Abstract
The Institute of Medicine recently concluded that-on average-medical residents make more serious medical errors and have more motor vehicle crashes when they are deprived of sleep. In the interest of public safety, society has required limitations on work hours in many other safety sensitive occupations, including transportation and nuclear power generation. Those who argue in favor of traditional extended duration resident work hours often suggest that there are inter- individual differences in response to acute sleep loss or chronic sleep deprivation, implying that physicians may be more resistant than the average person to the detrimental effects of sleep deprivation on performance, although there is no evidence that physicians are particularly resistant to such effects. Indeed, recent investigations have identified genetic polymorphisms that may convey a relative resistance to the effects of prolonged wakefulness on a subset of the healthy population, although there is no evidence that physicians are over-represented in this cohort. Conversely, there are also genetic polymorphisms, sleep disorders and other inter-individual differences that appear to convey an increased vulnerability to the performance-impairing effects of 24 hours of wakefulness. Given the magnitude of inter-individual differences in the effect of sleep loss on cognitive performance, and the sizeable proportion of the population affected by sleep disorders, hospitals face a number of ethical dilemmas. How should the work hours of physicians be limited to protect patient safety optimally? For example, some have argued that, in contrast to other professions, work schedules that repeatedly induce acute and chronic sleep loss are uniquely essential to the training of physicians. If evidence were to prove this premise to be correct, how should such training be ethically accomplished in the quartile of physicians and surgeons who are most vulnerable to the effects of sleep loss on performance without unacceptably compromising patient safety? Moreover, once it is possible to identify reliably those most vulnerable to the adverse effects of sleep loss on performance, will academic medical centers have an obligation to evaluate the proficiency of both residents and staff physicians under conditions of acute and chronic sleep deprivation? Should work-hour policy limits be modified to ensure that they are not hazardous for the patients of the most vulnerable quartile of physicians, or should the limits be personalized to enable the most resistant quartile to work longer hours? Given that the prevalence of sleep disorders has increased in our society overall, and increases markedly with age, how should fitness for extended duration work hours be monitored over a physician's career? In the spirit of the dictum to do no harm, advances in understanding the medical and genetic basis of inter-individual differences in the performance vulnerability to sleep loss should be incorporated into the development of work-hour policy limits for both physicians and surgeons.
After a comprehensive review of the medical and scientific literature as part of a year-long study initiated at the behest of the United States Congress and supported by the Agency for Healthcare Research and Quality, the Institute of Medicine (IOM) Committee on Optimizing Graduate Medical Trainee (Resident) Hours and Work Schedules to Improve Patient Safety concluded this past year that working for more than 16 consecutive hours without sleep is unsafe for both physicians in training (residents) and their patients (1). This latest IOM report builds on earlier work by the IOM Committee on Sleep Medicine and Research, which conducted an independent review of the adverse effects of sleep loss on neurobehavioral performance, learning, cognition, health and safety (2). The IOM recommendations are consistent with the recommendations promulgated by the Association for American Medical Colleges (AAMC) in 2001 that resident physician work shifts be limited to no more than 12 consecutive hours in all high intensity clinical settings, such as intensive care units (ICUs) and emergency departments (3).
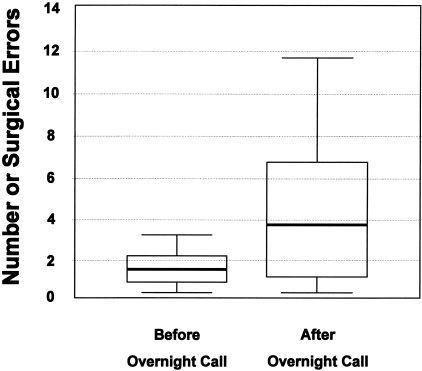
Nearly forty years ago, it was recognized that—on average—interns made twice as many mistakes detecting cardiac arrhythmias when they were sleep deprived as compared to when they were more well rested (4). More recently, laparoscopic surgical simulator studies have revealed that, on average, error rates doubled in residents when they performed simulated surgery after a night on call as compared to when they were more well rested (5) (Figure 1). In a meta-analysis cited by the IOM, which was conducted by Accreditation Council on Graduate Medical Education (ACGME) Senior Vice President of Field Activities, Dr. Ingrid Philibert, she concluded that after 24–30 hours of sleep deprivation, individual cognitive performance of the average person drops from the 50th to the 15th percentile of their performance when rested (6), and that clinical performance of physicians drops from the 50th to the 7th percentile of their performance when rested (6). There is also growing evidence that sleep plays an essential role in learning and memory consolidation, and that chronic sleep deprivation has many deleterious health consequences, including an increased risk of obesity, diabetes, depression and cardiovascular disease (2, 7–15).
Fig. 1.
Effect of Partial Sleep Loss during Hospital Call on Errors Committed by Surgeons during Simulated Surgery. Simulated laparoscopic surgery performance of surgical residents (median age 34) measured by a laparoscopic surgery simulator (task 6 of the MIST-VR, Mentice Medical Simulation, Gothenburg, Sweden) before and after a night on call (17.5 hours from 3:30 pm to 9 am; median reported sleep time 1.5 h; range 0–3 h). Horizontal bands indicate median number of errors on this task, boxes show 25th and 75th centiles, and whisker lines show the highest and lowest error values. Figure and legend reprinted with permission from: Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. BMJ 2001;323:1222–1223 (5).
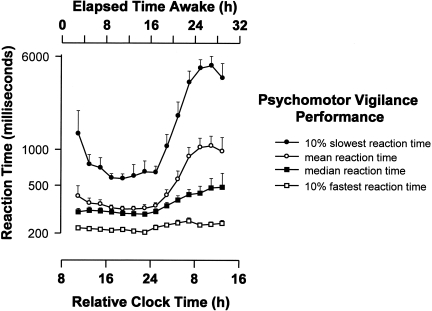
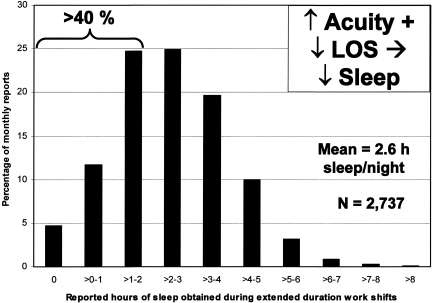
The conclusion that acute sleep loss impairs both cognitive and psychomotor performance and increases the risk of error among healthy young adults in both laboratory evaluations (16, 17) and in clinical settings (18) is consistent with previously published laboratory research on participant volunteers (2, 19, 20) and with the field data (21–26). As shown in Figure 2, sleep deprivation combined with misalignment of circadian phase creates a critical zone of vulnerability in the latter half of the night (17). Paradoxically, instead of slowing down to preserve accuracy as reaction time increases, sleep deprivation often leads people to make hasty decisions based on inadequate information (27). This results in increased rates of errors on selective attention tasks that require a search for targets in the visual field (27). Forty percent of interns working extended duration 'on call' shifts slept less than 2 hours in a 30-hour interval, and more than 15% obtained less than 1 hour of sleep during that interval (24) (Figure 3). Residents randomized to work extended duration (>24 hour) shifts experienced twice as many attentional failures (22) and—on average—made 36% more serious errors caring for patients in intensive care units, including 460% more serious diagnostic mistakes, as compared to the same physicians when they were scheduled to work no more than 16 consecutive hours in intensive care units (23). Nationwide, interns have a 168% increased risk of a motor vehicle crash driving home from extended duration (>24-hour) shifts (24) and were 73% more likely to suffer from a percutaneous injury when performing a procedure after their 20th hour of work than during shifts that averaged less than 12 hours in duration (26).
Fig. 2.
Impact of Acute Total Sleep Deprivation on Reaction Time Performance. Time course of psychomotor vigilance task (PVT) performance [mean, median, 10% slowest and fastest reaction times in milliseconds (logarithmic scale)] during more than 28 hours of continuously monitored wakefulness under constant environmental and behavioral conditions are shown averaged across 10 subjects ± standard error of the mean. All data are binned from 10-minute PVT tests administered at 2-hour intervals and expressed with respect to elapsed time since wake time (designated as a Relative Clock Hour of 8), which was scheduled at its habitual hour. Figure and legend reprinted with permission from: Cajochen C, Khalsa SBS, Wyatt JK, Czeisler CA, Dijk D-J. Am J Physiol 277: R640–R649, 1999 (17).
Fig. 3.
Sleep Duration in the Hospital. Average hours that interns reportedly slept in the hospital during extended duration (greater than 24 hour) on-call shifts as a percentage of 17,003 monthly survey reports collected from 2,737 resident physicians. Average hours of sleep obtained during extended duration shifts reported by interns for each week were averaged over the four weeks of each month to derive the value for each monthly survey report. Figure and legend reprinted with permission from: Barger LK, Cade BE, Ayas N, Cronin JW, Rosner B, Speizer FE, Czeisler CA, N Engl J Med 2005;352:125–134 (24).
On average, after being awake for 24 hours, impairment on a simple reaction time test in individuals who remain awake during testing is comparable with the impairment observed at a blood alcohol concentration of 0.10 g/dL (17, 28). In a survey of 2,737 resident physicians, our group found that one out of five first-year residents reported making a fatigue-related mistake that injured a patient, and one out of twenty first-year residents reported making a fatigue-related mistake that resulted in the death of a patient (25). In months when interns worked more than 5 extended duration (>24-hour) work shifts, the risks of such fatigue-related injuries and deaths increased by 700% and 300%, respectively.
Many within and outside the profession of medicine have hailed the IOM report as a long- overdue acknowledgement by the medical profession of the dangers of an obviously hazardous tradition (29). Yet, many conscientious physicians and surgeons argue vehemently that working for 24 consecutive hours is safe and that working such long hours is essential for the training of physicians and inherent in the practice of medicine (30, 31). What might be fueling such a passionate difference of opinion?
Wherever I have presented data on the average impact of sleep loss on performance, the most common question that I have been asked is about inter-individual differences. “Aren't some people more resistant to the effects of sleep loss than others?” The follow-up comments by such questioners usually reveals an underlying premise that individuals in certain professions (e.g., police officers, fire fighters, physicians, surgeons, aviators, special forces, astronauts) may be more resistant than the average person to the effects of acute sleep loss or chronic sleep restriction on performance. In fact, even among healthy young adults, there are profound inter-individual differences in the impact of sleep loss on performance (2, 32–38). In addition, healthy sleepers are likely to be much more resistant to the effects of sleep loss than the 50 to 70 million Americans who suffer from chronic disorders of sleep and wakefulness (2).
INTER-INDIVIDUAL DIFFERENCES IN TOLERANCE TO SLEEP LOSS IN HEALTHY YOUNG ADULTS
Differences in Vulnerability to Sleep Loss Due to Prior Sleep-wake History
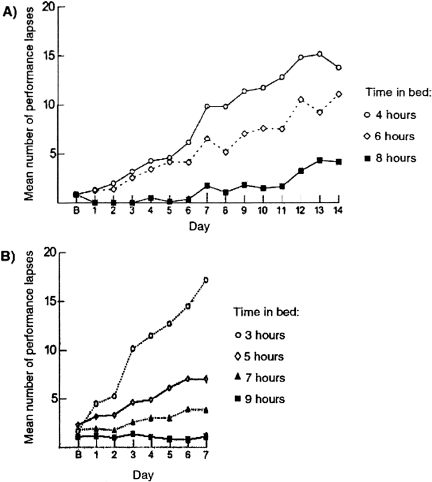
The ability to sustain attention, maintain cognitive performance and prevent attentional failures deteriorates when sleep is chronically restricted to 7 or fewer hours per night for a week or longer (2, 39–41) (Fig. 4). Many habitually short sleepers may be harboring substantial sleep debt, as reflected by higher sleep tendency, suggesting that their habitually short sleep durations may not reflect a reduced underlying sleep need (42, 43). Long work weeks together with commuting time often require those, who are not habitually short sleepers, to restrict the time available for sleep. This occurs to physicians during residency training, when weekly sleep durations are curtailed as weekly work hours increase (22, 24, 44). Yet, our data also reveal up to a two-hour difference in average nightly sleep durations among interns working the same number of hours per week in an ICU (22).
Fig. 4.
Repeated Nights of Sleep Loss Result in Cumulative Cognitive Impairment. Higher number of attentional performance failures on the PVT indicates poorer performance and more unstable alertness. Panel A shows the average number of lapses of attention recorded during 10- minute PVT tests administered eight times daily (every two hours) during 14 consecutive days when the amount of time in bed was limited to 4 hours, 6 hours or 8 hours per night. Lower panel shows the average number of lapses of attention recorded during 10-minute PVT tests administered four times daily during 7 consecutive days when the amount of time in bed was limited to 3 hours, 5 hours, 7 hours or 9 hours per night. Panel B signifies data from the baseline day. Figure and legend reprinted with permission from the 2006 IOM Report entitled: Sleep Deprivation and Sleep Disorders: An Unmet Public Health Problem (2).
There are many factors that may lead to the inter-individual differences in the average amount of sleep obtained by residents working the same number of hours in the hospital, including differences in their responsibilities outside the hospital. The very concept of residency has changed since it was first introduced in the U.S. at the Johns Hopkins University School of Medicine by Professor William Stewart Halsted in the 1890s. At the time, residents were required to live at the hospital and were discouraged from marrying (45). Today residents live outside the hospital, and many of these young physicians have families and become new parents during residency. The sleep of new mothers and fathers is often interrupted and curtailed by newborns, resulting in increased daytime fatigue (46, 47). Multiple births exacerbate post-partum sleep deprivation and daytime fatigue, particularly for fathers (48). Children with sleep or health problems may also lead to sleep fragmentation, sleep deprivation and increased daytime fatigue in parents (49, 50). Behaviorally induced insufficient sleep syndrome affects 7% of patients with excessive daytime sleepiness (EDS) in Japan and is associated with a high motor vehicle crash risk (51). Both acute and chronic sleep loss, whether caused by work schedules, family responsibilities, social activities or health problems, will degrade tolerance and increase performance vulnerability to the sleep deprivation imposed by extended duration (>24-hour) work shifts.
Moreover, preliminary evidence suggests that even after 8 to 12 hours of recovery sleep, individuals with a history of recent exposure to chronic sleep loss may be more vulnerable to the effects of re-exposure to sleep restriction, exhibiting nearly double the deterioration in performance on a vigilance task upon acute sleep restriction to 4 hours of time in bed as compared with their response to the same challenge when their recovery sleep was not preceded by exposure to chronic sleep debt (52). Thus, resident work schedules that cause chronic sleep restriction and provide for only one night of recovery sleep per week may generate a deterioration of performance that becomes progressively greater with additional weeks of sleep curtailment. Despite intermittent opportunities for recovery sleep, individuals exposed to such schedules may become increasingly vulnerable to the adverse effects of sleep loss on performance. Four weeks of scheduled sleep extension (14 hours per night) can reduced fatigue and increased energy levels, but only after an average of more than 30 hours of accumulated sleep debt has been repaid (53). Irregularity in the timing of sleep and wakefulness can impede circadian adaptation to altered work-rest schedules (54); loss of circadian entrainment, in turn, impairs cognitive performance (55). Thus, available evidence indicates that prior sleep-wake history, including exposure to both acute and chronic sleep restriction, to sleep extension, and to irregular sleep-wake schedules, may greatly affect an individual's resiliency in the face of sleep loss (2).
Age-related Differences in the Vulnerability to Sleep Loss
Thirty consecutive hours of sleep loss induces marked deterioration in neurobehavioral functions in health young participants (17). So long as participants remain awake, average reaction time after 24 hours without sleep is three times longer than when the same individuals are rested (17) (Figure 2). The risk of lapses of attention is much higher during the latter half of the night, with the slowest 10% of reaction times lengthening to nearly six seconds after 24 hours without sleep (17). Remarkably, the impact of a single night of acute sleep deprivation on such measures is much less pronounced in older participants over the age of 55 years (56–58) (Figure 5). This may be due to age-related loss of neurons in the ventro-lateral pre- optic (VLPO) area of the hypothalamus, which enables mammals to make the transition from wakefulness to sleep; age-related loss of VLPO neurons may also explain why the prevalence of insomnia rises with age. Because older people have greater difficulty obtaining recovery sleep at an adverse circadian phase (59), they are more vulnerable to the effects of a sequence of night shifts (60). Nonetheless, the data reveal that young adults are most severely affected by acute sleep deprivation; in fact, 55% of sleep-related motor vehicle crashes occur in drivers age 25 years or younger (61) (Figure 6). Thus, it is both ironic and alarming that young resident physicians in this most vulnerable age group are subjected to acute sleep deprivation during 30-hour shifts twice per week while caring for patients and these young physicians then attempt to commute home from work.
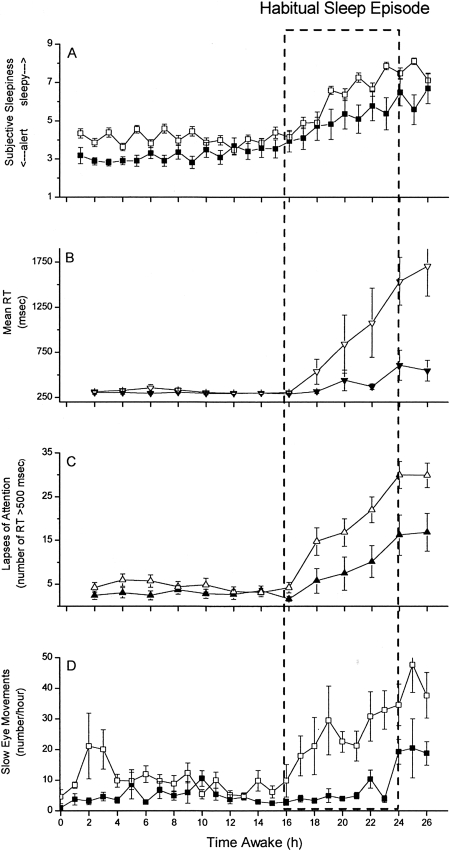
Fig. 5.
Subjective Sleepiness, Reaction Time, Lapses of Attention, and Attentional Failures Across 26 Hours of Wakefulness in Young and Older Participants. Group average data (+ standard error of the mean) are plotted with respect to time since scheduled awakening for 11 healthy older (mean age 68.1 ± 3.6 years; range 65 to 76 years; filled symbols) and 26 healthy young (mean age 21.9 ± 3.3 years; range 18 to 29 years; open symbols) adults. Dashed box indicates time of usual sleep episode. Subjective sleepiness ratings from the Karolinska Sleepiness Scale (KSS; scale range from 1=very alert to 9=very sleepy) are presented in Panel A. Mean reaction time (RT, in milliseconds) from each 10-minute PVT is presented in Panel B. As indicated in the first 16 hours of data, the RT on the PVT in well-rested individuals averages ∼250 milliseconds during the daytime, with minimal variability. The total number of lapses of attention (RT >500 milliseconds) from each 10-minute PVT are presented in Panel C. Well-rested individuals typically have very few (<5 per test administration) lapses of attention under these conditions. Attentional failures, defined as intrusions of slow eye movements (SEM) from continuous electro-oculographic recordings during EEG-verified wakefulness, were summed hourly and are presented in Panel D. Well-rested individuals typically have very few SEM under these conditions. Figure and legend reprinted with permission from: Duffy JD, Willson HJ, Wang W, Czeisler CA. J Am Geriatr Soc 2009; In Press. (56).
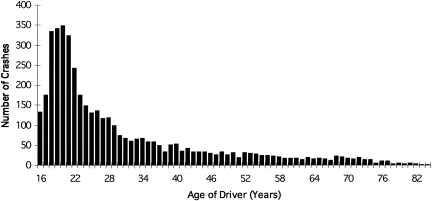
Fig. 6.
Age Distribution of Crashes in Which Driver Was Not Intoxicated But Judged to Have Been Asleep. Data from 4,333 fall asleep crashes in North Carolina, where standard crash report forms, as mandated by state law, include ‘fatigued’ and ‘asleep’ in driver condition. Data for years 1990-1992, inclusive. In 55% of fall-asleep crashes, driver was age 25 or younger. Peak age of crashes was at age 20 years. Figure and legend reprinted with permission from: Pack AI, Pack AM, Rodgman E, Cucchiara A, Dinges DF, Schwab CW. Accid Anal Prev 27: 769, 1995 (61).
Trait Differences in Vulnerability to Sleep Loss
There are also inter-individual differences in the vulnerability of well-rested, healthy young adults to sleep loss. In my laboratory, among healthy participants who have maintained a schedule of 8 hours of sleep at the same time each night for three weeks, we have found that approximately one-quarter of the participants account for roughly two-thirds of the attentional failures recorded during ∼30 hours of wakefulness. Interestingly, inter- individual differences in the vulnerability to sleep loss in healthy young adults appear to be task- dependent, such that reaction time in a sustained attention task is most affected by sleep loss in some individuals as compared to others, whereas sleep loss most adversely affects working memory in other individuals (32, 34). Van Dongen and his colleagues (32) have found that when subjects are brought back to the laboratory for repeat visits, the same ones remain particularly vulnerable while others remain resistant to the adverse effects of sleep loss on cognitive performance. While all the subjects studied had more lapses of attention when performing the psychomotor vigilance task (PVT, a measure of simple reaction time) after a night without sleep, some individuals averaged only 10 lapses per 20-minute PVT test (about one lapse of attention every 2 minutes), whereas other individuals experienced nearly 100 lapses of attention per 20-minute PVT test (nearly five lapses of attention every minute) (32) (Figure 7). In a relatively homogeneous sample of healthy young adults, these investigators reported that stable individual trait differences accounted for 67% to 92% of the variance in the cognitive performance decrement induced by sleep loss. These inter-individual differences appear to be trait differences, since they persist even when participants are either comparably sleep satiated (12 hours in bed per night for a week) or comparably sleep restricted (6 hours in bed per night for a week) before they stay awake all night (32).
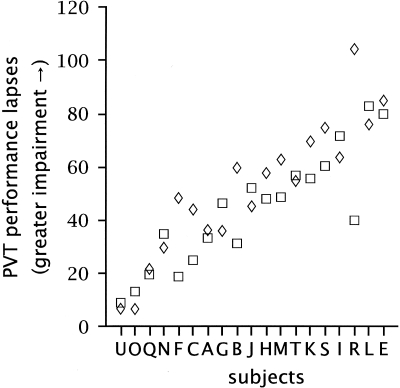
Fig. 7.
Neurobehavioral responses to total sleep deprivation after two different prior sleep extension conditions. Each episode of sleep deprivation followed a week during which time the participants spent 12 hours in bed each night. The average number of lapses on 20-minute PVT tests administered every two hours over the last 24 hours of total sleep deprivation is shown. The abscissa shows the 19 individual participants, who were arbitrarily assigned the labels A through U. The participants are ordered by the magnitude of their impairment (averaged over the 2 sleep deprivations), with the most resistant participants on the left and the most vulnerable participants on the right. Responses in the first exposure to sleep deprivation following 7 days of prior sleep extension (mean ± standard deviation of the average nightly sleep duration of 8.5 ± 1.0 hours) are marked by boxes; responses in the second exposure to sleep deprivation following 7 days of prior sleep extension (mean ± standard deviation of the average nightly sleep duration of 8.8 ± 1.3 hours) are marked by diamonds. The panel reveals that subjects differed substantially in their responses to acute sleep deprivation, while the responses were relatively stable within subjects between the two exposures to acute sleep deprivation. Figure and legend reprinted with permission from: Van Dongen HPA, Baynard MD, Maislin G, Dinges DF. Sleep 2004; 27:423–433 (32).
While the performance of most people deteriorates significantly when wakefulness extends beyond 16–18 hours, there are considerable inter-individual differences in the magnitude of this effect. There are likely also inter-individual differences in the effects of exposure to chronic partial sleep deprivation for several weeks.
Potential Genetic Vulnerability to Sleep Loss
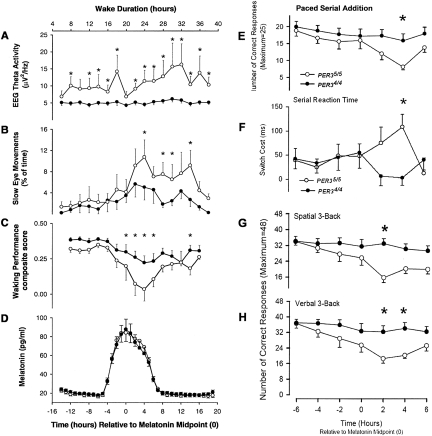
Genetic polymorphisms may account for differences in the tolerability to acute sleep loss. A variable length tandem repeat polymorphism in the PER3 gene (PER35/5) has been reported to confer a particular vulnerability to the performance- impairing effects of 24 hours of wakefulness in those with 5 rather than 4 copies of the sequence on both chromosomes (62, 63). This polymorphism is present in about 10–15% of the population. When individuals with the PER35/5 polymorphism are deprived of sleep at an adverse circadian phase, their cognitive performance at night is significantly worse than individuals with the PER34/4 genotype (63) (Figure 8). Individuals with the PER35/5 polymorphism reportedly experience significantly more attentional failures, as measured by slow rolling eye movements, than individuals with the PER34/4 polymorphism. Executive functioning of the brain's pre-frontal cortex is said to be particularly compromised by the PER35/5 polymorphism. On the other hand, a haplotype of the adenosine A2A receptor gene (ADORA2A), may be associated with resistance to the effects of sleep loss on performance (64). These preliminary data suggest that it may soon be possible to identify from a simple cheek swab a subset of individuals who will exhibit minimal perform degradation when deprived of sleep and another subset whose performance will deteriorate markedly when they are sleep deprived.
Fig. 8.
Polymorphism in Period Gene and Vulnerability to Sleep Loss. Left Hand Side: Deterioration of waking performance and increase of theta EEG activity and slow eye movements during sleep deprivation is greater in PER35/5 than in PER34/4 participants. Time course of central EEG theta (5–8 Hz) activity during wakefulness (Panel A), incidence of slow eye movement (SEMs) (percentage of 30 s epochs containing at least one SEM) (Panel B), and waking performance (composite performance score) (Panel C) are plotted relative to the timing of the plasma melatonin rhythm (Panel D) in ten PER35/5 (open symbols) and 14 PER34/4 (filled symbols) homozygotes. EEG theta activity, SEMs, and waking performance data were averaged per 2-hour intervals, relative to the midpoint of the melatonin rhythm. (* indicates a significant difference between genotypes, p < 0.05; upper abscissa indicates approximate wake duration.) Right Hand Side: Overnight performance on the paced visual serial addition task in PER35/5 and PER34/4 participants. Mean numbers of correct responses are plotted relative to the midpoint of the melatonin rhythm (Panel E). Overnight performance on the serial reaction time task in PER35/5 and PER34/4 participants. Mean switch costs, i.e., increase in time to respond to random rather than learned sequences of stimuli, are plotted relative to the melatonin midpoint. Higher values indicate poorer performance (Panel F). Overnight performance on spatial N-Back performance in relation to memory load in PER35/5 and PER34/4 participants. Mean numbers of correct responses, are plotted separately for the 1-, 2-, and 3-back, relative to the melatonin midpoint (Panel G). Overnight performance on verbal N-Back performance in relation to memory load in PER35/5 and PER34/4 participants. Mean numbers of correct responses are plotted separately for the 1-, 2-, and 3-back, relative to the melatonin midpoint (Panel H). * P < 0.05, Bonferroni corrected. Error bars represent the standard error of the mean. Figures and legends reprinted with permission from: Viola et al., Curr Biol, 2007 (62) (left hand side); and Groeger JA, Viola AU, Lo JC, von Schantz M, Archer SN, Dijk DJ. Sleep 2008;31:1159 (63) (right hand side).
FOOD, DRUGS AND PHARMACEUTICAL AGENTS
Soporific Agents
Sleepiness is one of the most frequent side effects of prescription medications, including some anti-depressants, statins, anti-hypertensive agents, hypnotics, anti-anxiety medications, pain management medications, anti-epileptic agents, muscle relaxants and medications to block stomach-acid secretion. Many common over-the-counter drugs have effects on sleep propensity. For example, diphenhydramine is sold under various trade names, including Benadryl® for relief of allergies. Few who take diphenhydramine for allergies realize that, because of its action as an anti-histamine, the very same drug—diphenhydramine—is also the active ingredient in many of the most popular over-the-counter sleeping pills in the U.S. It is marketed as an over-the-counter sleep aid under the trade names Unisom,® Nytol,® Sominex,® Tylenol Simply Sleep® and, with acetaminophen, in Tylenol PM.® Thus, many people attempting to control their allergies during the daytime may be unwittingly taking a sleeping pill on their way to work, and thereby greatly reducing their tolerance to sleep loss. Diphenhydramine affects the areas of the brain that control the transition from wakefulness to sleep and thereby impairs performance. Even some newer ‘non- sedating’ anti-histamines cause drowsiness in a substantial fraction of patients. Some decongestants, in turn, disrupt nocturnal sleep, which can increase sleep pressure the following day.
Stimulants and Drugs of Abuse
Stimulants and drugs of abuse can have a profound effect on the ability to sustain alertness and performance in the face of sleep loss. Caffeine is the most widely used psycho-active agent in the world. In fact, it is the most widely used drug in the world, fueling our 24/7 society. Because caffeine antagonizes the actions of adenosine (65), a neuromodulator that mediates the sleep-inducing effects of prolonged wakefulness (66), at the adenosine A2A receptor, it can mask some of the effects of sleep deprivation on sleep propensity. Caffeine is marketed in coffee, colas and so-called energy drinks, and mixed with alcoholic beverages to increase the amount of alcohol that consumers can drink before becoming overcome with alcohol-induced fatigue. Caffeine is also available in tablet form, gum and in energy bars. Caffeine enhances performance during the daytime, although we have found that chronic caffeine administration in subjects on a demanding schedule may increase sleepiness, perhaps due to interference with recovery sleep (67).
The National Transportation Safety Board (NTSB) found that plasma caffeine levels were highest in drivers involved in fatigue-related fatal crashes (68). The NTSB concluded that the high levels of caffeine were insufficient to save those drivers from the effects of fatigue, which the NTSB found to be the leading cause of heavy truck crashes, equal to the fraction of crashes caused by both drugs and alcohol combined (68). Moreover, while 80 to 90% of adults report regular consumption of caffeine-containing beverages and foods, some people who avoid or limit caffeine consumption report experiencing adverse effects of caffeine—such as anxiety, tachycardia, nervousness or tremors—even at low to moderate doses (69). Twin studies have yielded heritability estimates of up to 77% for caffeine use and for tolerance to the side effects of caffeine, leading to the recent discovery that a genetic polymorphism in the adenosine A2 receptor gene is associated with both reduced caffeine consumption (69) and with greater subjectively and objectively measured sensitivity to the adverse effects of caffeine on sleep (70). Thus, while most physicians use caffeine extensively to cope with sleep deprivation induced by extended duration work shifts and long work weeks, hundreds of thousands of physicians may be less able to avail themselves of the wake-promoting effects of caffeine due to a genetic variation in the adenosine 2A receptor that increases their sensitivity to the side effects of caffeine.
With 7.74% of children ages 4–17 diagnosed with attention-deficit hyperactivity disorder (ADHD) and 4.33% (6.2% of boys and 2.4% of girls) receiving ADHD medications, amphetamines and other stimulants used to treat ADHD are now readily available (71). An unintended consequence of this popularity is that one-third of college students illegally use the mixed amphetamine salts in Adderall to “stay awake to study” when academic workload is high (72). It is unknown whether the illegal use of such stimulant medications, which can induce insomnia, will persist when this cohort of students enters medical school and then residency training. If so, this trend would harken back to the man who established and directed the first surgical residency program at Johns Hopkins in the 1890s, as it was cocaine use (73) that enabled Professor William Stewart Halsted, who laid the foundation for present-day graduate medical education work-hour policies, to maintain his reputation as an indefatigable surgeon (74). Sedation, sleep deprivation and insomnia are common side effects of opiates, to which Dr. Halsted was also addicted (75–77). In healthy subjects, morphine and methadone decrease deep slow wave sleep (78). It is also likely that opiates increase the risk of attentional failures and degrade performance under conditions of sleep deprivation.
Alcohol, another major drug of abuse, is a central nervous system depressant that increases sleep propensity during wakefulness but disrupts sleep consolidation after sleep onset. One third of U.S. medical students regularly drink alcohol excessively (79). An estimated 4.9% of emergency medicine residents meet diagnostic criteria for alcoholism (80). An estimated 10–12% of practicing physicians in the United States develop a substance use disorder (81), with 50% of physicians in rehab centers treated for abuse of alcohol, 36% for opiates and 8% for stimulants (81).
These various agents, used commonly by a substantial fraction of practicing physicians and residents, have a profound effect on sleep-wake homeostasis and vulnerability to the effects of acute and chronic sleep loss on alertness and performance. Even low doses of alcohol synergistically interact with sleep loss to greatly increase sleep propensity, degrade driving performance and increase crash risk in a driving simulator (82–84). Healthy professional drivers who were awake for 18 to 21 hours and were exposed to legal low-dose alcohol (blood alcohol concentration of 0.03 g/dL) had significantly more attentional failures and greater variation in both lane position and speed in a driving simulator than they did when their blood alcohol concentration exceeded 0.05 g/dL—a level associated with significantly increased risk of a motor vehicle crash (85). Few programs have adopted the random drug testing procedures recently introduced for anesthesia residents, but not for practicing physicians, at the Massachusetts General Hospital in Boston (86). Therefore, physicians whose performance has been compromised by the use of such agents—even on non-work days—are not being identified and are being scheduled to work lengthy shifts of the same duration (e.g., 30 consecutive hours) and at the same frequency (e.g., twice per week) as those who have not used such agents. The practice of scheduling physicians to work for 30 consecutive hours without sleep does not take into account the widespread use among physicians of psychoactive agents that affect tolerance to sleep loss.
IMPACT OF MEDICAL CONDITION ON VULNERABILITY TO SLEEP LOSS
A number of medical conditions and/or the medications used to treat those conditions are associated with increased sleep tendency, increased risk of attentional failures and increased risk of sleep-related errors and accidents (87). These common conditions make it more difficult to sustain wakefulness during the day and increase performance vulnerability to sleep loss. For example, pregnancy is a non-pathologic condition that often interferes with sleep, particularly in the last trimester, induces fatigue and can greatly affect stamina and the ability to cope with sleep loss. Yet many residents are scheduled to work 30-hour shifts throughout pregnancy. Many infectious diseases also increase sleep tendency, including the common cold, mononucleosis and hepatitis. Other conditions, such as chronic pain and nocturnal asthma, cause chronic sleep loss that reduces tolerance to further sleep deprivation. Primary sleep disorders, such as narcolepsy and sleep apnea, as well as disturbances of sleep or wakefulness secondary to a medical condition or its treatment can affect tolerance to sleep loss. While there are 90 recognized sleep disorders (2), I have selected five to illustrate the issues raised by these common conditions, which collectively affect at least one out of five residents and practicing physicians in the U.S.
Sleep-related Breathing Disorders
Obstructive sleep apnea/hypopnea (OSAHS) syndrome results in sleep fragmentation and consequent daytime sleepiness. EDS is reported in about 80% of patients with OSAHS, 80 to 90% of whom remain undiagnosed and untreated; it often takes several years from the time a patient presents to a physician with symptoms until the correct diagnosis is made (2). Performance related to executive functions such as verbal fluency, planning and sequential thinking—the purview of the frontal cortex, an area of the brain that imaging studies has revealed to be exquisitely sensitive to sleep loss—is very adversely affected by OSAHS (88). Vigilance and the ability to sustain attention are also degraded in patients with OSAHS. Reaction times on a sustained attention task in patients with mild to moderate OSAHS have been reported to be comparable to or worse than those of a young adult with a blood alcohol concentration of 0.080 g/dL (89). Untreated patients with OSAHS perform much more poorly in a driving simulator (in terms of lane deviations, tracking errors, off-road events and collisions with obstacles) and are 6 to 10 times (i.e., about 500% to 1,000%) more likely to have an actual motor vehicle crash than people without OSAHS (90–103).
It is not known how many physicians in training or practicing physicians suffer from OSAHS. Snoring and the occurrence of attentional failures when driving a motor vehicle are cardinal symptoms of OSAHS (2). Obesity is the most prominent risk factor for OSAHS (2), though OSAHS can occur without obesity. Obesity confers such a high risk of OSAHS that Medical Advisory Board of the Federal Motor Carriers Safety Administration (FMCSA) has recommended mandatory objective OSAHS screening of all commercial motor vehicle drivers with a body mass index (BMI) greater than 30 kg/m2, as measured at the bi-annual commercial driver license (CDL) physical exam. Data from available cohorts suggest that at least 50,000 practicing physicians and up to 10,000 residents would be affected if similar criteria were applied to those seeking to obtain or renew a license to practice medicine, as an estimated 6% to 18% of practicing physicians (104–107) and 10% of residents (108) are obese (BMI > 30 kg/m2). Since long work hours leave little time for exercise, and sleep restriction increases ghrelin levels, decreases leptin levels and increases carbohydrate craving, appetite and weight (2), physicians in training gain weight during residency (109, 110), exacerbating the condition.
Thus, it is likely that a significant fraction of both residents and practicing physicians are at risk for OSAHS. Nearly two decades ago, the prevalence of OSAHS in the U.S. was estimated to be 4% of adult men and 2% of adult women (111). With the increased prevalence of obesity in the U.S. (112), the prevalence of OSAHS among both residents and practicing physicians is now at least 3 to 4 times higher. We have recently found that over one-third of a younger cohort of 4,471 North American employed law enforcement officers (mean age: 38.5 ± 8.3 years; mean BMI: 28.7 ± 4.6 kg/m2) screened with a sensitive and specific survey instrument were at high risk for OSAHS (113).
Fortunately, for the 10 to 20% of OSAHS patients who are correctly diagnosed and treated, there are a number of therapies available that can improve the subjective sleepiness associated with this condition. Splinting the airway open with continuous positive airway pressure (CPAP) treatment has been the standard treatment for this condition for more than two decades. While the symptoms of OSAHS, including the increased risk of motor vehicle crashes, are usually significantly improved with CPAP treatment (101, 114–116), less than half of patients diagnosed with OSAHS actually use CPAP for at least 4 hours on 70% of observed nights (117). Moreover, while the impact of CPAP treatment on the most widely used objective measure of daytime sleepiness, the Multiple Sleep Latency Test (MSLT), is statistically significant, it is rather modest in size, with the improvement in sleep latency averaging less than one minute (118).
Thus, a substantial fraction of patients who are compliant with CPAP therapy remain pathologically sleepy. Daily use of modafinil (200 mg.) has been demonstrated to be effective as an adjunct therapy in the treatment of residual subjective sleepiness in this population of OSAHS patients treated with CPAP (119). However, subjective sleepiness was only normalized (Epworth Sleepiness Scale < 10) in half of the modafinil-treated patients and objective sleep tendency, as measured by the MSLT, was not significantly improved by modafinil treatment (119). In summary, OSAHS is likely to have a substantial prevalence among physicians, and most physicians with this condition will remain impaired by EDS for the following reasons: lack of diagnosis and treatment in 80–90% of those affected, failure to comply with nasal CPAP treatment in half of those diagnosed, and residual impairment notwithstanding compliance with CPAP treatment and adjunctive pharmacotherapy in others (2). Increased sleepiness and neurobehavioral deficits due to chronic sleep loss and sleep fragmentation will likely render physicians afflicted with OSAHS much more susceptible to the adverse effects of acute and chronic sleep deprivation induced by extended duration work schedules typically required of both residents and practicing physicians.
Shift Work Disorder
Excessive sleepiness and/or insomnia that occur in association with work that is scheduled during hours usually reserved for sleep are symptomatic of a condition known as Circadian Rhythm Sleep Disorder (CRSD), Shift Work Type (Shift Work Disorder, SWD) (120). About 5 to 10% of such workers suffer chronically from moderate to severe SWD (121). Most employees nod off or fall asleep regularly while working at night or while commuting to or from night work (122). One-third of nurses who work at night report that they fall asleep in the hospital every week of night work (123), with half admitting that they fall asleep at the wheel while driving to or from the hospital; twice as many night-working nurses reported making medication errors or having motor vehicle crashes as compared to those who worked the day and evening shifts, but did not work at night (123).
Extended duration work shifts that routinely exceed 24 consecutive hours combined with very long work weeks that do not allow sufficient time for sleep can elicit attentional failures during work in most if not all employees (22), effectively inducing SWD in all workers in the same way that depriving people of food will eventually induce malnutrition in all people who are starved. Exposure to less extreme work schedules reveals considerable variability in the ability of individuals to cope with the demands of work schedules that require employees to work during times that they would otherwise be asleep. Continuous polysomnographic monitoring of 80 licensed commercial drivers without OSAHS during 7,500 hours of long-haul trucking operations revealed that more than half of the long-haul truck drivers experienced episodes of drowsy driving, mostly during night driving (124). Predictably, these episodes of drowsy driving were not distributed evenly across the 80 drivers. In fact, more than half of the drowsy driving episodes occurred in just 8 (10%) of the 80 drivers (124), revealing the differential vulnerability of this subset of drivers to the sleep deprivation and circadian misalignment associated with night driving. This is consistent with the differential vulnerability to nocturnal sleep loss observed in laboratory studies (32, 35– 37, 125, 126). A number of countermeasures and treatments can be effective in treating the symptoms of SWD, including improvements in work scheduling, use of properly timed exposure to light, administration of nutritional supplements such as caffeine and melatonin, and use of pharmacologic agents such as the wake-promoting therapeutic modafinil (22–24, 67, 122, 127–130), although none eliminate them.
Insomnia
Insomnia is the most common sleep disorder, with symptoms of disturbed sleep affecting most adults at some time in their lives (2). The clinical definition of insomnia is a chronic (> 6 months) complaint of latency to sleep onset or awakenings from sleep of at least a half hour occurring at least three times per week. The prevalence of insomnia associated with a daytime complaint of increased sleepiness or fatigue is 8% to 18% (131). Broadly, insomnia may be psychophysiologic or secondary to the sleep environment, an irregular sleep schedule, a sleep- related movement disorder, a psychiatric condition, a medical condition, a medication or substance, or a circadian rhythm sleep disorder (CRSD) such as SWD (2). Insomnia in non-depressed adults is a risk factor for depression (132). Insomnia syndrome, which includes increased daytime sleepiness, it is associated with an increased rate of work absenteeism, reduced productivity and increased risk of accidents (2, 133).
Narcolepsy
Narcolepsy is a neurological sleep disorder characterized by “EDS that typically is associated with cataplexy and other REM sleep phenomena such as sleep paralysis and hypnogogic hallucinations (120).” Cataplexy, a sudden muscular weakness often brought on by strong emotion, together with disturbed nocturnal sleep, are hallmarks of this condition, which affects about 5 out of every 10,000 adults in the U.S. Thus, an estimated 50 resident physicians and 400 practicing physicians in the United States have narcolepsy, assuming that the affected individuals have not self-selected out of the profession. Since narcolepsy often presents late in the second decade or in the third decade of life, affected individuals may already be in medical school or in a residency program before clinical symptoms are manifested. Despite the severity of sleep-wake impairment associated with this primary sleep disorder, it often takes more than five years from the time of symptom presentation to a physician until the correct diagnosis is made. Patients with narcolepsy often have difficulty sustaining attention during the day, and must rely on wake-promoting therapeutics to reduce EDS and minimize the occurrence of sleep attacks. This may be due to a deficiency of the hypothalamic neurotransmitter orexin (hypocretin), which is often associated with narcolepsy. Patients with narcolepsy perform poorer on a driving simulator and have a significantly higher rate of motor vehicle crashes than do control subjects (99).
Psychiatric Disorders
Sleep disturbance is often secondary to a number of psychiatric disorders and other conditions that may render affected individuals more susceptible to the effects of sleep deprivation. These include several medical and psychiatric disorders such as depression, which is common among practicing physicians and residents. A recent large-scale multi-site study revealed that 12% of medical students and residents had probable major depression, and 9.2% had probable mild/moderate depression (134). Burnout has been reported by half of medical students in two studies (135, 136); 11.2% of the medical students reported suicidal ideation; 20% of pediatric residents meet screening criteria for depression, and 74% meet the criteria for burnout (137).
Sleep disturbance is one of the most common symptoms of burnout, anxiety disorders and mood disorders, including depressive disorders and bipolar disorder. Burnout is also associated with persistent insomnia (138), with increased arousals and sleep fragmentation (139). Abrupt awakenings often occur in anxiety disorders, such as post-traumatic stress disorder, in which sleep is frequently disturbed by nightmares. Burnout, which is precipitated by occupational stress, induces EDS and mental fatigue throughout the day, which may be a consequence of the sleep disturbance (139). While insomnia is one of the most common features of mood disorders, hypersomnia can occur as well, especially during depressive episodes. Changes in sleep architecture, including reductions in deep slow wave sleep and earlier onset of REM sleep, occur in depression. Acute sleep deprivation can have an immediate anti-depressant effect, although its therapeutic effect is reversed as soon as recovery sleep is obtained. Sleep disturbance is one of the most common residual symptoms after effective treatment of depression, estimated to continue in up to 44% of patients in remission (131). For these reasons, the cognitive performance of physicians with mood disorders is likely to be more vulnerable to the effects of sleep loss and misalignment of circadian phase. Many of the medications used to treat these patients have side effects that affect sleep tendency. The adverse effects of depression and its treatment on sleep and consequent daytime functioning probably contribute the increased risk of medical errors in depressed pediatric residents, who make 6.2 times as many medication errors per resident month as residents who are not depressed (137).
ETHICAL CONSIDERATIONS
The evidence upon which the ACGME and the IOM have relied in developing resident work- hour policy limits has been largely derived from laboratory studies of healthy subjects who have passed physical examinations and volunteered to participate in relatively brief sleep restriction or sleep deprivation studies. In continuing to sanction every other work shift to be 30-consecutive hours in duration (Fig. 9) throughout years of residency training, the ACGME has implicitly assumed that the population of residents is entirely healthy, highly resistant to the effects of sleep deprivation, and free of concurrent family responsibilities, premises that are not accurate. Working such long hours that the risk of unnecessary patient injury rises sharply violates the ethical principle of nonmaleficence (140). The principle of beneficence requires implementation of safer work schedules that reduce risk by not scheduling physicians to care for patients after 16 hours without sleep. Exhausted physicians who have worked >16 consecutive hours without sleep should not endanger themselves and others by driving automobiles (141, 142). Since 24-hours of wakefulness degrades performance comparably to alcohol intoxication (28, 143–147), the principle of autonomy requires that physicians respect the right of the patient to be informed of the impairment and to withhold consent to the risk of receiving care from sleep-deprived providers (140). The principle of justice requires that those most vulnerable to sleep loss not shoulder a disproportionately large burden [i.e., a higher rate of fatigue-related errors, accidents and adverse health effects (2)] than those who are resistant to sleep loss (140), particularly for the convenience or financial benefit of teaching faculty or academic medical centers.
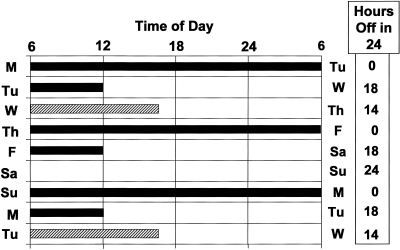
Fig. 9.
Illustration of Eight Consecutive Days of a Typical Resident Physician Work Schedule Sanctioned by Current 2009 ACGME Guidelines. Every other shift is 30 hours in duration on this schedule. Because each 30-hour shift includes two calendar days, this schedule results in the resident physician spending every third night in the hospital. Black bar indicates scheduled 30-hour shifts. Hatched bar indicates an 8- to 10-hour swing shift, when resident physicians are not scheduled to work overnight. Box on right indicates how many hours residents are not scheduled to work for each 24-hour interval, beginning at 6 am each day.
Systematic falsification of resident work-hour records (Fig. 10) (1, 148–150), which the IOM found to be widespread (1) and the ACGME recently admitted after years of official denial (151), violates the principle of truthfulness and honesty (140). It sets a regrettable precedent for trainees, especially when it occurs out of fear of reprisal from (152) or with the tacit approval, awareness or expectation of residency program directors or institutional leaders (151). Falsification of work-hour records by those who have caused harm have led to felony convictions, hefty fines and imprisonment, not only for those who falsified reports but for chief executives and supervisors complicit in the falsifications of work-hour records (153–163). At its core, resident work hour reform is an issue of human dignity for patient and physician (140). Burdening a physician with a workload that s/he cannot carry creates an ethical dilemma in which—on the one hand—the more hours that the physician works, the greater is the risk to both the patient and to the physician, whereas—on the other hand—the physician cannot ethically stop working and abandon a patient in need of medical care. Sleep deprivation should not be presented as a proxy for dedication.
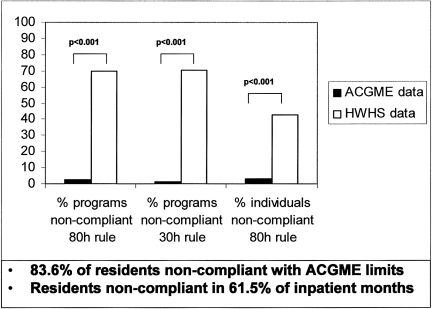
Fig. 10.
.Reported Non-Compliance with ACGME Work-Hour Limits as Reported by Residents Non-Confidentially to the ACGME vs. Confidentially to the Harvard Work-Hours Health and Safety (HWHS) Group. After the ACGME implemented the 2003 limits on resident physicians work hours, 83.6% of interns reported to the HWHS Group work hours that were in violation of the ACGME standards during 1 or more months. Working shifts greater than 30 consecutive hours was reported by 67.4% of interns (open bar, middle pair). Averaged over 4 weeks, 43.0% of interns reported working more than 80 hours weekly (open bar, right pair), and 43.7% reported not having 1 day in 7 off work duties (data not shown). Violations were reported during 61.5% of months during which interns worked exclusively in inpatient settings. Violations were reported to the HWHS Group from 85.4% of the 707 represented residency programs (open bar, left pair). During the same reporting period, the ACGME reported near-universal compliance with the ACGME standards (filled bars), claiming that only 5.0% of residency training programs were not compliant with the standards and that only 3.3% of surveyed residents reported violations of the 80-hour rule. Differences between the ACGME and HWHS Group data were statistically significant on each of these measures (p < 0.001). Data and legend from: Landrigan CP, Barger LK, Cade BE, Ayas NT, Czeisler CA. JAMA 2006;296:1063 (148) Figure courtesy of Christopher P. Landrigan, M.D., M.P.H.
Education about the impact of sleep deprivation and sleep disorders on performance, health and safety should be provided so that physicians and trainees understand that working when sleep deprived constitutes working in an impaired state, comparable to being under the influence of alcohol (1, 2, 17, 28, 82–84, 143–147). 30-hour work shifts are not necessary for training physicians, since GME programs in many other countries (1) (Table) and in the U.S. have eliminated them years ago, including the surgical residency training program at the Brigham and Women's Hospital in Boston. As in other safety-sensitive industries in the U.S. and in residency training in other countries (1, 164), work-hour limits for residents should be based on work hours that are safer for most people, not a select few (Table). Prior to the threat of federal legislation (151), many medical and surgical training programs in the U.S. required residents to work 120 to 140 hours per week (21, 24), as they currently do in El Salvador (165) (Table). 25 years ago, the tragic death of Libby Zion thrust the issue of resident work hours onto the national stage (166). As the IOM has recently concluded, now is the time for meaningful resident physician work-hour limits (1), which the federal government has the authority to implement (167) and for which the boards of directors of the Sleep Research Society and the National Sleep Foundation have endorsed enforcement by law or regulation (21, 168, 169)
Table.
Summary of work-hour regulations for various occupations in selected countries
| Current Work-Hour Regulations | |
|---|---|
| Occupation | Limits |
| U.S. Airplane Pilots (1–2 pilot airplanes): 1950s | <8 daily flight hours |
| <16 daily work hours | |
| >8–12 hours rest required (since 1985) | |
| <34 hours flight time per week | |
| U.S. Nuclear Power Plant Operators: 1982; 2009 | <16 consecutive work hours |
| <72 work hours per week | |
| >34 consecutive hours off every 9 days | |
| U.S. Railroad Operators: 1907, modified 1969 & 1976 | <12 work hours per day |
| >8–10 hours rest required per day | |
| U.S. Interstate Truck and Bus Drivers | <11 driving hours within a 14-hour interval |
| <14 consecutive hours from start to end of work | |
| >10 consecutive rest hours | |
| <60 work hours per 7 days; <70 work hours per 8 days | |
| >34 consecutive hours off between work weeks | |
| E.U. All Occupations (including resident physicians and practicing physicians): 2004; 2009 | <13 consecutive work hours |
| <56 work hours per week until 2009; 48 hours thereafter | |
| >11 hours rest time per day | |
| New Zealand Resident Physicians: 1985 | <16 consecutive work hours (labor agreement) |
| <72 work hours per week | |
| U.S. Resident Physicians | UNLIMITED: no federal laws of regulations |
| Self regulation by profession (ACGME): 2003 | |
| <30 consecutive work hours (two allowed per week) | |
| <80 work hours per week (averaged over 4 weeks) | |
| Monitoring by self-report (84% non-compliance) | |
| El Salvador Resident Physicians | UNLIMITED |
| Extended duration work shifts (36 hours) and long (>120 hours) work weeks common during internship | |
ACKNOWLEDGMENTS
The author acknowledges the cooperation of the National Residency Matching Program and Association of American Medical Colleges, which until 2006 provided access to medical student contact information for survey distribution, and wishes to thank Theresa L. Shanahan, M.D. for her advice, Laura K. Barger, Ph.D., Steven W. Lockley, Ph.D., Christopher P. Landrigan, M.D., M.P.H., Clark J. Lee, J.D., and Shantha W. Rajaratnam, Ph.D. for their thoughtful comments on the manuscript, Ms. Lorna Preston for editorial assistance on this manuscript, and collaborators Daniel Aeschbach, Ph.D., Erik K. Alexander, M.D., Najib T. Ayas, M.D., David W. Bates, M.D., Brian Cade, B.S., John W. Cronin, M.D., Erin Evans, B.A., James A. Gordon, M.D., M.P.A., Joel T. Katz, M.D., Craig M. Lilly, M.D., Conor O'Brien, B.A., Jeffrey M. Rothschild, M.D., Joseph M. Ronda, M.S., Frank E. Speizer, M.D., Peter H. Stone, M.D., Bernard A. Rosner, Ph.D. and Marshall Wolf, M.D. for their contributions to the work reviewed herein.
Supported in part by grants from the Agency for Healthcare Research and Quality (AHRQ) (RO1 HS12032; K08 HS13333; U18 HS015906; F32 HS14130); the National Institute of Occupational Safety and Health (RO1 OH07567); the National Institute on Aging (P01 AG09975; R01 AG06072); the National Heart, Lung, and Blood Institute (R01 HL52992;T32 HL07901; F33- HL-09588); National Institute of Mental Health (RO1 MH-45130); National Center for Research Resources (M01 RR02635); the Swiss National Foundation (823A-046640); the Wellcome Trust, United Kingdom (060018/B/99/Z); the US Air Force Office of Scientific Research (F49620-95-1- 0388); The Medical Foundation; The Harold Whitworth Pierce Charitable Trust; the Canadian Institutes of Health Research; the British Columbia Lung Association; the University of British Columbia; the University of Colorado; and by the Brigham and Women's Hospital and the Division of Sleep Medicine, Harvard Medical School. Dr. Czeisler is supported in part by the National Space Biomedical Research Institute, through the National Aeronautics and Space Administration (NCC 9–58).
Footnotes
Potential Conflicts of Interest: Dr. Czeisler is/was a consultant for: Actelion, Ltd.; Cephalon, Inc.; Delta Air Lines, Inc.; Eli Lilly and Co.; Garda Síochana Inspectorate; Global Ground Support; Johnson & Johnson; Koninklijke Philips Electronics, N.V.; Portland Trail Blazers; Respironics, Inc; Sanofi-Aventis Groupe; Sepracor, Inc.; Sleep Multimedia, Inc.; Somnus Therapeutics, Inc.; University of Wisconsin; Vanda Pharmaceuticals, Inc.; and Zeo, Inc.; and received royalties from McGraw Hill and Penguin Press. Dr. Czeisler owns an equity interest in Lifetrac, Inc.; Somnus Therapeutics, Inc.; Vanda Pharmaceuticals, Inc.; and Zeo, Inc. Dr. Czeisler has also received research support from Cephalon, Inc.; Tempur Pedic International, Inc; and Resmed, Inc. The Sleep and Health Education Program of the Harvard Medical School Division of Sleep Medicine has received support from Cephalon, Inc.; Takeda Pharmaceuticals North America, Inc.; Sanofi-Aventis Groupe; and Sepracor, Inc. Dr. Czeisler has received awards with monetary stipends from the American Clinical and Climatological Association; American Academy of Sleep Medicine; Association for Patient- Oriented Research; National Institute for Occupational Safety and Health and National Sleep Foundation; and the Sleep Research Society. Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, Dr. Czeisler has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms.
DISCUSSION
August, New York: The prevalence, you were saying, is about 25% of this polymorphism?
Czeisler, Boston: Polymorphism is about 10 to 15%. This is the first polymorphism that has been identified. There are a whole series of new genes that are being looked for, and there may be several other genetic markers that can identify the rest of that though.
August, New York: Are there any gender differences? You might expect that there would be in terms of traditionally women being awake during the night with feeding babies and things like that.
Czeisler, Boston: That is a good question. We have not looked into gender differences. That 25% most vulnerable cohort does not separate out based on gender or intelligence or any other of the usual suspects, race and so on, but it does seem to be related to this genetic marker.
Chapman, Jackson: There is a variation of alertness and our ability to do certain skills between generally 2 and 4 in the afternoon and 2 and 4 in the morning for those that work night shifts. When would that take into account some of the variability that you see?
Czeisler, Boston: The genetic vulnerability that I discussed is particularly when people are trying to function both sleep-deprived and at an adverse circadian phase. They don't seem to perform differently during the daytime when they are not at that most vulnerable phase.
Chapman, Jackson: So the circadian rhythm is not disrupted.
Czeisler, Boston: Right, so it's a combination of circadian misalignment and sleep deprivation that brings out this particular vulnerability, and there is a continuum as you saw from that other slide; but if you were trying to pick people who were resistant to sleep deprivation, you would be down to about 10 to 15% of the population, but that may be enough to fill the medical schools.
Boyer, New Haven: What about jet lag? Is there any relationship between the disruptions of circadian rhythm in jet lag and gentotype?
Czeisler, Boston: That's a great question. We just finished an unpublished study that we are in the process of submitting for publication in which we show that the same people who are most vulnerable to staying awake all night are actually less vulnerable to the dislocation associated with eastward travel across time zones. They tend to be morning types—well we are all morning types as we get older—but they tend to be morning types in their 20s, and they may be morning types because they are building up homeostatic sleep drive at a faster clip so that they go to bed earlier so that when they go to England, for example, they have much less difficulty sleep on British time than do people with the more common genotype.
Tweardy, Houston: Excellent talk Charles, and, in fact, this could open up a field of personalized genomic career advising as opposed to personalized genomic medicine. My question is, as our previous speaker, Steven, I am interested in pathophysiologic pathways, and I am curious to know how polymorphism links to the pathophysiology of sleep and its effects on sleep cycles.
Czeisler, Boston: Well no one knows the answer to that question. We are beginning to look to different populations, in Papua, New Guinea for example. This particular polymorphism is somewhere between 50 to 80% of the population, and we are looking to begin investigations in different regions of the world to try to understand how it might be related to the occurrence of sleep disorders. Certainly it may be directly related to the pathophysiology of shift work disorder, which is again a subset of the population that is about the same size that are particularly vulnerable not just to the performance impairments but also to adverse medical outcomes—cardiovascular disease and other issues associated with shift work.
Berl, Denver: If you are going to use this as a screening test for those vulnerable to this problem, do you have a sense of the sensitivity and specificity of this?
Czeisler, Boston: It's too early to say right now, and I am not actually advocating that it be used for a screening test. Just to clarify, my position is that the work hours should be designed in such a way as to accommodate the full range of the population, because I have not seen any evidence that these 30 hour shifts are critical for training; but I know that many people in this room would disagree with me.
REFERENCES
- 1.Ulmer C, Wolman DM, Johns MME, editors. Resident Duty Hours: Enhancing Sleep, Supervision, and Safety. Washington, D.C.: The National Academies Press; 2008. Institute of Medicine of the National Academies; pp. 1–322. [PubMed] [Google Scholar]
- 2.Colten HR, Alteveogt BM, editors. Washington, D.C.: National Academies Press; 2006. Institute of Medicine. Sleep disorders and sleep deprivation: An unmet public health problem. . ISBN:0-309-66012-2. 1–500. [PubMed] [Google Scholar]
- 3.Assuring Quality Patient care and Quality Education. Association of American Medical Colleges; 2001. AAMC; pp. 1–10. [PubMed] [Google Scholar]
- 4.Friedman RC, Bigger JT, Kornfield DS. The intern and sleep loss. N Engl J Med. 1971;285:201–3. doi: 10.1056/NEJM197107222850405. [DOI] [PubMed] [Google Scholar]
- 5.Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J. Laparoscopic performance after one night on call in a surgical department: prospective study. B M J. 2001;323:1222–3. doi: 10.1136/bmj.323.7323.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philibert I. Sleep loss and performance in residents and nonphysicians: a meta-analytic examination. Sleep. 2005;28(11):1392–402. doi: 10.1093/sleep/28.11.1392. [DOI] [PubMed] [Google Scholar]
- 7.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437(7063):1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 8.Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G. Sleep-dependent motor memory plasticity in the human brain. Neuroscience. 2005;133(4):911–917. doi: 10.1016/j.neuroscience.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44(1):121–33. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 10.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: Sleep-dependent motor skill learning. Neuron. 2002;35:205–11. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 11.Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: A multi-step process occurring during sleep. J Cognitive Neurosci. 2000;12(2):246–54. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- 12.Stickgold R, James L, Hobson JA. Visual discrimination learning requires sleep after training. Nat Neurosci. 2000;3(12):1237–8. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- 13.Hill S, Tononi G, Ghilardi MF. Sleep improves the variability of motor performance. Brain Res Bull. 2008;76(6):605–11. doi: 10.1016/j.brainresbull.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430(6995):78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 15.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300(24):2859–66. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinges DF, Orne MT, Whitehouse WG, Orne EC. Temporal placement of a nap for alertness: Contributions of circadian phase and prior wakefulness. Sleep. 1987;10(4):313–29. [PubMed] [Google Scholar]
- 17.Cajochen C, Khalsa SBS, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640–9. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 18.Veasey S, Rosen R, Barzansky B, Rosen I, Owens J. Sleep loss and fatigue in residency training. A Reappraisal. JAMA. 2002;288(9):1116–24. doi: 10.1001/jama.288.9.1116. [DOI] [PubMed] [Google Scholar]
- 19.Dinges DF, Kribbs NB. Performing while sleepy: Effects of experimentally-induced sleepiness. In: Monk TH, editor. Sleep, Sleepiness and Performance. Chichester, UK: John Wiley and Sons, Ltd.; 1991. –97.pp. 128 [Google Scholar]
- 20.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25(1):117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 21.Czeisler CA. The Gordon Wilson Lecture: Work hours, sleep and patient safety in residency training. Trans Am Clin Climatol Assoc. 2006;117:159–89. [PMC free article] [PubMed] [Google Scholar]
- 22.Lockley SW, Cronin JW, Evans EE, Cade BE, Lee CJ, Landrigan CP, et al. Effect of reducing interns' weekly work hours on sleep and attentional failures. N Engl J Med. 2004;351(18):1829–37. doi: 10.1056/NEJMoa041404. [DOI] [PubMed] [Google Scholar]
- 23.Landrigan CP, Rothschild JM, Cronin JW, Kaushal R, Burdick E, Katz JT, et al. Effect of reducing interns' work hours on serious medical errors in intensive care units. N Engl J Med. 2004;351(18):1838–48. doi: 10.1056/NEJMoa041406. [DOI] [PubMed] [Google Scholar]
- 24.Barger LK, Cade BE, Ayas NT, Cronin JW, Rosner B, Speizer FE, et al. Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med. 2005;352:125–34. doi: 10.1056/NEJMoa041401. [DOI] [PubMed] [Google Scholar]
- 25.Barger LK, Ayas NT, Cade BE, Cronin JW, Rosner B, Speizer FE, et al. Impact of extended-duration shifts on medical errors, adverse events, and attentional failures. PLoS Med. 2006;3(12):e487. doi: 10.1371/journal.pmed.0030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayas NT, Barger LK, Cade BE, Hashimoto DM, Rosner B, Cronin JW, et al. Extended work duration and the risk of self-reported percutaneous injuries in interns. JAMA. 2006;296(9):1055–62. doi: 10.1001/jama.296.9.1055. [DOI] [PubMed] [Google Scholar]
- 27.Horowitz TS, Cade BE, Wolfe JM, Czeisler CA. Searching night and day: a dissociation of effects of circadian phase and time awake on visual selective attention and vigilance. Psychol Sci. 2003;14(6):549–57. doi: 10.1046/j.0956-7976.2003.psci_1464.x. [DOI] [PubMed] [Google Scholar]
- 28.Dawson D, Reid K. Fatigue, alcohol and performance impairment. Nature. 1997;388:235. doi: 10.1038/40775. [DOI] [PubMed] [Google Scholar]
- 29.USA Today Editorial Board. Our view on doctors in training: Sleep deprivation studies fail to wake up teaching hospitals. Why are residents still working marathon shifts, endangering patients. USA Today. 2006 Dec 19; [Google Scholar]
- 30.Fischer JE. Surgeons: employees or professionals. Am J Surg. 2005;190(1):1–3. doi: 10.1016/j.amjsurg.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Leach DC. Opposing view: More hours, better doctors. Good patient care is complex; residents are students, not workers. USA Today. 2006 Dec 19; [Google Scholar]
- 32.Van Dongen HPA, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 33.Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regul Integr Comp Physiol. 2003;284:R280–90. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- 34.Frey DJ, Badia P, Wright KP., Jr. Inter- and intra-individual variability in performance near the circadian nadir during sleep deprivation. J Sleep Res. 2004;13(4):305–15. doi: 10.1111/j.1365-2869.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- 35.Van Dongen HPA, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: leitmotif for a research agenda. Sleep. 2005;28(4):479–96. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 36.Van Dongen HPA. Brain activation patterns and individual differences in working memory impairment during sleep deprivation. Sleep. 2005;28(4):386–8. [PubMed] [Google Scholar]
- 37.Van Dongen HPA. Shift work and inter-individual differences in sleep and sleepiness. Chronobiol Int. 2006;23(6):1139–47. doi: 10.1080/07420520601100971. [DOI] [PubMed] [Google Scholar]
- 38.Chuah YM, Venkatraman V, Dinges DF, Chee MW. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci. 2006;26(27):7156–62. doi: 10.1523/JNEUROSCI.0906-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 40.Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 41.Dinges DF. Sleep debt and scientific evidence. Sleep. 2004;27:1050–2. [PubMed] [Google Scholar]
- 42.Klerman EB, Dijk DJ. Interindividual variation in sleep duration and its association with sleep debt in young adults. Sleep. 2005;28:1253–9. doi: 10.1093/sleep/28.10.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinges DF. Can habitual sleep duration harbor sleep debt. Sleep. 2005;28:1209–10. doi: 10.1093/sleep/28.10.1209. [DOI] [PubMed] [Google Scholar]
- 44.Czeisler CA. Work hours and sleep in residency training. Sleep. 2004;27(3):371–2. [PubMed] [Google Scholar]
- 45.Farley P. Yale Medicine. Yale Medicine Magazine; 2004. Recreating the residency; pp. 30–36. [Google Scholar]
- 46.Gay CL, Lee KA, Lee SY. Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs. 2004;5(4):311–8. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SY, Lee KA. Early postpartum sleep and fatigue for mothers after cesarean delivery compared with vaginal delivery: an exploratory study. J Perinat Neonatal Nurs. 2007;21(2):109–13. doi: 10.1097/01.JPN.0000270627.73993.b0. [DOI] [PubMed] [Google Scholar]
- 48.Damato EG, Burant C. Sleep patterns and fatigue in parents of twins. J Obstet Gynecol Neonatal Nurs. 2008;37(6):738–49. doi: 10.1111/j.1552-6909.2008.00296.x. [DOI] [PubMed] [Google Scholar]
- 49.Martin J, Hiscock H, Hardy P, Davey B, Wake M. Adverse associations of infant and child sleep problems and parent health: an Australian population study. Pediatrics. 2007;119(5):947–55. doi: 10.1542/peds.2006-2569. [DOI] [PubMed] [Google Scholar]
- 50.McCann D. Sleep deprivation is an additional stress for parents staying in hospital. J Spec Pediatr Nurs. 2008;13(2):111–22. doi: 10.1111/j.1744-6155.2008.00142.x. [DOI] [PubMed] [Google Scholar]
- 51.Komada Y, Inoue Y, Hayashida K, Nakajima T, Honda M, Takahashi K. Clinical significance and correlates of behaviorally induced insufficient sleep syndrome. Sleep Med. 2008;9(8):851–6. doi: 10.1016/j.sleep.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 52.Banks S, Van Dongen H, Dinges D. Response to sleep restriction depends upon pre-existing sleep debt. Sleep Abstract Supplement[30] 2007:A119. [Google Scholar]
- 53.Wehr TA, Moul DE, Barbato G, Giesen HA, Seidel JA, Barker C, et al. Conservation of photoperiod-responsive mechanisms in humans. Am J Physiol. 1993;265:R846–57. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- 54.Horowitz TS, Cade BE, Wolfe JM, Czeisler CA. Efficacy of bright light and sleep/darkness scheduling in alleviating circadian maladaptation to night work. Am J Physiol Endocrinol Metab. 2001;281:E384–91. doi: 10.1152/ajpendo.2001.281.2.E384. [DOI] [PubMed] [Google Scholar]
- 55.Wright KP, Jr., Hull JT, Hughes RJ, Ronda JM, Czeisler CA. Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J Cognitive Neurosci. 2006;18:508–21. doi: 10.1162/jocn.2006.18.4.508. [DOI] [PubMed] [Google Scholar]
- 56.Duffy JF, Willson HJ, Wang W, Czeisler CA. Healthy older adults better tolerate sleep deprivation than young adults. J Am Geriatr Soc. 2009 doi: 10.1111/j.1532-5415.2009.02303.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adam M, Rétey JV, Khatami R, Landolt H-P. Age-related changes in the time course of vigilant attention during 40 hours without sleep in men. Sleep. 2006;29(1):55–7. doi: 10.1093/sleep/29.1.55. [DOI] [PubMed] [Google Scholar]
- 58.Blatter K, Graw P, Munch M, Knoblauch V, Wirz-Justice A, Cajochen C. Gender and age differences in psychomotor vigilance performance under differential sleep pressure conditions. Behav Brain Res. 2006;168(2):312–7. doi: 10.1016/j.bbr.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 59.Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol (Lond) 1999;516.2:611–27. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Folkard S. Shift work, safety, and aging. Chronobiol Int. 2008;25(2):183–98. doi: 10.1080/07420520802106694. [DOI] [PubMed] [Google Scholar]
- 61.Pack AI, Pack AM, Rodgman E, Cucchiara A, Dinges DF, Schwab CW. Characteristics of crashes attributed to the driver having fallen asleep. Accid Anal Prev. 1995;27(6):769–75. doi: 10.1016/0001-4575(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 62.Viola AU, Archer SN, James LM, Groeger JA, Lo JCY, Skene DJ, et al. PER3 Polymorphism Predicts Sleep Structure and Waking Performance. Curr Biol. 2007;17:1–6. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 63.Groeger JA, Viola AU, Lo JCY, von Shantz M, Archer SN, Dijk DJ. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep. 2008;31(8):1159–67. [PMC free article] [PubMed] [Google Scholar]
- 64.Bodenmann S, Hohoff C, Grietag C, Deckert J, Retey J, Landolt H-P. Genetic variation in the adenosine A2A receptor gene modulates performance on the psychomotor vigilance task. Sleep and Biological Rhythms. 2007;5:A47. [Google Scholar]
- 65.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 66.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjørkum AA, Greene RW, McCarley RW. Adenosine: A mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–8. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wyatt JK, Cajochen C, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Low-dose repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep. 2004;27(3):374–81. doi: 10.1093/sleep/27.3.374. [DOI] [PubMed] [Google Scholar]
- 68.Washington, DC: National Transportation Safety Board; 1990. National Transportation Safety Board. Safety Study: Fatigue, Alcohol, Other Drugs, and Medical Factors in Fatal-to-the-Driver Heavy Truck Crashes (Volume 1). NTSB/SS-90/01; pp. 1–181. [Google Scholar]
- 69.Cornelis MC, El Sohemy A, Campos H. Genetic polymorphism of the adenosine A2A receptor is associated with habitual caffeine consumption. Am J Clin Nutr. 2007;86(1):240–4. doi: 10.1093/ajcn/86.1.240. [DOI] [PubMed] [Google Scholar]
- 70.Retey JV, Adam M, Khatami R, Luhmann UF, Jung HH, Berger W, et al. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin Pharmacol Ther. 2007;81(5):692–8. doi: 10.1038/sj.clpt.6100102. [DOI] [PubMed] [Google Scholar]
- 71.Visser SN, Lesesne CA. Mental health in the United States: Prevalence of diagnosis and medication treatment for attention-deficit/hyperactivity disorder - United States, 2003. 9-2-2005;54(34):842–7. [PubMed] [Google Scholar]
- 72.DeSantis AD, Webb EM, Noar SM. Illicit use of prescription ADHD medications on a college campus: a multimethodological approach. J Am Coll Health. 2008;57(3):315–24. doi: 10.3200/JACH.57.3.315-324. [DOI] [PubMed] [Google Scholar]
- 73.Markel H. The accidental addict. N Engl J Med. 2005;352(10):966–8. doi: 10.1056/NEJMp048240. [DOI] [PubMed] [Google Scholar]
- 74.Cameron JL, Gordon TA, Kehoe MW, McCall N. William Stewart Halsted: letters to a young female admirer. Ann Surg. 2001;234(5):702–7. doi: 10.1097/00000658-200111000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nicholson B. Responsible prescribing of opioids for the management of chronic pain. Drugs. 2003;63(1):17–32. doi: 10.2165/00003495-200363010-00002. [DOI] [PubMed] [Google Scholar]
- 76.Zgierska A, Brown RT, Zuelsdorff M, Brown D, Zhang Z, Fleming MF. Sleep and daytime sleepiness problems among patients with chronic noncancerous pain receiving long-term opioid therapy: a cross-sectional study. J Opioid Manag. 2007;3(6):317–27. doi: 10.5055/jom.2007.0020. [DOI] [PubMed] [Google Scholar]
- 77.Wang D, Teichtahl H, Goodman C, Drummer O, Grunstein RR, Kronborg I. Subjective daytime sleepiness and daytime function in patients on stable methadone maintenance treatment: possible mechanisms. J Clin Sleep Med. 2008;4(6):557–62. [PMC free article] [PubMed] [Google Scholar]
- 78.Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3(1):33–6. [PubMed] [Google Scholar]
- 79.Frank E, Elon L, Naimi T, Brewer R. Alcohol consumption and alcohol counselling behaviour among US medical students: cohort study. BMJ. 2008;337:a2155. doi: 10.1136/bmj.a2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McBeth BD, Ankel FK. Don't ask, don't tell: substance use by resident physicians. Acad Emerg Med. 2006;13(8):893–5. doi: 10.1197/j.aem.2006.03.564. [DOI] [PubMed] [Google Scholar]
- 81.McLellan AT, Skipper GS, Campbell M, DuPont RL. Five year outcomes in a cohort study of physicians treated for substance use disorders in the United States. BMJ. 2008;337:a2038. doi: 10.1136/bmj.a2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horne JA, Reyner LA, Barrett PR. Driving impairment due to sleepiness is exacerbated by low alcohol intake. Occup Environ Med. 2003;60(9):689–92. doi: 10.1136/oem.60.9.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Banks S, Catcheside P, Lack L, Grunstein RR, McEvoy RD. Low levels of alcohol impair driving simulator performance and reduce perception of crash risk in partially sleep deprived subjects. Sleep. 2004;27(6):1063–7. doi: 10.1093/sleep/27.6.1063. [DOI] [PubMed] [Google Scholar]
- 84.Vakulin A, Baulk SD, Catcheside PG, Anderson R, van den Heuvel CJ, Banks S, et al. Effects of moderate sleep deprivation and low-dose alcohol on driving simulator performance and perception in young men. Sleep. 2007;30(10):1327–33. doi: 10.1093/sleep/30.10.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Howard ME, Jackson ML, Kennedy GA, Swann P, Barnes M, Pierce RJ. The interactive effects of extended wakefulness and low-dose alcohol on simulated driving and vigilance. Sleep. 2007;30(10):1334–40. doi: 10.1093/sleep/30.10.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fitzsimons MG, Baker KH, Lowenstein E, Zapol WM. Random drug testing to reduce the incidence of addiction in anesthesia residents: preliminary results from one program. Anesth Analg. 2008;107(2):630–5. doi: 10.1213/ane.0b013e318176fefa. [DOI] [PubMed] [Google Scholar]
- 87.Carter N, Ulfberg J, Nystrom B, Edling C. Sleep debt, sleepiness and accidents among males in the general population and male professional drivers. Accid Anal Prev. 2003;354:613–7. doi: 10.1016/s0001-4575(02)00033-7. [DOI] [PubMed] [Google Scholar]
- 88.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11(1):1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 89.Powell NB, Riley RW, Schechtman KB, Blumen MB, Dinges DF, Guilleminault C. A comparative model: Reaction time performance in sleep-disordered breathing versus alcohol-impaired controls. Laryngoscope. 1999;109:1648–54. doi: 10.1097/00005537-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 90.George CF, Nickerson PW, Hanly PJ, Millar TW, Kryger MH. Sleep apnoea patients have more automobile accidents. Lancet. 1987;2:447. doi: 10.1016/s0140-6736(87)90974-3. [DOI] [PubMed] [Google Scholar]
- 91.Findley LJ, Bonnie RJ. Sleep apnea and auto crashes: What is the doctor to do. Chest. 1988;94(2):225–6. doi: 10.1378/chest.94.2.225. [DOI] [PubMed] [Google Scholar]
- 92.Findley LJ, Unverzagt ME, Suratt PM. Automobile accidents involving patients with obstructive sleep apnea. Am Rev Respir Dis. 1988;138:337–40. doi: 10.1164/ajrccm/138.2.337. [DOI] [PubMed] [Google Scholar]
- 93.Findley LJ, Fabrizio M, Thommi G, Suratt PM. Severity of sleep apnea and automobile crashes. N Engl J Med. 1989;320(13):868–9. doi: 10.1056/nejm198903303201314. [DOI] [PubMed] [Google Scholar]
- 94.Findley LJ, Weiss JW, Jabour ER. Drivers with untreated sleep apnea: A cause of death and serious injury. Arch Intern Med. 1991;151:1451–2. [PubMed] [Google Scholar]
- 95.Haraldsson P-O, Carenfelt C, Tingvall C. Sleep apnea syndrome symptoms and automobile driving in a general population. J Clin Epidemiol. 1992;45(8):821–6. doi: 10.1016/0895-4356(92)90064-t. [DOI] [PubMed] [Google Scholar]
- 96.Findley LJ, Levinson MP, Bonnie RJ. Driving performance and automobile accidents in patients with sleep apnea. Clin Chest Med. 1992;13(3):427–35. [PubMed] [Google Scholar]
- 97.Gurubhagavatula I, Maislin G, Nkwuo JE, Pack AI. Occupational screening for obstructive sleep apnea in commercial drivers. Am J Respir Crit Care Med. 2004;170(4):371–6. doi: 10.1164/rccm.200307-968OC. [DOI] [PubMed] [Google Scholar]
- 98.George CF, Boudreau AC, Smiley A. Simulated driving performance in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154(1):175–81. doi: 10.1164/ajrccm.154.1.8680676. [DOI] [PubMed] [Google Scholar]
- 99.Findley L, Unverzagt M, Guchu R, Fabrizio M, Buckner J, Suratt P. Vigilance and automobile accidents in patients with sleep apnea or narcolepsy. Chest. 1995;108(3):619–24. doi: 10.1378/chest.108.3.619. [DOI] [PubMed] [Google Scholar]
- 100.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340(11):847–51. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 101.George CF. Sleep. 5: Driving and automobile crashes in patients with obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59(9):804–7. doi: 10.1136/thx.2003.007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hack MA, Choi SJ, Vijayapalan P, Davies RJ, Stradling JR. Comparison of the effects of sleep deprivation, alcohol and obstructive sleep apnoea (OSA) on simulated steering performance. Respir Med. 2001;95(7):594–601. doi: 10.1053/rmed.2001.1109. [DOI] [PubMed] [Google Scholar]
- 103.Pack AI, Maislin G, Staley B, Pack FM, Rogers WC, George CF, et al. Impaired performance in commercial drivers: role of sleep apnea and short sleep duration. Am J Respir Crit Care Med. 2006;174(4):446–54. doi: 10.1164/rccm.200408-1146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ajani UA, Lotufo PA, Gaziano JM, Lee IM, Spelsberg A, Buring JE, et al. Body mass index and mortality among US male physicians. Ann Epidemiol. 2004;14(10):731–9. doi: 10.1016/j.annepidem.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 105.Neser WB, Thomas J, Semenya K, Thomas DJ, Gillum RF. Obesity and hypertension in a longitudinal study of black physicians: the Meharry Cohort Study. J Chronic Dis. 1986;39(2):105–13. doi: 10.1016/0021-9681(86)90066-4. [DOI] [PubMed] [Google Scholar]
- 106.Frank E, Wright EH, Serdula MK, Elon LK, Baldwin G. Personal and professional nutrition- related practices of US female physicians. Am J Clin Nutr. 2002;75(2):326–32. doi: 10.1093/ajcn/75.2.326. [DOI] [PubMed] [Google Scholar]
- 107.La Puma J, Szapary P, Maki KC. Physicians recommendations for and personal use of low- fat and low-carbohydrate diets. Int J Obes (Lond) 2005;29(2):251–3. doi: 10.1038/sj.ijo.0802840. [DOI] [PubMed] [Google Scholar]
- 108.Mihalopoulos NL, Berenson GS. Cardiovascular risk factors among internal medicine residents. Prev Cardiol. 2008;11(2):76–81. doi: 10.1111/j.1751-7141.2008.07842.x. [DOI] [PubMed] [Google Scholar]
- 109.Arora R, Lettieri C, Claybaugh JR. The effects of residency on physical fitness among military physicians. Mil Med. 2004;169(7):522–5. doi: 10.7205/milmed.169.7.522. [DOI] [PubMed] [Google Scholar]
- 110.Gutgesell ME, Weltman A, Sowa C, Seip R, Bulatovic A, Woodson S. Fitness, body fat, and perceived stress in a group of primary care residents. Acad Med. 1992;67(4):286–7. doi: 10.1097/00001888-199204000-00026. [DOI] [PubMed] [Google Scholar]
- 111.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep- disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 112.CDC. Obesity trends among U.S. adults between 1985 and 2007. 2007. http://www.cdc.gov/nccdphp/dnpa/obesity/trend/maps/
- 113.Rajaratnam SM, Barger LK, Lockley SW, Cade B, O'Brien C, White D, et al. Screening for sleep disorders in North American police officers. Sleep 30[Abstract Supplement] 2007:A209. [Google Scholar]
- 114.Krieger J, Meslier N, Lebrun T, Levy P, Phillip-Joet F, Sailly JC, et al. Accidents in obstructive sleep apnea patients treated with nasal continuous positive airway pressure: a prospective study. The Working Group ANTADIR, Paris and CRESGE, Lille, France. Association Nationale de Traitement a Domicile des Insuffisants Respiratoires. Chest. 1997;112(6):1561–6. doi: 10.1378/chest.112.6.1561. [DOI] [PubMed] [Google Scholar]
- 115.Sassani A, Findley LJ, Kryger M, Goldlust E, George C, Davidson TM. Reducing motor- vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep. 2004;27(3):453–8. doi: 10.1093/sleep/27.3.453. [DOI] [PubMed] [Google Scholar]
- 116.Findley L, Smith C, Hooper J, Dineen M, Suratt PM. Treatment with nasal CPAP decreases automobile accidents in patients with sleep apnea. Am J Respir Crit Care Med. 2000;161(3 Pt 1):857–9. doi: 10.1164/ajrccm.161.3.9812154. [DOI] [PubMed] [Google Scholar]
- 117.Weaver TE, Kribbs NB, Pack AI, Kline LR, Chugh DK, Maislin G, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20(4):278–83. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 118.Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163(5):565–71. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 119.Pack AI, Black JE, Schwartz JR, Matheson JK. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164(9):1675–81. doi: 10.1164/ajrccm.164.9.2103032. [DOI] [PubMed] [Google Scholar]
- 120.Second Edition ed. Westchester, IL: American Academy of Sleep Medicine; 2005. The International Classification of Sleep Disorders; Diagnostic and Coding Manual. [Google Scholar]
- 121.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27(8):1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 122.Czeisler CA, Walsh JK, Roth T, Hughes RJ, Wright KP, Jr., Kingsbury L, et al. Modafinil for excessive sleepiness associated with shift work sleep disorder. N Engl J Med. 2005;353:476–86. doi: 10.1056/NEJMoa041292. [DOI] [PubMed] [Google Scholar]
- 123.Gold DR, Rogacz S, Bock N, Tosteson TD, Baum TM, Speizer FE, et al. Rotating shift work, sleep, and accidents related to sleepiness in hospital nurses. Am J Public Health. 1992;82:1011–4. doi: 10.2105/ajph.82.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mitler MM, Miller JC, Lipsitz JJ, Walsh JK, Wylie CD. The sleep of long-haul truck drivers. N Engl J Med. 1997;337(11):755–61. doi: 10.1056/NEJM199709113371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Van Dongen HPA, Maislin G, Dinges DF. Dealing with inter-individual differences in the temporal dynamics of fatigue and performance: importance and techniques. Aviat Space Environ Med. 2004;75(3) Suppl:A147–54. [PubMed] [Google Scholar]
- 126.Van Dongen HPA, Caldwell JA, Jr., Caldwell JL. Investigating systematic individual differences in sleep-deprived performance on a high-fidelity flight simulator. Behav Res Methods. 2006;38(2):333–43. doi: 10.3758/bf03192785. [DOI] [PubMed] [Google Scholar]
- 127.Czeisler CA, Moore-Ede MC, Coleman RM. Rotating shift work schedules that disrupt sleep are improved by applying circadian principles. Science. 1982;217:460–3. doi: 10.1126/science.7089576. [DOI] [PubMed] [Google Scholar]
- 128.Czeisler CA, Johnson MP, Duffy JF, Brown EN, Ronda JM, Kronauer RE. Exposure to bright light and darkness to treat physiologic maladaptation to night work. N Engl J Med. 1990;322:1253–9. doi: 10.1056/NEJM199005033221801. [DOI] [PubMed] [Google Scholar]
- 129.Czeisler CA, Dijk DJ. Use of bright light to treat maladaption to night shift work and circadian rhythm sleep disorders. J Sleep Res. 1995;4(Suppl 2):70–3. doi: 10.1111/j.1365-2869.1995.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 130.Wyatt JK, Dijk DJ, Ritz-De Cecco A, Ronda JM, Czeisler CA. Sleep facilitating effect of exogenous melatonin in healthy young men and women is circadian-phase dependent. Sleep. 2006;29:609–18. doi: 10.1093/sleep/29.5.609. [DOI] [PubMed] [Google Scholar]
- 131.4th ed. Philadelphia: W.B. Saunders; 2005. Principles and Practice of Sleep Medicine. [Google Scholar]
- 132.Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci. 2008;10(3):329–36. doi: 10.31887/DCNS.2008.10.3/dnutt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Daley M, Morin CM, LeBlanc M, Gregoire JP, Savard J, Baillargeon L. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med. 2008 doi: 10.1016/j.sleep.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 134.Goebert D, Thompson D, Takeshita J, Beach C, Bryson P, Ephgrave K, et al. Depressive symptoms in medical students and residents: a multischool study. Acad Med. 2009;84(2):236–41. doi: 10.1097/ACM.0b013e31819391bb. [DOI] [PubMed] [Google Scholar]
- 135.Dyrbye LN, Thomas MR, Massie FS, Power DV, Eacker A, Harper W, et al. Burnout and suicidal ideation among U.S. medical students. Ann Intern Med. 2008;149(5):334–41. doi: 10.7326/0003-4819-149-5-200809020-00008. [DOI] [PubMed] [Google Scholar]
- 136.Dyrbye LN, Thomas MR, Huntington JL, Lawson KL, Novotny PJ, Sloan JA, et al. Personal life events and medical student burnout: a multicenter study. Acad Med. 2006;81(4):374–84. doi: 10.1097/00001888-200604000-00010. [DOI] [PubMed] [Google Scholar]
- 137.Fahrenkopf AM, Sectish TC, Barger LK, Sharek PJ, Lewin D, Chiang VW, et al. Rates of medication errors among depressed and burnt out residents: prospective cohort study. BMJ. 2008;336(7642):488–91. doi: 10.1136/bmj.39469.763218.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vela-Bueno A, Moreno-Jimenez B, Rodriguez-Munoz A, Olavarrieta-Bernardino S, Fernandez-Mendoza J, de la Cruz-Troca JJ, et al. Insomnia and sleep quality among primary care physicians with low and high burnout levels. J Psychosom Res. 2008;64(4):435–42. doi: 10.1016/j.jpsychores.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 139.Ekstedt M, Soderstrom M, Akerstedt T, Nilsson J, Sondergaard HP, Aleksander P. Disturbed sleep and fatigue in occupational burnout. Scand J Work Environ Health. 2006;32(2):121–31. doi: 10.5271/sjweh.987. [DOI] [PubMed] [Google Scholar]
- 140.University of Washington School of Medicine. Ethics in Medicine. http://depts.washington.edu/bioethx/tools/princpl.html. 2-27-2009.
- 141.Moore RT, Kaprielian R, Auerbach J. Asleep at the wheel. Report of the Massachusetts commission on drowsy driving. 2-1-2009. [Google Scholar]
- 142.Moore RT. An act relative to health care provider transportation. Senate Docket No. 1901. 1-14-2009. [Google Scholar]
- 143.Falleti MG, Maruff P, Collie A, Darby DG, McStephen M. Qualitative similarities in cognitive impairment associated with 24 h of sustained wakefulness and a blood alcohol concentration of 0.05% J Sleep Res. 2003;12(4):265–74. doi: 10.1111/j.1365-2869.2003.00363.x. [DOI] [PubMed] [Google Scholar]
- 144.Powell NB, Schechtman KB, Riley RW, Li K, Troell R, Guilleminault C. The road to danger: the comparative risks of driving while sleepy. Laryngoscope. 2001;111(5):887–93. doi: 10.1097/00005537-200105000-00024. [DOI] [PubMed] [Google Scholar]
- 145.Williamson AM, Feyer AM. Moderate sleep deprivation produces impairments in cognitive and motor performance equivalent to legally prescribed levels of alcohol intoxication. Occup Environ Med. 2000;57:649–55. doi: 10.1136/oem.57.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lamond N, Dawson D. Quantifying the performance impairment associated with fatigue. J Sleep Res. 1999;8(4):255–62. doi: 10.1046/j.1365-2869.1999.00167.x. [DOI] [PubMed] [Google Scholar]
- 147.Arnedt JT, Owens J, Crouch M, Stahl J, Carskadon MA. Neurobehavioral performance of residents after heavy night call vs after alcohol ingestion. JAMA. 2005;294(9):1025–33. doi: 10.1001/jama.294.9.1025. [DOI] [PubMed] [Google Scholar]
- 148.Landrigan CP, Barger LK, Cade BE, Ayas NT, Czeisler CA. Interns' compliance with accreditation council for graduate medical education work-hour limits. JAMA. 2006;296(9): 1063–70. doi: 10.1001/jama.296.9.1063. [DOI] [PubMed] [Google Scholar]
- 149.Carpenter RO, Austin MT, Tarpley JL, Griffin MR, Lomis KD. Work-hour restrictions as an ethical dilemma for residents. Am J Surg. 2006;191(4):527–32. doi: 10.1016/j.amjsurg.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 150.Cull WL, Mulvey HJ, Jewett EA, Zalneraitis EL, Allen CE, Pan RJ. Pediatric residency duty hours before and after limitations. Pediatrics. 2006;118(6):e1805–11. doi: 10.1542/peds.2006-0210. [DOI] [PubMed] [Google Scholar]
- 151.Nasca TJ. Open letter to the GME community. 2-16-2009. [Google Scholar]
- 152.Madsen T. The New Physician. The New Physician; 2004. A whistleblower's story: Reflections on making that report. [Google Scholar]
- 153.Fresno, California: U.S. District Court; California trucking company safety director and four drivers sentenced for their role in a false driver's log book scheme. 7-21-2008. [Google Scholar]
- 154.Abington, VA: U.S. District Court; Two sentenced in case involving falsification of truck drivers' logs. 3-11-1998. [Google Scholar]
- 155.Federal Court, MN: Trucking company, owner sentenced in driver log-fraud case. 1-22-1999. [Google Scholar]
- 156.Bangor, ME: U.S. District Court; Trucking firm and president sentenced in hours-of-service violations. 7-24-2002. [Google Scholar]
- 157.Philadelphia, PA: U.S. District Court; Truck driver imprisoned for falsifying his driver's log. 12-16-2003. [Google Scholar]
- 158.Sioux Falls, SD: U.S. District Court; South Dakota trucking firm and president ordered to pay over $325,000 in driver logbook falsification case. 9-22-2005. [Google Scholar]
- 159.Lynchburg, VA: U.S. District Court; Former executive, dispatcher, and two drivers of Virginia trucking company sentenced for their roles in falsifying drivers' logbooks. 1-18-2006. [Google Scholar]
- 160.Williamsport, PA: U.S. District Court; Former owner and dispatcher for Pennsylvania trucking company sentenced in logbook falsification case. 3-14-2006. [Google Scholar]
- 161.Brunswick, GA: U.S. District Court; Virginia truck driver gets jail time for falsifying logbooks. 8-11-2006. [Google Scholar]
- 162.Fresno, CA: U.S. District Court; 2009. Four truck drivers sentenced in logbook falsification case. [Google Scholar]
- 163.Easton, PA: State Court; Pennsylvania truck driver sentenced to 2 years in prison for vehicular homicide relating to hours-of-service violations. 3-7-2007. [Google Scholar]
- 164.Cappuccio FP, Bakewell A, Taggart FM, Ward G, Ji C, Sullivan JP, et al. Implementing a 48 h EWTD-compliant rota for junior doctors in the UK does not compromise patients' safety: assessor-blind pilot comparison. Q J Med. 2009 doi: 10.1093/qjmed/hcp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fernandez-Taylor KR. Excessive Work Hours of Physicians in Training in El Salvador: Putting Patients at Risk. PLoS Medicine. 2007;4(7):e205 1–3. doi: 10.1371/journal.pmed.0040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lerner BH. A life-changing case for doctors in training. The New York Times. 2009 Mar 3; [Google Scholar]
- 167.Lee CJ. Federal regulation of hospital resident work hours: Enforcement with real teeth. J Health Care Law & Policy. 2006;9(1):1–55. [Google Scholar]
- 168.Moore RT. An act relative to safe work hours for physicians in training and protection of patients. Senate Docket No. 1178. 12-30-2008. [Google Scholar]
- 169.Czeisler CA. President's Message. SRS Bulletin. 2005;11(2):4. [Google Scholar]