Abstract
Cystic Fibrosis (CF) is a common autosomal recessive disease that affects multiple organs. The lack of an animal model with manifestations like those typically found in humans has slowed understanding of its pathogenesis. Therefore, because of the similarities between human and swine anatomy, biochemistry, physiology, size, and genetics, we chose to develop a porcine model of CF. We used homologous recombination in primary cultures of porcine fibroblasts to disrupt the CFTR gene and then used those cells as nuclear donors for somatic cell nuclear transfer. After crossing heterozygous pigs, we produced CFTR−/− pigs. The newborn CFTR null piglets manifested meconium ileus, pancreatic destruction, early focal biliary cirrhosis, and gall bladder abnormalities that were very similar to those observed in humans with CF. At birth, there were no abnormalities in the airway epithelium or submucosal glands and no evidence of inflammation, consistent with findings in the newborn human. We hope that this porcine model will help elucidate the pathogenesis of CF and thereby lead to the development of new mechanism-based therapies.
The term “cystic fibrosis of the pancreas” was coined in 1938 by Dorthy Andersen (1). The hereditary nature of CF and its autosomal recessive pattern of transmission were described soon after. In 1989, investigators discovered the gene that is mutated in CF and named its product the cystic fibrosis transmembrane conductance regulator (CFTR) (2). We now know CF to be a common, lethal genetic disease, with a carrier rate of approximately 5% in the Caucasian population (3,4).
Since the first descriptions of CF, physicians and scientists have discovered that the disease affects many organs (for reviews, see (3,4)). Early reports focused on destruction of the exocrine pancreas and resulting pancreatic insufficiency because of its severity and an incidence of approximately 85%. Appreciation for the frequency and severity of lung dysfunction soon followed, and today airway infection and inflammation currently cause most of the mortality. Meconium ileus, an intestinal obstruction, occurs in approximately 15% of newborns. Patients also lose salt in their sweat; this observation forms the basis of the diagnostic sweat Cl− test. CF also causes gall bladder disease, male infertility, chronic sinusitis and CF-related diabetes mellitus. Liver disease that begins as focal biliary cirrhosis is currently the second leading cause of death.
Progress and Hypotheses in CF Research
Recent years have brought substantial advances in our knowledge of the genetic, molecular and cellular basis of CF (3–6). We know that >1000 different mutations in the CFTR gene are associated with CF. We have learned that CFTR is an anion channel regulated by phosphorylation of its R domain and by ATP binding and enzymatic activity (ATPase and adenylate kinase activity) by its two nucleotide-binding domains (7, 8). Although there are exceptions, the protein localizes predominantly in the apical membrane of involved epithelia. Much has also been learned about how CF-associated mutations alter protein function, thereby disrupting Cl− transport across CF epithelia. In addition, studies have suggested that CFTR may alter the function of other transport processes (9).
Despite these advances, our understanding of the pathogenesis of CF is limited. This is true for all CF organs, but is especially the case for the lung disease. Numerous hypotheses have been invoked to explain the pathogenesis of lung disease. In each case there are data that support and often data that counter the hypotheses. Here we list a few of the hypotheses; for references to the primary literature and further description, the reader may wish to consult several reviews (3, 5, 6, 10–15). a) Defective CFTR-mediated anion transport has been hypothesized to increase airway surface liquid salt concentration, thereby impairing killing by airway antimicrobials. b) Abnormally increased ENaC-dependent Na+ absorption has been proposed to dehydrate airway surface liquid, thereby impairing mucociliary clearance. c) Defective CFTR-mediated transport of HCO3− has been proposed to cause defective alkalinization of airway surface liquid impairing mucociliary clearance and bacterial killing. d) Loss of CFTR channel function in submucosal glands has been proposed to produce secretions with increased viscosity that impair mucociliary clearance. e) Loss of CFTR has been hypothesized to cause an intrinsic hyper-inflammatory state that predisposes to lung disease. In addition, an overly exuberant inflammatory response to infection or other challenges is hypothesized to influence the course of CF lung disease. f) Defective CFTR Cl− channel function has been hypothesized to impair acidification of intracellular organelles, including the Golgi complex, thereby altering protein glycosylation, increasing bacterial binding and altering other functions. g) Lack of CFTR Cl− channels has been proposed to impair acidification of macrophage and neutrophil phagosomes and hence reduce bacterial killing. h) Lack of CFTR on the cell surface is hypothesized to disrupt bacterial binding and hence their endocytosis and killing. i) Reduced oxygenation of airway surface liquid is proposed to decrease the ability to eliminate bacteria from the lung. j) Ceramide accumulation in CF is proposed to contribute to the pathogenesis of the lung disease. k) Loss of CFTR may decrease thiocyanate (SCN−) transport to the apical surface thereby impairing production of bactericidal hypothiocyanite (OSCN−). l) CFTR is suggested to regulate a number of other membrane and soluble proteins, and the lack of this interaction in CF is hypothesized to lead to the disease or exacerbate its manifestations.
Learning which of these hypotheses contribute to disease is important, because the results may suggest mechanism-based therapies and new strategies for treating CF lung disease. The same is true for understanding pathogenesis in other organs affected by CF.
Current Models of CF
Clinical and histopathological studies of patients have long guided thoughts about CF. These have had an enormous impact in improving survival and the quality of life for patients. However, for understanding the pathogenesis of the disease, clinical studies have limitations, because observations are often obtained long after the onset of disease. Moreover, many studies cannot be done in humans. As a result, the onset of changes in the fetus, infants and patients at an early stage of disease are difficult and/or impossible to discern or investigate. Later in the course of disease, secondary changes resulting from infection, inflammation and other manifestations may obscure the initiating factors.
To study and understand the complex and varied functions of airway epithelia, investigators have developed cell culture models (16–19). Human tracheal, bronchial and nasal airway epithelial cells are seeded on permeable membrane supports and grown at the air-liquid interface. Over the course of approximately 2 weeks, the cells differentiate into a polarized, pseudo-stratified epithelium containing ciliated cells, goblet cells, non-ciliated columnar cells and basal cells. These in vitro models exhibit many properties that are comparable to in vivo human airway epithelia. These models have the important advantage of flexibility, control of experimental conditions and opportunities for intervention. They also allow the study of epithelial function in the absence or presence of other cells such as macrophages, fibroblasts and cells of the immune system. As a result, in vitro models have proven exceptionally valuable for mechanistic studies in CF. However, in vitro models are limited in that CF involves not just cells, but also organs and indeed, the whole organism.
Mice, with targeted mutations in their CFTR gene (knock-outs and knock-ins of various human mutations) have been developed with the hope of generating an organism that mimics the clinical phenotype of CF patients. However, during their limited lifespan, mice with targeted CFTR modifications do not develop the airway disease that typically occurs in humans with CF (20, 21). Mice also fail to develop the pancreatic, vas deferens, liver, and gallbladder disease typically observed in patients (20–22). Moreover, while intestinal abnormalities occur in CFTR gene-targeted mice, the phenotype differs from the meconium ileus in newborn humans. Many hypotheses have arisen to explain why CF mice fail to reproduce the airway, pancreatic and other disease manifestations found in humans, but they remain untested. Thus, while gene-targeted mouse models have taught us much about CF and have been invaluable for the field of research, they have been limited in helping researchers understand the pathogenesis of CF.
Potential of a Porcine Model of CF
The failure to answer persistent research questions in CF and the inability to offer better treatments to patients, has led investigators to search for a new animal model. The sheep (23), monkey (24), ferret (25), and pig (26) have each been studied in an attempt to develop a new CF animal model. Each has advantages as a model.
We chose to investigate the pig as a CF model for several reasons (27). First, swine are important for biomedical research on many human diseases. Their organs share many anatomical, histological, physiological, biochemical, immune and inflammatory responses with human organs. This similarity is evidenced by the effort to develop porcine organs for xenotransplantation (28, 29). Second, the porcine lung is an established model for the normal human lung, for assessing several disease states (including infection), and for exploring therapeutics. Third, the porcine lifespan offers an opportunity for investigating disease pathogenesis and the efficacy of treatments. Fourth, the size of a porcine CF model may allow testing of many interventions used in humans. Fifth, the reproductive characteristics and relatively fast maturation rate of swine are favorable for a model.
Strategy to Develop a Pig With a Targeted CFTR Gene
Two developments have allowed the production of gene-targeted animals to become a standard procedure in mice. First, embryonic stem (ES) cells are available for mice. Unfortunately, ES cells that can form chimeras and contribute to the germ line had not been successfully developed for any other species (30) until the recent publication of work with rat ES cells (31). Second, the ability to genetically modify the genome has benefitted from the availability of numerous approaches that can be adapted to mouse ES cells. In contrast, the gene targeting efficiency of classical homologous recombination is extremely low in somatic cells (23, 32). The development of somatic cell nuclear transfer (SCNT) and the production of a cloned sheep in 1997 (33) provided a strategy to overcome these obstacles to producing a gene-targeted pig.
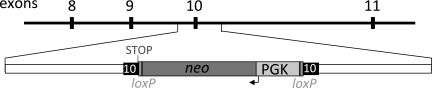
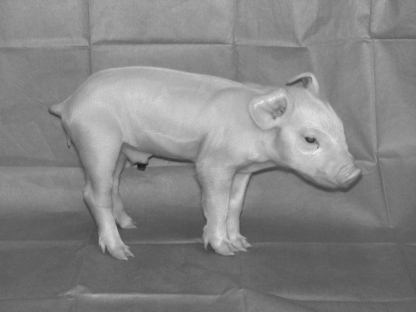
To generate pigs with a targeted CFTR gene, we used fetal fibroblasts because they are one of the few cell types known to be suitable for nuclear transfer. We initially delivered the targeting vector using nuclear microinjection and electroporation. However, many cells had random, non-homologous integrations, and we did not detect a cell with correct targeting. We then turned to an adeno-associated virus (AAV) vector. AAV vectors have been used to deliver targeting vectors to cell lines and primary cells (34, 35). They have the advantage of delivering single-stranded DNA to the nucleus; the amount of DNA per cell is small; and they can infect fibroblasts. We designed a “null” targeting construct to disrupt CFTR exon 10 with a neomycin resistance cassette (NeoR) (Figure 1); exon 10 is required to produce a functional CFTR protein. After screening infected cells, we identified fibroblasts with a targeted CFTR allele (26). To produce CFTR+/− heterozygote pigs, we used the targeted fetal fibroblasts as nuclear donors for transfer to enucleated oocytes. The resulting embryos were then transferred to surrogate sows. These animals delivered male CFTR+/− piglets; Figure 2 shows the first heterozygote piglet.
Fig. 1.
Schematic of targeting constructs for CFTR-null homologous recombination. Exons 8–11 of pig CFTR are depicted in black boxes. NeoR contains a neomycin resistance cDNA driven by the PGK promoter and flanked by loxP sites. The engineered stop codon is indicated. Adapted from (26), with permission.
Fig. 2.
Photo of the first CFTR+/− piglet taken at one day of age. From (26), with permission.
Production of CFTR−/− Piglets
Once the CFTR+/− males reached sexual maturity, they sired numerous litters of heterozygote offspring, both males and females. The heterozygotes were then mated to produce CFTR+/+, CFTR+/−, and CFTR−/− piglets in a ratio not significantly different from that expected for a Mendelian trait (36). These results suggest that as in humans and mice, disrupting the CFTR gene did not reduce survival to birth. In the CFTR−/− pigs, northern blot and quantitative RT- PCR did not detect normal CFTR transcripts, and immunoprecipitation and immunocytochemistry did not detect CFTR protein.
Meconium Ileus in Newborn CFTR−/− Piglets
In humans, the earliest manifestation of CF is meconium ileus, an intestinal obstruction that occurs in approximately 15% of CF infants (37,38). The obstruction is variably located throughout the small intestine and colon, but often occurs near the ileocecal junction (39). Distal to the site of obstruction, the small bowel and/or colon appear small and sometimes atretic. Intestinal perforation in utero or following birth occurs in some infants.
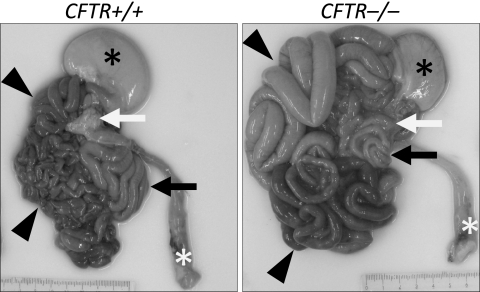
We found that all CFTR−/− piglets developed meconium ileus (36). The site of obstruction ranged from the mid small intestine to the proximal colon. Proximal to the obstruction, the small intestine was distended with dark green meconium and proximal to that with gas. Distal to the obstruction and extending through the descending colon, the intestine was stenotic to atretic, consistent with the microcolon found in patients with meconium ileus. Figure 3 shows a representative photomicrograph of the gastrointestinal system.
Fig. 3.
Gross appearance of gastrointestinal tract. Piglets were fed colostrum and milk- replacer for 30–40 h and then euthanized. Stomach (black*), small intestine (arrowheads), pancreas (white arrow), rectum (white*), and spiral colon (black arrow) are indicated. From (36), with permission.
The changes of meconium ileus replicate findings in humans with CF (3, 39) with one difference: the penetrance of meconium ileus was 100% in the CFTR−/− piglets, whereas it is approximately 15% in human CF. The reasons for the more severe presentation include a restricted genetic background in these pigs vs. humans and anatomical or physiological differences between the two species.
Pancreatic Disease in CFTR−/− Piglets
Approximately 85% of patients with CF have exocrine pancreatic insufficiency, the incidence is even higher in patients with two alleles that severely disrupt CFTR function (3, 4, 37). The classical description includes loss of exocrine lobular parenchyma with increased amounts of connective tissue (40). Gross inspection of porcine CFTR−/− pancreas revealed an organ that lacked the creamy, white lobular pattern of wild-type pancreas (Figure 3).
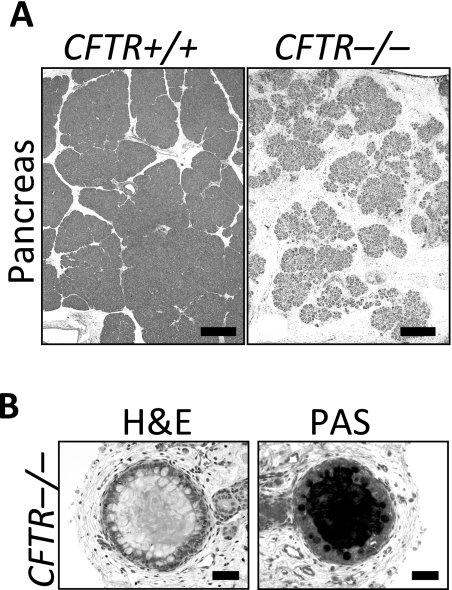
On microscopic examination, the parenchyma was reduced to small, degenerative lobules with a corresponding increase in the intervening loose adipose and myxomatous connective tissue stroma (Figure 4). Residual acini had markedly reduced amounts of eosinophilic zymogen granules. Centroacinar spaces, ductules and ducts were variably dilated by inspissated eosinophilic material. Sometimes the luminal eosinophilic material formed concentrically lamellar concretions. Pancreatic endocrine tissue was relatively intact and spared from alteration. These changes parallel those observed in humans with CF.
Fig. 4.
Panel A: Loss of parenchyma in the CFTR−/− pancreas. H&E stain. Bars, 500 μm. Panel B: Ducts within the CFTR−/− pancreas. H&E stain, left; PAS stain, right. Bars, 50 μm. From (36), with permission.
Liver Disease and Gall Bladder Abnormalities in CFTR−/− Piglets
The most common hepatic disease in CF is focal biliary cirrho-sis (41, 42). Although it can be found in early childhood and the majority of adults, the disease often remains clinically silent until overt complications emerge related to portal hypertension or cirrhosis. Most areas of the porcine CFTR−/− liver had a normal appearance like that of their CFTR+/+ and CFTR+/− littermates. However, we observed infrequent, mild to moderate hepatic lesions, including focal portal changes composed of chronic cellular inflammation and bile ductule hyperplasia on a background of increased portal connective tissue.
Cholelithiasis occurs in 15–30% of patients, and a small gallbladder is a common autopsy finding (3). We found that porcine CFTR−/− gallbladders were small and often filled with congealed bile. Microscopically, luminal contents of gallbladders were composed of bile and variable amounts of mucus. The biliary ducts had inspissated eosinophilic material in mucosal recesses with luminal changes similar to those seen in the gallbladder.
Lack of Airway Epithelial and Submucosal Gland Abnormalities in CFTR−/− Piglets
In neonatal CFTR−/− piglets, the lungs lacked microscopic evidence of cellular inflammation in the airways or parenchyma. The airway epithelium and submucosal glands appeared similar in all three genotypes, and we observed no evidence of dilated or plugged submucosal gland ducts. Moreover, bronchoalveolar lavage in newborns revealed no significant differences between cell counts or IL-8 levels in the three genotypes.
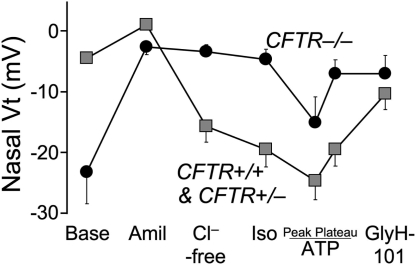
Despite the absence of morphological abnormalities, the lack of CFTR produced abnormalities in electrolyte transport characteristic of those observed in CF airway epithelia (Figure 5). We measured transepithelial voltage (Vt) across nasal epithelia as is frequently used as an in vivo assay of CFTR function in humans (43). The baseline nasal Vt was more electrically negative in CFTR−/− piglets than in their littermates. Adding amiloride to the mucosal surface to inhibit ENaC Na+ channels reduced Vt in all genotypes. Subsequent perfusion of the apical surface with a solution containing a low Cl− concentration hyperpolarized Vt in wild-type and heterozygous animals, and adding isoproterenol to increase cellular levels of cAMP produced a small additional hyperpolarization. In contrast, there was no response to these interventions in the CFTR−/− epithelia. Perfusion with ATP to activate P2Y2 receptors and Ca2+-activated Cl− channels produced an additional Vt hyperpolarization. Finally, perfusion with the CFTR inhibitor, GlyH- 101 (44), depolarized Vt in CFTR+/+ and CFTR+/−, but not CFTR−/− piglets. Although data from newborn humans with CF is not available for a direct comparison, these results are qualitatively similar to those obtained in children and adults with CF and indicate the lack of CFTR Cl− channel activity in the CFTR−/− piglets.
Fig. 5.
Average in vivo nasal voltage (Vt) measured in newborn pigs. After baseline measurements, the following agents/solutions were sequentially added to the epithelial perfusate: amiloride (100 μM), Cl−-free solution, isoproterenol (10 μM), ATP (100 μM), and GlyH-101 (100 μM). Data from 4 CFTR+/+ and 4 CFTR+/− piglets were not statistically different and were combined and compared to data from 5 CFTR−/− piglets. Values of baseline nasal Vt for CFTR−/− piglets differed from the controls, as did the changes in Vt induced by adding amiloride, a Cl−-free solution, and GlyH-101. Data are mean ± SEM. From (36), with permission.
Concluding Comments
The phenotype of the CFTR−/− piglets is similar to that of newborn humans with CF. Abdominal lesions dominated the initial presentation in both, with meconium ileus, pancreatic destruction, early focal biliary cirrhosis and abnormalities of the gall bladder and bile ducts. The newborn pigs did not show evidence of airway inflammation or infection. Determining whether airway disease develops with time and how it might progress should prove very interesting.
Finding that disrupting the porcine CFTR gene reproduces several of the major phenotypes observed in humans with CFTR mutations provides hope that this model will provide investigators with new opportunities to better understand the disease and to develop novel prevention and treatment strategies.
Footnotes
Potential Conflicts of Interest: Dr. Welsh owns shares in Exemplor Genetics, which has licensed CFTR-gene targeted pigs.
DISCUSSION
High, Philadelphia: Beautiful work! So, how are you doing on breeding additional pigs?
Welsh, Iowa City: The penetrance of meconium ileus is 100% in the CFTR −/− pig. We are doing surgery to relieve the intestinal obstruction, but that and the post-op care are a substantial effort. I think that in the long run, it will be best to find a non-surgical way to circumvent the meconium ileus. We are taking several apporaches. One strategy is to out-cross the CFTR-targeted pigs to pigs that have substantially different genetics. A second apporach is to make a transgenic pig that is a knockout for CFTR, but expresses CFTR selectivity in the intestine. There are other strategies we are also considering.
High, Philadelphia: Do you know whether the delta 508 pigs have meconium ileus at the same rate? Do you know that yet?
Welsh, Iowa City: In very preliminary experiments it looks like the answer may be yes for CFTR−/ΔF508 pigs.
Chapman, Jackson: Very fascinating talk! Do you think these techniques could be used for looking at the genetics of the phenotypic cystic fibrosis patients that we see in adults but without the genotypic?
Welsh, Iowa City: That is a very good question. There is enormous variability in patients. For example, if you have one child with CF that has meconium ileus, and then you have a second child with CF, the chances that the second child will also have meconium ileus are only about 30%. This suggests that despite having the same CFTR mutations there are genetic modifers with substantial consequences for the meconium ileus phenotype. Genetic modifiers also affect the lung disease. So use of pigs with variations in their CF phenotype might provide a way to discover genetic modifiers. If one could discover genetic modifiers that either make the disease better or make the disease worse, those might become targets for development of new therapeutics.
Alexander, Atlanta: I want to congratulate you on a spectacular tour de force. Wonderful! I wanted to ask at the cell level, what do you now understand is wrong and why the chloride transporter has such a profound effect?
Welsh, Iowa City: Well, I still do not understand the pathogenesis of the disease. That is why I said I think this is still just the beginning. However, I am very hopeful that future studies will help us understand how the loss of CFTR leads to disease in the lung and in other organs.
Baum, New York: I just want to add my praise to the work, but I wanted to raise the chicken and egg conundrum that you raised, being an infectious disease specialist. This would seem to give you a real opportunity to do serial bacterial cultures, since the bacteriology of this disease is pretty specific. It is either Pseudomonas or strep milleri now, and are you doing serial bacterial cultures to see if you can get organisms before you get the major inflammation?
Welsh, Iowa City: In CF lung disease, one thinks about Pseudomonas aeruginosa. However, the first organisms involved are often Haemophilus influenzae and Staphylococcus aureus, and Staph. aureus remains a big problem. Pseudomonas, Burkholderia, Stenotrophomonas, and other organisms that colonize the CF lung are mostly opportunistic pathogens. Patients with CF clearly have a host defense defect, but it is present only in the lung. That defect is relatively mild compared to that of a child with severe combined immunodeficiency (SCID), as children with CF may not present with a respiratory infection until two months of age or two years of age or even later. Perhaps it takes two hits to trigger severe progressive lung disease. For example, a viral infection or another insult combined with the impaired host defense defect might initiate severe lung disease. However, I will add that there are many ideas about the mechanisms responsible for the onset of the lung disease.
REFERENCES
- 1.Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease. A clinical and pathologic study. Am J Dis Child. 1938;56:344–99. [Google Scholar]
- 2.Riordan JR, Rommens JM, Kerem BS, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 3.Welsh MJ, Ramsey BW, Accurso F, Cutting GR. Cystic Fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogelstein B, editors. The Metabolic and Molecular Basis of Inherited Disease. 8 ed. New York: McGraw-Hill; 2001. pp. 5121–89. [Google Scholar]
- 4.Davis PB, Drumm ML, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–56. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- 5.Quinton P. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev. 1999;79:S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- 6.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 7.Randak C, Welsh MJ. An intrinsic adenylate kinase activity regulates gating of the ABC transporter CFTR. Cell. 2003;115:837–50. doi: 10.1016/s0092-8674(03)00983-8. [DOI] [PubMed] [Google Scholar]
- 8.Gadsby DC, Vergani P, Csanady L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–83. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwiebert EM, Benos DJ, Egan ME, Stutts J, Guggino WB. CFTR is a conductance regulator as well as a chloride channel. Physiol Rev. 1999;79:S145–S66. doi: 10.1152/physrev.1999.79.1.S145. [DOI] [PubMed] [Google Scholar]
- 10.Wine JJ. The genesis of cystic fibrosis lung disease. J Clin Invest. 1999;103:309–12. doi: 10.1172/JCI6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verkman AS. From the farm to the lab: the pig as a new model of cystic fibrosis lung disease. Am J Physiol Lung Cell Mol Physiol. 2008;295:L238–9. doi: 10.1152/ajplung.90311.2008. [DOI] [PubMed] [Google Scholar]
- 12.Guggino WB, Banks-Schlegel SP. Macromolecular interactions and ion transport in cystic fibrosis. Am J Respir Crit Care Med. 2004;170:815–20. doi: 10.1164/rccm.200403-381WS. [DOI] [PubMed] [Google Scholar]
- 13.Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–58. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 14.Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol Rev. 1999;79:S215– S55. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- 15.Quinton PM. Cystic fibrosis: lessons from the sweat gland. Physiology (Bethesda) 2007;22:212–25. doi: 10.1152/physiol.00041.2006. [DOI] [PubMed] [Google Scholar]
- 16.Karp PH, Moninger T, Weber SP, et al. An in vitro model of differentiated human airway epithelia: methods and evaluation of primary cultures. In: Wise C, editor. Epithelial Cell Culture Protocols. Totowa, NJ: Humana Press, Inc.; 2002. pp. 115–37. [DOI] [PubMed] [Google Scholar]
- 17.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. In: Picot J, editor. Methods in Molecular Medicine: Human Cell Culture Protocols. 2nd ed. Totowa, NJ: Humana Press Inc; 2004. pp. 183–206. [DOI] [PubMed] [Google Scholar]
- 18.Gruenert DC, Finkbeiner WE, Widdicombe JH. Culture and transformation of human airway epithelial cells. Am J Physiol. 1995;268:L347–L60. doi: 10.1152/ajplung.1995.268.3.L347. [DOI] [PubMed] [Google Scholar]
- 19.Whitcutt MJ, Adler KB, Wu R. A biphasic chamber system for maintaining polarity of differentiation of cultured respiratory tract epithelial cells. In Vitro Cell Dev Biol. 1988;24:420–8. doi: 10.1007/BF02628493. [DOI] [PubMed] [Google Scholar]
- 20.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- 21.Guilbault C, Saeed Z, Downey GP, Radzioch D. Cystic fibrosis mouse models. Am J Respir Cell Mol Biol. 2007;36:1–7. doi: 10.1165/rcmb.2006-0184TR. [DOI] [PubMed] [Google Scholar]
- 22.Clarke LL, Gawenis LR, Franklin CL, Harline MC. Increased survival of CFTR knockout mice with an oral osmotic laxative. Lab Anim Sci. 1996;46:612–8. [PubMed] [Google Scholar]
- 23.Williams SH, Sahota V, Palmai-Pallag T, Tebbutt SJ, Walker J, Harris A. Evaluation of gene targeting by homologous recombination in ovine somatic cells. Mol Reprod Dev. 2003;66:115–25. doi: 10.1002/mrd.10340. [DOI] [PubMed] [Google Scholar]
- 24.Wine JJ, Glavac D, Hurlock G, et al. Genomic DNA sequence of Rhesus (M. mulatta) cystic fibrosis (CFTR) gene. Mamm Genome. 1998;9:301–5. doi: 10.1007/s003359900753. [DOI] [PubMed] [Google Scholar]
- 25.Sun X, Ziying Y, Yaling Y, et al. AAV targeted disruption of the CFTR gene in cloned ferrets. J Clin Invest. 2008;118:1578–83. doi: 10.1172/JCI34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers CS, Hao Y, Rokhlina T, et al. Production of CFTR null and ΔF508 heterozygous pigs by AAV-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest. 2008;118:1571–7. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers CS, Abraham WM, Brogden KA, et al. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L240–63. doi: 10.1152/ajplung.90203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu X, Dor FJ, Cooper DK. Pig-to-non-human primate heart transplantation: immunologic progress over 20 years. J Heart Lung Transplant. 2007;26:210–8. doi: 10.1016/j.healun.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Cooper DK, Ezzelarab M, Hara H, Ayares D. Recent advances in pig-to-human organ and cell transplantation. Expert Opin Biol Ther. 2008;8:1–4. doi: 10.1517/14712598.8.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Capecchi MR. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat Rev Genet. 2005;6:507–12. doi: 10.1038/nrg1619. [DOI] [PubMed] [Google Scholar]
- 31.Buehr M, Meek S, Blair K, et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–98. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Sedivy JM, Dutriaux A. Gene targeting and somatic cell genetics–a rebirth or a coming of age? Trends Genet. 1999;15:88–90. doi: 10.1016/s0168-9525(98)01689-8. [DOI] [PubMed] [Google Scholar]
- 33.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KHS. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–3. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 34.Hirata R, Chamberlain JS, Dong R, Russell DW. Targeted transgene insertion into human chromosomes by adeno-associated virus vectors. Nat Biotechnol. 2002;20:735–8. doi: 10.1038/nbt0702-735. [DOI] [PubMed] [Google Scholar]
- 35.Porteus MH, Cathomen T, Weitzman MD, Baltimore D. Efficient gene targeting mediated by adeno-associated virus and DNA double-strand breaks. Mol Cell Biol. 2003;23:3558–65. doi: 10.1128/MCB.23.10.3558-3565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers CS, Stoltz DA, Meyerholz DK, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–41. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Ped. 1993;122:1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- 38.Mushtaq I, Wright VM, Drake DP, Mearns MB, Wood CB. Meconium ileus secondary to cystic fibrosis. The East London experience. Pediatr Surg Int. 1998;13:365–9. doi: 10.1007/s003830050341. [DOI] [PubMed] [Google Scholar]
- 39.Oppenheimer EH, Esterly JR. Pathology of cystic fibrosis review of the literature and comparison with 146 autopsied cases. Perspect Pediatr Pathol. 1975;2:241–78. [PubMed] [Google Scholar]
- 40.Imrie JR, Fagan DG, Sturgess JM. Quantitative evaluation of the development of the exocrine pancreas in cystic fibrosis and control infants. Am J Pathol. 1979;95:697–708. [PMC free article] [PubMed] [Google Scholar]
- 41.Wilschanski M, Durie PR. Pathology of pancreatic and intestinal disorders in cystic fibrosis. J R Soc Med. 1998;91 Suppl 34:40–9. doi: 10.1177/014107689809134s07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oppenheimer EH, Esterly JR. Hepatic changes in young infants with cystic fibrosis: possible relation to focal biliary cirrhosis. J Pediatr. 1975;86:683–9. doi: 10.1016/s0022-3476(75)80351-9. [DOI] [PubMed] [Google Scholar]
- 43.Standaert TA, Boitano L, Emerson J, et al. Standardized procedure for measurement of nasal potential difference: an outcome measure in multicenter cystic fibrosis clinical trials. Pediatr Pulmonol. 2004;37:385–92. doi: 10.1002/ppul.10448. [DOI] [PubMed] [Google Scholar]
- 44.Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol. 2004;124:125–37. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]