Abstract
Malignancy is a dreaded complication following organ transplantation. Immunosuppressive therapy-induced impairment of the host immune system is the prevailing hypothesis for the high incidence and aggressive progression of post-transplant neoplasm. We summarize our observations supporting an autonomous cellular mechanism for cyclosporine and tacrolimus associated metastases. Cyclosporine conferred tumor invasiveness by a direct effect on the tumor cells and promoted metastases in T-, B-, and NK cell deficient SCID- beige mice, and anti-TGF-β antibodies reduced metastases. Tacrolimus, another calcineurin inhibitor widely used in transplantation, induced TGF-β secretion by tumor cells and promoted metastases in the SCID- beige mice. The immunosuppressive macrolide rapamycin reversed an invasive phenotype to a non-invasive one, reduced circulating levels of TGF-β1 and prevented tumor growth and metastases in the immocompetant BALB/c mice and in the SCID-beige mice. Our studies, in addition to demonstrating a cell autonomous mechanism for tumor progression, advance TGF-β blockade as an anti-tumor strategy.
The first successful organ transplantation was performed on December 23, 1954, and transplantation of allogeneic cells and organs has advanced from a highly experimental therapy to a safe and consistently successful treatment modality in just 5 decades (1, 2). Improved understanding of the immune response, refinement in surgical skills, better methods of organ preservation, and the development and clinical application of potent immunosuppressive drugs and therapeutic polyclonal and monoclonal antibodies have all contributed to the stellar outcome in the clinical setting (3).
The primary goal of immunosuppressive therapy in organ graft recipients is to prevent immune rejection of the transplanted organ, and the 2005 UNOS Scientific Renal Transplant Registry reported that less than 30% of the renal transplants undergo acute rejection in the first year of transplantation (4). However, the current method of usage of immunosuppressive is associated with a heightened risk of post-transplantation malignancy (5–7).
Post-transplant malignancy is characterized by a high incidence of lymphoma and atypical malignancy, particularly of the skin, and aggressive metastatic progression. The primary hypothesis for the increased frequency and metastasis is impairment of the organ graft recipient's immune system by the drugs used to prevent graft rejection, although direct evidence for the failure of an immunosurveillance system to be the cause is scant. Nevertheless, the current guiding principle for the clinical management is a prompt reduction or discontinuation of immunosuppressive drugs once the post-transplant malignancy is diagnosed, with the understanding that such a strategy may precipitate allograft rejection and graft failure.
We summarize here our findings that support a host immune cell-independent and a tumor cell autonomous mechanism for calcineurin inhibitor (CNI)-associated tumor progression in the organ graft recipient (8–12). We posit that the commonly used CNI, cyclosporine and tacrolimus, have a direct effect on the tumor cells and confer invasiveness, and facilitate metastases. It is worth emphasizing that our data suggest that the calcineurin inhibitors promote metastatic spread of the pre-existing tumor cells rather than convert a non-malignant cell into a cancer cell.
In this report, we also summarize our studies demonstrating that the macrolide rapamycin converts a malignant cell with an invasive phenotype to a noninvasive one in a cell autonomous fashion, blocks tumor cell growth, and prevents metastasis (9, 10, 12). Our provocative observations that a higher immunosuppression burden can be associated with tumor regression (9, 10, 12) are also included in this overview.
MATERIALS AND METHODS
Cell lines and Reagents and culture.
Human lung adenocarcinoma cells, A-549 cells (ATCC CCL 185, American Type Culture Collection, Rockville, MD), human bladder transitional cell carcinoma cells (ATCC HTB4, T24), mink lung epithelial cells, CCL-64 (ATCC), mouse mammary gland epithelial cells, NMuMG (ATCC), Lewis lung carcinoma cells (ATCC), and mouse KLN-205 (gift of Robert Korst, NY), a NSCLC line originally induced in a DBA/2 mouse (13), were all grown in minimum essential medium (MEM) supplemented with 10% fetal bovine serum, at 37°C in a 5% CO2/95% air atmosphere. Murine renal adenocarcinoma cells (a gift from Dr. R. H. Wiltrout, National Cancer Institute, Bethesda MD), were maintained by in vivo serial passages in syngeneic BALB/c mice. For the in-vitro studies, the cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2 and grown in minimal essential medium, supplemented with 10% fetal bovine serum, 1 mM L-glutamine and penicillin/streptomycin (100 IU/50 μg/ml) (Gibco, Grand Island, New York). A well-characterized human renal carcinoma cell line 786-O was obtained from ATCC (14) and an additional human renal carcinoma cell line Porson was a kind gift of Dr. D. Nanus (Weill Medical College of Cornell University, New York, NY). Human renal cancer cells (RCC) were cultured in minimal essential medium (MEM) supplemented with 10% FBS and penicillin/streptomycin/L-glutamine (100IU/50μg/ml/2mM) (Life Technologies, Inc., Grand Island, NY) and at 37°C and maintained in a 5%CO2/95% air atmosphere. For the in vitro studies, the cells were harvested when they reached 70–80% confluence, washed with PBS and re-suspended in MEM/10%FBS. Murine
Reagents.
Cyclosporin A (CsA) was purchased from Novartis Pharmaceutics (Summit, New Jersey). Tacrolimus was a gift from Fujisawa USA (Deerfield, Illinois). Rapamycin was a gift from Dr. S. Seghal, Wyeth Laboratories, Philadelphia, Pennsylvania. 1D11.16 mAbs (15) directed at TGF- was a gift from Dr B. Pratt (Genzyme Corporation, Framingham MA).
Scanning electron microscopy.
Cells were seeded at a density of 105 on 12-mm-round glass cover slips, and grown for 72 h in the absence or presence of CsA, tacrolimus or rapamycin To assess the ability of TGF-β specific antibody to inhibit CsA-mediated effects, A-549 cells were incubated in the presence of both CsA and mAbs (Genzyme, Boston MA) that recognized TGF- β1, β2 and β3, were fixed with PBS, pH 7.4, containing 2.0% glutaraldehyde, and processed as previously described (16). Samples were examined using a JEOL 25SIII electron microscope.
Mice and in-vivo tumor models.
SCID-beige mice (Taconic, Germantown, New York), BALB/c mice (Charles River Laboratories, Kingston, New York) and DBA/2 mice (Taconic) were maintained in our animal facility and were used at age of 8–10 weeks. Tumor progression and the effect of CsA, tacrolimus or rapamycin were assessed using the subcutaneous growth model, the intrarenal model and/or the pulmonary metastasis model (17). The number of pulmonary metastases was determined following endotracheal insufflation of lungs with 15% India ink solution and bleaching the collected lungs in Fekete's solution (17).
RESULTS
Cyclosporine confers an invasive phenotype in a cell autonomous fashion.
CsA treatment of A-549 cells, a non-transformed human pulmonary adenocarcinoma cell line (18), resulted in striking morphological changes (8). Scanning electron microscopic examination revealed that untreated A-549 cells display a cuboidal epithelial and non-invasive phenotype (Figure 1a and b), whereas CsA-conditioned cells exhibit marked membrane ruffling and formation of numerous pseudopodia (Figure 1c and d).
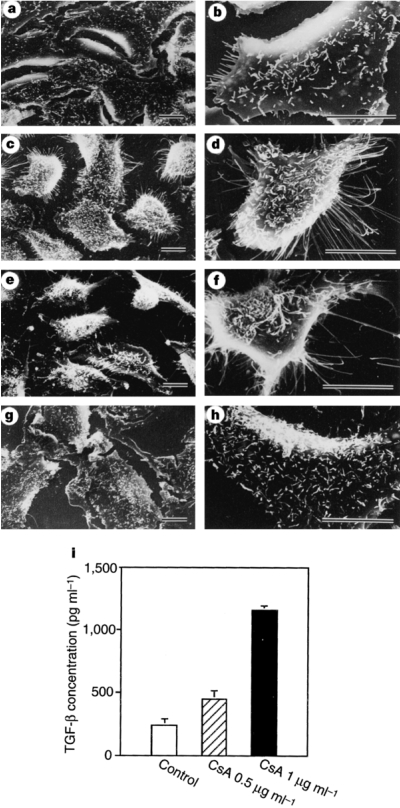
Fig. 1.
CsA induces A-549 cells to acquire an invasive phenotype. Scanning electron microscopic photographs of A-549 cells grown on glass cover slips and incubated for 72h with: none (control cells, Panel A and Panel B); 1 μg/ml CsA (Panel C and Panel D); 2 ng/ml recombinant TGF-β1 protein (Panel E and Panel F); 1 μg/ml CsA plus 30 μg/ml anti-TGF-β mAbs (Panel G and Panel H). Note that CsA-conditioned cells, in a fashion similar to TGF-β1-treated cells, display membrane ruffling as well as acquire exploratory pseudopodia, and that anti-TGF-β mAbs prevent CsA-induced morphological alterations. Bars indicate 10 μm. Panel I: illustrates TGF-β protein concentrations (mean ± SD) in supernatants obtained from untreated or CsA-treated A-549 cells. The cells were incubated for 72 h, in the absence or presence of 0.5 μg or 1.0 μg/ml CsA, and a sandwich ELISA was used to quantify TGF-β1 protein levels. (Reprinted from Hojo et al (8) with permission).
The phenotypic changes observed following CsA treatment appeared TGF-β dependent. CsA, in a concentration-dependent manner, stimulated TGF-β1 protein secretion by A-549 cells (Figure 1i); Anti-TGF-β mAbs (1D11.16 IgG1 mAbs), in contrast to control IgG1 mAbs, prevented CsA-induced morphological alterations (Figure 1g and h); and recombinant TGF-β1 protein induced morphological alterations in A-549 cells that were similar to those elicited by CsA (Figure 1e and f).
Rapamycin converts an invasive phenotype into a non-invasive phenotype.
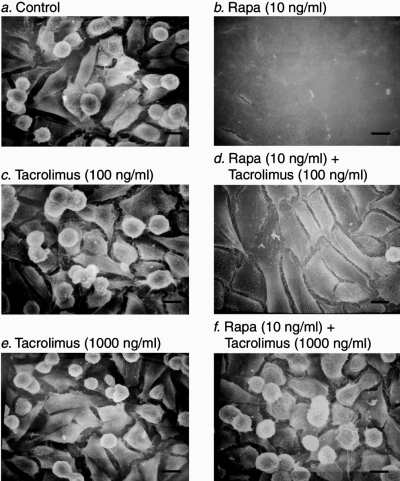
We selected a sub-clone of murine renal cancer cells with an invasive phenotype and examined the effect of rapamycin (9). Figure 2 illustrates that whereas the untreated, control renal cancer cells are spindle- or dome-shaped cells with exploratory pseudopodia, and lack cell-to-cell contact (Figure 2a), rapamycin treated cells have lost their spherical shape, and have become cuboidal, formed cell- to-cell adhesion and display a cobblestone appearance (Figure 2b).
Fig. 2.
Conversion of renal cancer cells from an invasive phenotype to a non-invasive phenotype by rapamycin. Scanning electron microscope photographs of murine renal adenocarcinoma cells grown on glass cover slips and incubated for 72 h with: nothing (control cells, Panel A); 10 ng/ml rapamycin (Panel B); 100 ng/ml tacrolimus (Panel C); 10 ng/ml rapamycin and 100 ng/ml tacrolimus (Panel D); 1000 ng/ml tacrolimus (Panel E); 10 ng/ml rapamycin and 1000 ng/ml tacrolimus (Panel F). Note that rapamycin treatment induces phenotypic transition of renal cancer cells from spindle shaped cells with exploratory pseudopodia to cuboidal shaped cells that form cell-to-cell adhesion and display a cobblestone appearance, and that tacrolimus prevents the rapamycin-induced morphological alterations. Scale bars, 10 μm. (Reprinted from Luan et al (9) with permission).
We investigated whether the phenotypic conversion induced by rapamycin is contingent upon FKBP12 binding. Since tacrolimus also binds FKBP12, we treated the renal cancer cells with rapamycin in the presence of molar excess of tacrolimus. Rapamycin binding of FKBP12 protein was essential for its ability to convert from an invasive to a non-invasive phenotype since tacrolimus prevented the morphologic alterations induced by rapamycin (Figure 2d and f).
Cyclosporine increases pulmonary metastases.
We used murine renal adenocarcinoma cells (19), and two different tumor cell lines, one of murine origin (Lewis lung carcinoma cells (20)) and the other of human origin (human bladder transitional carcinoma cells (21)) as the tumor inoculum, and T-cell, B-cell and NK-cell deficient SCID-beige mice (mice homozygous for both SCID and beige mutations (22)) as the tumor-bearing host. We used the SCID-beige mice rather than immunocompetant mice since we wanted to examine the effect CsA independent of its effects on the host immune system (8).
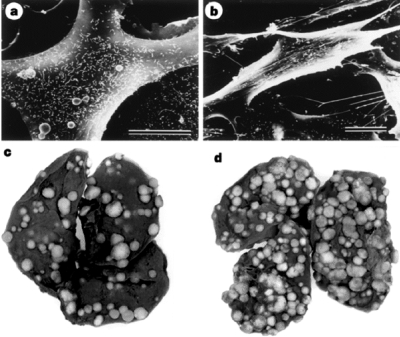
CsA treatment resulted in phenotypic alterations in murine renal cell adenocarcinoma cells (Figure 3a and b). Very importantly, CsA increased the number of murine renal cell cancer metastases in SCID-beige mice. Representative lungs, illustrating that the number of pulmonary metastases was higher in CsA-treated mice compared to untreated mice, are shown in Figure 3c and d. Data from four separate experiments demonstrated that CsA treatment results in a significant increase in the number of renal cell cancer pulmonary metastases from 241 ± 22 (mean ± SEM, n = 21) in the control SCID-beige mice to 338 ± 26 (n = 18) in the CsA-treated mice (P = 0.007, t test). The number of pulmonary metastases resulting from murine Lewis lung carcinoma cells (LCC) increased from 11 ± 8 (n = 9 mice) in the control mice to 28 ± 4 (n = 8 mice) with CsA treatment (P = 0.003) and the number of pulmonary metastases resulting from human bladder transitional cancer cells increased from 63 ± 18 (n = 9 mice) in the control mice to 138 ± 21 (n = 9 mice) in CsA-treated mice (P = 0.01).
Fig. 3.
CsA induces renal cancer cells to acquire an invasive phenotype and promotes tumor growth in vivo. Scanning electron microscopic photograph of murine renal adenocarcinoma cells incubated for 72 h in the absence (Panel A) or presence of 1 μg/ml CsA (Panel B). Bars indicate 10 μm. Representative lungs, retrieved from untreated mice (Panel C) and from CsA-treated mice (Panel D), are shown to illustrate CsA-associated increase in renal cell cancer pulmonary metastasis in SCID-beige mice. (Reprinted from Hojo et al (8) with permission).
We investigated the effect of anti-TGF-β mAbs (1D11.16 IgG1 mAbs) to determine whether in-vivo tumor progression resulting from CsA was dependent upon TGF-β. Anti-TGF- β mAbs but not control IgG1 mAbs prevented the CsA-induced increase in the number of metastases. The number of pulmonary metastases was 350 ± 22 (mean ± SEM, n = 12) in the control mice, 441 ± 20 (n = 10) in CsA-treated mice, 284 ± 34 (n = 8) in the CsA plus anti-TGF-β mAbs treated mice and 490 ± 26 (n = 4) in CsA plus control IgG1 mAbs treated mice (P = 0.0005, one-way ANOVA). The reduction in the number of metastases found following administration of anti-TGF-β mAbs to CsA-treated mice was significant at P < 0.01 by ANOVA (Bonferoni P value). In contrast, there was no significant difference between the number of metastases found in CsA-treated mice and that found in mice treated with CsA plus control IgG1 (P > 0.05).
Tacrolimus increases murine renal adenocarcinoma cells pulmonary metastases in syngeneic BALB/c mice.
Murine renal adenocarcinoma cells (50,000 cells in 0.5ml of PBS) were injected in the tail vein of BALB/c mice and randomly assigned to the following treatment groups: (a) untreated control, (b) treatment with 2mg/kg of tacrolimus, and (c) treatment with 4mg/kg of tacrolimus (11). Tacrolimus was administered by the subcutaneous route and on every other day starting one day prior to tumor inoculation. The mice were killed on days 17 to 19 after tumor inoculation and the number of pulmonary metastasis was counted. The mean (+SE) number of pulmonary metastases was 197 ± 16 in the untreated mice (n = 18 mice), 281 ± 26 in the mice treated with 2mg/kg of tacrolimus (n = 17 mice) and 339 ± 25 in the mice treated with 4mg/kg of tacrolimus (n = 21 mice) (P = 0.0004, ANOVA). Multiple comparisons of all possible pairs of experimental groups showed that the number of pulmonary metastasis in mice treated with 4mg/kg of tacrolimus was significantly higher compared with that of control mice (Bonferoni P < 0.001). None of the other pair-wise comparisons were significant (P > 0.05).
Tacrolimus increases murine renal adenocarcinoma cell pulmonary metastasis in SCID-beige mice.
Murine renal adenocarcinoma cells (100,000 cells in 0.5ml of PBS) were injected in the tail vein of SCID-beige mice and randomly assigned to the following treatment groups: (a) untreated control, (b) treatment with 2mg/kg of tacrolimus, and (c) treatment with 4mg/kg of tacrolimus (11). Tacrolimus was administered by the subcutaneous route and on every other day starting one day prior to tumor inoculation. The mice were killed on day 17 after tumor inoculation and the number of pulmonary metastases was counted. The mean (+ SE) number of pulmonary metastases was 117 ± 18 in the untreated SCID-beige mice (n = 12 mice), 137 ± 19 in the mice treated with 2mg/kg of tacrolimus (n = 9 mice) and 216 ± 29 in the mice treated with 4mg/kg of tacrolimus (n = 7 mice) (P < 0.01, ANOVA). Multiple comparisons of all possible pairs of experimental groups showed that the number of pulmonary metastases in mice treated with 4 mg/kg of tacrolimus was significantly higher compared with the number of metastases observed in the control mice (Bonferoni P < 0.05). None of the other pair-wise comparisons of number of metastases were significant (P > 0.05).
Rapamycin prevents tumor cell growth and metastases.
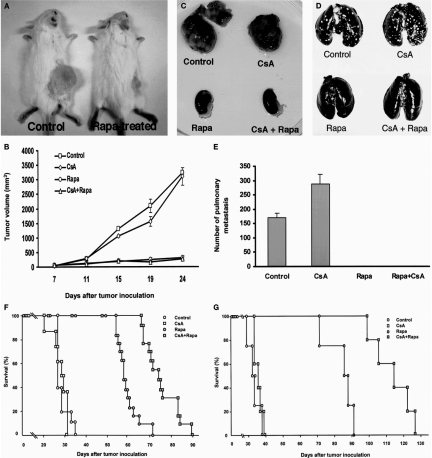
Subcutaneous implantation of renal cancer cells resulted in the formation of well-defined tumor in the untreated, syngeneic BALB/c mice, and in mice treated with CsA whereas there was minimal tumor growth in mice treated with rapamycin or rapamycin plus CsA (Figure 4a and b) (9).
Fig. 4.
In-vivo anti-tumor efficacy of rapamycin. (Panel A and Panel B) Rapamycin inhibits in-vivo growth of renal cancer cells in BALB/c mice. Murine renal adenocarcinoma cells (105 cells in 0.1 ml PBS) were injected subcutaneously (s.c.) into flanks of mice and treatment with rapamycin (2 mg/kg/day, oral) was initiated when the tumor volume was 50 mm3. Cyclosporine (20 mg/kg) was injected subcutaneously every other day. A representative result showing tumor growth in untreated mice and the lack of such tumor growth in rapamycin treated mice is shown in Panel A. The picture was taken 24 days after tumor inoculation. In Panel B, tumor volume (mean ± SE) measured in untreated mice (n = 3), cyclosporine-treated mice (n = 4), and the lack of tumor growth in rapamycin-treated (n = 4) or rapamycin and cyclosporine-treated mice (n = 4) is shown. Subcutaneous implantation of renal cancer cells resulted in the formation of well-defined tumor in the untreated BALB/c mice, and the mean (+SE) tumor volume was 3230 ± 21 mm3 24 days after implantation. In contrast, there was minimal tumor growth in mice treated with rapamycin, and the tumor volume was only 308 ± 66 mm3 (P < 0.01, Panel A and Panel B). Rapamycin was also effective in preventing tumor growth in mice treated with cyclosporine. The tumor volume was 3103 ± 305 mm3 at 24 days after implantation in cyclosporine treated mice, and was only 268 ± 55 mm3 in mice treated with cyclosporine plus rapamycin (P < 0.001). (Panel C) Rapamycin inhibits intrarenal growth of renal cancer cells in BALB/c mice. Murine renal adenocarcinoma cells (5 × 104 cells in 0.1 ml PBS) were injected under the renal capsule following exposure of the kidney through a flank incision. Rapamycin (2 mg/kg, oral) was started 3 days after tumor inoculation and was administered daily until the mice were killed. Cyclosporine (20 mg/kg, s.c.) was initiated one day prior to tumor inoculation and was administered every other day until the mice were killed. The tumor inoculated BALB/c mice were killed 20 days after tumor inoculation and the kidneys were retrieved. Exuberant tumor growth was observed in untreated (control) or cyclosporine (CsA) treated mice and there was no visible tumor in rapamycin (Rapa) treated or cyclosporine and rapamycin (CsA + Rapa) treated mice. (Panel D, and Panel E) Rapamycin prevents pulmonary metastasis in BALB/c mice. Murine renal adenocarcinoma cells (5 × 104 cells in 0.5 ml PBS) were injected in the tail vein of BALB/c mice and rapamycin and cyclosporine were administered as described in Panel C. Representative lungs retrieved 20 days after tumor inoculation of untreated mice (control), cyclosporine-treated mice (CsA), rapamycin-treated (Rapa), and cyclosporine and rapamycin-treated (CsA + Rapa) mice are shown in Panel D to illustrate the increase in renal cell cancer pulmonary metastasis in cyclosporine-treated mice and the absence of metastasis in rapamycin- or rapamycin and cyclosporine-treated mice. In Panel E, the number of pulmonary metastases in untreated control mice (n = 26), cyclosporine-treated mice (n = 24), rapamycin-treated mice (n = 14) and rapamycin and cyclosporine-treated mice (n = 9) is shown. The mice were killed 20-24 days after tumor inoculation and the number of metastasis was counted. The mean (+SE) number of renal cancer pulmonary metastases was 171 ± 15 in the control mice, and cyclosporine treatment increased the number of metastases to 287 ± 33 (P < 0.01). In striking contrast, there were no metastases in mice treated with rapamycin, or with rapamycin and cyclosporine. Results (mean ± SE) are from 5 experiments. Panel F: Rapamycin prolongs the survival of tumor-inoculated BALB/c mice. Murine renal adenocarcinoma cells (5 × 104 cells in 0.5 ml PBS) were injected in the tail vein of BALB/c mice and rapamycin and cyclosporine were administered as described in Panel C. Deaths were recorded on a daily basis. The mean duration of survival was 28.6 ± 0.9 days in the control untreated mice (n = 10), 27.8 ± 1.3 days in cyclosporine-treated mice (n = 8), 67.7 ± 2.7 days in rapamycin-treated mice (n = 13), and 75.2 ± 2.1 days in rapamycin and cyclosporine-treated mice (n = 13). Comparison of treatment effects in the four experimental groups showed that the null hypothesis of equal group means should be rejected (P < 0.0001, ANOVA). Multiple comparisons of all possible pairs of experimental groups showed that the mean survival time of rapamycin treated group is significantly longer than that of control mice (P < 0.001) and cyclosporine-treated mice (P < 0.001). The mean survival time of mice treated with both rapamycin and cyclosporine was higher compared to control mice (P < 0.001), cyclosporine- treated mice (P < 0.001), or rapamycin-treated mice (P < 0.001). Panel G: Rapamycin prolongs the survival of tumor-inoculated SCID-beige mice. Murine renal adenocarcinoma cells (5 × 104 cells in 0.5 ml PBS) were injected in the tail vein of SCID-beige mice and rapamycin and cyclosporine were administered as described in panel c. Deaths were recorded on a daily basis. The mean duration of survival was 34 ± 2.1 days in the control untreated mice (n = 4), 34.8 ± 1.2 days in cyclosporine-treated mice (n = 5), 84 ± 4 days in rapamycin-treated mice (n = 4), and 114 ± 5.2 days in rapamycin and cyclosporine-treated mice (n = 5). The survival time of rapamycin treated mice was longer compared to control mice (P < 0.001) or cyclosporine treated mice (P < 0.001); the survival time of rapamycin plus cyclosporine treated mice was longer compared to control mice (P < 0.001), cyclosporine treated mice Epub 2008 Jul 22 (P < 0.001), or rapamycin treated mice (P < 0.01). (Reprinted from Luan et al (9) with permission).
Rapamycin was effective in the intrarenal tumor model (9). Inoculation of renal cancer cells under the renal capsule resulted in the formation of multiple tumor nodules in untreated or CsA-treated mice, and there was a virtual absence of tumor nodules in mice treated with rapamycin or rapamycin plus CsA (Figure 4c).
Rapamycin, in addition to inhibiting local tumor growth, prevented metastatic spread (9). As anticipated, cyclosporine treatment increased the number of pulmonary metastases of renal cancer cells in the syngeneic BALB/c mice, and there were no metastases in mice treated with rapamycin, or with rapamycin and CsA (Figure 4d and e).
Rapamycin increased the survival of tumor-inoculated mice (9). Rapamycin prolonged the survival of tumor-inoculated BALB/c mice (Figure 4f) and the SCID-beige mice (Figure 4g), and its anti-tumor efficacy was evident in CsA-treated BALB/c or SCID-beige mice as well (Figure 4f and g).
Rapamycin was also effective in the SCID-beige mice inoculated with the T24 human bladder transitional cancer cells (9). The mean (+SE) duration of survival was 27 ± 1.6 days (n = 6 mice) in the control mice, 27 ± 1 day (n = 6 mice) in the mice treated with CsA, 39 ± 2 days (n = 6 mice) in the mice treated with rapamycin, and 40 ± 2 days (n = 7) in the mice treated with CsA plus rapamycin (P < 0.0001). Multiple comparisons of all possible pairs of experimental groups showed that the mean survival time of rapamycin-treated group was significantly longer than that of control mice (P < 0.01) and CsA-treated mice (P < 0.01). The survival time of SCID-beige mice treated with CsA plus rapamycin was longer than that of control mice (P < 0.01) and mice treated with CsA alone (P < 0.02).
Rapamycin reduces human renal cancer cell pulmonary metastasis in SCID-beige mice.
Human renal cancer cells RCC-786-O (2 × 105 cells in 0.5 ml PBS) were injected in the tail vein of SCID-beige mice, and the tumor inoculated mice were untreated (controls), treated with CsA 20mg/kg/every other day, treated with rapamycin 2mg/kg/day, or treated with CsA 20mg/kg/every other day and rapamycin 2mg/kg/day (10). The mice were killed 30+/−3 days after tumor inoculation and the number of metastasis was counted.
The number of pulmonary metastases (mean ± SE) in untreated control mice was 344 ± 32 (n = 8 mice), 478 ± 35 in cyclosporine-treated mice (n = 10 mice), 156 ± 28 in rapamycin-treated mice (n = 11 mice) and 92 ± 20 in rapamycin and CsA-treated mice (n = 13) mice (P < 0.0001, ANOVA). Multiple comparisons of all possible pairs of experimental groups showed that the mean number of pulmonary metastasis in rapamycin treated group is significantly lower than that of control mice (P < 0.0001) and CsA-treated mice (P < 0.0001). The mean number of pulmonary metastasis in mice treated with both rapamycin and CsA was also significantly lower compared to control mice (P < 0.0001), CsA-treated mice (P < 0.0001), but not rapamycin-treated mice (P = 0.098).
Rapamycin prolongs the survival of human renal cancer cell-inoculated SCID-beige mice.
Human renal cancer cells RCC-786-O (2 × 105 cells in 0.5 ml PBS) were injected in the tail vein of SCID-beige mice, and the mice were treated using the regimen described above until death (10). The mean duration of survival was 54 ± 3 days in the control mice (n = 13 mice), 42 ± 2 days in CsA-treated mice (n = 16 mice), 108 ± 8 days in rapamycin-treated mice (n = 8 mice), and 109 ± 5 days in rapamycin and CsA-treated mice (n = 9 mice) (P < 0.0001, ANOVA). Multiple comparisons of all possible pairs of experimental groups showed that the mean survival time of rapamycin treated group is longer than that of control mice (P < 0.0001) and cyclosporine-treated mice (P < 0.0001). The mean survival time of mice treated with both rapamycin and cyclosporine was higher compared to control mice (P < 0.0001), cyclosporine treated mice (P < 0.0001), but not rapamycin-treated mice (P = 0.83).
Rapamycin prevents the growth of murine KLN-205 NSCLC cells in-vivo.
Lung cancer claims over 150,000 lives each year in the United States (23). We used two models of in vivo tumor progression of murine KLN-205 Non-Small Cell Lung Cancer cell line to investigate the effect of Rapamycin (12). Murine KLN-205 cells (2 × 105 cells) were injected subcutaneously into syngeneic DBA/2 mice. The mice were untreated (control), treated with rapamycin (2mg/kg/day), CsA (20mg/kg/every other day), or (rapamycin, 2mg/kg/day + CsA, 20mg/kg/every other day) once the tumor was palpable (50mm3). Subcutaneous tumor growth was quantified by measuring the volume of the tumors. Rapamycin, either alone or in combination with CsA blocked subcutaneous tumor growth; the tumor volume was 1290 ± 173 mm3 at 29 days after injection in the untreated control and 246 ± 80 mm3 in rapamycin treated mice (P < 0.001).
Rapamycin reduces distant metastases of KLN-205 NSCLC cells.
Prior studies have shown that subcutaneous KLN-205 can metastasize to distant sites (24). To evaluate pulmonary metastases from a distant primary, the subcutaneous model described above was utilized. Murine non-small cell lung cancer KLN-205 cells (2 × 105 cells) were injected into the subcutaneous tissue of syngeneic DBA/2 mice and treated for 37 days using the regimen described above. Pulmonary metastases were visualized as filling defects following intra-tracheal infusion of India ink (17). Whereas 8 of 8 (100%) of untreated mice showed pulmonary metastasis, only 1 of 6 mice treated with rapamycin developed pulmonary metastasis following subcutaneous implantation of KLN-205 cells. Furthermore, rapamycin was effective in reducing the development of pulmonary metastasis in CsA-treated mice.
DISCUSSION
CsA and tacrolimus can promote tumor cell metastases independent of their immunosuppressive effects on host T-, B- and NK cells.
In our studies, we investigated the hypothesis that two widely used calcineurin inhibitors, CsA and tacrolimus, independent of any effects on the host immune system, would program non-invasive malignant cells to acquire an invasive phenotype and promote metastatic spread. We formulated our hypothesis on the basis of: (i) our observations that CsA as well as tacrolimus promote the transcription and functional expression of the TGF-β1 gene (11, 25), and (ii) the experimental findings of others that TGF-β can promote tumor cell invasion and metastatic potential (18, 26, 27).
CsA-treated non-transformed malignant cells, human lung adenocarcinoma cells A-549 and murine renal adenocarcinoma cells, acquired an invasive phenotype under in- vitro conditions that allow no possible involvement of the host immune system, and CsA promoted tumor cell metastases in T-, B- or NK cell deficient SCID-beige mice. Tacrolimus-induced impairments in the functioning of host T-, B- or NK cells did not appear to be an absolute requirement for tumor progression, since the drug increased the number of pulmonary metastases not only in the immunocompetant BALB/c mice but also in the immunodeficient SCID-beige mice.
CsA and tacrolimus associated tumor progression is TGF-beta dependent.
TGF-β1 has been implicated in the acquisition of tumor invasiveness and metastatic spread (26–29). High grade and biologically aggressive tumors have been shown to contain significantly higher levels of TGF-β1 than better differentiated tumors, and the dynamic relationship between levels of TGF- β1 protein and TGF-β receptors is considered significant in tumor progression (28–32).
In our study, monoclonal antibodies directed at TGF-β prevented CsA induced phenotypic alterations from a noninvasive phenotype to an invasive one. Anti-TGF-β monoclonal antibodies also prevented the metastatic spread of murine renal adenocarcinoma cells in SCID-beige mice. These observations, and our additional findings that tacrolimus promotes metastatic spread only at the high dosage (4mg/kg) required to induce TGF-β, support the hypothesis that this multifunctional cytokine plays a central role in CsA and tacrolimus associated tumor progression. The source of TGF-β is likely to be the tumor cells themselves, but the possibility that TGF-β may be derived from other sources can not be excluded in our in-vivo studies of tumor progression. Additional support for the hypothesis that TGF-β hyperexpression contributed to metastatic spread is also provided by the observation that blockade of TGF-β signaling with the use of a dominant negative TGF-β receptor prevents breast cancer metastasis to the bone in a mouse model (33). These findings, together, raise the possibility that TGF-β blockade may be of value in the clinical setting to prevent tumor progression.
Anti-tumor efficacy of rapamycin.
We found rapamycin to be highly effective as an anti-tumor agent in every tumor cell type studied (9, 10, 12). The anti-tumor efficacy of rapamycin has also been noted by others. Guba et al (34) reported that rapamycin constrains metastatic tumor growth of CT-26 mouse colon adenocarcinoma cells in BALB/c mice. Majewski et al (35) observed that RAD, an analogue of rapamycin, inhibits subcutaneous growth of Epstein-Barr virus-positive B cells in SCID mice. It is noteworthy that the new observations regarding the anti- tumor efficacy of rapamycin were anticipated almost two decades ago by the report of Eng et al. (36).
Mechanisms for the anti-tumor efficacy of rapamycin.
Rapamycin is an effective inhibitor of proliferation of hematopoietic and lymphoid cells, and its anti-proliferative effect may contribute to its anti-tumor efficacy. We found that human renal cancer cells (10) and mouse non-small cell lung cancer cells (12) were both growth-arrested at the G1 checkpoint by rapamycin. We also found that rapamycin up-regulates E-cadherin expression in murine renal adenocarcinoma cells (9). E-cadherin is important for contact-dependent growth inhibition, and impaired expression or function of E-cadherin has been noted in several human carcinomas (37, 38). On the other hand, restoration of E-cadherin expression results in a transition from a malignant and invasive phenotype to a non-invasive and benign one (39, 40). Forced expression of E-cadherin has also been shown to up-regulate the CDK inhibitor, p27kip1 (41). Upregulation of p27kip1 and reduced expression of cyclin D1 have been associated with cell cycle arrest at G1 phase (42), whereas loss of p27kip1 or over-expression of cyclin D1 has been linked to tumor growth and metastatic progression (43, 44). In this regard, we found that rapamycin not only up-regulated the expression of E-cadherin but also increased p27kip1 and reduced cyclin D1 of murine renal cancer cells (9).
Rapamycin's inhibitory effect on cytokines such as TGF-β1 and VEGF-A may also contribute to its anti-tumor efficacy. We found that circulating levels of TGF-β1 protein in tumor inoculated SCID-beige are significantly reduced by rapamycin (66 ± 5 pg/ml in treated mice vs. 40 ± 4 pg/ml in controls, P = 0.001) (10). Rapamycin reduced circulating levels of VEGF-A also in these mice but in a less effective manner (69 ± 4 pg/ml vs. 56 ± 8 pg/ml P > 0.05) (10).
Translation from the bench to the bedside.
Long-term immunosuppressive therapy is associated with a heightened incidence of malignancy and greater tumor invasiveness (5–7). The immunosuppressive drugs used to prevent organ graft rejection are thought to facilitate malignancy development and progression by impairing recipients' immune surveillance system. Such reasoning has led to the clinical practice of prompt reduction or withdrawal of immunosuppressive drugs, although it is well understood that such withdrawal may precipitate graft rejection and irreversible failure.
Our identification of an autonomous cellular mechanism involving TGF-β in the pathogenesis of metastases associated with CsA and tacrolimus advance the idea that TGF-β blockade is worthy of consideration for the management of post-transplant neoplasm. Our additional findings that rapamycin can function as an anti-tumor agent independent of its effects on host T-, B-, and NK cells unlinks immunosuppression potential from anti-tumor efficacy. We are pleased to note that our interpretation of our pre-clinical studies to mean that rapamycin would be of value as an anti-tumor agent in humans is beginning to find support not only in the post-transplantation setting (45–47) but also in patients suffering from renal cell carcinoma and lung cancers (48).
ACKNOWLEDGEMENTS
This work was supported in part by an award from NIH/NIAID RO1 AI 26932 to MS. We thank Dr. P. August for critical review and Ms. M. Trantino for the meticulous preparation of the manuscript.
Abbreviations:
- CsA
cyclosporin A
- TGF-β
transforming growth factor beta
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Mitch, Houston: Thank you Suthan, very interesting and exciting. You may have mentioned, I didn't hear, what happens to calcineurin activity with the rapamycin?
Suthanthiran, New York: This is a fascinating and unresolved issue. To date, it has not been reported that rapamycin affects calcineurin activity. As you know, rapamycin binds the cytosolic protein FKBP12, and the rapamycin/FKBP12 complex binds mammalian target 06 rapamycin (mTOR) and not calcineurin. There is, however, a very interesting possibility that the biological effects of calcineurin could be altered by rapamycin, because it turns out that mTOR is a serine threonine kinase, whereas calcineurin is a serine threonine phosphatase. So the balance between the serine kinase and serine phosphatase may be perturbed by rapamycin targeting mTOR and inhibiting its kinase activity. I am raising this possibility, but as I mentioned, a direct molecular interaction between rapamycin and calcineurin has not been reported.
Gersh, Rochester: I found that EMT fascinating as a cardiologist, because these are the compounds used in drug-eluding coronary stents, and what I wanted to ask you and this may be a naïve question, are the mechanisms the same because they do prevent intimal and medial proliferation, there is no question?
Suthanthiran, New York: This is an important issue. In our studies, rapamycin was anti-proliferative, not only for lymphoid cells but also for non-lymphoid cells. In cell cycling studies, rapamycin blocks G1 to S transition in a number of cell types. This anti-proliferative effect may be very useful when incorporated into the cardiac stents. However, there may be a price to pay in terms of tissue repair and remodeling.
Gersh, Rochester: Well, your last slide actually illustrated that very nicely, because the problem with coronary stents is they are so effective that they prevent endothelial healing and repair, so that these same stents are vulnerable to late coronary thrombosis. So it is a little bit the yin and yang as you pointed out.
Suthanthiran, New York: You are exactly right. It may be very interesting to put a bare metal stent and give systemic rapamycin. That study I am not sure has been done, to compare whether a bare metal stent plus rapamycin is equivalent to rapamycin incorporated in the stents.
Gersh, Rochester: That study is being done with rapamycin being given systemically. I do not know the results but someone is doing that. That would be interesting.
Weir, Baltimore: Well Suthan, great theories as always! Would you provide some opportunity to link the TGF beta story with VEGF inhibitor and, of course, also maybe comment on is there any existing data with an anti-TGF beta drug like RAS inhibitors, where we have some registry data on their use and whether or not there is an association with reduced incidence of malignancy?
Suthanthiran, New York: Thank you, Matt. We did find that rapamycin blocked the production both TGF beta and VEGF. As you well know, VEGF has also been implicated in tumor progression. In our tumor models, both ACE inhibitors and AII 1 receptor blockers reduced tumor cell pulmonary metastases. I am not aware of data regarding the impact of RAS inhibitors on malignancy in the clinic. My prediction would be that they would be useful in preventing metastatic spread rather than in reducing the incidence of malignancy.
Alexander, Atlanta: I congratulate you on a fascinating study. I was curious, given some of the mechanisms that you discovered. Have you specifically looked at effects on migration or angiogenesis as potential contributory processes?
Suthanthiran, New York: Thank you, Dr. Alexander. In our experiments examining the effect of cyclosporine on tumor cells we found that cyclosporine treatment of tumor cells results in their acquisition of motility, and the treated cells display exploratory pseudopodia. I should also mention that the tumor promoting effects we saw with cyclosporine could be blocked with an antibody to TGF beta. So I think that the increased migration, the epithelial to mesenchymal transition and the fibrosis associated with cyclosporine may all be linked by a common and TGF beta-dependent mechanism. There is also a very interesting story about TGF beta and the microRNA 200 family members of considerable significance to EMT and tumor progression.
Southwick, Gainesville: Actually, my question is related to the same thing. I was very struck by the marked formation of pseudopodia or filopodia, depending on what you call them. There are a number of contractile proteins related to that formation. Have the expression of those been looked at with cyclosporine exposure?
Suthanthiran, New York: It would be important to look at them, but we have not investigated these important issues.
Wilson, Durham: I am a clinician and a gastroenterologist, and one comment I wanted to ask is that, have you looked at your patient registry regarding the incidence of preventable cancers like colon cancer? The reason I bring it up is because I've just recently diagnosed fairly aggressive cancers in two transplant survivors, one long-term survivor (a 5 year plus survivor of kidney transplant), one for heart transplant, and as you know with colon cancer, we develop very specific preventive protocols that are based on prediction of what cell growth is likely to be; and to date, we don't have any modifications of that in the transplanted patient population. Now that they are living longer, the query is can we develop those kinds of protocols that would direct our care?
Suthanthiran, New York: You bring up a very important issue. As you know, most of the focus of the transplant clinician has been on immunologic aspects, and we have not done a very good job with the long term care. We follow them very intensely in the first year of transplantation but not with the equal vigor in the latter years. Very interestingly, cancer in transplant patients is not the same as in the general population. The common cancers are not very much increased. The incidence of common cancers like stomach, colon or lung cancer is increased only modestly, whereas the incidence of Kaposi's sarcoma and lymphomas is increased markedly. Also, squamous cell cancer tends to be very aggressive in the transplant population. We need to develop robust and specific protocols for malignancy surveillance.
Wilson, Durham: The behavior of these few patients that we have seen has been very atypical so in an attempt to prevent cancer, you have to stage your interventions at the right level.
Suthanthiran, New York: This is also a very important point, because the development of malignancy and its progression are two different issues, and we need to develop not only effective preventive strategies but also mechanistic approaches to prevent tumor progression. Our data suggest that TGF blockade may be of value in reducing tumor progression.
REFERENCES
- 1.Sayegh MH, Carpenter CB. Transplantation 50 years later-progress, challenges, and promises. N Engl J Med. 2004;351:2761–6. doi: 10.1056/NEJMon043418. [DOI] [PubMed] [Google Scholar]
- 2.Morris PJ. Transplantation-a medical miracle of the 20th century. N Engl J Med. 2004;351:2678–80. doi: 10.1056/NEJMp048256. [DOI] [PubMed] [Google Scholar]
- 3.Suthanthiran M, Strom TB. Renal transplantation. N Engl J Med. 1994;331:365–376. doi: 10.1056/NEJM199408113310606. [DOI] [PubMed] [Google Scholar]
- 4.Cecka JM. The UNOS Scientific Renal Transplant Registry. In: Cecka JM, Terasaki PI, editors. Clinical Transplants 2005. Los Angeles: UCLA Tissue Typing Laboratory; 2005. pp. 1–16. [Google Scholar]
- 5.London NJ, Farmerry SM, Will EJ, Davison AM, Lodge JPA. Risk of neoplasia in renal transplant patients. Lancet. 1995;346:403–406. doi: 10.1016/s0140-6736(95)92780-8. [DOI] [PubMed] [Google Scholar]
- 6.Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation 2005. 15 Oct;80(2) Suppl:S254–64. doi: 10.1097/01.tp.0000186382.81130.ba. [DOI] [PubMed] [Google Scholar]
- 7.Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222–30. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 8.Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, Shimbo T, Suthanthiran M. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature (Lond.) 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 9.Luan F, Hojo M, Maluccio M, Yamaji K, Suthanthiran M. Rapamycin blocks tumor progression: unlinking immunosuppression from anti-tumor efficacy. Transplantation. 2002;73:1565–72. doi: 10.1097/00007890-200205270-00008. [DOI] [PubMed] [Google Scholar]
- 10.Luan FL, Ding R, Sharma VK, Chon WJ, Lagman M, Suthanthiran M. Rapamycin is an effective inhibitor of human renal cancer metastasis. Kidney Int. 2003;63:917–26. doi: 10.1046/j.1523-1755.2003.00805.x. [DOI] [PubMed] [Google Scholar]
- 11.Maluccio M, Sharma V, Lagman M, Vyas S, Yang H, Li B, Suthanthiran M. Tacrolimus enhances TGF-[β]1 expression and tumor progression in a dose-dependent fashion. Transplantation. 2003;76:597–602. doi: 10.1097/01.TP.0000081399.75231.3B. [DOI] [PubMed] [Google Scholar]
- 12.Boffa DJ, Luan FL, Thomas D, Yang H, Sharma VK, Lagman M, Suthanthiran M. Rapamycin inhibits the growth and metastatic progression of non-small cell lung cancer. Clin Ca Res. 2004;10:293–300. doi: 10.1158/1078-0432.ccr-0629-3. [DOI] [PubMed] [Google Scholar]
- 13.Nettesheim P, Hammons A.S. Induction of squamous cell carcinoma in the respiratory tract of mice. J Natl Cancer Inst. 1971;47:697–701. [PubMed] [Google Scholar]
- 14.Iliopoulos O, Kibel A, Gray S, Kaelin WG., Jr. Tumour suppression by the human von Hippel-Lindau gene product. Nat Med. 1995;1:822–826. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- 15.Dasch JR, Pace DR, Waigell W, Inenaga D, Ellingsworth L. Monoclonal antibodies recognizing transforming growth factor-β. Bioactivity neutralization and transforming growth factor-2 affinity purification. J Immunol. 1989;142:1536–1541. [PubMed] [Google Scholar]
- 16.Cohen-Gould L, Robinson TF, Factor SM. Intrinsic connective tissue abnormalities in the heart muscle of cardiomyopathy Syrian hamsters. Am J Pathol. 1987;127:327–334. [PMC free article] [PubMed] [Google Scholar]
- 17.Asano T, Khanna A, Lagman M, Li B, Suthanthiran M. Immunostimulatory therapy with anti-CD3 monoclonal antibodies and recombinant interleukin-2: Heightened in vivo expression of mRNA encoding cytotoxic attack molecules and immunoregulatory cytokines and regression of murine renal cell carcinoma. J Urol. 1997;157:2396–2401. doi: 10.1016/s0022-5347(01)64787-6. [DOI] [PubMed] [Google Scholar]
- 18.Mooradian DL, McCarthy JB, Komanduri KV, Furcht LT. Effects of transforming growth factor.-1 on human pulmonary adenocarcinoma cell adhesion, motility, and invasion in vitro. J Natl Cancer Inst. 1992;84:523–527. doi: 10.1093/jnci/84.7.523. [DOI] [PubMed] [Google Scholar]
- 19.Murphy GP, Hrushesky WJ. A murine renal cell carcinoma. J Natl Cancer Inst. 1973;50:1013–1025. doi: 10.1093/jnci/50.4.1013. [DOI] [PubMed] [Google Scholar]
- 20.Bertram JS, Janik P. Establishment of a cloned line of Lewis Lung Carcinoma cells adapted to cell culture. Cancer Lett. 1980;11:63–73. doi: 10.1016/0304-3835(80)90130-5. [DOI] [PubMed] [Google Scholar]
- 21.Bubenik J, Baresova M, Viklicky V, Jakoubkova J, Sainerova H, Donner J. Established cell line of urinary bladder carcinoma (T24) containing tumour-specific antigen. Int J Cancer. 1973;11:765–773. doi: 10.1002/ijc.2910110327. [DOI] [PubMed] [Google Scholar]
- 22.Mosier DE, Stell KL, Gulizia RJ, Torbett BE, Gilmore GL. Homozygous scid/scid:beige/beige mice have low levels of spontaneous or neonatal T cell-induced B cell generation. J Exp Med. 1993;177:191–194. doi: 10.1084/jem.177.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Atlanta: American Cancer Society; Estimated new cancer cases and deaths for all sites, United States, in: Cancer Facts and Figures-2001; pp. 5–14. [Google Scholar]
- 24.Kaneko T, LePage G.A. Growth characteristics and drug responses of a murine lung carcinoma in vitro and in vivo. Cancer Res. 1978;38:2084–90. [PubMed] [Google Scholar]
- 25.Li B, Sehajpal PK, Khanna A, Vlassara H, Cerami A, Suthanthiran M. Differential regulation of transforming growth factor and interleukin 2 genes in human T cells: Demonstration by usage of novel competitor DNA constructs in quantitative polymerase chain reaction. J Exp Med. 1991;174:1259–1262. doi: 10.1084/jem.174.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welch DR, Fabra A, Nakajima M. Transforming growth factor-β stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc Natl Acad Sci USA. 1990;87:7678–7682. doi: 10.1073/pnas.87.19.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caulin C, Scholl FG, Frontelo P, Gamallo C, Quntanilla M. Chronic exposure of cultured transformed mouse epidermal cells to transforming growth factor-β1 induces an epithelial-mesenchymal transdifferentiation and a spindle tumoral phenotype. Cell Growth & Differ. 1995;6:1027–1035. [PubMed] [Google Scholar]
- 28.Erickson AC, Barcellos-Hoff MH. The not-so innocent bystander: the microenvironment as a therapeutic target in cancer. Expert Opin Ther Targets. 2003;7:71–88. doi: 10.1517/14728222.7.1.71. [DOI] [PubMed] [Google Scholar]
- 29.Teicher BA. Malignant cells, directors of the malignant process: role of transforming growth factor-beta. Cancer Metastasis Rev. 2001;20:133–43. doi: 10.1023/a:1013177011767. [DOI] [PubMed] [Google Scholar]
- 30.Wikstrom P, Stattin P, Franck-Lissbrant I, Damber JE, Bergh A. Transforming growth factor beta 1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate. 1998;37:19–29. doi: 10.1002/(sici)1097-0045(19980915)37:1<19::aid-pros4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Niki M, Toyoda M, Nomura E, Shinohara H, Nakamura M, Nishiguchi K, Tanigawa N. Expression of transforming growth factor beta (TGF-beta) may contribute, in part, to the variations in histogenesis and the prevalence of peritoneal dissemination in human gastric carcinoma. Gastric Cancer. 2000;3:187–192. doi: 10.1007/pl00011716. [DOI] [PubMed] [Google Scholar]
- 32.Maehara Y, Kakeji Y, Kabashima A, Emi Y, Watanabe A, Akazawa K, Baba H, Kohnoe S, Sugimachi K. Role of transforming growth factor-beta 1 in invasion and metastasis in gastric carcinoma. J Clin Oncol. 1999;17:607–14. doi: 10.1200/JCO.1999.17.2.607. [DOI] [PubMed] [Google Scholar]
- 33.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massagué J, Mundy GR, Guise TA. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 35.Majewski M, Korecka M, Kossev P, et al. The immunosuppressive macrolide RAD inhibits growth of human Epstein-Barr virus-transformed B lymphocytes in vitro and in vivo: A potential approach to prevention and treatment of posttransplant lymphoproliferative disorders. Proc Natl Acad Sci U S A. 2000;97:4285–90. doi: 10.1073/pnas.080068597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eng Cp, Sehgal SN, Vezina C. Activity of rapamycin (AY-22,989) against transplanted tumors. J Antibiot (Tokyo) 1984;37:1231–7. doi: 10.7164/antibiotics.37.1231. [DOI] [PubMed] [Google Scholar]
- 37.Birchmeier W, Weidner KM, Hulsken J, Behrens J. Molecular mechanisms leading to cell junction (cadherin) deficiency in invasive carcinomas. Semin Cancer Biol. 1993;4:231–9. [PubMed] [Google Scholar]
- 38.Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–11. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- 39.Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–19. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 40.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–3. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 41.St Croix B, Sheehan C, Rak JW, Flrenes VA, Slingerland JM, Kerbel RS. E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1) J Cell Biol. 1998;142:557–71. doi: 10.1083/jcb.142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawamata S, Sakaida H, Hori T, Maeda M, Uchiyama T. The upregulation of p27Kip1 by rapamycin results in G1 arrest in exponentially growing T-cell lines. Blood. 1998;91:561–9. [PubMed] [Google Scholar]
- 43.Hashemolhosseini S, Nagamine Y, Morley SJ, Desrivières S, Mercep L, Ferrari S. Rapamycin inhibition of the G1 to S transition is mediated by effects on cyclin D1 mRNA and protein stability. J Biol Chem. 1998;273:14424–9. doi: 10.1074/jbc.273.23.14424. [DOI] [PubMed] [Google Scholar]
- 44.Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–21. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- 45.Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med. 2005;352:1317–23. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- 46.Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80:883–9. doi: 10.1097/01.tp.0000184006.43152.8d. [DOI] [PubMed] [Google Scholar]
- 47.Kauffman HM, Cherikh WS, McBride MA, Cheng Y, Hanto DW. Post-transplant denovo malignancies in renal transplant recipients: the past and present. Transpl Int. 2006;19:607–20. doi: 10.1111/j.1432-2277.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- 48.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A. RECORD-1 Study Group. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]