Abstract
The hypertonic inner medulla poses challenges to the cells that inhabit this area of the nephron. We employed discovery tools including proteomics and genomics to identify proteins that subserve the adaptive response. The γ subunit of the Na/K-ATPase is critical to the survival of cells in hypertonic conditions, as silencing it increases osmosensitvity, and overexpression increases osmotolerance. The inner medullary collecting duct (IMCD) has high transepithelial resistance (TER). Proteins responsible for tight junction integrity are upregulated in hypertonic states. Multi PDZ protein 1 (MUPP1), a PDZ scaffolding protein, targets Claudin 4 to the tight junction. The silencing of either of these proteins decreases TER and renders the epithelium leaky. The accumulation of inert osmolytes is integral to the adaptive response. The genes involved are regulated by the transcription factor Tonicity Enhancer Binding Protein. An osmoregulated nuclear protein Nup88 is critical to the retention of this transcription factor in the nucleus and to the generation of the osmolytes. In summary, IMCD cells bring forth a coordinated response to hypertoncity that is necessary for cell survival and function of these cells in anisotonic conditions.
Introduction
Homer Smith elegantly narrated in his classic book From Fish to Philosopher, how the kidney played a central role in making possible the adaptation of living organisms in their transition from an aqueous saline to an arid terrestrial environment (1). What made such a transition possible was the evolution of an excretory organ endowed with the ability to conserve water. This conservation required that the urine be concentrated to a level much greater than that of its surrounding tissues. The ability to do so stemmed from the development of a loop like structure, now designated as the Loop of Henley, that serving as a countercurrent multiplier and acting in parallel with an equally designed vascular countercurrent exchanger, allows for the generation of a marked cortico-medullary tonicity gradient, while maintaining a small gradient at any given level of renal tissue (2). In this manner terrestrial organisms have the capacity to excrete highly concentrated urines that range from 1200 mOsm/Kg H2O in humans to levels that approach 5000 mOsm/Kg H2O in some desert rodents (3). The corollary to this evolutionary process is that the cells that inhabit the inner medulla of mammalian kidneys need to adapt to such an inhospitable environment, a process that is critical to the viability of these cells and therefore the integrity and operation of the renal concentrating mechanism. This adaptation appears to be initially mediated by the activation of ion transport systems (4) and subsequently by the generation of inert organic osmolytes such as sorbitol, inositol and betaine (5–8). However, it has been recognized that in addition to the proteins involved in the transport and synthesis of these osmolytes, the maintenance of cell viability calls forth the coordinated upregulation of many other proteins, such as heat shock proteins (9), cyclooxygenase 2 (10), the atrial natriuretic peptide receptor (11), and the cystic fibrosis transmembrane conductance regulator (CFTR) (12), to name a few. In fact, a genomic analysis reveals that hypertonicity brings about the up- and down-regulation of hundreds of genes (13, 14). The present review will describe the use of genomic and proteomic approaches to discover proteins that we felt were critical in making it possible for inner medullary cells to meet three challenges posed by their surrounding, markedly hypertonic environment.
CHALLENGE 1: THE MAINTENANCE OF CELLULAR BIOENERGETICS—THE γ SUBUNIT OF NA/K-ATPASE
Analysis of gene chip data that allowed us to compare cells in isotonic conditions (300mOsm/Kg H2O) to those we adapted to chronically survive at 600 and 900 mOsm/Kg H2O, revealed that the most upregulated gene message was for the γ subunit of Na/K-ATPase. This protein is a member of the FXYD family of proteins (15), also designated as FXYD 2, which is almost exclusively expressed in the kidney (16–18). We confirmed the observation made with the gene chip with protein expression and found that the protein is virtually absent in isotonic conditions and markedly upregulated in hypertonic settings (19). This observation explained the previously reported absence of the protein in cultured renal cells, as these were studied exclusively at isotonic condition. In fact, when the cells that had adapted to hypertonicity were returned to 300mOsm/Kg, protein synthesis ceased and the decay kinetics revealed a T1/2 of 17 hours. Studies were also performed in vivo in mice during ad lib water intake compared to mice given D5W as drinking solution, a maneuver that decreased their urinary osmolality from 3066 to 499 mOsm/Kg. This brought about a highly significant decrement in the abundance of the γ subunit in the inner medulla of such mice.
The above experiments were performed by the addition of NaCl to increase the tonicity of the media. The observed upregulation with NaCl could not be mimicked with either mannitol, an effective nonelectrolyte solute, or with urea which does not set up an effective osmotic gradient. A subsequent series of substitution experiments for sodium with choline chloride or for chloride with sodium acetate pointed to chloride as the mediator of the γ subunit expression, in contrast to the alpha or beta subunit of the pump that is sodium dependent. This was confirmed by the failure of a blocker of sodium entry into the cell, namely amiloride, to alter the protein's expression, in marked contrast to the effect of a chloride channel blocker to profoundly affect the synthesis of the subunit, an intervention that was associated with a marked decrement in cell survival (20).
The signaling pathways that control the synthesis of the γ subunit were also investigated, particularly as we had previously shown that all three (ERK, JNK and P38) of the MAP kinase pathways are activated by hypertonicty (21) and that JNK kinase activation in particular is critical to the response of renal medullary cells to osmotic stress (22). We observed that tonicity-mediated up-regulation of the γ subunit was insensitive to inhibitors of ERK kinase and P38 MAP kinase. In contrast, the pharmacologic inhibition of JNK kinase, as well as the use of dominant negative mutants of this kinase, were associated with failure to synthesize the subunit and with a marked decreased in cell survival. A similar effect on γ subunit synthesis and cell survival was noted when the PI3 kinase pathway was inhibited (19). Subsequent experiments determined that the effect of JNK kinase in the regulation of the protein's synthesis occurrs at the transcriptional level, while the effect of PI3 kinase is at the translational level (23).
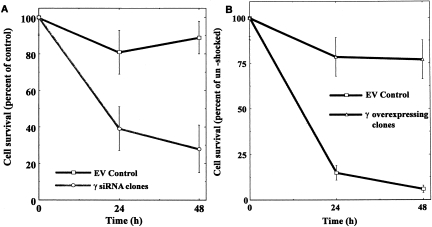
In all of the above described settings (Chloride substitution, JNK inhibition, PI3 kinase inhibition) the failure to generate the γ subunit was associated with a decrement in cell survival under these stress conditions. While such observations strongly suggested a central role for the protein in protecting the viability of inner medullary cells, these were only associations. Clearly, more direct evidence was needed. To this end, we developed clones of these cells that either over-expressed the protein or were silenced for it (24). The osmosensitivity of the cells was tested by acutely exposing the transfected over-expressing clones to osmolalities that are above those usually tolerated by empty vector controls (>550 mOsm/Kg H2O). As depicted in Figure 1, such clones evidenced greater osmotolerance, as they withstood the stress of 675 mOsm/Kg with minimal loss of viability, while the empty vector controls were unable to do so. Conversely, as also depicted in Figure 1, in complementary experiments, the cells that were silenced for the protein, when exposed to tonicities well tolerated by empty vector controls, rapidly lost viability. Taken together, these studies strongly support a critical role for the γ subunit of Na/K-ATPase in allowing cells in the inner medulla to withstand osmotic stress.
Fig. 1.
Panel A: Effect of silencing the γ subunit of Na/K-ATPase on cell survival upon exposure to sublethal hypertonic stress (550 mOsm/Kg H2O). While empty vector (EV) controls, (squares) withstand this stress well, the silenced cells (circles) display significantly decreased survival ability. Panel B: Effect of overexpressing the γ subunit of Na/K-ATPase on cell survival in cells exposed to lethal osmotic stress (675 mOsm/Kg H2O). The empty vector controls (squares) cannot withstand such a stress, the overexpressing cells (triangles) have high levels of survival. Adapted from reference 24.
The mechanism whereby the γ subunit provides osmoprotection has not been fully elucidated. The subunit appears to decrease the activity of Na/K-ATPase, at any given intracellular sodium concentration by decreasing its affinity for sodium (25, 26). Since the adaptive process requires the entry of sodium coupled with the various aforementioned osmolytes, and the prevailing extracellular concentration of sodium is high, cellular sodium concentration is increased in this setting. Such a change in cation concentration would increase the activity of the pump, thereby depleting the energy stores of the cell by diminishing ATP levels. Instead, by decreasing the affinity for sodium, the γ subunit allows the pump to operate at its usual activity level despite the higher sodium concentrations, thereby preserving the bioenergetics of the cell.
CHALLENGE 2. PRESERVATION OF HIGH EPITHELIAL RESISTANCE IN THE INNER MEDULLA—MUPP1 AND CLAUDIN 4
The inner medullary collecting duct of the mammalian nephron, in contrast to the “leaky” proximal tubule, is characterized by low sodium permeability and by a large transepithelial resistance. This characteristic is important in the final control of sodium excretion that occurs almost exclusively by transcellular movement via the epithelial sodium channel and not in paracellular pathway. This is thus a “tight” epithelium. When we examined the differential expression of protein in our antibody array approach to proteomics (27) and gene array, we found that two proteins involved intimately with the tight junction complex, and therefore critical to maintaining the tight characteristics of this segment of the nephron, were markedly upregulated. These proteins were MUPP1 and Claudin 4 (28, 29).
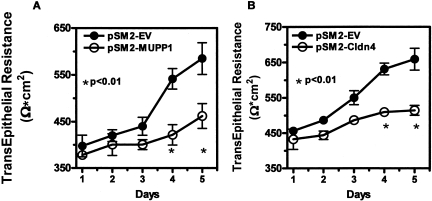
MUPP1 is located at the tight junction of epithelial and endothelial cells where it acts as a scaffolding protein. It interacts with integral proteins, including claudin and junctional adhesion molecules (30) that together with occludins form tight junction strands (31, 32). In addition to confirming the findings seen in the antibody array with quantitative PCR and Western blots, we found that MUPP1 is upregulated by water deprivation in the papilla of mice and much more abundant in the human renal papilla than in the cortex (28). The significance of this increment in MUPP1 expression was assessed by silencing cells for this protein. While such a maneuver had only a minor impact on cell survival, it had profound consequences on the maintenance of transepithelial resistance. This is depicted in the left panel of Figure 2. The transepithelial resistance (TER) was measured daily after cells previously adapted to hypertonicy were seeded on filters. While empty vector control cells progressively increased TER as they achieved confluence, those that were silenced for MUPP1 failed to do so. This was also reflected in a greater paracellular flux of 4kDa fluorescence isotheocyanide (FITC) dextran in the silenced cells (28). We thus concluded that the upregulation of MUPP1 is critical in the maintenance of tight junction integrity, allowing the preservation of the permeability of the tight epithelium.
Fig. 2.
Effect of silencing MUPP1 Panel A and Claudin 4 Panel B on transepithelial resistance (TER) in monolayers of inner medullary collecting duct cells previously adapted to 550 mOsm/Kg H2O, compared to empty vector (EV) control. Under both circumstances the silenced cells generated a significantly lower TER. Adapted from reference 28 and 29.
As mentioned above, our gene chip data also led us to observe the up-regulation of another important tight junction protein, namely claudin 4 (29). This was of interest because two claudins, 1 and 8, had been reported to interact with MUPP1 (31, 33), and claudin 4 was primarily localized to the high resistance segments of the nephron, including the medullary collecting duct (34, 35). A review of our data regarding the claudins revealed that claudin 4 was in fact the most robustly up-regulated member of the family when cells adapted to 600mOsm/Kg were compared to cells living in isotonic condition. In fact, silencing Claudin 4 had effects on TER similar to those we observed with MUPP 1 silenced cells, as depicted in Figure 2. We found that MUPP1 and Claudin 4 co-precipitate in immunoprecipitates and co-localize at the tight junction, as assessed by confocal microspcopy (29). Accompanying studies demonstrated that MUPP1 is necessary for the proper trafficking of claudin 4 to the tight junction; as in MUPP1-deficient cells, claudin 4 was targeted to the lysosome where it was rapidly degraded. In summary, we believe that MUPP1/Claudin 4 up-regulation plays a critical role in maintaining tight junction phenotype in the inner medulla under hypertonic stress.
CHALLENGE 3: ACCUMULATION OF COMPATIBLE OSMOLYTES-NUP88
The accumulation of inert compatible osmolytes is mediated by the enhanced activity of aldose reductase (36) to generate sorbitol, the sodium-myoinositol transporter (36), to accumulate inositol, the betaine/GABA transporter (37), to generate betaine, and the taurine transporter (38), to generate taurine, among others. What these transporters and aldose reductse have in common is that their synthesis involves the transcription of genes that are targets of the tonicty enhancer-binding protein (TonEBP) (6). Under hypertonic stress, TonEBP is rapidly translocated from the cytoplasm to the nucleus where it enhances the transcription of these genes, allowing for the accumulation of the osmolytes. In turn, one of the mechanisms involved in the regulation of TonEBP is nucleo-cytoplasmic trafficking (39–42). In this regard, employing the antibody array we found a marked upregulation of Nup-88 (nucleoporin-88) (43). This protein is a component of the nuclear pore complex in the nuclear membrane involved precisely in the nucleocytoplasmic trafficking of different molecules, including transcription factors (44–47).
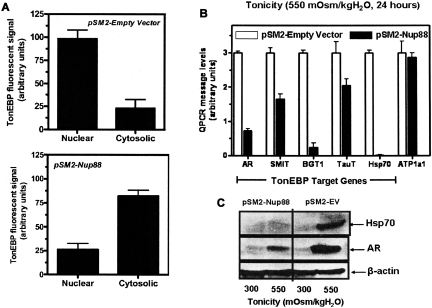
We confirmed the array data with quantitative PCR and western blots for Nup88 and determined its regulation by hydration in mice and its preferential localization to the papilla in human tissue (43). In order to study its effect on TonEBP trafficking, a TonEBP-green fluorescent protein (GFP) construct was prepared. The transcription factor was found to move to the nucleus within 30 minutes of an acute osmotic stress. However, the retention of TonEBP in the nucleus proved to be Nup88-dependent. As is illustrated in Figure 3, panel A, after 8 hours, in empty vector controls, the factor was almost completely retained in the nucleus. In contrast, in Nup88-silenced cells, most of the fluorescence had left the nucleus and returned to the cytoplasm. The significance of this failure to retain the transcription factor in the nucleus is reflected in the marked decrement in message for several TonEBP target genes and down-regulation of the expression of the protein, as illustrated inn Figure 3 B and C, respectively. We therefore feel that the up-regulation of this nucleoporin plays a critical role in allowing the transcription of target genes that are essential for cell adaptation to hypertonicity.
Fig. 3.
Panel A: Effect of silencing Nup 88 on the retention of TonEBP in the nucleus. The upper panel depicts that most of the fluorescent signal for the transcription factor is in the nucleus in the empty vector control, while the lower panel shows that the fluorescence is primarily in the cytoplasm in the silenced cells following 8 hours of exposure to hypertonicity. Panel B: Quantitative PCR for target genes of TonEBP in empty vector controls (open bars) and Nup 88 silenced cells (solid bars) following acute exposure to hypertonicity. Note the decrement in message for all target genes but not for the control alpha subunit of Na K ATPase that is not a target gene for TonEBP. Panel C: A representative Western blot for aldose reductase (AR) and Hsp 70 protein expression in empty vector (EV) and Nup 88 silenced cells. The β actin is a loading control. With permission, from reference 43.
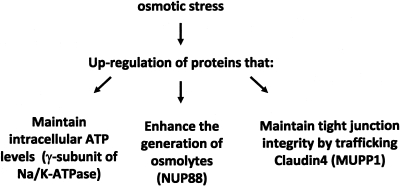
In summary, as depicted in Figure 4, the cells of the mammalian inner medulla muster a variety of adaptive responses. These responses subserve equally diverse functions, all directed at either the maintenance of viability, or at least function, of the cells in this environment. Thus, the presence of the γ subunit in the kidney, by altering the kinetic of the Na/K-ATPase pump allows the cell to preserve its source of energy (ATP) despite higher than normal intracellular sodium concentrations. This protein appears particularly critical to survival, and is a major determinant of osmotolerance. Other proteins such a MUPP1 and Claudin 4 preserve the integrity of the tight junction and thereby high TER that characterizes this segment of the nephron. While this may not be critical to cell viability, it is of great importance in the control of sodium balance. Finally, the generation of inert osmolytes is central to the adaptive response. Many of the genes that generate these osmolytes are under the control of the transcription factor TonEBP. Hypertonicity upregulates Nup88, a nuclear membrane protein involved in nucleocytoplasmic trafficking. Its presence is critical to the retention of TonEBP in the nucleus for the ongoing transcription of its target genes. This protein is therefore a component of the adaptive response that allows for accumulation of inert organic solutes, a central process in the osmoadaptive response. Taken together, the picture that emerges is that of a coordinated response involving numerous pathways that make it possible for inner medullary collecting duct cells to live and operate normally in this inhospitable environment.
Fig. 4.
Coordinated upregulation of proteins to maintain cellular viability and function in IMCD cells.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grants DK-19928 and DK-66544 (to T.B.).
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Mitch, Houston: Thank you Tom for using a variety of techniques to uncover a rather difficult bit of physiology. My question is that some time ago, a lot of these osmolytes were shown in the brain under certain conditions, and I wondered if you had insights into what is regulating the production and decreased elimination of these under conditions in brain cells as well as kidney cells?
Berl, Denver: Yeah, well, that's where the next step should take us because of much subtle changes in osmolality. We kind of use the big club when cells are exposed to 500, 600, 900 miliosmoles. The changes that the brain sustains are more than 10, 20 and, sometimes in extreme situations, 30 or 40 miliosmoles, but some of these adaptive mechanisms clearly occur in the brain, because people who have measured by NMR techniques these organic osmolytes have been found to be modulated, we used to call them idiogenic osmoles in the old days, but NMR techniques have shown the accumulation of all of the organic compounds I outlined, and particularly in the human brain, there is accumulation of inositol. Now these are very important in the protection of cell volume, because if they didn't occur and didn't accumulate or leave the brain in the hypotonic condition, the changes in cell volume that would occur would be incompatible with the constraints of the brain in a fixed skull. On the other hand, the yin yang, to use the TGF beta analogy of having too much or too little, is that when you lose some of these osmolytes from the brain in the adaptation to hypotonic states and then correct the hypotonic state, the reaccumulation of these osmolytes is slow, and that may underlie the pathogenesis of osmotic demyelination. So I think some of this work has to be taken to neural tissue and neural cells and to more subtle changes in osmolality; but the adaptation or failure to adapt that occurs in some young women who develop cerebral edema is also something that deserves some study. There is literature to the fact that premenopausal women don't have the best adaptive mechanisms and are much more prone to cerebral edema when they become hypotonic. So this sort of big club study at 1200 miliosmoles has to be looked at to more subtle changes in neural tissue.
Billings, Baton Rouge: Tom, that was very interesting. Could you comment on the paraneoplastic syndromes of inappropriate ADH and where the anti-mitotic modulation of this phenomenon that is so commonly seen with lung cancer patients how that weaves into this picture?
Berl, Denver: Well yes. You are referring to the fact that the most common cause of the syndrome of inappropriate ADH are the small cell lung cancers. These are tumors that secrete ADH or ADH-like substances and are in clinical medicine, the most common causes of hyponatremia. The patients who have that have undergone this adaptive mechanism, because it takes five to 10 days for some of these osmolytes to leave the brain and we have not studied adaptation to hypotonicity; but I think the opposite of what I have just shown you most likely happens; but most of the patients at least when they have modest decrements in serum sodium are essentially asymptomatic because they have adapted to this process. Now there is a lot of question about whether some of these mildly hyponatremic patients are truly asymptomatic. There is now data suggesting that they have a higher incidence of falls and fractures, particularly from the European literature, which underlies the emergence of vasopressin antagonists which are probably the drugs that you are going to be hearing about in the next year or two-a lot more because the FDA just approved an oral vasopressin antagonist. These are all called “vaptans.” There is an IV form that is available; but the question of whether most of the patients with paraneoplastic syndromes have any symptoms has been somewhat debated, because we have all seen patients walking and talking with sodiums of 128; but what some of the groups have found is that their gait is not entirely normal, and that their risk of falling and fractures is markedly enhanced, especially in the elderly individuals. So the push is, should we correct what we call asymptomatic hyponatremia? And I think we need more studies to assess who should and who shouldn't be treated with these drugs that are going to be out there, and you will hear about them more often than you would like.
REFERENCES
- 1.Smith H. From fish to philosopher: the story of our internal environment. Boston: Little, Brown; 1953. [Google Scholar]
- 2.Sands JM, Kokko JP. Countercurrent system. Kidney Int. 1990;38:695–9. doi: 10.1038/ki.1990.261. [DOI] [PubMed] [Google Scholar]
- 3.Berl T, Schrier RW. Disorders of Water Metabolism. In: Schrier RW, editor. Renal and Electrolyte Disorders. 6 ed. Philadelphia: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 4.Chamberlin ME, Strange K. Anisosmotic cell volume regulation: a comparative view. Am J Physiol. 1989;257:C159–73. doi: 10.1152/ajpcell.1989.257.2.C159. [DOI] [PubMed] [Google Scholar]
- 5.Burg MB, Kwon ED, Kultz D. Regulation of gene expression by hypertonicity. Annu Rev Physiol. 1997;59:437–55. doi: 10.1146/annurev.physiol.59.1.437. [DOI] [PubMed] [Google Scholar]
- 6.Burg MB, Kwon ED, Kultz D. Osmotic regulation of gene expression. FASEB J. 1996;10:1598–606. doi: 10.1096/fasebj.10.14.9002551. [DOI] [PubMed] [Google Scholar]
- 7.Ferraris JD, Burg MB. Tonicity-dependent regulation of osmoprotective genes in Mammalian cells. Contrib Nephrol. 2006;152:125–41. doi: 10.1159/000096320. [DOI] [PubMed] [Google Scholar]
- 8.Handler JS, Kwon HM. Regulation of the myo-inositol and betaine cotransporters by tonicity. Kidney Int. 1996;49:1682–3. doi: 10.1038/ki.1996.246. [DOI] [PubMed] [Google Scholar]
- 9.Neuhofer W, Muller E, Grunbein R, et al. Influence of NaCl, urea, potassium and pH on HSP72 expression in MDCK cells. Pflugers Arch. 1999;439:195–200. doi: 10.1007/s004249900164. [DOI] [PubMed] [Google Scholar]
- 10.Yang T, Huang Y, Heasley LE, et al. MAPK mediation of hypertonicity-stimulated cyclooxygenase-2 expression in renal medullary collecting duct cells. The Journal of Biological Chemistry. 2000;275:23281–6. doi: 10.1074/jbc.M910237199. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Gardner DG. Osmoregulation of natriuretic peptide receptor signaling in inner medullary collecting duct. A requirement for p38 MAPK. The Journal of Biological Chemistry. 2002;277:6037–43. doi: 10.1074/jbc.M111117200. [DOI] [PubMed] [Google Scholar]
- 12.Baudouin-Legros M, Brouillard F, Cougnon M, et al. Modulation of CFTR gene expression in HT-29 cells by extracellular hyperosmolarity. Am J Physiol Cell Physiol. 2000;278:C49–56. doi: 10.1152/ajpcell.2000.278.1.C49. [DOI] [PubMed] [Google Scholar]
- 13.Rivard C, Capasso JM, Heasley LE, et al. A genomic analysis of the effects of adaptation to hypertonicity in inner medullary collecting duct (IMCD3) cells. J Am Soc Nephrol. 2003;14:254. [Google Scholar]
- 14.Tian W, Cohen DM. Urea stress is more akin to EGF exposure than to hypertonic stress in renal medullary cells. American Journal of Physiology. 2002;283:F388–98. doi: 10.1152/ajprenal.00031.2002. [DOI] [PubMed] [Google Scholar]
- 15.Garty H, Karlish SJ. Role of FXYD proteins in ion transport. Annu Rev Physiol. 2006;68:431–59. doi: 10.1146/annurev.physiol.68.040104.131852. [DOI] [PubMed] [Google Scholar]
- 16.Arystarkhova E, Wetzel RK, Sweadner KJ. Distribution and oligomeric association of splice forms of Na(+)-K(+)-ATPase regulatory gamma-subunit in rat kidney. American Journal of Physiology. 2002;282:F393–407. doi: 10.1152/ajprenal.00146.2001. [DOI] [PubMed] [Google Scholar]
- 17.Farman N, Fay M, Cluzeaud F. Cell-specific expression of three members of the FXYD family along the renal tubule. Ann N Y Acad Sci. 2003;986:428–36. doi: 10.1111/j.1749-6632.2003.tb07225.x. [DOI] [PubMed] [Google Scholar]
- 18.Fuzesi M, Gottschalk KE, Lindzen M, et al. Covalent cross-links between the gamma subunit (FXYD2) and alpha and beta subunits of Na,K-ATPase: modeling the alpha-gamma interaction. The Journal of Biological Chemistry. 2005;280:18291–301. doi: 10.1074/jbc.M500080200. [DOI] [PubMed] [Google Scholar]
- 19.Capasso JM, Rivard C, Berl T. The expression of the gamma subunit of Na-K-ATPase is regulated by osmolality via C-terminal Jun kinase and phosphatidylinositol 3-kinase-dependent mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13414–9. doi: 10.1073/pnas.231309198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capasso JM, Rivard CJ, Enomoto LM, et al. Chloride, not sodium, stimulates expression of the gamma subunit of Na/K-ATPase and activates JNK in response to hypertonicity in mouse IMCD3 cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6428–33. doi: 10.1073/pnas.1130871100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berl T, Siriwardana G, Ao L, et al. Multiple mitogen-activated protein kinases are regulated by hyperosmolality in mouse IMCD cells. Am J Physiol. 1997;272:F305–11. doi: 10.1152/ajprenal.1997.272.3.F305. [DOI] [PubMed] [Google Scholar]
- 22.Wojtaszek PA, Heasley LE, Siriwardana G, et al. Dominant-negative c-Jun NH2-terminal kinase 2 sensitizes renal inner medullary collecting duct cells to hypertonicity-induced lethality independent of organic osmolyte transport. The Journal of Biological Chemistry. 1998;273:800–4. doi: 10.1074/jbc.273.2.800. [DOI] [PubMed] [Google Scholar]
- 23.Capasso JM, Rivard CJ, Berl T. Synthesis of the Na-K-ATPase gamma-subunit is regulated at both the transcriptional and translational levels in IMCD3 cells. American Journal of Physiology. 2005;288:F76–81. doi: 10.1152/ajprenal.00026.2004. [DOI] [PubMed] [Google Scholar]
- 24.Capasso JM, Rivard CJ, Berl T. Silencing and overexpression of the gamma-subunit of Na-K-ATPase directly affect survival of IMCD3 cells in response to hypertonic stress. American Journal of Physiology. 2006;291:F1142–7. doi: 10.1152/ajprenal.00077.2006. [DOI] [PubMed] [Google Scholar]
- 25.Therien AG, Karlish SJ, Blostein R. Expression and functional role of the gamma subunit of the Na, K-ATPase in mammalian cells. The Journal of Biological Chemistry. 1999;274:12252–6. doi: 10.1074/jbc.274.18.12252. [DOI] [PubMed] [Google Scholar]
- 26.Arystarkhova E, Wetzel RK, Asinovski NK, et al. The gamma subunit modulates Na(+) and K(+) affinity of the renal Na-K-ATPase. The Journal of Biological Chemistry. 1999;274:33183–5. doi: 10.1074/jbc.274.47.33183. [DOI] [PubMed] [Google Scholar]
- 27.Anderson K, Potter A, Baban D, et al. Protein expression changes in spinal muscular atrophy revealed with a novel antibody array technology. Brain. 2003;126:2052–64. doi: 10.1093/brain/awg208. [DOI] [PubMed] [Google Scholar]
- 28.Lanaspa MA, Almeida NE, Andres-Hernando A, et al. The tight junction protein, MUPP1, is up-regulated by hypertonicity and is important in the osmotic stress response in kidney cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13672–7. doi: 10.1073/pnas.0702752104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanaspa MA, Andres-Hernando A, Rivard CJ, et al. Hypertonic stress increases claudin-4 expression and tight junction integrity in association with MUPP1 in IMCD3 cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15797–802. doi: 10.1073/pnas.0805761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeansonne B, Lu Q, Goodenough DA, et al. Claudin-8 interacts with multi-PDZ domain protein 1 (MUPP1) and reduces paracellular conductance in epithelial cells. Cell Mol Biol (Noisy-le-grand) 2003;49:13–21. [PubMed] [Google Scholar]
- 31.Koval M. Claudins—key pieces in the tight junction puzzle. Cell Commun Adhes. 2006;13:127–38. doi: 10.1080/15419060600726209. [DOI] [PubMed] [Google Scholar]
- 32.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–35. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 33.Hamazaki Y, Itoh M, Sasaki H, et al. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. The Journal of Biological Chemistry. 2002;277:455–61. doi: 10.1074/jbc.M109005200. [DOI] [PubMed] [Google Scholar]
- 34.Balkovetz DF. Claudins at the gate: determinants of renal epithelial tight junction paracellular permeability. American Journal of Physiology. 2006;290:F572–9. doi: 10.1152/ajprenal.00135.2005. [DOI] [PubMed] [Google Scholar]
- 35.Kiuchi-Saishin Y, Gotoh S, Furuse M, et al. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–86. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- 36.Miyakawa H, Woo SK, Dahl SC, et al. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2538–42. doi: 10.1073/pnas.96.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyakawa H, Woo SK, Chen CP, et al. Cis- and trans-acting factors regulating transcription of the BGT1 gene in response to hypertonicity. Am J Physiol. 1998;274:F753–61. doi: 10.1152/ajprenal.1998.274.4.F753. [DOI] [PubMed] [Google Scholar]
- 38.Ito T, Fujio Y, Hirata M, et al. Expression of taurine transporter is regulated through the TonE (tonicity-responsive element)/TonEBP (TonE-binding protein) pathway and contributes to cytoprotection in HepG2 cells. Biochem J. 2004;382:177–82. doi: 10.1042/BJ20031838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeon US, Kim JA, Sheen MR, et al. How tonicity regulates genes: story of TonEBP transcriptional activator. Acta Physiol (Oxf) 2006;187:241–7. doi: 10.1111/j.1748-1716.2006.01551.x. [DOI] [PubMed] [Google Scholar]
- 40.Woo SK, Lee SD, Kwon HM. TonEBP transcriptional activator in the cellular response to increased osmolality. Pflugers Arch. 2002;444:579–85. doi: 10.1007/s00424-002-0849-2. [DOI] [PubMed] [Google Scholar]
- 41.Cha JH, Woo SK, Han KH, et al. Hydration status affects nuclear distribution of transcription factor tonicity responsive enhancer binding protein in rat kidney. J Am Soc Nephrol. 2001;12:2221–30. doi: 10.1681/ASN.V12112221. [DOI] [PubMed] [Google Scholar]
- 42.Tong EH, Guo JJ, Huang AL, et al. Regulation of nucleocytoplasmic trafficking of transcription factor OREBP/TonEBP/NFAT5. The Journal of Biological Chemistry. 2006;281:23870–9. doi: 10.1074/jbc.M602556200. [DOI] [PubMed] [Google Scholar]
- 43.Andres-Hernando A, Lanaspa MA, Rivard CJ, et al. Nucleoporin 88 (Nup88) is regulated by hypertonic stress in kidney cells to retain the transcription factor tonicity enhancer-binding protein (TonEBP) in the nucleus. The Journal of Biological Chemistry. 2008;283:25082–90. doi: 10.1074/jbc.M802381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hutten S, Kehlenbach RH. Nup214 is required for CRM1-dependent nuclear protein export in vivo. Mol Cell Biol. 2006;26:6772–85. doi: 10.1128/MCB.00342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roth P, Xylourgidis N, Sabri N, et al. The Drosophila nucleoporin DNup88 localizes DNup214 and CRM1 on the nuclear envelope and attenuates NES-mediated nuclear export. J Cell Biol. 2003;163:701–6. doi: 10.1083/jcb.200304046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uv AE, Roth P, Xylourgidis N, et al. members only encodes a Drosophila nucleoporin required for rel protein import and immune response activation. Genes Dev. 2000;14:1945–57. [PMC free article] [PubMed] [Google Scholar]
- 47.Xylourgidis N, Roth P, Sabri N, et al. The nucleoporin Nup214 sequesters CRM1 at the nuclear rim and modulates NFkappaB activation in Drosophila. J Cell Sci. 2006;119:4409–19. doi: 10.1242/jcs.03201. [DOI] [PubMed] [Google Scholar]