Abstract
Heart failure decompensation is dominated by congestive symptoms from elevated pulmonary and systemic venous pressures. In dilated heart failure, forward flow is optimal at near-normal filling pressures, with minimized mitral regurgitation. Tailored therapy to reduce filling pressures improves symptoms acutely. However, monitored reduction of filling pressures during hospitalization did not translate into improved outcome during the ESCAPE trial. Data recently analyzed from the COMPASS trial indicates that 1) ambulatory filling pressures are far higher than clinically suspected, 2) filling pressures begin to increase over 3 weeks before heart failure events, and 3) events occurring during weight-based management show mismatch between changes in weight and changes in filling pressures. Accumulated days of high filling pressures increases risk continuously above left-sided filling pressures of 15 mmHg. The challenge is to intensify not only acute management of heart failure but ambulatory surveillance to allow early intervention and reduce re-hospitalization.
For over two thousand years, the description of clinical heart failure, or “dropsy”, has emphasized the appearance and complaints typical of fluid overload, or congestion. Much intervening work has emphasized the role and quantification of myocardial contractility and the ability of the heart to distribute sufficient blood flow to meet metabolic needs. However, the clinical portrait of heart failure remains etched by symptoms of elevated pulmonary venous and systemic venous filling pressures. Many patients with advanced heart failure live with excessive intracardiac filling pressures, which often reflect incomplete therapy of fluid overload during hospitalization and the limitations of weight-based management to identify recurrent fluid retention at home. Whether the left ventricular ejection fraction is reduced or normal, the achievement and maintenance of freedom from congestion could lighten the symptomatic burden and may improve prognosis for heart failure patients, as well as decreasing the burden of heart failure on the national health care system.
Role of Cardiac Filling Pressures
The risk of rehospitalization and mortality in heart failure relates consistently to elevated right and left-sided filling pressures (1). The prognostic contribution of cardiac output-related measurements are much lower and in some experiences not significant. The natriuretic peptides, which are currently the most powerful single prognostic biomarkers, are closely, although not linearly, related to elevated intracardiac pressures (2). Echocardiographic parameters of dynamic valvular regurgitation and abnormal left ventricular filling patterns predict outcome (3) both at baseline and when they are changed by therapies. The robust clinical correlates of outcome are New York Heart Association Class IV symptoms. These are defined as those which occur at rest or during minimal activity, all of which can be attributed to pulmonary or systemic venous congestion. Clinical assessment of congestion, including signs such as jugular venous pressure elevation (4, 5) and symptoms such as orthopnea (5), can be shown to predict outcome. Reduction of measured filling pressures has been associated with improvements in prognosis (1) and the ability to maintain freedom from clinical congestion at one month after hospital discharge is associated with better outcome than predicted at the time of hospital admission (5, 6).
Optimal Filling Pressures and Flow
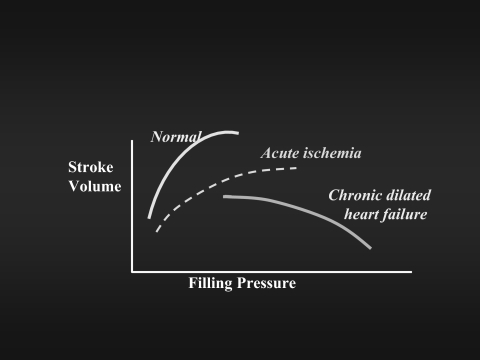
To what degree can elevated filling pressures of chronic heart failure be reduced? The fundamental Frank-Starling relationship has been translated loosely into the dependence of stroke volume on ventricular filling pressures. This curve shifts rightward in acute myocardial ischemia. Three-fold elevations of filling pressures commonly occur in chronic dilated heart failure and were at one time assumed to be necessary to preserve stroke volume and cardiac output. In seeking the compromise level at which congestive symptoms could be relieved without compromising forward flow, it was discovered that cardiac output is often maximal at near-normal levels of filling pressures in chronic dilated heart failure (7). Multiple mechanisms contribute to this “inverse Starling curve” (Figure 1), including reduced myocardial oxygen consumption, improved coronary arterial and venous perfusion gradients, and released pericardial constraint and right-left ventricular interdependence. Arithmetically, however, the increase in forward stroke volume is most attributable to the reduction in mitral regurgitation, which is frequently reduced by about half during therapy tailored to decrease filling pressures to near-normal levels (8, 9).
Fig. 1.
Conceptualized version of the relationship between left ventricular filling pressures and stroke volume. The normal relationship is depicted by the solid line on the far left. The dotted line is as described for acute myocardial infarction (20), in which the heart is acutely stiff, but not dilated. The line drawn to the far right shows that for heart failure with low ejection fraction and chronic left ventricular dilation; the stroke volume is better as the filling pressures are reduced toward normal levels (7).
Vigorous reduction of filling pressures has been associated not only with decreases in mitral regurgitation and improvements in ventricular filling patterns (9), but also improvement in right ventricular function. Reduction of filling pressures is often accompanied by some reduction in systemic vascular resistance, with rapid improvement in exercise flow reserve (10). Therapy to decrease filling pressures is also associated with decreases in circulating norepinephrine and endothelin levels (11), although renin levels may increase in some patients. The degree of reduction in pulmonary capillary wedge pressures correlates modestly with the degree of improvement in symptoms. Therapy to decrease elevated filling pressures in heart failure can decrease the severity of periodic apnea during sleep. However, further therapy beyond that needed to relieve resting symptoms has been associated with improvements in peak oxygen consumption and overall exercise capacity (12).
Targets for Therapy
Clearly, vigorous reduction of filling pressures is beneficial at multiple levels of function. How effective are we at achieving relief of congestion? We rely heavily on clinical assessment of elevated left and right sided filling pressures. Expert heart failure clinicians can estimate right atrial pressure reasonably well from jugular venous pressure (13). Although the symptom of orthopnea is helpful for the identification of pulmonary capillary wedge pressures of ≥ 30 mm Hg, it is often difficult to diagnose elevated left sided filling pressures specifically. The most helpful clinical sign of pulmonary capillary wedge pressures > 22 mmHg is right atrial pressure >10 mm Hg (13). Thus our ability to detect and track elevated left sided filling pressures during expert clinical management is limited by the degree to which they parallel jugular venous pressures.
Current Outpatient Management
What happens after heart failure hospitalization? Most patients leave the hospital with symptomatic improvement but without complete reduction of filling pressures to optimal levels. This is sometimes due to an optimistic plan to “continue diuresis at home”, which is rarely achievable. However, regardless of the therapy patients receive during heart failure hospitalization, the rate of re-hospitalization is 30–60% during the next 6 months. Close clinical follow up, such as is done in academic heart failure management programs, includes follow-up at 1–2 weeks, at least monthly for 3 months, and then every 2–3 months as needed, with frequent phone calls from the heart failure nurses. A cornerstone of management is daily weight measurement, typically with plans to increase diuretic briefly to treat a 2 lb weight gain. One study of disease management after discharge demonstrated 320 contacts in 32 patients during the first 3 month period, with 191 interventions (14); the largest fraction of these interventions was changes in diuretic doses made on the basis of daily weights at home. Why is this intense management not adequate to prevent re-admission?.
High Ambulatory Filling Pressures Drive Events
The recent COMPASS study, enabling continuous monitoring of right ventricular and estimated pulmonary artery diastolic pressures in 274 patients, has provided new information about what happens during ambulatory daily life in patients with recent hospitalizations for advanced heart failure (15). Three major surprises are how high the daily filling pressures remained despite apparently intensive management, how slowly the filling pressures rose prior to heart failure decompensation events, and how poorly the weights reflected changes in filling pressures during extended outpatient follow-up.
The median daily pulmonary artery diastolic pressure was 27 mmHg. Excluding the periods around events, the risk of subsequent heart failure events was clearly and continuously related to the level of chronic median filling pressures, with no threshold or shoulder level once the median daily pressure was over 14 mm Hg. The right ventricular end-diastolic pressures and estimated pulmonary artery diastolic pressures changed similarly and had similar predictive power for events, which was less for the median daily pulmonary artery systolic pressure.
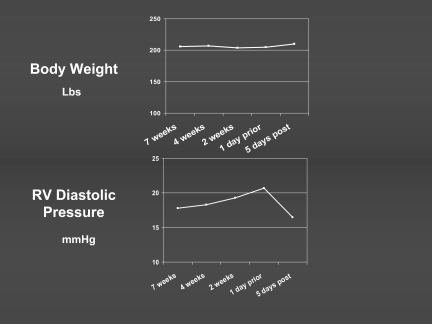
The heart failure hospitalizations were associated with an average of 12–15% increase from baseline filling pressures (16). The increases occurred gradually, with an overall interval of 29 ± 22 days of elevation prior to an event for the patients with low ejection fraction heart failure. Weight-based management was routine in this study, but those events that occurred despite this management occurred in the setting of a mismatch between weight change and change in filling pressures (Figure 2). The average weight change at the time of an event was only 1.1 ± 3.4%. There was no significant relationship between the weight change and change in filling pressure (R2=0.0074) during the period prior to the event. Even in those patients in whom weight did increase at the time of an event, this was detected only in the few days immediately prior. This has led to a re- evaluation of the assumed relationship between weights and overall fluid status. This relationship is likely strong during periods of several days, during which dry weight is unlikely to fluctuate substantially. However, during weeks to months after a baseline assessment, weight increase can occur without a change in fluid balance if patients have improved appetite or a decrease in activity without decrease in oral intake. Conversely, gradual fluid retention frequently occurs in the absence of weight gain as hepatosplanchnic congestion decreases appetite and gastro-intestinal absorption, and the pro-inflammatory state increases cachexia.
Fig. 2.
Changes in weight and filling pressure during the weeks prior to a “hypervolemic” heart failure event for 163 adjudicated events in 90 patients in the COMPASS trial including ambulatory hemodynamic monitoring. The daily weight did not increase detectably, whereas there was a rise in diastolic filling pressure beginning over 3 weeks prior to the event, and decreasing sharply during therapy for the event.
Congestion Dominates All Ejection Fraction Heart Failure
The extensive research done during the past 30 years into therapies for heart failure has focused almost exclusively on heart failure with low ejection fraction. However, approximately half of the over one million annual heart failure hospitalizations are in patients with preserved ejection fraction (17). The information from the COMPASS trial includes 70 patients with heart failure and preserved ejection fraction, in whom the relationship between risk and events parallels that for patients with reduced ejection fraction. The average filling pressures both at baseline and during events are 2–3 mm Hg lower in patients with heart failure and preserved ejection fraction, which may be explained by the relatively lower oncotic pressure in this population (18). The overall time at elevated pressures prior to events is slightly shorter (19 ± 17 days) than for heart failure with low ejection fraction, as described above, but is still almost 3 weeks (16). Thus, the differences in demographics and pathophysiology of the clinical heart failure syndrome with preserved ejection fraction do not diminish the fundamental importance of identifying and addressing elevated intracardiac filling pressures for all patients with a history of decompensated heart failure.
How Can We Do Better?
Multiple approaches are now being tested for convenient monitoring of outpatient filling pressures. We may evolve to a management strategy where patients with recurrent hospitalizations enter a strategy of real-time intervention based on supervised self- monitoring of filling pressures in much the same way, although less frequently, as diabetics adjust insulin dosages to blood glucose levels.
Heart failure currently affects 37% of the Medicare population (19). Patients with heart failure account for almost half of all Medicare inpatient costs. The tide of increasing age of the population will bring in with it an even greater burden of heart failure. At the same time, most patients with heart failure are managed in primary care settings, where multiple other conditions are managed at the same time. There are fewer than 1000 cardiologists specially trained in the care of heart failure patients. Chronic care will require extension of physician manpower by an increasing phalanx of nurse practitioners, many of whom could provide care for concomitant co-morbidities, such as diabetes that affects 30–50% of heart failure patients. The information we have gained about the importance of gradual changes in filling pressures, not identified by changes in weight, should lead to new strategies for surveillance and intervention to maintain optimal filling pressures during chronic heart failure management.
FINANCIAL SUPPORT
Portions of this research have been supported by the American Heart Association - Greater Los Angeles Affiliate, Ahmanson Foundation, Fannie Rippel Foundation, William T Young Foundation, and the National Heart, Lung, and Blood Institute. The engineers and project leaders at Medtronics Inc. have persevered for over 20 years to realize the vision of monitoring daily ambulatory pressures to learn how patients can live better with heart failure.
ACKNOWLEDGMENTS
This report was submitted with grateful appreciation for the strong collaboration with the heart failure nurses and physicians dedicated to the compassion and scholarly approach to the care of these patients at UCLA Medical Center, Los Angeles, and subsequently with the Advanced Heart Disease Program at Brigham and Women's Hospital. Specific recognition goes to the heart failure/transplant fellows through the past 25 years who contributed their efforts to relieve congestion before this was a recognized field of specialty.
Footnotes
Potential Conflicts of Interest: Dr. Stevenson has been a consultant for and received honoraria from Medtonics, Inc.
DISCUSSION
Wolf, Boston: Lynne that was a very nice talk. I thought you should know that the model of our society is continuous improvement without change, but I did have a question; and that is: in this study during the three weeks when the patients were getting increased wedge pressure, what was happening to their weight? In my practice, I think that one of the things that is useful when you talked about monitoring and looking for signs of, worsening heart failure is just to have patients weigh themselves and record it every day.
Stevenson, Boston: I wish that were true. Actually, one of the most profound things we found from this trial was, in fact, the lack of relationship of change in filling pressures to change in weights. It was almost random. Having said that, in the study that we did, we would respond to a change in weight in general by increasing diuretics. So we were already doing that, and the changes that we saw in addition to therapy modulated by weights. So, in fact, when you have a patient who has an event and didn't have a change in weight, the filling pressure, in general, would have shown you that it was going to happen.
Luke, Cincinnati: My question was the same really as Marshall's, but you didn't mention the word diuretic until just now, and I think some cardiologists think diuretic is a bad word; Derived from dialysis and not perfect, the ideal dry weight concept has been useful and seems to me empirically to work clinically; but you are telling us even in individual patients, we have optimized them and teach them how to use the weight at home and stop or use the diuretics accordingly. That doesn't work now based on your data?
Stevenson, Boston: Well, how many people here weigh themselves regularly? My question is how many people find that their weight goes up or down by a couple of pounds within a week? Most of us do. So if you were increasing diuretics based on that two pound weight gain, you might, in fact, be risking your renal function.
Luke, Cincinnati: Well I would say that all rapid changes in weight are fluid.
Stevenson, Boston: I can tell you from what we've seen now by monitoring people like this, that many of the rapid changes in weight are not fluid, but they are a big meal; they are constipation. That's one of the biggest changes that we've found by monitoring the filling pressures, is how often we were wrong in terms of their weights. In terms of dialysis, I agree with you. The ideal dry weight is certainly a target. We frequently see patients referred to us who are on chronic dialysis who are either dizzy standing up or still short of breath because they are being dialyzed to a target weight that is about six months old.
Calkins, Baltimore: Lynne, I enjoyed your talk. That was fantastic! Maybe you can comment on two things. One is a theme of what Marshall brought up in terms of monitoring patients with heart failure. I know for a while there was a big push on using Swans and admitting patients to the hospital, and that was a very popular thing to do; and then it switched towards sort of looking at weight, and now there are these implanted, heart failure monitors. Where do you think all of this is going, and what do you think the right answer is? The second question is, who should be following patients with heart failure? Should they all be seen in heart failure clinics and followed continuously in a heart failure clinic, or should internists be doing it, or general cardiologists? I would just be interested in your comments on that point as well.
Stevenson, Boston: The first question in terms of when we should be using a monitor like this - the majority of heart failure patients respond relatively well to a very careful program with sodium restriction, monitoring their weight and adjusting their diuretics accordingly. If you have a patient who has been hospitalized twice for heart failure despite what we call heart failure management, that is a person in whom we need to have a better indication of filling pressures on a regular basis. In terms of who should be following this, right now 95% of patient's with heart failure are cared for in general medicine clinics, and there are only about 900 heart failure cardiologists in the country. So it is not going to be us. What we need to look at for heart failure, as well as for many other chronic diseases, is nurse or nurse practitioner-driven models where they are seeing patients most of the time and then referring those who continue to resist their efforts.
Berl, Denver: Well thanks for emphasizing the importance for ideal filling pressure, and you didn't call it the descending limb of the Starling curve, but I think that is what you implied. I want you to address the issue of the relative merits of using diuretics versus ultrafiltration. There has been some data that there is less neurohumoral response to the ultrafiltration and a more lasting control of filling pressures in at least symptoms of patients who get ultrafiltration as opposed to the usual diuretics. Do you have a view on that?
Stevenson, Boston: That is a very intriguing question. First of all, you cannot manage heart failure without diuretics. Diuretics, in general, are underused in the community in terms of relieving congestion. As far as ultrafiltration, it is very interesting where this is now. Instead of actually having to use the renal team, there is a small device that can be hooked up through two catheters to a patient on a general medical service to remove fluid quickly, often up to 500 cc an hour. Very intriguing data about it. There is concern that the very patients who were refractory to diuretics may also be at high risk of renal dysfunction from removing fluid with ultrafiltration, and I think its role has yet to be decided through investigation in a number of different ways.
August, New York: On the renal consult service, every month we have a handful of patients with advanced heart failure who after vigorous diuresis developed renal failure; and so could you comment on the renal response to aggressive fluid removal, because that is a real challenge in end-stage patients?
Stevenson, Boston: That is a definite challenge that is called the cardio-renal syndrome. It occurs in about 25% of advanced heart failure patients where you can't diurese them as much as you want because renal function deteriorates, and we don't have an answer for that. There are a number of interesting approaches. One is adenosine receptor antagonists which are under investigation. Interestingly, as many patients improve their renal function as have a worsening of renal function with diuresis, and part of that may be related to intraperitoneal pressure that can impair renal flow. When you diurese a patient who has a lot of extra fluid, you actually decrease the pressure on the kidneys themselves, and that has been shown sometimes to improve output. But you are absolutely right! You have identified one of the biggest roadblocks we have right now to keeping people more comfortable with heart failure.
Glassock, Laguna Naguel: Very brief question about the value of monitoring the inferior vena caval diameter with ultrasound as a method of assessing congestion and following it in response—is that a tool that is being used, and what is its sensitivity and specificity?
Stevenson, Boston: Looking at the IVC is relatively similar to looking at the jugular venous pressure, and it has been validated for that. I would propose that if Marshall could train everyone in the country, we could do jugular venous pressures cheaper than we could do serial echos. Having said that, a lot of echocardiographers aren't being very careful to read the IVC. So be very careful in your own institution. When it says it is normal, it often isn't. But you are very right. It is quite closely correlated, and one could probably substitute one for the other.
Gersh, Rochester: Just a quick comment. I really enjoyed your talk as well and the point you made about converting some cardiac death into death due to heart failure; and you have talked before about the fact that class IV heart failure is a contraindication to ICD placement, and I think you are absolutely correct. What we are seeing is the following: I think it is well-established that appropriate ICD dischargers are an adverse prognostic factor. In other words, the ventricular fibrillation or VT episode is a marker of deterioration, and more and more, I find myself being faced with the issue of deactivating the ICD in patients that are dying of heart failure; and so we have taken them through the arrhythmic phase of their disease and now the arrhythmic phase is simply a manifestation of end-stage disease, and I think this is happening; and we are faced with this increasingly. It is a very difficult decision to make but I do think that deactivating a defibrillator for some patients is probably the best thing to do when they are in refractory heart failure.
Stevenson, Boston: I would certainly agree. Several trials have shown us that once you have that first ICD firing, that you move on to a much lower curve of prognosis. One of the most important things, at the time of implantation, even if you have selected the patients well, to acquaint them with the possibility that it may be appropriate someday to turn it off. Jaime Curtis and I were talking about this last night. If they hear that before they get there, it is often much easier to have that discussion. Interestingly, I used to be very concerned about turning all these gentle deaths into electrical storms. Patients who have never had a device firing during life rarely have one as they are dying. However, it would obviously be better if we could could turn off the ICD, and often that discussion gives us a chance to go into that issue of how would you like your death to occur, and what do you want to accomplish before you get there?
REFERENCES
- 1.Stevenson LW, Tillisch JH, Hamilton M, et al. Importance of hemodynamic response to therapy in predicting survival with ejection fraction less than or equal to 20% secondary to ischemic or nonischemic dilated cardiomyopathy. Am J Cardiol. 1990;66(19):1348–54. doi: 10.1016/0002-9149(90)91166-4. [DOI] [PubMed] [Google Scholar]
- 2.Gardner RS, Ozalp F, Murday AJ, et al. N-terminal pro-brain natriuretic peptide. A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. 2003;24(19):1735–43. doi: 10.1016/j.ehj.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Patel JB, Borgeson DD, Barnes ME, et al. Mitral regurgitation in patients with advanced systolic heart failure. J Card Fail. 2004;10(4):285–91. doi: 10.1016/j.cardfail.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Drazner MH, Rame JE, Stevenson LW, et al. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med. 2001;345(8):574–81. doi: 10.1056/NEJMoa010641. [DOI] [PubMed] [Google Scholar]
- 5.Lucas C, Johnson W, Hamilton MA, et al. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J. 2000;140(6):840–7. doi: 10.1067/mhj.2000.110933. [DOI] [PubMed] [Google Scholar]
- 6.Rogers JG, Hellkamp A, Young J.B., et al. Low congestion score one month after hospitalization predicts better function and survival (Abstract) Journal Am Coll Cardiol. 2007;49 in press. [Google Scholar]
- 7.Stevenson LW, Tillisch JH. Maintenance of cardiac output with normal filling pressures in patients with dilated heart failure. Circulation. 1986;74(6):1303–8. doi: 10.1161/01.cir.74.6.1303. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson LW, Brunken RC, Belil D, et al. Afterload reduction with vasodilators and diuretics decreases mitral regurgitation during upright exercise in advanced heart failure [see comments] J Am Coll Cardiol. 1990;15(1):174–80. doi: 10.1016/0735-1097(90)90196-v. [DOI] [PubMed] [Google Scholar]
- 9.Rosario LB, Stevenson LW, Solomon SD, et al. The mechanism of decrease in dynamic mitral regurgitation during heart failure treatment: importance of reduction in the regurgitant orifice size. J Am Coll Cardiol. 1998;32(7):1819–24. doi: 10.1016/s0735-1097(98)00461-6. [DOI] [PubMed] [Google Scholar]
- 10.Johnson W, Lucas C, Stevenson LW, et al. Effect of intensive therapy for heart failure on the vasodilator response to exercise. J Am Coll Cardiol. 1999;33(3):743–9. doi: 10.1016/s0735-1097(98)00631-7. [DOI] [PubMed] [Google Scholar]
- 11.Johnson W, Omland T, Collins CM, Stevenson LW. Neurohormonal activation rapidly decreases after intravenous vasodilator and diuretic therapy for Class IV heart failure. Circulation. 1998;98:1–780 (abstr). doi: 10.1016/s0735-1097(02)01814-4. [DOI] [PubMed] [Google Scholar]
- 12.Chomsky DB, Lang CC, Rayos G, et al. Treatment of subclinical fluid retention in patients with symptomatic heart failure: effect on exercise performance. J Heart Lung Transplant. 1997;16(8):846–53. [PubMed] [Google Scholar]
- 13.Drazner MH, Hellkamp AS, Leier CV, et al. Value of clinician assessment of hemodynamics in advanced heart failure. The ESCAPE trial. Circ Heart Fail. 2008;1:170– 7. doi: 10.1161/CIRCHEARTFAILURE.108.769778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah MR, Flavell CM, Weintraub JR, et al. Intensity and focus of heart failure disease management after hospital discharge. Am Heart J. 2005;149(4):715–21. doi: 10.1016/j.ahj.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Bourge RC, Naftel DC, Costanzo-Nordin MR, et al. Pretransplantation risk factors for death after heart transplantation: a multiinstitutional study. The Transplant Cardiologists Research Database Group. J Heart Lung Transplant. 1993;12(4):549–62. [PubMed] [Google Scholar]
- 16.Zile MR, Bennett TD, St John Sutton M, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118(14):1433–41. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 17.Sweitzer NK, Lopatin M, Yancy CW, et al. Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (> or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. Am J Cardiol. 2008;101(8):1151–6. doi: 10.1016/j.amjcard.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arques S, Ambrosi P, Gelisse R, et al. Hypoalbuminemia in elderly patients with acute diastolic heart failure. J Am Coll Cardiol. 2003;42(4):712–6. doi: 10.1016/s0735-1097(03)00758-7. [DOI] [PubMed] [Google Scholar]
- 19.Page R, Hogan C, Strongin K, et al. Medicare beneficiaries with mild to severe heart failures see 15–23 different providers annually (abstract) Circulation. 2008;118(18):S1030. [Google Scholar]
- 20.Russell RO, Jr., Rackley CE, Pombo J, et al. Effects of increasing left ventricular filling. Pressure in patients with acute myocardial infarction. J Clin Invest. 1970;49(8):1539–50. doi: 10.1172/JCI106371. [DOI] [PMC free article] [PubMed] [Google Scholar]