Abstract
Ventricular tachycardia (VT) is a life-threatening arrhythmia that is common to all forms of heart disease and an important cause of sudden death. Ventricular scars from infarction or replacement fibrosis provide a substrate for reentry that is a common cause. Understanding the pathophysiologic link between ventricular scars and ventricular tachycardia informs approaches to identify patients at risk, has led to development of methods to ablate the arrhythmia substrate that can be applied even in severe heart disease, and suggests future diagnostic and therapeutic strategies.
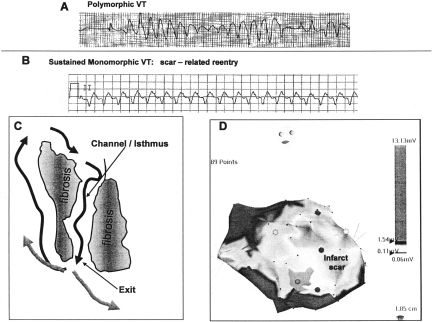
Sustained ventricular tachycardia (VT) is an important cause of many of the 150,000 to 300,000 out of hospital sudden deaths that occur annually in the US, but also has less catastrophic presentations, including syncope and palpitations, that are sometimes harbingers of a future fatal event. The electrocardiographic characteristics of VT indicate its potential mechanism and cause. Polymorphic VT (Figure 1, panel A) has a continually changing QRS morphology, indicating that the sequence of ventricular activation is changing on a beat to beat basis. Polymorphic VT is due to irregular activation waves spiraling throughout the ventricles. This type of activation can occur in the absence of a structural arrhythmia substrate (e.g., induced by electrical shock in a previously healthy individual), and often degenerates to ventricular fibrillation. This sequence is commonly induced by acute myocardial ischemia, as well as genetically determined ion channel abnormalities that cause sudden death, such as the long QT syndrome and Brugada syndrome.
Fig. 1.
Polymorphic VT is shown in Panel A. Monomorphic VT is shown in Panel B. A schematic for reentry in a region of scar is shown in Panel C. Reentry wavefronts are indicated by black arrows. Fibrosis creates a channel for conduction through the scar. A gray scale rendition of a voltage map of the left ventricle as viewed from the right oblique projection is shown in Panel D. This map was created by moving a mapping catheter over the endocardial surface of the left ventricle and plotting the peak to peak electrogram amplitude. An extensive anterior wall infarct is present, indicated by low voltage (lighter shades of gray). The basal portion of the ventricle has normal amplitude (darker shade of gray). Exit regions for VT were identified along the border zones (interface between low voltage and normal voltage regions) in this patient.
Monomorphic VT, in which each QRS is similar to the preceding and following QRS indicates that ventricular activation is the same from beat to beat (Figure 1 panel B). These VTs are associated with a fixed arrhythmia substrate. It may be a focus of automaticity in some, relatively benign idiopathic VTs, or, more commonly, a region of scar that provides the substrate for reentry (1).
Understanding the anatomy and electrophysiology of ventricular scars can facilitate recognition of patients at risk for VT, provides an understanding of therapeutic options and limitations, and suggests avenues for future investigations.
REENTRY IN VENTRICULAR SCARS
Myocardial infarction is the most common cause of ventricular scars, but scars also occur in nonischemic cardiomyopathies due to replacement fibrosis, which is a poorly understood consequence of many cardiomypathies. Scars are also created in the course of cardiac surgical repairs, such as surgical correction of Tetralogy of Fallot (2). Ventricular scars consist of regions of dense fibrosis with collagen and fibrocytes, but also contain regions with surviving myocyte bundles (3, 4). The dense fibrosis creates conduction block that can define borders of reentry circuits (Figure 1, panel C). Fibrosis also contributes to the creation of slow conduction that is necessary for reentry (3). At the microscopic level, fibrosis separates myocyte bundles, forcing the excitation wavefront to take a circuitous course through the bundles. In addition, conductivity between cells can be diminished. This uncoupling of myocyte bundles, and to some extent the myocytes within the bundles, slows conduction, although action potentials and ion channels in the myocytes can be relatively normal. Slow conduction and fibrous anatomic barriers set the stage for reentry.
The relatively fixed anatomic nature of the substrate for reentrant VT contributes to several features of scar related reentrant VT. VT is monomorphic, because ventricular activation outside the scar proceeds from a stable location, which is typically the exit of a group of surviving myocyte bundles, that form a channel for conduction through the scar (Figure 1, panel C). A single scar is often able to support multiple different circuits, however, so a single patient can have different morphologies of monomorphic VT. This substrate for reentry remains stable over time, so that patients are subject to recurrent episodes of VT over years. Antiarrhythmic drug therapy has poor efficacy. Available drugs act on ion channels to delay recovery from excitation or slow conduction. These effects may slow conduction further in the scar, but often fail to prevent reentry, which may be largely anatomically determined.
THE IMPLANTABLE DEFIBRILLATOR
Implantable cardioverter defibrillators (ICDs) effectively terminate VT episodes, either by delivering a high voltage shock for cardioversion, or by applying a burst of rapid pacing that interrupts reentry. Of patients who survive an episode of sustained VT and have an ICD implanted, 40% to 60% will have another episode of VT detected by the ICD withing two years. Of patients who receive an ICD for sudden death prophylaxis, because they are recognized to have a significant risk of sudden death (often an old infarct scar), approximately 20% will have an episode of VT within 3 to 5 years (5–8). While ICDs are life-saving, shocks reduce quality of life. In addition, episodes of VT predict increased risk for subsequent heart failure and mortality even though sinus system is restored by an ICD (5, 8). Antiarrhythmic drug therapy can reduce VT episodes but with disappointing efficacy and a problematic incidence of side effects (6). Catheter ablation has an important role to reduce VT episodes and can be life-saving when VT is incessant (1, 9, 10).
CATHETER MAPPING AND ABLATION
Catheter ablation seeks to locate reentry circuits and interrupt them, usually with radiofrequency burns. There are several challenges. Ablation lesions are relatively small, 5 to 10 mm in diameter, compared to the size of the scars and reentry circuits, which are typically over 10 to 30 cm in circumference (1, 11). One approach to this problem is to identify the critical isthmus in the reentry circuit where a small lesion can interrupt the circuit. Previously this approach required mapping during the induced VT, an approach that is difficult when the VT was poorly tolerated. Even when one channel is identified for ablation, most scars contain multiple channels and potential reentry circuits. In many patients these problems can often be overcome by mapping techniques that identify the reentry circuit substrate during stable sinus rhythm.
Substrate Mapping
As a consequence of reduced myocyte bundles, scars can be delineated based on the amplitude of electrograms recorded during catheter mapping. Development of technology to create three-dimensional anatomic maps that display electrogram amplitude (voltage maps) nicely demonstrates the location of the scar as a low voltage region (Figure 1, panel D) (1, 4, 12, 13). Furthermore, to define potential reentry circuit channels and slow conduction, areas of dense fibrosis can be identified as electrically unexcitable regions that have a high pacing threshold (4). Slow conduction can be identified from multiple component signals that are evidence of asynchronous slow conduction through uncoupled myocyte bundles. The region of the scar that contains the exit of a reentry circuit channel with the surrounding myocardium can be identified by pacing along the border as the region where pacing produces a QRS configuration similar to that of VT (14, 15). Identification of the reentry substrate allows it to be targeted for ablation during stable sinus rhythm. Additional mapping during brief episodes of VT can be focused on likely substrate areas if desired.
Ablation of Post-Infarction VT
The greatest experience for ablation is for VT due to prior myocardial infarction in patients who are having frequent episodes of VT despite antiarrhythmic drug therapy. Two multicenter trials and a number of single center series have reported over 1000 patients treated with catheter ablation (1, 16, 17). These patients have relatively severe heart disease with a left ventricular ejection fraction that typically averages below 0.32. Catheter ablation reduces frequent episodes of VT in over 70% of patients and abolishes all episodes of VT in about half of patients. The procedure related mortality is approximately 3%, often due to failure to control VT when the procedure fails. Minor complications occur in about 10% of patients, most commonly related to vascular access.
Scars in Nonischemic Heart Disease
Smaller series have reported ablation of scar-related VT in nonischemic heart disease (18–20). Ablation is more difficult, because the scars are often intramural or epicardial. Ablation has been facilitated by the development of percutaneous epicardial mapping and ablation techniques that employ a subxyphoid pericardial approach to access the pericardial space (21, 22).
The techniques for substrate mapping have also been applied to ablation of scar-related VT after repair of tetralogy of Fallot (2), and in arrhythmogenic right ventricular dysplasia (23).
When Ablation Fails
Failure of catheter ablation is now often due to anatomic constraints. The reentry circuits are located deep within the myocardium, or are in close proximity to a coronary artery and protected, or simply greatly exceed the size of ablation lesions. Transcoronary ethanol ablation has been used to approach some of these VTs, but is dependent on the ability to identify the vascular supply to the reentry circuit, and likely has greater risks (24).
Mapping and ablation technologies to deal with these limitations are in development. Use of needle electrodes deployed through a steerable catheter for intramural mapping and ablation appears feasible in animal models (25, 26).
Imaging of Ventricular Scar to Predict Risk
The link between ventricular scar and arrhythmia risk suggests that identification and characterization of scar with imaging may allow prediction of arrhythmia risk. In magnetic resonance images, scar is identified by delayed washout of gadolinium contrast (27, 28). In myocardial infarction survivors, the size of the “border zone” region of scar was a marker for late mortality (28). The characteristics of the scar were also related to the presence of ventricular tachycardia in another study (29). In patients with nonischemic cardiomyopathy who have VT, MR imaging usually shows scar (27). Ongoing trials will clarify the clinical utility of cardiac scar imaging.
THERAPY DIRECTED AT SCARS AND IMPROVING VENTRICULAR FUNCTION
Although ICDs prevent sudden deaths, and recurrent VT can usually be controlled by ablation, arrhythmia control is only part of the problem. Episodes of VT are a marker for increased risk for progressive heart failure and death, even when VT is promptly terminated by the ICD. Thus, exploration of biological therapies to improve myocardial function are of great interest. There is also evidence that infarct scars are subject to ongoing remodeling with continual degradation and deposition of the fibrous matrix. Furthermore, it appears that fibroblasts and myofibroblasts may influence the electrophysiologic properties of infarct scars. These cells have a membrane potential and if coupled to myocytes, can slowly transmit the excitation wavefront, or may serve as current sinks that can impede conduction (30, 31). As with antiarrhythmic drug therapy, modification of electrophysiologic properties in scars has the potential for beneficial, as well as adverse, proarrhythmic, effects (32, 33). An indication that this concern is relevant emerged as frequent VT in patients who had skeletal myoblasts injected into old infarct ventricular scars at the time of coronary artery bypass surgery.
CONCLUSIONS
Ventricular scars are a major cause of ventricular tachycardia in many forms of heart disease. Methods to identify and characterize scar hold promise for identifying patients at risk. Despite implantable defibrillators, which provide protection from sudden death, recurrent VT predicts increased risk of mortality and negatively affects quality of life. Whether VT is simply a marker for deteriorating cardiac function, or directly contributes to the adverse outcomes is not clear. Reentry circuits in scars can be targeted for ablation by identifying the substrate during stable sinus rhythm, but methods to target intramural reentry circuis are needed. Therapies that alter scar remodeling and improve ventricular function hold promise, but have potential proarrhythmic effects requiring careful evaluation as these therapies move into clinical trials.
Footnotes
Potential Conflicts of Interest: Grants Received: Biosense Webster Inc.; Honororia: Medtime, St. Jude, Biosense Webster, Boston Scientific; Patents pending: needle electrode to Brigham and Women's Hospital
DISCUSSION
Mackowiak, Baltimore: Bill, thank you very much. That was a very nice presentation. What percentage of implantable defibrillators actually fire once they have been put into place?
Stevenson, Boston: People fall into two groups. The first are those who are at risk for having VT, but they haven’t yet had an event, the so called primary prevention group, and they usually have a defibrillator placed because they have depressed ventricular function, often with heart failure. Defibrillators detect a spontaneous arrhythmia in that group at a rate of about 5 to 7% per year, less in some populations. Depending on how the device is programmed, often a rapid VT can be terminated without a shock, can be terminated with rapid pacing. Often that will be asymptomatic, and that's probably two-thirds or so of patients. So shocks occur at a rate of about 2% per year. The second group are patients who are resuscitated from an arrest and then get their defibrillator. They have a much higher risk of recurrent episodes of about 40% over two years.
Brenner, San Diego: What happens with the stem cell therapy? Do they actually incorporate into the myocytes or do they have some other unclear beneficial effect?
Stevenson, Boston: It appears that very few of them survive in the myocardium there is the potential that they alter that fibrous matrix, and that may still have adverse or beneficial electrophysiologic effects.
Friesinger, Nashville: Thank you very much. I have two questions of a general nature. In the 70s and 80s, we had some enthusiasm for drugs. The lack of efficacy and the side effects led us away from that. We now have a new word in the Lexicon—“channelopathies.” So we have developed a lot of new knowledge, but I assume that doesn’t play out with these microcircuits in reference to what you speak of, So I was curious about that, and maybe a guide to how much we are doing with drugs would be what sales have done in the last few years. The second question has to do with the other approach—the anti-tachycardic pacing for these kinds of arrhythmias. My colleagues at Vanderbilt have some enthusiasm for that. So I would be interested in your view on that also.
Stevenson, Boston: Now we know a lot more about ion channels, and we know a lot more about ion channelopathies, but still pharmacologic approaches for scar-related VTs is disappointing. It seems like it is such a structural problem. Some drugs slow conduction, which can promote re-entry unless they slow it to the point of failing to conduct, and it seems like you can’t really do that without getting a fair amount of toxicity. Others prolong the recovery time, which is a bit more effective; but still efficacy has been poor. Recognizing now that it is more of a structural problem, there are some drugs on the horizon that alter myocyte coupling. There is hope for future pharmacologic therapy of atrial arrhythmias, and scar-related VTs. Anti-tachycardia pacing is routinely used with defibrillators and is very important because it does allow many people to avoid a painful shock for their VT. The group at Vanderbilt was instrumental in a trial that showed that even with very rapid VTs, it is worth trying a burst of rapid pacing to try and terminate VT before a shock.
REFERENCES
- 1.Stevenson WG, Soejima K. Catheter ablation for ventricular tachycardia. Circulation. 2007;115(21):2750–60. doi: 10.1161/CIRCULATIONAHA.106.655720. [DOI] [PubMed] [Google Scholar]
- 2.Zeppenfeld K, Schalij MJ, Bartelings MM, Tedrow UB, Koplan BA, Soejima K, Stevenson WG. Catheter ablation of ventricular tachycardia after repair of congenital heart disease: electroanatomic identification of the critical right ventricular isthmus. Circulation. 2007;116(20):2241–52. doi: 10.1161/CIRCULATIONAHA.107.723551. [DOI] [PubMed] [Google Scholar]
- 3.de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, Lahpor JR. Slow conduction in the infarcted human heart. Zigzag course of activation. Circulation. 1993;88(3):915–26. doi: 10.1161/01.cir.88.3.915. [DOI] [PubMed] [Google Scholar]
- 4.Soejima K, Stevenson WG, Maisel WH, Sapp JL, Epstein LM. Electrically unexcitable scar mapping based on pacing threshold for identification of the reentry circuit isthmus: feasibility for guiding ventricular tachycardia ablation. Circulation. 2002;106(13):1678–83. doi: 10.1161/01.cir.0000030187.39852.a7. [DOI] [PubMed] [Google Scholar]
- 5.Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Reddy RK, Marchlinski FE, Yee R, Guarnieri T, Talajic M, Wilber DJ, Fishbein DP, Packer DL, Mark DB, Lee KL, Bardy GH. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359(10):1009–17. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connolly SJ, Dorian P, Roberts RS, Gent M, Bailin S, Fain ES, Thorpe K, Champagne J, Talajic M, Coutu B, Gronefeld GC, Hohnloser SH. Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. Jama. 2006;295(2):165–71. doi: 10.1001/jama.295.2.165. [DOI] [PubMed] [Google Scholar]
- 7.Schron EB, Exner DV, Yao Q, Jenkins LS, Steinberg JS, Cook JR, Kutalek SP, Friedman PL, Bubien RS, Page RL, Powell J. Quality of life in the antiarrhythmics versus implantable defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation. 2002;105(5):589–94. doi: 10.1161/hc0502.103330. [DOI] [PubMed] [Google Scholar]
- 8.Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, Daubert JP, McNitt S, Andrews ML, Elkin AD. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110(25):3760–5. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 9.Brugada J, Berruezo A, Cuesta A, Osca J, Chueca E, Fosch X, Wayar L, Mont L. Nonsurgical transthoracic epicardial radiofrequency ablation: an alternative in incessant ventricular tachycardia. J Am Coll Cardiol. 2003;41(11):2036–43. doi: 10.1016/s0735-1097(03)00398-x. [DOI] [PubMed] [Google Scholar]
- 10.Bansch D, Oyang F, Antz M, Arentz T, Weber R, Val-Mejias JE, Ernst S, Kuck KH. Successful catheter ablation of electrical storm after myocardial infarction. Circulation. 2003;108(24):3011–16. doi: 10.1161/01.CIR.0000103701.30662.5C. [DOI] [PubMed] [Google Scholar]
- 11.de Bakker JM, van Capelle FJ, Janse MJ, van Hemel NM, Hauer RN, Defauw JJ, Vermeulen FE, Bakker de Wekker PF. Macroreentry in the infarcted human heart: the mechanism of ventricular tachycardias with a “focal” activation pattern. J Am Coll Cardiol. 1991;18(4):1005–14. doi: 10.1016/0735-1097(91)90760-7. [DOI] [PubMed] [Google Scholar]
- 12.Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101(11):1288–96. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 13.Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin K, Kralovec S, Sediva L, Ruskin JN, Josephson ME. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357(26):2657–65. doi: 10.1056/NEJMoa065457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunckhorst CB, Delacretaz E, Soejima K, Maisel WH, Friedman PL, Stevenson WG. Identification of the ventricular tachycardia isthmus after infarction by pace mapping. Circulation. 2004;110(6):652–9. doi: 10.1161/01.CIR.0000138107.11518.AF. [DOI] [PubMed] [Google Scholar]
- 15.Kottkamp H, Wetzel U, Schirdewahn P, Dorszewski A, Gerds-Li JH, Carbucicchio C, Kobza R, Hindricks G. Catheter ablation of ventricular tachycardia in remote myocardial infarction: substrate description guiding placement of individual linear lesions targeting noninducibility. J Cardiovasc Electrophysiol. 2003;14(7):675–81. doi: 10.1046/j.1540-8167.2003.02541.x. [DOI] [PubMed] [Google Scholar]
- 16.Calkins H, Epstein A, Packer D, Arria AM, Hummel J, Gilligan DM, Trusso J, Carlson M, Luceri R, Kopelman H, Wilber D, Wharton JM, Stevenson W. Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy: results of a prospective multicenter study. Cooled RF Multi Center Investigators Group. J Am Coll Cardiol. 2000;35(7):1905–14. doi: 10.1016/s0735-1097(00)00615-x. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson WG, Wilber DJ, Natale A, et al. Multicenter trial of irrigated radiofrequency catheter ablation guided by electroanatomic mapping for the treatment of recurrent ventricular tachycardia after myocardial infarction: the Thermocool VT ablation trial. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.108.788604. in press. [DOI] [PubMed] [Google Scholar]
- 18.Soejima K, Stevenson WG, Sapp JL, Selwyn AP, Couper G, Epstein LM. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. J Am Coll Cardiol. 2004;43(10):1834–42. doi: 10.1016/j.jacc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Hsia HH, Callans DJ, Marchlinski FE. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation. 2003;108(6):704–10. doi: 10.1161/01.CIR.0000083725.72693.EA. [DOI] [PubMed] [Google Scholar]
- 20.Eckart RE, Hruczkowski TW, Tedrow UB, Koplan BA, Epstein LM, Stevenson WG. Sustained ventricular tachycardia associated with corrective valve surgery. Circulation. 2007;116(18):2005–11. doi: 10.1161/CIRCULATIONAHA.107.703157. [DOI] [PubMed] [Google Scholar]
- 21.Sosa E, Scanavacca M. Epicardial mapping and ablation techniques to control ventricular tachycardia. J Cardiovasc Electrophysiol. 2005;16(4):449–52. doi: 10.1046/j.1540-8167.2005.40710.x. [DOI] [PubMed] [Google Scholar]
- 22.Zei PC, Stevenson WG. Epicardial catheter mapping and ablation of ventricular tachycardia. Heart Rhythm. 2006;3(3):360–3. doi: 10.1016/j.hrthm.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Verma A, Kilicaslan F, Schweikert RA, Tomassoni G, Rossillo A, Marrouche NF, Ozduran V, Wazni OM, Elayi SC, Saenz LC, Minor S, Cummings JE, Burkhardt JD, Hao S, Beheiry S, Tchou PJ, Natale A. Short- and long-term success of substrate-based mapping and ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia. Circulation. 2005;111(24):3209–16. doi: 10.1161/CIRCULATIONAHA.104.510503. [DOI] [PubMed] [Google Scholar]
- 24.Sacher F, Sobieszczyk P, Tedrow U, Eisenhauer AC, Field ME, Selwyn A, Raymond JM, Koplan B, Epstein LM, Stevenson WG. Transcoronary ethanol ventricular tachycardia ablation in the modern electrophysiology era. Heart Rhythm. 2008;5(1):62–8. doi: 10.1016/j.hrthm.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Sapp JL, Cooper JM, Soejima K, Sorrell T, Lopera G, Satti SD, Koplan BA, Epstein LM, Edelman E, Rogers C, Stevenson WG. Deep myocardial ablation lesions can be created with a retractable needle-tipped catheter. Pacing Clin Electrophysiol. 2004;27(5):594–9. doi: 10.1111/j.1540-8159.2004.00492.x. [DOI] [PubMed] [Google Scholar]
- 26.Thiagalingam A, Pouliopoulos J, Barry MA, Boyd AC, Eipper V, Yung T, Ross DL, Kovoor P. Cooled needle catheter ablation creates deeper and wider lesions than irrigated tip catheter ablation. J Cardiovasc Electrophysiol. 2005;16(5):508–15. doi: 10.1046/j.1540-8167.2005.40540.x. [DOI] [PubMed] [Google Scholar]
- 27.Nazarian S, Bluemke DA, Lardo AC, Zviman MM, Watkins SP, Dickfeld TL, Meininger GR, Roguin A, Calkins H, Tomaselli GF, Weiss RG, Berger RD, Lima JA, Halperin HR. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112(18):2821–5. doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113(23):2733–43. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marban E, Tomaselli GF, Lima JA, Wu KC. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115(15):2006–14. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res. 2006;98(6):801–10. doi: 10.1161/01.RES.0000214537.44195.a3. [DOI] [PubMed] [Google Scholar]
- 31.Zlochiver S, Munoz V, Vikstrom KL, Taffet SM, Berenfeld O, Jalife J. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophys J. 2008;95(9):4469–80. doi: 10.1529/biophysj.108.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abraham MR, Henrikson CA, Tung L, Chang MG, Aon M, Xue T, Li RA, B OR, Marban E. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res. 2005;97(2):159–67. doi: 10.1161/01.RES.0000174794.22491.a0. [DOI] [PubMed] [Google Scholar]
- 33.Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, Bel A, Sarateanu S, Scorsin M, Schwartz K, Bruneval P, Benbunan M, Marolleau JP, Duboc D. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41(7):1078–83. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]