Summary
Allergic diseases are often triggered by environmental allergens that induce dominant type2 immune responses, characterized by the infiltrated TH2 lymphocytes, eosinophils, and elevated TH2 cytokines. In addition to TH2 type immune responses, epithelial stress and injury linked to tissue remodelling are often observed, suggesting that epithelial cells may play important role in regulating allergic responses. Dendritic cells (DCs), the professional antigen-presenting cells with the capabilities of sampling allergens, are considered as the key player on instructing TH2 immune responses. Whether inflamed epithelium can regulate innate immunity, such as macrophages and DCs, which in turns instruct adaptive immunity has long been hypothesized. Studies of TSLP (thymic stromal lymphopoietin), an epithelial cells-derived cytokine, that can strongly activate DCs, provide important evidences that the epithelial barrier can trigger allergic diseases by regulating immune responses. The finding that OX40/OX40L interactions are the molecular trigger responsible for the induction and maintenance of TH2 responses by TSLP-activated DCs provides a plausible molecular explanation for TSLP-mediated allergy. Recent progresses in characterizing the proinflammatory IL-17 cytokine family have added an additional layer of complexity on the regulation of allergic inflammation. TSLP-DCs can induce a robust expansion of TH2 memory cells and strengthen functional attributes by upregulating their surface expression of IL-17RB (IL-25R), the receptor for cytokine IL-17E (IL-25), a distinct member of IL-17 cytokine family. IL-17E (also know as IL-25) produced by epithelial cells, and other innate cells, such as eosinphils, basophils, and mast cells, are shown to regulate adaptive immunity by enhancing TH2 cytokine productions. These exciting findings expand our knowledge of the complex immunological cascades that result in allergic inflammation and may provide novel therapeutic approaches for the treatments of allergic diseases.
Introduction
Allergic responses induced by dysregulated type-2 immune response to environmental allergens often cause harmful symptoms such as asthma and atopy. The epithelial barrier in the local mucosal surface such as airway, skin, and gastrointestinal has long been hypothesized to play important roles in the initiation of allergic response by secreting various chemokines, cytokines and growth factors, which in turns regulate innate immune cells. The idea that the epithelial barrier and altered innate immunity are fundamental to the onset of allergic diseases is supported by the findings that thymic stromal lymphopoietin (TSLP) represent a key molecule at the epithelial cell-dendritic cell (DC) interface to initiate allergic inflammation. Upon exposure to allergens or virus infections, proinflammatory cytokines, such as TNF-α; and IL-1β, trigger strong TSLP production by human airway epithelial cells and human keratinocytes. In turn, TSLP endows DCs to induce the differentiation of inflammatory TH2 cells and the expansion and activation of allergen specific TH2 memory cells. In addition, understanding the maintenance and regulation of long-lived allergen-specific human TH2 memory cells and their molecular signatures may provide novel therapeutic approaches for the treatments of allergic diseases. Recent advances demonstrate that cytokines TSLP and IL-25, as well as OX40L/OX40, a member of tumor-necrosis factor (TNF)/TNF receptor superfamily seem to be important contributors in the maintenance of TH2 memory pool during the pathogenesis of allergic inflammation. In this review, we discuss how these three factors contribute to the onset and maintenance of allergic response by regulating innate and adaptive immunity.
TSLP and TSLPR
TSLP, a distant paralog of IL-7, is a type I cytokine that is part of the IL-2 “cytokine family” (IL-2, IL-4, IL-7, IL-9, IL-13, IL-15, IL-21, and TSLP) [1]. Murine TSLP was first identified as a factor expressed in the supernatants of thymic stromal cell line that could support the development of B cells [2]. The activities of mouse TSLP overlap with those of IL-7, which can stimulate the proliferation of thymocytes and facilitate B lymphopoiesis in cultures of fetal liver and bone marrow lymphocyte precursors. Subsequent characterization and cloning revealed that the activity was a result of four-helix bundle cytokine with three potential sites for N-linked carbohydrate addition and seven cysteine residues [1]. Human orthologue of TSLP was later discovered in a computational screen of genomic database. While human TSLP shares poor homology with that of mouse at only 43% amino acid identity, sequence prediction of human TSLP cDNA revealed a similar four-helix structured cytokine with two N-glycosylation sites and six cysteine residues. Both mouse and human TSLP are expressed predominantly by epithelial cells, especially in the lung, skin, and gut, and exert similar biological functions as discussed below.
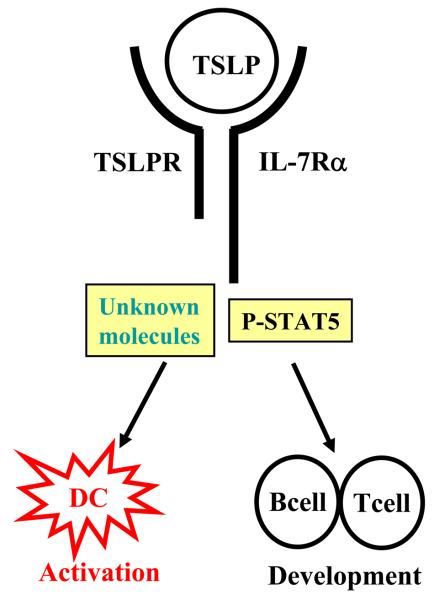
TSLPR is an atypical type I cytokine receptor and exhibits a remarkable 24% identity to the common γ receptor chain (γc) [3]. Similar to other type I cytokine receptor, TSLPR contains the conserved box1 region which is important for the binding of Janus family tyrosine kinase (JAKs). TSLP was found to signal through a heterodimeric receptor complex that consists of a new member of the hemopoietin family termed TSLPR and IL-7Rα chain [4;5]. TSLP binds TSLPR with low affinity, but the addition of IL-7Rα chain greatly enhances the binding affinity [3;4]. While human and mouse TSLPR share only 39% amino acid identity, expression of TSLPR transcript can be detected in early B and T cell progenitors, peripheral CD4+ T cells, mast cells, DCs and myeloid cells in both species [6]. Therefore, TSLP may exert various biological functions by acting on cell lineages in different immunological context [7]. In humans, myeloid DCs were found to express the highest level of TSLPR in both transcript and protein levels among the examined hematopoietic cell lineages, suggesting that myeloid DCs are the primary responding cells to this epithelial-derived cytokine. Molecular studies in T cells or transfected cell line showed that signaling through the TSLPR/IL-7Rα heterodimeric complex results in the activation of signal transducer and activator of transcription (STAT)-3 and STAT-5 [3;5;8]. However, the comprehensive analyses of signaling pathways activated by TSLP within primary myeloid DCs have not been reported (Fig.1).
Figure 1. TSLP and TSLPR structure and function.
TSLP was discovered by its biological activity to promote B and T cell development. In the periphery, TSLP strongly activates myeloid dendritic cells that upregulate their surface TSLPR expression rapidly. The TSLPR is a heterodimeric complex composed of TSLPR and IL-7Rα chains. In T cell lines, TSLP stimulation induced strong activation and phosphorylation of STAT5, whereas unidentified molecules or pathways may exist downstream of TSLPR signaling in DCs.
Regulation of TSLP Expression during Allergic Inflammation
During allergic inflammation, epithelial cells, keratinocyotes, and stromal cells are the primary producers of TSLP [9]. Recent findings show that proinflammatory cytokines TNF-α and IL-1β can synergize with TH2 cytokines (IL-4 and IL-13) to induce TSLP production by human epithelial cells and keratinocytes [10]. Moreover, TLR3 ligand such as a viral double-strand intermediate RNA (dsRNA) produced during rhinovirus replication can augment TSLP production by TH2 cytokines-primed epithelial cells [11]. The ligands for TLR2, TLR8, and TLR9 can trigger the production of TSLP by human primary epithelial cell lines [12]. In a murine allergic rhinitis model, TSLP production by nasal epithelial cells can be induced by mast cells expressing FcεRI. In addition to structural cells, basophils also have the potential to produce TSLP for the initiation of type-2 immune response in the papain induced mouse allergic model. These findings suggest that epithelial derived TSLP expression may be regulated by the inflammatory stimuli produced by innate or adaptive immune cells or some TLR ligands during allergic inflammation.
The molecular mechanism to control TSLP gene expression induced by these inflammatory stimuli or TLR ligands has recently been addressed. One study suggested that the activation of NF-κB signaling pathway plays a key role on the induction of TSLP gene expression by the inflammatory stimuli [12]. The others demonstrated that retinoid X receptor (RXR)α or RXRβ can pair with vitamin D receptor and act as a transcriptional repressor to inhibit TSLP gene expression in the mouse skin keratinocytes [13;14]. These studies may have the potential to provide molecular links between allergen exposure and TSLP induction during allergic immune response.
DCs bridge innate and adaptive responses through effects mediated by TSLP
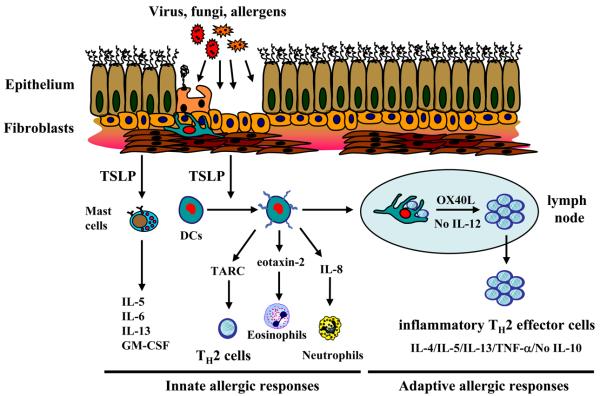
Since the identification of TSLP cytokine in mouse, many studies have been focused on the effect of TSLP on promoting B and T cell development [6;15;16]. The findings that human TSLP can strongly activate peripheral blood CD11c+ immature DCs in culture provide a novel biological function of TSLP, linking this epithelial-derived cytokine to DC-mediated allergic immune responses. DCs are the professional antigen presenting cells that have the capability to sense invaded pathogens and tissue stress, then present antigen to T cells, thereby bridging the innate and adaptive immunity. Human DCs express high level of TSLPR and respond to TSLP rapidly by upregulating their surface expression of MHC class II, CD40, CD80, CD86, and the DC-associated activation marker, DC-lamp [17]. Unlike other DC stimuli such as CD40L and TLR ligands, TSLP does not stimulate mDCs to produce the TH1-polarizing cytokine IL-12 or the proinflammatory cytokines TNF-α, IL-1β, and IL-6. To identify the unique molecular features of mDCs induced by TSLP but not other stimuli, microarray analysis of mDCs activated with different stimuli were performed [18]. TSLP does not induce mDCs to express mRNA encoded IL-12 family members, such as IL-12, IL-23, and IL-27, nor the type I IFNs, the cytokines known to induce TH1 differentiation. Interestingly, TSLP triggers mDCs to produce an array of distinct chemokines, including eotaxin-2, IL-8, as well as TARC and I-309,which are important for recruiting eosinphils, neutrophils, and TH2 cells, respectively [19]. These findings suggest that one of the most important features of TSLP cytokine is to endow mDCs to become capable of inducing type-2 immune response, while losing the capability of producing TH1 polarizing cytokines. At the site of allergic inflammation; such as the airway, intestine, and skin, DCs are in close contact with epithelial cells. TSLP may thus mediate the crosstalk between epithelial cells and DCs during allergic inflammation, thus leading to allergic diseases [6]. Indeed, strong TSLP expression by keratinocytes in the atypical layer of the epidermis was found in the lesional skins of patients with chronic atopic dermatitis, providing the first evidence for the association of TSLP expression and allergic diseases. A recent study further demonstrates that the severity of asthma is correlated with the elevation of TSLP expression in the airway of asthmatic subjects [20]. Moreover, studies in vivo using genetic approaches in mice showed that tissue-specific over expression of TSLP in lung and skin triggers allergic response leading to asthma and atopic dermatitis respectively, whereas mice with gene disruption of TSLPR fail to develop airway inflammation in the allergen-challenged animal model [21]. Together, these studies suggest that TSLP produced by epithelial cells can activate DCs to create a TH2 permissive microenvironment leading to asthma and atopy (Fig. 2).
Figure 2. TSLP initiate innate and adaptive allergic responses.
Invaded pathogens or allergens can trigger mucosal epithelial cells or skin cells (keratinocytes, fibroblasts, and mast cells) to produce TSLP. TSLP can initiate innate allergic responses by activating immature DCs to produce chemokines IL-8, eotaxin-2, and Th2 attarcting chemokine TARC. TSLP can also costimulate mast cells to produce IL5, IL13, GM-CSF, and IL-6. TSLP-activated mDCs can migate into the draining lymph nodes to initiate adaptive allergic responses. TSLP-activated DCs express OX40L, which triggers the differentiation of naïve CD4+ T cells into inflammatory Th2 cells and the expansion of allergen-specific TH2 memory cells.
TSLP-activated DCs induce inflammatory TH2 cells and maintain TH2 memory pool
Studies in mice suggest that TSLP may exert direct effects on CD4+ T cells. During T cell development in thymus, TSLP may modestly affect thymocyte growth in vitro and support an overall population expansion of peripheral lymphocyte in vivo by injection of TSLP [16]. While TSLPR-deficient (Tpte2−/−) mice have normal T and B cell development [7], Tpte2−/−IL2rg−/− mice have more deleterious phenotype than that in IL2rg−/− mice, suggesting a non-redundant influence of TSLP on T lymphocyte development [16]. Indeed, CD4+ T cells from TSLPR-deficient mice expanded less efficiently than wild type CD4+ T cells in irradiated hosts, indicating that TSLP may play a direct role on T cell homeostasis [16]. In humans, pre-activated but not naïve CD4+ T cells upregulate their TSLPR expression and become responsive to TSLP stimulation [22]. Following TCR engagement, TSLP augments the proliferative response of the pre-activated CD4+ T cells by activating STAT5 and inducing STAT5 target genes in vitro [22]. In addition, studies in mice suggest that the epithelial or basophil-derived TSLP can directly induce TH2 differentiation of naïve CD4+ T cells in the presence of anti-CD3 stimulation in vitro [23]. While these studies propose that TSLP produced by innate immunity can trigger allergic response by directly targeting activated T cells in the absence of DCs, other studies in human and mice suggest that DCs are the primary cells responsible for TSLP-induced allergy.
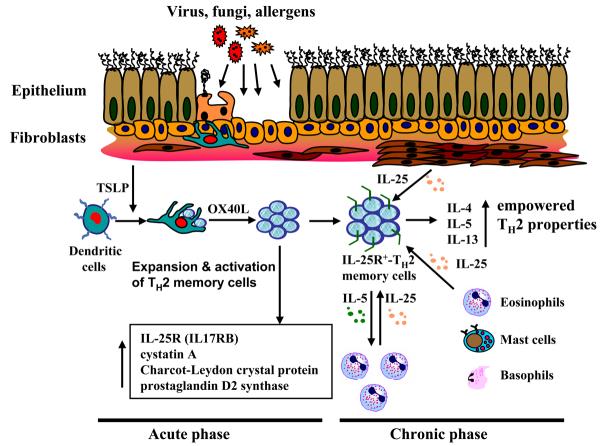
In humans, TSLP-activated mDCs rapidly upregulate their surface TSLPR expression at a much higher level compared to that on pre-activated CD4+ T cells [24]. Mature DCs induced by TSLP mediate the differentiation of naïve CD4+ T cells into a unique type of TH2 cells that produce classic TH2 cytokines IL-4, IL-5, and IL-13, and large amount of TNF-α, but little IL-10 (Fig. 2). TSLP-activated DCs can also trigger CD8+ T cells to differentiate into proallergic cytotoxic T cells that produce IL-13 [25]. Similar studies in mice also demonstrated that TSLP functions on the maturation and activation of DCs, which in turn, induce the differentiation of naïve CD4+ T cells to TH2 cells were also observed [21;26]. In addition, TSLP-activated DCs can induce a robust expansion of human TH2 memory cells while maintaining their central memory phenotype and TH2 commitments [27]. TH2 memory cells expanded by TSLP-activated DCs undergo further TH2 polarization and express proallergic genes such as IL-25R (IL17RB), cystatin A, Charcot-Leydon crystal protein and prostaglandin D2 synthase (Fig. 3) [27]. While some studies in patients with atopic dermatitis observed elevated TSLP expression in inflamed skin [17;27], others demonstrated that these patients often exhibit significantly increased frequency of circulated CRTH2+CD4+ TH2 memory cells in blood [28]. Recently, we further showed that patients with AD, but not TH1-mediated inflammatory skin diseases, exhibited strong TSLP expression together with accumulated CRTH2+CD4+TH2 memory cells clustering with DC-LAMP+DCs within dermis of their lesional skin [27]. Together, these studies led us to propose that at the sites of allergen exposures, DCs activated by epithelial-derived TSLP or other stimuli may function not only in priming TH2-mediated immune responses but also in sustaining the allergic inflammation by inducing the expansion and further TH2 polarization of allergen-specific TH2 memory T cells, thereby contributing to the maintenance and relapse of TH2-mediated allergic diseases [27;29-31]. The potential for TSLP to provide a new therapeutic target for the treatment of allergic disorders is compelling, and elucidating the mechanisms that regulate TSLP expression and the effects of TSLP on orchestrating the immune response toward a TH2 phenotype should facilitate this quest.
Figure 3. OX40L and IL-25 (IL-17E) maintain chronic allergic responses.
DCs activated by TSLP can expand and activate resident Th2 memory cells that upregulates the expression of proallergic molecules, including IL-25R, cystatin A, Charcot-Leydon crystal protein, and prostaglandin D2 synthase. During chronic allergic inflammation, infiltrated mast cells, eosinophils, basophils, and injured structural cells can produce IL-25 that can further enhance TH2 cytokines production, in particular IL-5 and IL-13 by DC-activated TH2 memory cells.
OX40/OX40L: the molecular trigger for TSLP-DCs induced allergic responses
In an attempt to identify the molecular mechanisms by which TSLP-activated DCs induce allergic inflammation, OX40-ligand (OX40L) was identified to be the key molecule expressed by TSLP-activated DCs using microarray analyses. OX40L, originally termed glycoprotein 34 kDa (GP34), and its cognate receptor OX40, belong to tumor-necrosis factor (TNF) and TNF receptor superfamily [32;33]. OX40 is preferentially expressed on activated CD4 T cells, whereas OX40L is mainly expressed by antigen-presenting cells (APC), including activated DCs, B cells, macrophages, and Langerhan cells, as well as T cells and endothelial cells. Unlike other costimulatory molecules; such as CD28 that plays an important role on T cell priming, OX40 is not constitutively expressed on naïve T cells but is induced at 24 to 48 h after engagement of TCR, with peak levels between day 2 to day 5 in vivo after antigen immunization. OX40-deficient CD4+ T cells proliferate initially, but cannot survive long-term due to lower expression of anti-apoptotic proteins such as Bcl-2, Bcl-xL, and Bfl-1. Therefore, OX40/OX40L interaction was shown to be crucial for T-cell activation, and survival and the generation of memory T cells from activated effector T cells [34].
The interaction between OX40 and OX40L is implicated in a number of inflammatory diseases, in particular allergic inflammation. In the studies of allergen-induced animal models, mice lacking OX40 or OX40L exhibit significant impairments of TH2 responses and diminished lung inflammation, implicating the involvement of OX40 signaling on triggering allergic response [35;36]. The finding that OX40L expression on TSLP-activated DCs plays an important role for the induction of inflammatory TH2 cells provides the molecular basis for the understanding of OX40/OX40L interaction on the induction of allergic responses. Blockade of OX40 and OX40L interactions using a neutralizing antibody against OX40L inhibited the production of TH2 cytokines and TNF-α and enhanced the production of IL-10 by the differentiating CD4+ T cells cocultured with TSLP-activated DCs. OX40L-induced inflammatory TH2 cell differentiation depends on the absence of IL-12, as OX40L is incapable of triggering inflammatory TH2 cell differentiation in the presence of IL-12. Thus TSLP-activated DCs may create a TH2-permissive microenvironment by up-regulating OX40L without inducing the production of TH1-polarizing cytokines. Moreover, OX40L expressed by TSLP-DCs also plays an important role on driving the expansion of TH2 memory cells by contributing to the prolonged cognate formation during T cell-DC interaction [27]. Loss of OX40 signaling during T cell-DC interaction induces the expression of p14ARF and other CDK inhibitors in CD4+ TH2 memory cells, which results in cell cycle arrest or cell senescence [27]. Triggering OX40 signaling in TH2 memory cells by their ligands on TSLP-DCs is indispensable for the reactivation and homeostatic maintenance of the TH2 memory pool by controlling their entrance into cell cycle, thereby linking the critical role of OX40L in the pathogenesis of allergic inflammation in animal models [36]. Together, these studies provide the plausible explanations for the functions of OX40L on inducing the generations of inflammatory TH2 cells and the maintenance of TH2 memory pool during TSLP-DCs mediated allergic response [37].
The pivotal role of OX40L on TSLP-induced allergic inflammation is further demonstrated in a recent study using both mouse and non-human primate models in vivo. To address the role of OX40/OX40L interactions in contributing to allergic response in both animal models, the chimeric hamster-mouse mAb and a fully humanized mAb against mouse, and human OX40L, respectively, were generated [38]. The efficacy of these mAb treatments was demonstrated by their ability in abolishing the interactions of OX40/OX40L and the depletion of OX40L-positive DCs [38]. These invaluable anti-OX40L antibodies prove to be efficacious in inhibiting antigen driven TH2 inflammation in mouse and non-human primate model of asthma. Administrations of these anti-OX40L antibodies resulted in a significant reduction of TH2 cytokines, and antigen-specific IgE and IgG1, and the loss of infiltrated eosinophils and CD4+ effector/memory T cells [38]. Interestingly, the treatments resulted in only moderate reduction of TH2 inflammatory response during a primary effector response, whereas a significant decrease of re-activation and infiltration of memory CD4+ T cells, TH2 cytokine production, and antigen-specific serum IgE levels were observed during antigen recall response. These results demonstrated the critical roles of OX40/OX40L interaction on the maintenance and re-activation of TH2 memory responses during TSLP-DCs mediated allergic responses (Fig. 3). Targeting the TSLP-DC-induced inflammatory TH2 cells or allergen-specific TH2 memory cells by blocking OX40/OX40L interaction may be one of the new therapeutic approaches for the prevention and treatment of human allergic diseases.
Strong induction of IL-17RB, a cognate receptor for IL-25 (IL-17E), in TH2 memory cells by TSLP-DCs
Studies from patients and animal experimental models indicate that memory-like TH2 cells that reside in the lungs during disease remission can become activated effectors upon allergen exposure and are one of the principle cells responsible for the exacerbation of allergic diseases. One of the unique features of TSLP-induced allergic responses is the maintenance and activation of TH2 memory cells. TSLP-DCs induce strong up-regulation of IL-17RB (IL-25R) expression by activated TH2 memory cells, but not in other T cell subsets [39], thereby linking TSLP-driven TH2 responses to the functions of IL-17E (IL-25), a distinct member of IL-17 cytokine family. IL-17RB serves as co-receptor for both IL-17B and IL-17E (IL-25), with higher binding avidity to IL-17E (also known as IL-25) [40]. IL-25 belongs to a recent discovered IL-17 cytokine family, which consist of IL-17A, and five additional family members designated as IL-17B-F [41-43]. IL-17A identified from activated T cell clones (originally named as CTLA-8) is the prototypic family member [44-46]. Among the IL-17 cytokine family, the expression and functions of IL-17A, IL-17F and IL-17E (IL-25) are better characterized. IL-17F shares the greatest similarity with IL-17A (55% identity), whereas IL-17E (IL-25) is the most distant (17%) [47]. Studies of IL-17 receptor family (IL-17RA-E) revealed additional complexities in the regulations and biological functions of IL-17 cytokine family. IL-17RA, the cognate receptor for IL-17A, is ubiquitously expressed [48]. However, the biological activity of IL-17A or IL-17F is dependent on the heterodimeric complex composed of IL-17RA and IL17RC [49]. In asthmatic patients, IL-17A expression was increased in the lungs, sputum, bronchoalveolar lavage (BAL) fluids or sera, and the severity of airway hypersensitivity in patients correlates with the level of IL-17A expression, suggesting that IL-17 cytokines play important roles in driving allergic inflammation [50]. Indeed, IL-17A and/or IL-17F can orchestrate local inflammation by inducing the release of proinflammatory cytokines such as TNF-α, IL-1β, G-CSF, and IL-6, as well as chemokines CXCL1/Gro-α, CXCL2 and CXCL8/IL-8 production by human bronchial fibroblast, epithelial, and airway smooth muscle cells, as well as venous endothelial cells in vitro [51-53]. Studies in mice showed that IL-17R-deficient mice exhibit not only reduced neutrophil but also eosinophil recruitments [54], whereas IL-17A−/− mice exhibited reduced TH2 responses to antigen sensitization [55]. As summarized in Table I, these studies suggest that IL-17A/F can trigger lung inflammation by stimulating innate immunity to mediate neutrophil recruitments, implicating the potential role of IL-17A/F on the pathogenesis of severe asthma mediated by neutrophilia.
Table I.
IL-17 cytokine family and functional effects on allergy
| Ligands | Receptors | Functions | References |
|---|---|---|---|
| IL-17A | IL-17RA | IL-6, IL-8, IL-11, Gro-α, G-CSF and GM-CSF ↑ | [53] |
| IL-17F | IL-17RA/C | MUC5AC and MUC5B ↑ | [52] |
| Airway hyper-reactivity ↑ | [55] | ||
| Neutrophilia ↑ | [51] | ||
| Severity of asthma ↑ | [55] | ||
| IL-17E | IL-17RB | IL-4, IL-5, IL-13, IgE, and eotaxin ↑ | [39,56,58,59,60.64] |
| Mucus secretion ↑ | [60,61] | ||
| Airway hyper-reactivity ↑ | [56,62,64] | ||
| Eosinophilia ↑ | [56,58,60,64] | ||
| Severity of asthma ↑ | [56,60,62,64] |
Distinct from other IL-17 cytokine family members, IL-25 (IL-17E) was first described as a TH2 cell-derived cytokine [56]. Human IL-25 shares 80% identity with mouse transcript and exhibits as two isoforms [56]. The isoform 1 has an extended N-terminal sequence and is expressed at high level in the testis, trachea and brain, whereas isoform 2 is mainly expressed in the testis, prostate, and spleen. In mice, IL-25 transcript can be found in the gastrointestinal tract and uterus, and IL-25R is expressed in the lymphoid organs, lung, and liver [57]. Recent studies demonstrated that the expression of IL-25 transcript could be found in the mast cells activated by IgE cross-linking [58], alveolar macrophage and lung epithelial cells stimulated with allergens in mice [59]. In humans, bioactive IL-25 protein was found to be secreted by activated eosinophils and basophils [39]. Interestingly, these cells obtained from allergic patients produce more substantial amounts of IL-25 after activation. These studies suggest that multiple cellular sources of IL-25 may exist during allergic responses (Fig. 3).
IL-25 enhances allergic response through multiple pathways
Compared with other IL-17 cytokine family members, IL-25 is distinctive with low sequence homology, unique expression pattern, and unique functions in evoking type-2 immune responses in animal studies [56;60-62]. Systemic administration of IL-25 protein [56;62] or overexpression of IL-25 [60;61] induces elevated TH2 cytokine and eotaxin productions, which result in eosinophilia, increased serum IgE, mucus hyperplasia, and other pathological changes in many tissues. Moreover, administrations of neutralizing antibody against IL-25 in an experimental model of allergic asthma resulted in significantly reduced levels of IL-5, IL-13 and serum IgE production, the infiltration of TH2 cells and eosinophils, and prevented airway hyperresponsiveness [63]. These in vivo studies imply that IL-25 may play a pivotal role in the development of TH2-mediated allergic inflammation.
The function of IL-25 on type-2 immunity, which play protective role in defense against parasitic infection was further elucidated by recent studies in animal models using helminth infection. In the absence of IL-25, mice infected with Trichuris muris, the gastrointestinal parasite, failed to develop a lymphocyte dependent protective type2 immunity to expel chronic parasitic infection [64]. In an other study, IL-25 was found to trigger the non-B or T, c-kit+ cells for the rapid clearance of N. brasiliensis acute infection [65]. Using allergen-induced allergic animal models, one study showed that the administrations of recombinant IL-25 proteins can induce acute lung inflammation mediated by the unidentified IL-5-producing non-B or T cells [62], whereas the other demonstrated that the enforced expression of IL-25 in lung resulted in the amplification of allergic inflammation driven by CD4+T cells and STAT6 signaling pathway [66]. These findings suggest that IL-25 can enhance type-2 immune responses by regulating either CD4+ T cells or non-B or T, c-kit+ cells depending on experimental models.
The finding that IL-25 receptor (IL-25R or IL-17BR) is highly expressed on CD4+ TH2 memory cells in humans has provided direct evidence that IL-25 can function directly on CD4+ T cells to mediate enhanced type-2 immune response [39]. Indeed, IL-25 co-stimulates the proliferation of the TH2 memory cells, and enhances their TH2 polarization and cytokine productions, in particular IL-5, by upregulating the gene expression of the transcription factors, GATA-3, c-MAF, and junB in an IL-4 independent manner [39]. A parallel study in mouse showed that IL-25 treatment during T cell differentiation can enhance TH2 cytokine productions and inhibit IFN-γ production, indicative of the TH2 polarizing function [59]. Together, these results suggest that IL-25 may amplify allergic immune responses by inducing the local expansion and augmented effector functions of TH2 memory/effector cells. On the contrary to the T cell derived proinflammatory cytokine IL-17A and IL-17F, which induce the productions of pro-inflammatory cytokines/chemokines from a variety of cell types during the onset of allergic inflammation, IL-25 (IL-17E) produced by activated eosinophils, basophils, and mast cells may exert a critical role in maintaining the functional capacity and maintenance of IL-25R-expressing allergen-specific TH2 memory cells, thus propagating a positive feed back loop between innate effectors and adaptive immunity leading to the amplification of allergic inflammation (Fig. 3).
Conclusions
TSLP is a cytokine with pleiotropic functions. TSLP appears to function in CD4+ T cell homeostasis in the peripheral mucosa associated lymphoid tissues under normal physiological conditions, whereas aberrant TSLP expression by epithelial cells or keratinocytes has been shown to play a critical role in inducing allergic inflammatory diseases, such as asthma and atopic dermatitis. Invaded pathogens, allergens or other environmental factors that can induce proinflammatory cytokine productions have the potential to trigger the strong TSLP expression in the epithelium layer. The RXRα or RXRβ that can pair with vitamin D receptor to act as a transcriptional repressor to control TSLP gene expression may be one of the crucial factors to control the steady state level of TSLP production. TSLP targets mDCs to create a TH2 permissive microenvironment at local inflammatory sites. TSLP-activated DCs are potent inducer in the generation of inflammatory TH2 cells and the maintenance and activation of TH2 memory cells via their surface OX40L expression. TSLP-activated DCs induce activated TH2 memory/effector cells to express the high level of surface IL-25R, thereby responding to the stimulation of IL-25 to produce elevated TH2 cytokines. The eosinophil/basophil lineage, particularly eosinophils, appears to be the potential producers of the cytokine IL-25. This raises the possibility that eosinophils, through the production of IL-25, may exert a critical role in maintaining the functional capacity of allergen-specific T central memory cells in allergic inflammation. These recent exciting findings have not only advanced our understandings of complex allergic responses but also provided important pointers towards novel approaches in the prevention and therapy for allergic diseases.
References
- 1.Leonard WJ. Cytokines and immunodeficiency diseases. Nat Rev Immunol. 2001;1:200–8. doi: 10.1038/35105066. [DOI] [PubMed] [Google Scholar]
- 2.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–8. [PubMed] [Google Scholar]
- 3.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, Ziegler SF, Leonard WJ, Lodish HF. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 4.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG, Ziegler SF, Morrissey PJ, Paxton R, Sims JE. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–70. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, Zurawski SM, Johnston J, Liu YJ, Spits H, de Waal MR, Kastelein RA, Bazan JF. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–43. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 6.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt RW, Omori M, Zhou B, Ziegler SF. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol. 2006;7:709–14. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- 8.Quentmeier H, Drexler HG, Fleckenstein D, Zaborski M, Armstrong A, Sims JE, Lyman SD. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia. 2001;15:1286–92. doi: 10.1038/sj.leu.2402175. [DOI] [PubMed] [Google Scholar]
- 9.Soumelis V, Liu YJ. Human thymic stromal lymphopoietin: a novel epithelial cell-derived cytokine and a potential key player in the induction of allergic inflammation. Springer Semin Immunopathol. 2004;25:325–33. doi: 10.1007/s00281-003-0152-0. [DOI] [PubMed] [Google Scholar]
- 10.Bogiatzi SI, Fernandez I, Bichet JC, Marloie-Provost MA, Volpe E, Sastre X, Soumelis V. Cutting Edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol. 2007;178:3373–7. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- 11.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–7. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007;104:914–9. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Messaddeq N, Teletin M, Pasquali JL, Metzger D, Chambon P. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc Natl Acad Sci U S A. 2005;102:14795–800. doi: 10.1073/pnas.0507385102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A. 2006;103:11736–41. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vosshenrich CA, Cumano A, Muller W, Di Santo JP, Vieira P. Thymic stromal-derived lymphopoietin distinguishes fetal from adult B cell development. Nat Immunol. 2003;4:773–9. doi: 10.1038/ni956. [DOI] [PubMed] [Google Scholar]
- 16.Al-Shami A, Spolski R, Kelly J, Fry T, Schwartzberg PL, Pandey A, Mackall CL, Leonard WJ. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–68. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, Waal-Malefyt RR, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 18.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–73. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–90. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 21.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–39. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. 2007;178:6720–4. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 23.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–8. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol. 2007;120:238–44. doi: 10.1016/j.jaci.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Gilliet M, Soumelis V, Watanabe N, Hanabuchi S, Antonenko S, Waal-Malefyt R, Liu YJ. Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells. J Exp Med. 2003;197:1059–63. doi: 10.1084/jem.20030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–53. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 27.Wang YH, Ito T, Wang YH, Homey B, Watanabe N, Martin R, Barnes CJ, McIntyre BW, Gilliet M, Kumar R, Yao Z, Liu YJ. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–38. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Cosmi L, Annunziato F, Iwasaki M, Galli G, Manetti R, Maggi E, Nagata K, Romagnani S. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. European Journal of Immunology. 2000;30:2972–9. doi: 10.1002/1521-4141(200010)30:10<2972::AID-IMMU2972>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Jahnsen FL, Moloney ED, Hogan T, Upham JW, Burke CM, Holt PG. Rapid dendritic cell recruitment to the bronchial mucosa of patients with atopic asthma in response to local allergen challenge. Thorax. 2001;56:823–6. doi: 10.1136/thorax.56.11.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambrecht BN, Hoogsteden HC, Pauwels RA. Dendritic cells as regulators of the immune response to inhaled allergen: recent findings in animal models of asthma. Int Arch Allergy Immunol. 2001;124:432–46. doi: 10.1159/000053778. [DOI] [PubMed] [Google Scholar]
- 31.Julia V, Hessel EM, Malherbe L, Glaichenhaus N, O'Garra A, Coffman RL. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity. 2002;16:271–83. doi: 10.1016/s1074-7613(02)00276-5. [DOI] [PubMed] [Google Scholar]
- 32.Paterson DJ, Jefferies WA, Green JR, Brandon MR, Corthesy P, Puklavec M, Williams AF. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24:1281–90. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 33.Godfrey WR, Fagnoni FF, Harara MA, Buck D, Engleman EG. Identification of a human OX−40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. J Exp Med. 1994;180:757–62. doi: 10.1084/jem.180.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4:420–31. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 35.Hoshino A, Tanaka Y, Akiba H, Asakura Y, Mita Y, Sakurai T, Takaoka A, Nakaike S, Ishii N, Sugamura K, Yagita H, Okumura K. Critical role for OX40 ligand in the development of pathogenic Th2 cells in a murine model of asthma. Eur J Immunol. 2003;33:861–9. doi: 10.1002/eji.200323455. [DOI] [PubMed] [Google Scholar]
- 36.Salek-Ardakani S, Song J, Halteman BS, Jember AG, Akiba H, Yagita H, Croft M. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med. 2003;198:315–24. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang YH, Liu YJ. OX40-OX40L interactions: a promising therapeutic target for allergic diseases? J Clin Invest. 2007;117:3655–7. doi: 10.1172/JCI34182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, Zhang J, Diehl L, Austin CD, Meng YG, Tan M, Bullens SL, Seeber S, Fuentes ME, Labrijn AF, Graus YMF, Miller LA, Schelegle ES, Hyde DM, Wu LC, Hymowitz SG, Martin F. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. 2007 doi: 10.1172/JCI33559. Ref Type: Generic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–47. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian E, Sawyer JR, Largaespada DA, Jenkins NA, Copeland NG, Shaughnessy JD., Jr Evi27 encodes a novel membrane protein with homology to the IL17 receptor. Oncogene. 2000;19:2098–109. doi: 10.1038/sj.onc.1203577. [DOI] [PubMed] [Google Scholar]
- 41.Lee J, Ho WH, Maruoka M, Corpuz RT, Baldwin DT, Foster JS, Goddard AD, Yansura DG, Vandlen RL, Wood WI, Gurney AL. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem. 2001;276:1660–4. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- 42.Starnes T, Robertson MJ, Sledge G, Kelich S, Nakshatri H, Broxmeyer HE, Hromas R. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol. 2001;167:4137–40. doi: 10.4049/jimmunol.167.8.4137. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Chen J, Huang A, Stinson J, Heldens S, Foster J, Dowd P, Gurney AL, Wood WI. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc Natl Acad Sci U S A. 2000;97:773–8. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–6. [PubMed] [Google Scholar]
- 45.Yao Z, Maraskovsky E, Spriggs MK, Cohen JI, Armitage RJ, Alderson MR. Herpesvirus saimiri open reading frame 14, a protein encoded by T lymphotropic herpesvirus, binds to MHC class II molecules and stimulates T cell proliferation. J Immunol. 1996;156:3260–6. [PubMed] [Google Scholar]
- 46.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, it-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das MB, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–74. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 48.Yao Z, Spriggs MK, Derry JM, Strockbine L, Park LS, VandenBos T, Zappone JD, Painter SL, Armitage RJ. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine. 1997;9:794–800. doi: 10.1006/cyto.1997.0240. [DOI] [PubMed] [Google Scholar]
- 49.Toy D, Kugler D, Wolfson M, Vanden BT, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–9. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 50.Kolls JK, Kanaly ST, Ramsay AJ. Interleukin-17: an emerging role in lung inflammation. Am J Respir Cell Mol Biol. 2003;28:9–11. doi: 10.1165/rcmb.2002-0255PS. [DOI] [PubMed] [Google Scholar]
- 51.Linden A, Laan M, Anderson GP. Neutrophils, interleukin-17A and lung disease. Eur Respir J. 2005;25:159–72. doi: 10.1183/09031936.04.00032904. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003;278:17036–43. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 53.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–52. [PubMed] [Google Scholar]
- 54.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–87. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 56.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 57.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–74. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 58.Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, Saito Y, Iwamoto I. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood. 2003;101:3594–6. doi: 10.1182/blood-2002-09-2817. [DOI] [PubMed] [Google Scholar]
- 59.Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–17. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan G, French D, Mao W, Maruoka M, Risser P, Lee J, Foster J, Aggarwal S, Nicholes K, Guillet S, Schow P, Gurney AL. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J Immunol. 2001;167:6559–67. doi: 10.4049/jimmunol.167.11.6559. [DOI] [PubMed] [Google Scholar]
- 61.Kim MR, Manoukian R, Yeh R, Silbiger SM, Danilenko DM, Scully S, Sun J, DeRose ML, Stolina M, Chang D, Van GY, Clarkin K, Nguyen HQ, Yu YB, Jing S, Senaldi G, Elliott G, Medlock ES. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100:2330–40. doi: 10.1182/blood-2002-01-0012. [DOI] [PubMed] [Google Scholar]
- 62.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–53. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 63.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120:1324–31. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 64.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–9. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–16. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, Suto A, Suzuki K, Watanabe N, Saito Y, Tokuhisa T, Iwamoto I, Nakajima H. IL-25 enhances allergic airway inflammation by amplifying a T(H)2 cell-dependent pathway in mice. J Allergy Clin Immunol. 2006;118:606–14. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]