402. [DOI] [PubMed] [Google Scholar]
- 15.Fuerst TR, Earl PL, Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987;7:2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skiadopoulos MH, Tatem JM, Surman SR, Mitcho Y, Wu SL, Elkins WR, Murphy BR. The recombinant chimeric human parainfluenza virus type 1 vaccine candidate, rHPIV3-1cp45, is attenuated, immunogenic, and protective in African green monkeys. Vaccine. 2002;20:1846–1852. doi: 10.1016/s0264-410x(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 17.Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82:2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brideau RJ, Oien NL, Lehman DJ, Homa FL, Wathen MW. Protection of cotton rats against human parainfluenza virus type 3 by vaccination with a chimeric FHN subunit glycoprotein. J Gen Virol. 1993;74:471–477. doi: 10.1099/0022-1317-74-3-471. [DOI] [PubMed] [Google Scholar]
- 19.Power UF, Plotnicky H, Blaecke A, Nguyen TN. The immunogenicity, protective efficacy and safety of BBG2Na, a subunit respiratory syncytial virus (RSV) vaccine candidate, against RSV-B. Vaccine. 2003;22:168–176. doi: 10.1016/s0264-410x(03)00570-x. [DOI] [PubMed] [Google Scholar]
- 20.Spriggs MK, Murphy BR, Prince GA, Olmsted RA, Collins PL. Expression of the F and HN glycoproteins of human parainfluenza virus type 3 by recombinant vaccinia viruses: Contributions of the individual proteins to host immunity. J Virol. 1987;61:3416–3423. doi: 10.1128/jvi.61.11.3416-3423.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu SL, Abrams K, Barber GN, Moran P, Zarling JM, Langlois AJ, Kuller L, Morton WR, Benveniste RE. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science. 1992;255:456–459. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- 22.Elango N, Prince GA, Murphy BR, Venkatesan S, Chanock RM, Moss B. Resistance to human respiratory syncytial virus (RSV) infection induced by immunization of cotton rats with a recombinant vaccinia virus expressing the RSV G glycoprotein. Proc Natl Acad Sci(USA) 1986;83:1906–1910. doi: 10.1073/pnas.83.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooney EL, Collier AC, Greenberg PD, Coombs RW, Zarling J, Arditti DE, Hoffman MC, Hu SL, Corey L. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet. 1991;337:567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- 24.Wyke Coelingh KL, Winter CC, Tierney EL, London WT, Murphy BR. Attenuation of bovine parainfluenza virus type 3 in nonhuman primates and its ability to confer immunity to human parainfluenza virus type 3 challenge. J Infect Dis. 1988;157:655–662. doi: 10.1093/infdis/157.4.655. [DOI] [PubMed] [Google Scholar]
- 25.Tang RS, Spaete RR, Thompson MW, MacPhail M, Guzzetta JM, Ryan PC, Reisinger K, Chandler P, Hilty M, Walker RE, Gomez MM, Losonsky GA. Development of a PIV-vectored RSV vaccine: Preclinical evaluation of safety, toxicity, and enhanced disease and initial clinical testing in healthy adults. Vaccine. 2008;26:6373–6382. doi: 10.1016/j.vaccine.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Durbin AP, Karron RA. Progress in the development of respiratory syncytial virus and parainfluenza virus vaccines. Clin Infect Dis. 2003;37:1668–1677. doi: 10.1086/379775. [DOI] [PubMed] [Google Scholar]
- 27.Ishida N, Homma M. Sendai virus. Adv Virus Res. 1978;23:349–383. doi: 10.1016/s0065-3527(08)60103-7. [DOI] [PubMed] [Google Scholar]
- 28.Tai FH, Chiu S, Ma CC. Seroepidemiologic studies of Sendai virus infection in Taiwan. Taiwan I Hsueh Hui Tsa Chih. 1967;66:312–318. [PubMed] [Google Scholar]
- 29.Stark JE, Heath RB. The development of antibodies against Sendai virus in childhood. Arch Gesamte Virusforsch. 1967;20:438–444. doi: 10.1007/BF01275224. [DOI] [PubMed] [Google Scholar]
- 30.Ely KH, Ahmed M, Kohlmeier JE, Roberts AD, Wittmer ST, Blackman MA, Woodland DL. Antigen-specific CD8+ T cell clonal expansions develop from memory T cell pools established by acute respiratory virus infections. J Immunol. 2007;179:3535–3542. doi: 10.4049/jimmunol.179.6.3535. [DOI] [PubMed] [Google Scholar]
- 31.Sealy R, Surman S, Hurwitz JL, Coleclough C. Antibody response to influenza infection of mice: different patterns for glycoprotein and nucleocapsid antigens. Immunology. 2003;108:431–439. doi: 10.1046/j.1365-2567.2003.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyland L, Sangster M, Sealy R, Coleclough C. Respiratory virus infection of mice provokes a permanent humoral immune response. J Virol. 1994;68:6083–6086. doi: 10.1128/jvi.68.9.6083-6086.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou S, Doherty PC, Zijlstra M, Jaenisch R, Katz JM. Delayed clearance of sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol. 1992;149:1319–1325. [PubMed] [Google Scholar]
- 34.Anderson MJ, Pattison JR, Cureton RJR, Argent S, Heath RB. The role of host responses in the recovery of mice from Sendai virus infection. J Gen Virol. 1980;46:373–379. doi: 10.1099/0022-1317-46-2-373. [DOI] [PubMed] [Google Scholar]
- 35.De Waal LP, Kast WM, Melvold RW, Melief CJM. Regulation of the cytotoxic T lymphocyte response against Sendai virus analyzed with H-2 mutants. J Immunol. 1983;130:1090–1096. [PubMed] [Google Scholar]
- 36.Kast WM, Bronkhorst AM, De Waal LP, Melief CJM. Cooperation between cytotoxic and helper T lymphocytes in protection against lethal Sendai virus infection. J Exp Med. 1986;164:723–738. doi: 10.1084/jem.164.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kast WM, Roux L, Curren J, Blom HJ, Voordouw AC, Meloen RH, Kolakofsky D, Melief CJ. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc Natl Acad Sci USA. 1991;88:2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyn D, Gill DS, Scroggs RA, Portner A. The nucleoproteins of human parainfluenza virus type 1 and Sendai virus share amino acid sequences and antigenic and structural determinants. J Gen Vir. 1991;72:983–987. doi: 10.1099/0022-1317-72-4-983. [DOI] [PubMed] [Google Scholar]
- 39.Gorman WL, Gill DS, Scroggs RA, Portner A. The hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus type 1 and sendai virus have high structure-function similarity with limited antigenic cross-reactivity. Virology. 1990;175:211–223. doi: 10.1016/0042-6822(90)90201-2. [DOI] [PubMed] [Google Scholar]
- 40.Smith FS, Portner A, Leggiadro RJ, Turner EV, Hurwitz JL. Age-related development of human memory T-helper and B-cell responses toward parainfluenza virus type-1. Virology. 1994;205:453–461. doi: 10.1006/viro.1994.1665. [DOI] [PubMed] [Google Scholar]
- 41.Slobod KS, Leggiadro RJ, Presbury G, Smith FS, Hurwitz JL. Peripheral T cell receptor repertoire among CD4+ and CD8+ subsets during acute infectious mononucleosis. Viral Immunol. 1994;7:151–153. doi: 10.1089/vim.1994.7.151. [DOI] [PubMed] [Google Scholar]
- 42.Sangster M, Smith FS, Coleclough C, Hurwitz JL. Human parainfluenza virus-type 1 immunization of infant mice protects from subsequent Sendai virus infection. Virology. 1995;212:13–19. doi: 10.1006/viro.1995.1448. [DOI] [PubMed] [Google Scholar]
- 43.Hurwitz JL, Soike KF, Sangster MY, Portner A, Sealy RE, Dawson DH, Coleclough C. Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine. 1997;15:533–540. doi: 10.1016/s0264-410x(97)00217-x. [DOI] [PubMed] [Google Scholar]
- 44.Slobod KS, Shenep JL, Lujan-Zilbermann J, Allison K, Brown B, Scroggs RA, Portner A, Coleclough C, Hurwitz JL. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine. 2004;22:3182–3186. doi: 10.1016/j.vaccine.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 45.Takimoto T, Hurwitz JL, Coleclough C, Prouser C, Krishnamurthy S, Zhan X, Boyd K, Scroggs RA, Brown B, Nagai Y, Portner A, Slobod KS. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J Virol. 2004;78:6043–6047. doi: 10.1128/JVI.78.11.6043-6047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takimoto T, Hurwitz JL, Zhan X, Krishnamurthy S, Prouser C, Brown B, Coleclough C, Boyd K, Scroggs RA, Portner A, Slobod KS. Recombinant Sendai virus as a novel vaccine candidate for respiratory syncytial virus. Viral Immunol. 2005;18:255–266. doi: 10.1089/vim.2005.18.255. [DOI] [PubMed] [Google Scholar]
- 47.Zhan X, Hurwitz JL, Krishnamurthy S, Takimoto T, Boyd K, Scroggs RA, Surman S, Portner A, Slobod KS. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine. 2007;25:8782–8793. doi: 10.1016/j.vaccine.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhan X, Slobod KS, Krishnamurthy S, Luque LE, Takimoto T, Jones B, Surman S, Russell CJ, Portner A, Hurwitz JL. Sendai virus recombinant vaccine expressing hPIV-3 HN or F elicits protective immunity and combines with a second recombinant to prevent hPIV-1, hPIV-3 and RSV infections. Vaccine. 2008;26:3480–3488. doi: 10.1016/j.vaccine.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voges B, Vallbracht S, Zimmer G, Bossow S, Neubert WJ, Richter K, Hobeika E, Herrler G, Ehl S. Recombinant Sendai virus induces T cell immunity against respiratory syncytial virus that is protective in the absence of antibodies. Cell Immunol. 2007;247:85–94. doi: 10.1016/j.cellimm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Bousse T, Matrosovich T, Portner A, Kato A, Nagai Y, Takimoto T. The long noncoding region of the human parainfluenza virus type 1 f gene contributes to the read-through transcription at the m-f gene junction. J Virol. 2002;76:8244–8251. doi: 10.1128/JVI.76.16.8244-8251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuerst TR, Niles EG, Studier FW, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsung K, Yim JH, Marti W, Buller RM, Norton JA. Gene expression and cytopathic effect of vaccinia virus inactivated by psoralen and long-wave UV light. J Virol. 1996;70:165–171. doi: 10.1128/jvi.70.1.165-171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 54.Dave VP, Allan JE, Slobod KS, Smith SF, Ryan K, Powell U, Portner A, Hurwitz JL. Viral cross-reactivity and antigenic determinants recognized by human parainfluenza virus type 1-specific cytotoxic T-cells. Virology. 1994;199:376–383. doi: 10.1006/viro.1994.1135. [DOI] [PubMed] [Google Scholar]
- 55.Ito Y, Tsurudome M, Hishiyama M, Yamada A. Immunological interrelationships among human and non-human paramyxoviruses revealed by immunoprecipitation. J Gen Virol. 1987;68:1289–1297. doi: 10.1099/0022-1317-68-5-1289. [DOI] [PubMed] [Google Scholar]
- 56.Nishio M, Tsurudome M, Bando H, Ito Y. Immunological relationships of simian virus 41 (SV41) to other paramyxoviruses and serological evidence of SV41 infection in human populations. J Gen Virol. 1990;71( Pt 9):2093–2097. doi: 10.1099/0022-1317-71-9-2093. [DOI] [PubMed] [Google Scholar]
- 57.Hurwitz JL. Development of recombinant Sendai virus vaccines for prevention of human parainfluenza and respiratory syncytial virus infections. Pediatr Infect DIs J. 2008;27:S126–S128. doi: 10.1097/INF.0b013e318168b780. [DOI] [PubMed] [Google Scholar]
- 58.Mitchell I, Tough S, Gillis L, Majaesic C. Beyond randomized controlled trials: a “real life” experience of respiratory syncytial virus infection prevention in infancy with and without palivizumab. Pediatr Pulmonol. 2006;41:1167–1174. doi: 10.1002/ppul.20507. [DOI] [PubMed] [Google Scholar]
- 59.Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, Kimpen JL. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 60.Siber GR, Leszcynski J, Pena-Cruz V, Ferren-Gardner C, Anderson R, Hemming VG, Walsh EE, Burns J, McIntosh K, Gonin R. Protective activity of a human respiratory syncytial virus immune globulin prepared from donors screened by microneutralization assay. J Infect Dis. 1992;165:456–463. doi: 10.1093/infdis/165.3.456. [DOI] [PubMed] [Google Scholar]
- 61.Groothuis JR, Simoes EA, Levin MJ, Hall CB, Long CE, Rodriguez WJ, Arrobio J, Meissner HC, Fulton DR, Welliver RC. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 62.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 63.Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. The PREVENT Study Group. Pediatrics. 1997:93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 64.Null D, Jr, Pollara B, Dennehy PH, Steichen J, Sanchez PJ, Givner LB, Carlin D, Landry B, Top FH, Jr, Connor E. Safety and immunogenicity of palivizumab (Synagis) administered for two seasons. Pediatr Infect DIs J. 2005;24:1021–1023. doi: 10.1097/01.inf.0000183938.33484.bd. [DOI] [PubMed] [Google Scholar]
- 65.Saez-Llorens X, Castano E, Null D, Steichen J, Sanchez PJ, Ramilo O, Top FH, Jr, Connor E. Safety and pharmacokinetics of an intramuscular humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. The MEDI-493 Study Group. Pediatr Infect DIs J. 1998;17:787–791. doi: 10.1097/00006454-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 66.Sangster M, Hyland L, Sealy R, Coleclough C. Distinctive kinetics of the antibody-forming cell response to Sendai virus infection of mice in different anatomical compartments. Virology. 1995;207:287–291. doi: 10.1006/viro.1995.1079. [DOI] [PubMed] [Google Scholar]
- 67.Murphy K, Travers P, Walport M. Janeway’s Immunobiology. Garland Science; New York, NY: 2008. [Google Scholar]
- 68.Gray D. A role for antigen in the maintenance of immunological memory. Nat Rev Immunol. 2002;2:60–65. doi: 10.1038/nri706. [DOI] [PubMed] [Google Scholar]
- 69.Ogasawara T, Emoto M, Kiyotani K, Shimokata K, Yoshida T, Nagai Y, Yoshikai Y. Sendai virus pneumonia: evidence for the early recruitment of gamma delta T cells during the disease course. J Virol. 1994;68:4022–4027. doi: 10.1128/jvi.68.6.4022-4027.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmed R. Understanding immunological memory to improve vaccination strategies. Keystone Meeting Presentation: HIV Vaccine Development: Immunological and Biological Challenges; Banff Canada. 2003. [Google Scholar]
- 72.Yu X, Tsibane T, McGraw PA, House FS, Keefer CJ, Hicar MD, Tumpey TM, Pappas C, Perrone LA, Martinez O, Stevens J, Wilson IA, Aguilar PV, Altschuler EL, Basler CF, Crowe JE., Jr Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature. 2008;455:532–536. doi: 10.1038/nature07231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maruyama M, Lam KP, Rajewsky K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature. 2000;407:636–642. doi: 10.1038/35036600. [DOI] [PubMed] [Google Scholar]
- 74.Bousse T, Chambers RL, Scroggs RA, Portner A, Takimoto T. Human parainfluenza virus type 1 but not Sendai virus replicates in human respiratory cells despite IFN treatment. Virus Res. 2006;121:23–32. doi: 10.1016/j.virusres.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 75.Skiadopoulos MH, Surman SR, Riggs JM, Elkins WR, St CM, Nishio M, Garcin D, Kolakofsky D, Collins PL, Murphy BR. Sendai virus, a murine parainfluenza virus type 1, replicates to a level similar to human PIV1 in the upper and lower respiratory tract of African green monkeys and chimpanzees. Virology. 2002;297:153–160. doi: 10.1006/viro.2002.1416. [DOI] [PubMed] [Google Scholar]
- 76.Lee MS, Greenberg DP, Yeh SH, Yogev R, Reisinger KS, Ward JI, Blatter MM, Cho I, Holmes SJ, Cordova JM, August MJ, Chen W, Mehta HB, Coelingh KL, Mendelman PM. Antibody responses to bovine parainfluenza virus type 3 (PIV3) vaccination and human PIV3 infection in young infants. J Infect Dis. 2001;184:909–913. doi: 10.1086/323150. [DOI] [PubMed] [Google Scholar]
- 77.Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, Dormitzer M, O’Grady J, Koenig S, Tamura JK, Woods R, Bansal G, Couchenour D, Tsao E, Hall WC, Young JF. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 78.Murphy BR, Hall SL, Kulkarni AB, Crowe JEJ, Collins PL, Connors M, Karron RA, Chanock RM. An update on approaches to the development of respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3) vaccines. Virus Res. 1994;32:13–36. doi: 10.1016/0168-1702(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 79.Haller AA, Miller T, Mitiku M, Coelingh K. Expression of the surface glycoproteins of human parainfluenza virus type 3 by bovine parainfluenza virus type 3, a novel attenuated virus vaccine vector. J Virol. 2000;74:11626–11635. doi: 10.1128/jvi.74.24.11626-11635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmidt AC, McAuliffe JM, Huang A, Surman SR, Bailly JE, Elkins WR, Collins PL, Murphy BR, Skiadopoulos MH. Bovine parainfluenza virus type 3 (BPIV3) fusion and hemagglutinin-neuraminidase glycoproteins make an important contribution to the restricted replication of BPIV3 in primates. J Virol. 2000;74:8922–8929. doi: 10.1128/jvi.74.19.8922-8929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmidt AC, McAuliffe JM, Murphy BR, Collins PL. Recombinant bovine/human parainfluenza virus type 3 (B/HPIV3) expressing the respiratory syncytial virus (RSV) G and F proteins can be used to achieve simultaneous mucosal immunization against RSV and HPIV3. J Virol. 2001;75:4594–4603. doi: 10.1128/JVI.75.10.4594-4603.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmidt AC, Wenzke DR, McAuliffe JM, St Claire M, Elkins WR, Murphy BR, Collins PL. Mucosal immunization of rhesus monkeys against respiratory syncytial virus subgroups A and B and human parainfluenza virus type 3 by using a live cDNA-derived vaccine based on a host range-attenuated bovine parainfluenza virus type 3 vector backbone. J Virol. 2002;76:1089–1099. doi: 10.1128/JVI.76.3.1089-1099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tao T, Skiadopoulos MH, Davoodi F, Surman SR, Collins PL, Murphy BR. Construction of a live-attenuated bivalent vaccine virus against human parainfluenza virus (PIV) types 1 and 2 using a recombinant PIV3 backbone. Vaccine. 2001;19:3620–3631. doi: 10.1016/s0264-410x(01)00101-3. [DOI] [PubMed] [Google Scholar]
- 84.Tao T, Skiadopoulos MH, Davoodi F, Riggs JM, Collins PL, Murphy BR. Replacement of the ectodomains of the hemagglutinin-neuraminidase and fusion glycoproteins of recombinant parainfluenza virus type 3 (PIV3) with their counterparts from PIV2 yields attenuated PIV2 vaccine candidates. J Virol. 2000;74:6448–6458. doi: 10.1128/jvi.74.14.6448-6458.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng X, Zhou H, Tang RS, Munoz MG, Jin H. Chimeric subgroup A respiratory syncytial virus with the glycoproteins substituted by those of subgroup B and RSV without the M2-2 gene are attenuated in African green monkeys. Virology. 2001;283:59–68. doi: 10.1006/viro.2001.0894. [DOI] [PubMed] [Google Scholar]
- 86.Whitehead SS, Hill MG, Firestone CY, St Claire M, Elkins WR, Murphy BR, Collins PL. Replacement of the F and G proteins of respiratory syncytial virus (RSV) subgroup A with those of subgroup B generates chimeric live attenuated RSV subgroup B vaccine candidates. J Virol. 1999;73:9773–9780. doi: 10.1128/jvi.73.12.9773-9780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jin H, Clarke D, Zhou HZ, Cheng X, Coelingh K, Bryant M, Li S. Recombinant human respiratory syncytial virus (RSV) from cDNA and construction of subgroup A and B chimeric RSV. Virology. 1998;251:206–214. doi: 10.1006/viro.1998.9414. [DOI] [PubMed] [Google Scholar]
- 88.Buchholz UJ, Granzow H, Schuldt K, Whitehead SS, Murphy BR, Collins PL. Chimeric bovine respiratory syncytial virus with glycoprotein gene substitutions from human respiratory syncytial virus (HRSV): effects on host range and evaluation as a live-attenuated HRSV vaccine. J Virol. 2000;74:1187–1199. doi: 10.1128/jvi.74.3.1187-1199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hall SL, Sarris CM, Tierney EL, London WT, Murphy BR. A cold-adapted mutant of parainfluenza virus type 3 is attenuated and protective in chimpanzees. J Infect Dis. 1993;167:958–962. doi: 10.1093/infdis/167.4.958. [DOI] [PubMed] [Google Scholar]
- 90.Karron RA, Wright PF, Newman FK, Makhene M, Thompson J, Samorodin R, Wilson MH, Anderson EL, Clements ML, Murphy BR. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in healthy infants and children. J Infect Dis. 1995;172:1445–1450. doi: 10.1093/infdis/172.6.1445. [DOI] [PubMed] [Google Scholar]
- 91.Karron RA, Wright PF, Hall SL, Makhene M, Thompson J, Burns BA, Tollefson S, Steinhoff MC, Wilson MH, Harris DO. A live attenuated bovine parainfluenza virus type 3 vaccine is safe, infectious, immunogenic, and phenotypically stable in infants and children. J Infect Dis. 1995;171:1107–1114. doi: 10.1093/infdis/171.5.1107. [DOI] [PubMed] [Google Scholar]
- 92.Karron RA, Makhene M, Gay K, Wilson MH, Clements ML, Murphy BR. Evaluation of a live attenuated bovine parainfluenza type 3 vaccine in two- to six-month-old infants. Pediatr Infect DIs J. 1996;15:650–654. doi: 10.1097/00006454-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 93.Girard MP, Cherian T, Pervikov Y, Kieny MP. A review of vaccine research and development: human acute respiratory infections. Vaccine. 2005;23:5708–5724. doi: 10.1016/j.vaccine.2005.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]