Abstract
Oxidative stress is implicated in the pathogenesis of many neurodegenerative diseases, including Parkinson's disease and Alzheimer's disease. The depletion of glutathione (GSH) a powerful antioxidant renders cells particularly vulnerable to oxidative stress. Isolated neuronal and glial cell culture studies suggest that glia rather than neurons have greatest reserves of GSH, implying that neurons are most sensitive to oxidative stress. However, pathological in vivo studies suggest that GSH associated enzymes are elevated in neurons rather than astrocytes. The active, reduced form of GSH is rapidly degraded thus making it difficult to identify the location of GSH in post-mortem tissue. Therefore, to determine whether GSH is more highly expressed in neurons or astrocytes we perfused mouse brains with a solution containing NEM which reacts with the sulfhydryl group of GSH, thus locking the active form in situ, prior to immunostaining with an anti-GS-NEM antibody. We obtained brightfield and fluorescent digital images of sections stained with DAPI and antibodies directed against GS-NEM, glial fibrillary acidic protein (GFAP) in regions containing the hippocampus, striatum, frontal cortex, midbrain nuclei, cerebellum and reticular formation neurons. GSH was most abundant in neurons and white matter in all brain regions, and only in occasional astrocytes lining the third and fourth ventricles. High levels of GSH in neurons and white matter, suggests astrocytes rather than neurons may be particularly vulnerable to oxidative stress.
Keywords: Glutathione, White Matter, Mouse Brain, Neurons, Astrocytes, Oxidative Stress
1 Introduction
Oxidative stress and the formation of toxic oxidant species such as hydrogen peroxide are implicated in the pathogenesis of a number of neurodegenerative diseases including Parkinson's disease, Alzheimer's disease and dementia with lewy bodies (Andersen, 2001;Behl and Holsboer, 1998;Halliwell, 2001;Przedborski, 2005). Oxidative stress is triggered by an imbalance between the production of reactive species and the ability of a cell to produce antioxidants to metabolize these species. The oxidative capacity of a cell is therefore dependent on its ability to produce antioxidants such as glutathione (GSH) (Ault and Lawrence, 2003). During oxidative stress the reduced form of GSH is rapidly oxidized to either a disulfide form (GSSG) or to form mixed disulfides with other thiol-containing reactants (GSSR) (Fig. 1). In vitro isolated cell culture studies suggest that astrocytes and oligodendrocytes have increased GSH reserves compared with neurons (Hirrlinger et al., 2002;McTigue and Tripathi, 2008;Raps et al., 1989). This data apparently was used to substantiate in vivo studies suggesting that increased astrocyte activity attributed to the increased levels of GSH related proteins in post-mortem AD and murine brains (Aksenov et al., 1998;Dasgupta et al., 2005). However, many in vivo studies, which measure the active form of GSH itself, rely on analysis of tissue homogenates, which contain a mixture of cells, including astrocytes, neurons, oligodendrocytes, endothelial cells, smooth muscle cells, and microglia to verify increased astroglial synthesis of GSH. Therefore, it cannot be stated with certainty that GSH, which may well be increased in response to oxidative stress, is synthesized by astrocytes rather than neurons in vivo.
Figure 1.
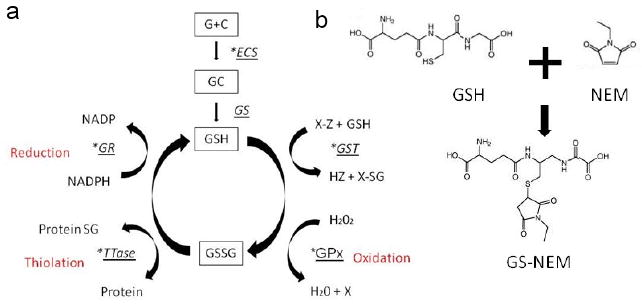
a This diagram shows enzymes marked with an astrix which are associated with Glutathione activity which has been described in vivo by other research groups as an index of GSH synthesis. GSH synthesis is catalysed by gamma-glutamylcysteine synthase (gamma-ECS) and glutathione synthase (GS). GSH is a cofactor of by Glutathione peroxidase (GPX) used to reduce hydrogen peroxidase H2O2. GSH is also a substrate for Glutathione-S-Transferase a family of phase II detoxification enzymes. Oxidised glutathione (GSSG) can reversibly react with protein thiols either spontaneously or enzymatically to give protein-glutathione mixed disulphides. This thiolation can alter enzyme activity and is a method of rapidly altering enzyme activity in response to oxidative stress. Oxidised glutathione is reduced back to glutathione by the NADPH-dependent enzyme glutathione reductase (GR).1b) This diagram shows the reaction between GSH and NEM to produce GS-NEM the molecule to which an antibody was generated.
Furthermore, there are problems with using immunohistochemistry to determine the intracellular location of the active form of GSH in post-mortem brain tissue. GSH is rapidly metabolized during post-mortem delays in the collection, freezing and fixation of brain tissue. In addition, the fixation process itself can alter the binding of particular antibodies to the constitutively expressed form of GSH, thus making it difficult to accurately interpret immunostained sections (Philbert et al., 1995). However, despite the problems associated with measuring the active form of GSH, pathological studies on enzymes related to the metabolism of GSH indirectly suggest that neurons rather than astrocytes contain greater reserves of GSH.
Thioltransferase (TTase) is a neuroprotective enzyme involved in cellular recovery from oxidative stress. TTase can reverse the formation of mixed protein-GSH disulfides, thus decreasing the likelihood of misfolded protein aggregate formation during oxidative stress. One pathological study found that mRNA for TTase is most highly expressed in hippocampal neurons, Purkinje cells in the cerebellum, and reticular formation midbrain neurons in addition to thalamic and striatal neurons rather than astrocytes in the rat brain (Balijepalli et al., 1999). Additional studies have found that glutathionine S-transferase (GST) which catalyzes the conjugation of reactive electrophilic compounds to GSH, is more highly expressed in neurons rather than astrocytes, and in the adult rat brain is expressed in both neurons and astrocytes (Beiswanger et al., 1995; Philbert et al., 1995). Thus studies on enzymes indirectly associated with the activity of GSH suggest by proxy that GSH is highly expressed in vivo in neurons rather than astrocytes; however, this has not been conclusively demonstrated by the use of an antibody directed against the active reduced form of GSH.
Therefore, to determine the precise neuroanatomical location of the active form of GSH in the unperturbed adult mouse brain, we perfused mouse brains with a solution containing N-ethylmaleimide (NEM), a low molecular mass molecule which reacts rapidly and with high affinity with sulfhydryl groups including those found on GSH (Fig. 1). We then used a combination of immunohistochemistry and immunofluorescence staining techniques with an antibody specific to the GS-NEM, which does not recognize NEM adducts with other thiols (Ault and Lawrence, 2003;Burchiel et al., 1997;Messina, 1987;Palace and Lawrence, 1995;Pedersen-Lane et al., 2007). In summary, we describe in situ the location of the active form of GSH in the mouse central nervous system, using a direct and dynamic immunohistochemistry-based imaging technique.
2. Results
Anatomical localization of GS-NEM with brightfield staining
In the sections stained with DAB, it was clear that the reduced form of GSH (GS-NEM) was abundant in both neurons and white matter tracts but did not stain astrocytic cells (Figure 2a and b). The corpus callosum, and striatal fibers were clearly immunoreactive to the GS-NEM antibody as shown in Figure 2a. Neurons in the hippocampus in the cornu-amos (CA) neuronal subfields and neurons in the dentate gyrus in addition to white matter tissues of the external capsule, thalamus were also immunoreactive to the GS-NEM antibody as can be seen in figure 2b. We noted that neuronal cell bodies in both the hippocampus, and the pyramidal cortical layer in addition to the parietal cortex were clearly immunoreactive for the GS-NEM and that the staining was particularly dense in the nucleus rather than the cell stroma (Figures 2c and 2d). This suggested that the reduced form of GSH is constitutively synthesized in neurons and readily diffuses into the nucleus to protect DNA from oxidative damage. In order to optimize the staining process, we tried a number of alternative staining methods. However, incubation of tissue sections without the anti-GS-NEM antibody revealed no staining, while incubation with the GS-NEM antibody but without having previously perfused the animals with the NEM containing solution also revealed no staining. This suggested that perfusion of brain tissue with both the NEM and PFA solution was necessary in order to achieve good immunoreactivity with the anti-GS-NEM antibody; this is consistent with previous in vitro straining of macrophages (Ault and Lawrence, 2003). Analysis of the DAB immunostained sections strongly suggested that the active form of GSH is predominantly synthesized by neurons and oligodendrocytes rather than astrocytes. Therefore, in order to further determine whether the active form of GSH was only in white matter and neurons, or if it was present in astrocytes as suggested with in vitro cell culture studies, we next co-labeled sections with DAPI, GS-NEM and an antibody directed against GFAP, an astrocyte-specific protein. In addition, to demonstrate specificity of the GS-NEM antibody, we used the antibody on tissue treated with PFA, PFA and NEM, and PBS only and found no non-specific staining on western blots, but that the antibody stained neuronal N27 cells which were fixed with PFA-NEM, but not those which were only fixed with PFA (supplemental Fig. 1 and 2).
Figure 2.
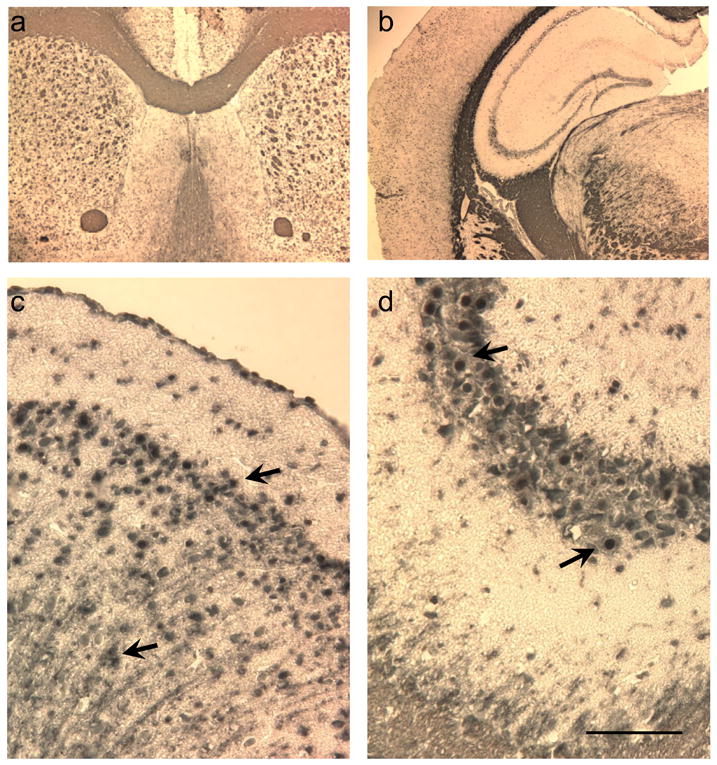
Photomicrographs illustrate immunoreactivity of the anti-GS-NEM antibody in mouse brain sections. a) Low mangification photomicrograph shows that the myelinated fibers within the caudate putamin, corpus callosum and anterior commissure are highly immunoreactive to the antibody (∼Bregma 0.62mm). b) Low mangification photomicrograph shows that thalamic white matter, the corpus callosum and dentate granule and hippocampal CA1 neurons are highly immunoreactive (IR) to the anti-GSH antibody (∼Bregma -2.06mm). c) Higher magnification photomicrograph is taken of the lateral parietal cortex and shows that external and internal cortical granular, pyramidal and molecular neurons, in addition to their output axons are highly IR with the anti-GS-NEM antibody. d) Higher mangification photomicrograph was taken of the hippocampal CA3 neurons. Arrows point to neuronal nucleus which is appears more densely stained than the neuronal stroma. Bar corresponds to 50μm and applies to figures c and d.
Anatomical co-localization of GS-NEM and GFAP
Cerebellum
The cerebellum contains a variety of cell types; purkinje cells, granule cells, astrocytes, white matter, and therefore is a good region to select to better determine if there is regional variability in the neuroanatomical colocalisation of GS-NEM. We colabelled sections with DAPI, GFAP and GS-NEM antibody and found that there was a regional variation in the intracellular location of the GS-NEM antibody. The GS-NEM antibody was clearly visible within neurons, particularly neuronal nuclei of stellate neurons, granule cells and purkinje cells in addition to myelinated axons within the cerebellum. Figure 3a shows astrocytic fibers identified with the Alexa-568 conjugated GFAP antibody in red and GS-NEM IR in green. The GS-NEM was most prominent in neuronal cell bodies, rather than astrocytic fibers or astrocytic cell bodies which did not colocalise with the GS-NEM antibody in this region. The inset region in figure 3a shows the level in the cerebellum at which the photomicrographs were taken ∼Bregma -6mm. Figures 3b, c, and d are of the same tissue sections showing DAPI stained granule neurons, GSNEM stained granule neurons, white matter and occasional stellate cells, and GFAP identified astrocytic fibers. Figure 3e is a merged composition of 3bc and d, and illustrates that the regions which were most dense in astrocytic fibers were also mostly sparse in GS-NEM immunoreactivity.
Figure 3.
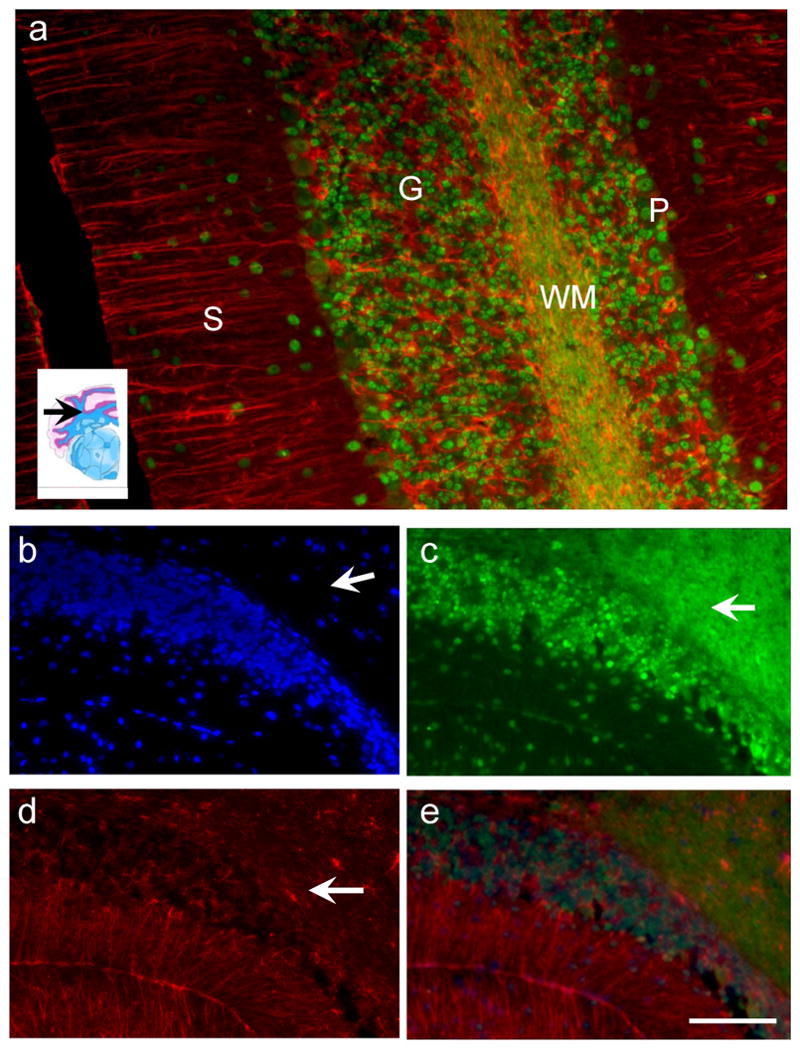
Fluorescent photomicrographs were taken of cerebellar tissues stained with DAPI (blue), the GS-NEM antibody (Green) and the anti-GFAP antibody (Red). a) photomicrograph illustrates the regional specificity of both the GS-NEM and GFAP antibodies. Picture shows the red astrocytic (GFAP) fibres in the stellate layer (S) in the cerebellum, the granular (G) and purkinje (P) cells are clearly IR to the GS-NEM antibody, while the white matter fibers (WM) contain mostly GS-NEM. There does not appear to be colocalisation of GS-NEM and GFAP. Photomicrographs b-e were taken of the same section. b) DAPI shows cell nuclei in a cerebellar slice. c) Shows GS-NEM IR in the same slice- arrows point to white matter and nuclei stained prominently with GSH. d) Shows same section stained with GFAP, arrow points to astrocytes. e) a composite merged image of bcd) clearly there is a regional specificity of both GS-NEM and GFAP immunoreactivity. Bar applies to b-e and is 70μm.
Hippocampus and Reticular Formation Neurons
It was clear that neurons in particularly were highly immunoreactive for the GS-NEM antibody therefore to further determine whether this staining pattern was region specific we analysed sections from a variety of coronal levels within the mouse brain. Figure 4 illustrates the intra neuronal and intra-nucleus staining of the GS-NEM antibody in both hippocampal CA1 neurons, in addition to reticular formation neurons in the brainstem (4e and 4f) in addition to neurons in the interposterior cerebellar nucleus (4g). Additionally we noted that white matter tracts, were also very immunoreactive to the GS-NEM antibody, yet the astrocytes in both the hippocampus and reticular formation did not colocalise with GS-NEM as demonstrated in figures 4a-d. The inset photomicrograph in 4a shows the level at which the panel of photomicrographs were taken, approximately Bregma -1.40mm. The arrows in figures 4b,c and d show an astrocyte nestled around a neuron which is IR to the GS-NEM antibody and illustrates the specificity of the antibody staining.
Figure 4.
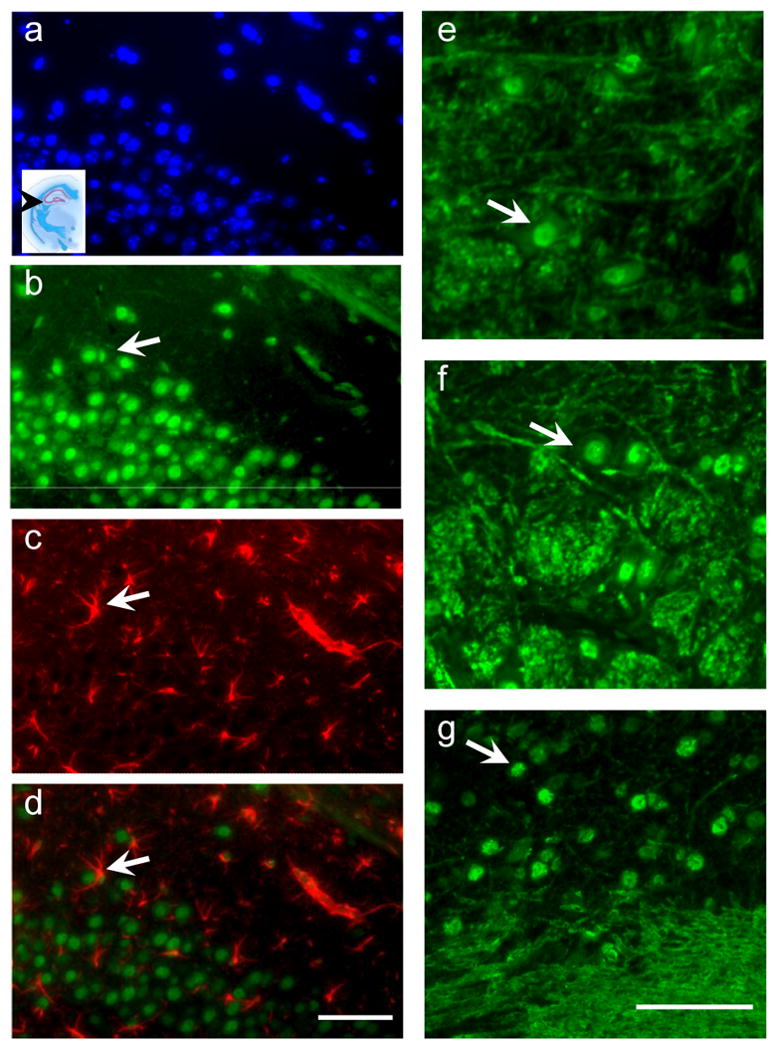
Fluorescent photomicrographs taken of hippocampal tissue stained with a) DAPI (blue), b) GS-NEM (green), c) GFAP (red), and d) a merged image of a-c. Panel of photomicrographs a-d illustrate that GSH was more prominent in neurons rather than astrocytes in the hippocampus. Bar is 60μm. Adjacent panel of photomicrographs e f and g are immunostained with GS-NEM showing the neuronal and white matter specificity of the antibody staining in e) and f) brainstem reticular formation nuclei and d) the interior cerebellar nuclei. Bar is 60μm.
Lining of Ventricles
One of the regions which is most vulnerable to toxicant induced oxidative stress is the lining of the ventricles where cells are exposed to molecules in the cerebrospinal fluid. Therefore we examined sections containing the ventricles in order to determine if cells lining the ventricles- ependymal cells in particular were highly immunoreactive for the GS-NEM antibody. We noted that while the ependymal cuboidal cells lining the opening of the third ventricle showed intense immunoreactivity for the GS-NEM antibody (Figure 5c) the astrocytes lining the third ventricle did not contain high levels of GS-NEM (Figure 5d). There was only occasional colocalisation between the GS-NEM antibody and the GFAP immunoreactive cells at the third and fourth ventricles as shown in figures 4d-f. The small degree of colocalisation between GS-NEM (green) and GFAP (red) is demonstrated by the yellow color where the arrows are pointing.
Figure 5.
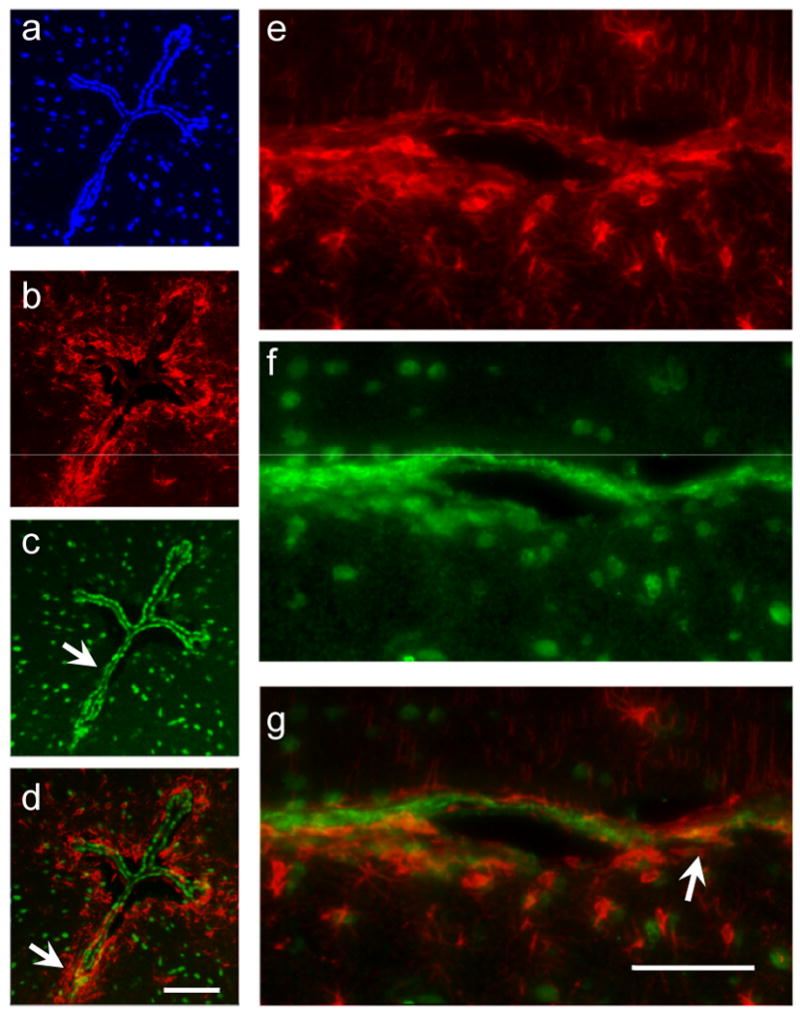
Panel of photomicrographs illustrate the density of GS-NEM and GFAP at the lining of the IV and III ventricles. Photomicrographs a-d are taken at the opening of the III ventricle (∼Bregma -3.08mm). a) DAPI, b) GFAP c) GS-NEM and d) a merged composite image of a-c. At the III ventricle there is no colocalisation of astrocytes (GFAP-red) and GSH containing cells (Green). Photomicrographs e-g are taken at the IVth ventricle (∼Bregma – 5.4mm). e) section stained with GFAP, arrow points to astrocyte. f) section stained with GSH; arrow points to a cell IR to GS-NEM, g) merged image shows colocalisation of GSH and GFAP in astrocytes. Arrows points to astrocytes which contain both GS-NEM and GFAP as identified by the yellow color on the merged photomicrographs. Bar is 40 μm.
3 Discussion
This study strongly suggests that the active form of GSH is most abundant in neurons and myelinated axons in a variety of brain regions including hippocampal CA1 neurons, frontal cortex pyramidal neurons and reticular formation neurons in the unperturbed adult mouse brain. Furthermore, in the aforementioned brain regions, there was a lack of colocalisation of GS-NEM with astrocytic proteins strongly suggesting that despite the many in vitro cell culture studies which describe greater antioxidant reserve in astrocytes, in the unperturbed brain astrocytes actually contain very low levels of GSH. This study therefore implies there is less GSH in neuroglia, astrocytes in particular, than previously suspected which would suggest that astrocytes may actually be more susceptible than neurons to oxidative stress induced cell death. A recent study did report that treatment of cultured hippocampal slices with hydrogen peroxide induced more cell death in glial cells than neurons (Feeney et al., 2008). Astrocytes may then be sacrificed at the expense of neurons or white matter tracts in order to enhance neuronal survival at the early stages of an initial oxidative stress assault. Alternatively, it is known that GSH is unable to cross the blood brain barrier, suggesting that exogenous antioxidants are not bioavailable to neurons on a constitutive basis, thus necessitating a higher level of constitutive antioxidant synthesis in neurons as compared with astrocytes. As such neurons would be primed for an oxidative stress associated insult and ready to respond without necessitating anti-oxidant synthesis via astroglia.
We found that GFAP colocalised with the GS-NEM antibody in occasional cells lining the fourth and third ventricles. Astrocytes lining of the ventricles may be more active in metabolizing neurotoxic species than those in the parenchyma, due to their persistent exposure to substances contained in the cerebrospinal fluid (CSF) in the ventricles. Thus they may have a higher tonic level of GSH synthesis than astrocytes in the parenchyma which are not exposed to CSF. Studies on the neuroanatomical location of GST isoforms in the developing rat brain found at post-natal day 10, astrocytes lining the choroid plexus were immunoreactive for GST, while at later time points, oligodendrocytes and neurons but not astrocytes were immunoreactive for GSH (Beiswanger et al., 1995). Our findings echo the aforementioned studies in the developing rat brain, and suggest that in the adult brain, neurons and oligodendrocytes, but only a few astrocytes lining the ventricles constitutively, express high levels of GSH.
Whether a greater reserve of reduced GSH actually affords neurons greater protection from neurotoxic insults remains to be determined. It is possible that a greater metabolic demand of neurons necessitates a greater oxidative capacity and GSH synthesis. While we describe the use of a technique to directly image the dynamic location of GSH in the unperturbed mouse brain, it is likely that with age or in response to a toxic event, stroke or hemorrhage the expression of GSH may be altered in the brain (Schulz et al., 2000). GSH peroxidase (GPx) is an enzyme which uses GSH to convert H2O2 to H2O. and is reportedly increased in microglia and astrocytes of patients with dementia with Lewy Bodies and Parkinson's disease compared to control patients (Power and Blumbergs, 2008). Increased GPx in neuroglia in neurodegenerative diseases suggests that GSH synthesis may increase in response to injury. It could be argued that the limited immunoreactivity found in astrocytes for the GS-NEM antibody may be related to the limited ability of NEM to penetrate astrocytic membranes rather than neuronal membranes. However we have previously used this antibody in flow-cytometric experiments to determine GSH activity in leukocytes, in addition to T-cells and B-cells in culture, which suggests that the antibody readily penetrates a variety of cell membranes (Ault and Lawrence, 2003;Pedersen-Lane et al., 2007). The GS-NEM antibody is also sensitive to concentration dependent changes in GSH levels. Using flow cytometry studies have found that the antibody fluorescence increases in lymphocytes which are activated and GSH levels are high, but does not increase when the cells activated but treated with an inhibitor of γ-glutamylcysteine synthase (Burchiel et al., 1997). In addition the specificity of the GS-NEM antibody has been demonstrated in immunoelectron studies, where it has been shown to specifically bind to GSH uniformly throughout the cell but not the endoplasmic reticululm (Ault and Lawrence, 2003). Furthermore, previous studies using rat brains to determine location of GSH-related enzymes, TTase and GST describe a similar pattern of immunoreactivity in neurons and white matter rather than astrocytes.
We suggest that a toxicant may transiently increase the synthesis of GSH in neuroglia, but it is also likely that with age, neurons and neuroglia may have an altered and perhaps reduced capacity to synthesize antioxidants thus rendering the aged brain more susceptible to neurodegenerative processes (Harry and Kraft, 2008). This study describes GSH levels in the unperturbed adult mouse brain- and it is plausible that GSH synthesis would be altered within both neurons and glia. Thus in an adult animal, a toxicant might induce a transient elevation in GSH, but in an aged animal this increase might not be precipitated. Such a temporal shift may be important because defects in astrocytic anti-oxidant activity with age would have consequences for additional astrocytic functions such as glucose metabolism, the synthesis of neuronal growth factors and recycling of glutamate, and maintenance of the blood brain barrier; all of which could contribute to neurodegenerative processes; rather than the uncontrolled release of neurotoxic reactive oxygen or nitrogen species from defective aged neuroglia. Therefore, we suggest that it is important to study oxidative stress responses in vivo, by availing of new techniques to measuring the active form of antioxidants in situ in relation to both age and neurotoxic stressors in order to better understand cell and region specific mechanisms of oxidative stress mediated cell death.
4 Experimental Procedure
Animals
New Zealand White mice were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained in our AAALAC-approved animal facility of Wadsworth. Mice were housed in clear Plexiglas cages (29 × 18 × 12.5 cm) with stainless steel wire lids with or without filter tops, in a temperature (21–23 °C) controlled room, and maintained on a 12:12 h light:dark cycle (lights on at 7:00AM). All of the mice assayed and used for these studies were 10–16 week of age. All procedures were IACUC approved.
Tissue preparation
Animals were euthanized with CO2, and perfused with warmed phosphate buffered saline (PBS pH 7.4) followed by cold perfusion with a solution containing 10mM NEM, a low-molecular-mass molecule that penetrates cells easily and reacts with sulfhydryl groups [the 8.1-GSH antibody is specific for GS-NEM and does not recognize NEM adducts with other thiols] and 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4). The brains were removed, and immediately submersion fixed in a 4% PFA solution containing 10 mM NEM for 24 h at 4 °C. Subsequently, tissue was micro-dissected into 2 mm coronal rostra-caudal blocks followed by a further 4 hr submersion fixation in the PFA thiol solution prior to tissue processing and paraffin embedding. As a control measure, additional animals were perfused with a solution containing only 4% PFA to determine the specificity of antibody staining. Furthermore to determine whether or not perfusion with the NEM solution was necessary, additional brain tissue was perfused with 4% PFA then immersion fixed with a solution containing both the 10mM NEM and 4% PFA. For immunohistochemical (IHC) and immunofluorescence (IFC) staining, tissue blocks containing the hippocampus, corpus callosum, striatum, thalamus, cerebellum, brainstem and midbrain nuclei were selected. Brain tissue regions were selected using a mouse brain atlas (Franklin, 2008). A series of 6 μm sections were cut from each block using a rotary microtome, and sections then mounted onto charged glass tissue slides
Immunohistochemistry
Tissue sections were heated for 40minutes at 56°C, cooled to room temperature then cleared in xylenes and rehydrated through graded alcohols; 100%, 95%, 70%, and 50%, prior to use. For optimum antigen retrieval, sections were pretreated in a 10 mM pH 6.0 citrate buffer and heated in a microwave for 10 min full power. To quench endogenous peroxidase activity sections were immersed in a 0.3% H2O2 solution for 15 min. To block non-specific antigen binding, sections were pre-incubated for 30 min with a blocking solution containing horse serum, goat serum and 0.01% BSA. For identification of the GSH using brightfield microscopy, a mouse monoclonal IgG1 antibody (8.1GSH; Assay Designs, Ann Arbor, MI) directed against the glutathione adduct with N-ethylmaleimide (GS-NEM) was titrated initially using a series of dilutions before the 1:200 dilution was chosen (Messina, 1987). Tissue sections were incubated with the antibody, which was suspended in PBS and 0.1% BSA, incubated for 1hour min at room temperature. Sections were then washed and incubated with a secondary biotinylated anti-mouse antibody (1:1000 Vector ABC kit) followed by 3 washes in PBS, and incubation with an avidin-biotin solution for 1 hour (ABC kit Vector Labs). Sections were then washed 3 times and visualized with 3, 3′-diaminobenzidine (DAB) as the chromogen. Sections were rinsed in deionized water, dehydrated through graded alcohols and cleared in xylenes prior to mounting with DPX (Sigma, St. Louis, MO).
Immunofluorescence
For colocalisation experiments, sections were incubated with either, an alexa-488 conjugated mouse monoclonal IgG1 antibody (8.1-GSH; StressGen, Victoria, BC, Canada) directed against the glutathione adduct with N-ethylmaleimide (GS-NEM), a mouse monoclonal anti-glial-fibrillary acid protein antibody (GFAP Millipore 1:500 suspended in PBS), a mixture of both antibodies, or neither antibody- as a negative control, for 24h at 4°C in darkness. Sections were then washed for 2hrs in PBS. The sections stained with anti-mouse GFAP were then incubated for 24hours in darkness at 4°C with a solution containing an Alexa 568 anti-mouse antibody 1:200 PBS for a further 24 hours. All sections were then washed three times in darkness for 2hours then rapidly dehydrated through graded alcohols and cleared in xylenes. Sections were mounted using vectashield Hard Set™ mounting medium with DAPI: 4′,6-diamidino-2-phenylindole a fluorescent stain which binds strongly to DNA (Vector Laboratories), cover-slipped and stored in darkness at 4°C until images were taken.
Digital Image Acquisition
Digital photo-micrographs of areas of interest were generated using a Nikon (50i) microscope using a 4×, 10× and 20× lens coupled to a CCD camera with image grabber software. For fluorescence microscopy, images were obtained using the UV 2E/C DAPI fluorescence filter cube for DAPI identified cell nuclei, the FITC B-2EC1 fluorescence filter cube for the green Alexa 488 GS-NEM antibody and the TRITC G-2E/C fluorescence filter cube for the 568 Alexa-fluorophore tagged anti-GFAP antibody. Images were taken using the three filter cubes of the same region of interest to demonstrate colocalisation where appropriate, and merged using Adobe Photoshop CS3.
Supplementary Material
Acknowledgments
We acknowledge assistance from the Advanced Light Microscopy core at the Wadsworth Center. This research was supported in part by NIEHS R01ES01467502 to RF Seegal.
Footnotes
Disclosure statement. All authors have neither conflict of interest, nor any financial, personal or other relationships with other people or organizations within the last three years which may inappropriately bias or influence this work. All animal work was approved by the Wadsworth Center IACUC committee.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Aksenov MY, Tucker HM, Nair P, Aksenova MV, Butterfield DA, Estus S, Markesbery WR. The expression of key oxidative stress-handling genes in different brain regions in Alzheimer's disease. J Mol Neurosci. 1998;11:151–164. doi: 10.1385/JMN:11:2:151. 12. [DOI] [PubMed] [Google Scholar]
- Andersen JK. Do alterations in glutathione and iron levels contribute to pathology associated with Parkinson's disease? Novartis Found Symp. 2001;235:11–20. 112. [PubMed] [Google Scholar]
- Ault JG, Lawrence DA. Glutathione distribution in normal and oxidatively stressed cells. Experimental Cell Research. 2003;285:9–14. doi: 10.1016/s0014-4827(03)00012-0. [DOI] [PubMed] [Google Scholar]
- Balijepalli S, Tirumalai PS, Swamy KV, Boyd MR, Mieyal JJ, Ravindranath V. Rat brain thioltransferase: regional distribution, immunological characterization, and localization by fluorescent in situ hybridization. J Neurochem. 1999;72:1170–1178. doi: 10.1046/j.1471-4159.1999.0721170.x. [DOI] [PubMed] [Google Scholar]
- Behl C, Holsboer F. Oxidative stress in the pathogenesis of Alzheimer's disease and antioxidant neuroprotection. Fortschr Neurol Psychiatr. 1998;66:113–121. doi: 10.1055/s-2007-995246. [DOI] [PubMed] [Google Scholar]
- Beiswanger CM, Diegmann MH, Novak RF, Philbert MA, Graessle TL, Reuhl KR, Lowndes HE. Developmental changes in the cellular distribution of glutathione and glutathione S-transferases in the murine nervous system. Neurotoxicology. 1995;16:425–440. [PubMed] [Google Scholar]
- Burchiel SW, Kerkvliet NL, Gerberick GF, Lawrence DA, Ladics GS. Assessment of immunotoxicity by multiparameter flow cytometry. Fundam Appl Toxicol. 1997;38:38–54. doi: 10.1006/faat.1997.2325. [DOI] [PubMed] [Google Scholar]
- Dasgupta A, Das S, Sarkar PK. Thyroid hormone stimulates gamma-glutamyl transpeptidase in the developing rat cerebra and in astroglial cultures. J Neurosci Res. 2005;82:851–857. doi: 10.1002/jnr.20657. 1. [DOI] [PubMed] [Google Scholar]
- Feeney CJ, Frantseva MV, Carlen PL, Pennefather PS, Shulyakova N, Shniffer C, Mills LR. Vulnerability of glial cells to hydrogen peroxide in cultured hippocampal slices. Brain Res. 2008;1198:1–15. doi: 10.1016/j.brainres.2007.12.049. 1. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, P G. The mouse brain in stereotaxic coordinates. Academic Press Elsevier; New York: 2008. p. 351. [Google Scholar]
- Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- Harry GJ, Kraft AD. Neuroinflammation and microglia: considerations and approaches for neurotoxicity assessment. Expert Opin Drug Metab Toxicol. 2008;4:1265–1277. doi: 10.1517/17425255.4.10.1265. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirrlinger J, Resch A, Gutterer JM, Dringen R. Oligodendroglial cells in culture effectively dispose of exogenous hydrogen peroxide: comparison with cultured neurones, astroglial and microglial cells. J Neurochem. 2002;82:635–644. doi: 10.1046/j.1471-4159.2002.00999.x. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- Messina JP, M J, L DA. Production characterization of monoclonal antibodies to thiol-modified glutathione. In: Cerutti PA, Nygaard MG, Simic MG, editors. Anticarcinogenesis and Radiation Protection. Plenum Press; NY, USA: 1987. pp. 407–412. [Google Scholar]
- Palace GP, Lawrence DA. Phospholipid metabolism of lymphocytes with inhibited glutathione synthesis using L-buthionine-S,R-sulfoximine. Free Radic Biol Med. 1995;18:709–720. doi: 10.1016/0891-5849(94)00193-n. [DOI] [PubMed] [Google Scholar]
- Pedersen-Lane JH, Zurier RB, Lawrence DA. Analysis of the thiol status of peripheral blood leukocytes in rheumatoid arthritis patients. J Leukoc Biol. 2007;81:934–941. doi: 10.1189/jlb.0806533. [DOI] [PubMed] [Google Scholar]
- Philbert MA, Beiswanger CM, Manson MM, Green JA, Novak RF, Primiano T, Reuhl KR, Lowndes HE. Glutathione S-transferases and gamma-glutamyl transpeptidase in the rat nervous systems: a basis for differential susceptibility to neurotoxicants. Neurotoxicology. 1995;16:349–362. [PubMed] [Google Scholar]
- Power JH, Blumbergs PC. Cellular glutathione peroxidase in human brain: cellular distribution, and its potential role in the degradation of Lewy bodies in Parkinson's disease and dementia with Lewy bodies. Acta Neuropathol. 2008 doi: 10.1007/s00401-008-0438-3. 1. [DOI] [PubMed] [Google Scholar]
- Przedborski S. Pathogenesis of nigral cell death in Parkinson's disease. Parkinsonism Relat Disord. 2005;11 1:S3–S7. doi: 10.1016/j.parkreldis.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Raps SP, Lai JC, Hertz L, Cooper AJ. Glutathione is present in high concentrations in cultured astrocytes but not in cultured neurons. Brain Res. 1989;493:398–401. doi: 10.1016/0006-8993(89)91178-5. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


