Abstract
For the human brain, in vitro models that accurately represent what occurs in vivo are lacking. Organotypic models may be the closest parallel to human brain tissue outside of a live patient. However, this model has been limited primarily to rodent-derived tissue. We present an organotypic model to maintain intraoperatively collected human tumor and non-tumor explants ex vivo for a prolonged period of time (∼ 11 days) without any significant changes to the tissue cytoarchitecture as evidenced through immunohistochemistry and electron microscopy analyses. The ability to establish and reliably predict the cytoarchitectural changes that occur with time in an organotypic model of tumor and non-tumor human brain tissue has several potential applications including the study of cell migration on actual tissue matrix, drug toxicity on neural tissue, and pharmacological treatment for brain cancers, among others.
Keywords: organotypic, explant, brain, human, electron microscopy, immunohistochemistry
1. Introduction
In vivo models are currently the gold standard for experimental research; however, they are not always practical in terms of time, cost, availability, and applicability to human disease. Organotypic human cultures, however, may be the closest representation of in vivo human brain tissue outside of a live patient (Gahwiler et al. 1997; Tanaka et al. 1994). These explants are intraoperatively obtained brain tissues that would have otherwise been discarded, and are maintained ex vivo.
The preparation of organotypic cultures has primarily been limited to rodent-based research (Cavaliere et al. 2006; Holopainen 2005; Holtkamp et al. 2005; Stoppini et al. 1991; Tanaka et al. 1994; Valster et al. 2005). Preparation of adult human brain cultures has only been reported twice in the literature (Jung et al. 1999; Jung et al. 2002). These studies used these explants as the matrices to evaluate glioma cell migration; however, no structural analyses were conducted, and preservation of the tissue cytoarchitecture was inadequate. Furthermore, to our knowledge, there have been no reports in the literature of using this model to maintain brain tumor tissue from either rodents or humans.
The ability to establish and reliably predict the cytoarchitectural changes with time in an organotypic model of tumor and non-tumor human brain tissue can have several potential applications. Normal human explants, as with rodents, can be used to study cell migration (Jung et al. 2002; Valster et al. 2005), drug toxicity (Eyupoglu et al. 2006; Oest et al. 2006), and various disease models including epilepsy (Duveau et al. 2005; Hanson et al. 2006), stroke (Cavaliere et al. 2006; Cimarosti et al. 2006; Shirakawa et al. 2006), and Alzheimer's disease (Shahani et al. 2006; Stein et al. 2004). In addition, tumor explants can be used to study cytoarchitecture, tumor and non-tumor cell migration, and the efficacy of various pharmacologic interventions. This human-derived model can ultimately provide a more accurate clinical correlation to human conditions than a rodent based model.
2. Methods
2.1. Tissue Collection and Organotypic Culture Preparation
Permission to utilize human brain tissue was obtained from the Johns Hopkins Hospital Institutional Review Board (Approval #B0508080102). Both adult and pediatric tumor and non-tumor brain tissue samples were obtained from patients undergoing craniotomy for tumor, trauma, arteriovenous malformation, aneurysm, or epilepsy. For the purposes of this study, non-tumor cortical tissue was only utilized and examined from the frontal and anterior temporal lobes. All samples were part of the planned resections and tissue was only collected after appropriate diagnosis was made by frozen section and sufficient specimen was available for final permanent pathology as per an attending senior pathologist. After resection, the harvested tissue was immediately placed into ice cold Hank's balanced salt solution (Gibco, Gaithersburg, MD) supplemented with glucose (6.44 mg/ml) (HBSS). The tissue was then taken directly from the operating room, and, under sterile conditions in a laminar flow hood, cut with a scalpel into rectangular slices 5-20 millimeters in length and 1-2 millimeters in width. The slices were further sectioned into 350 μm-thick slices using a McIlwain tissue chopper (Lafayette Instruments, Lafayette, IN) and placed into a Petri dish containing HBSS. The brain slices were gently separated with sterile forceps and individually transferred with sterile, polyethylene transfer pipettes onto 30-mm Millicell inserts (Millipore, Billerica, MA) within 6-well Costar plates (Corning, Acton, MA).
Before placing the inserts into 6-well plates, 1 ml of medium containing 25% heat inactivated horse serum (Gibco), 25% HBSS supplemented with glucose (25.8 mg/ml), 49% Minimal essential medium (MEM) (Sigma, St. Louis, MO) supplemented with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (Sigma, St. Louis, MO) (12 mg/ml), and 1% 0.2M glutamine (Gibco) was placed into each well. The final pH of the medium was maintained between 7.2 and 7.4 with the addition of sodium hydroxide droplets (Sigma). The medium was stored at 4°C for up to one week and filtered prior to use. The medium from the wells was changed every other day for non-tumor tissue and every day for tumor tissue, making sure not to deposit medium onto the membrane with each exchange. The brain slices were incubated at 37°C and 5% CO2, and checked daily with an inverted light microscope for viability.
2.2. Immunohistochemistry Tissue Preparation for Laser Confocal Microscopy Analysis
The medium in the wells were removed, and the tissue on the Millicell inserts were submerged and fixed with phosphate-buffered 4% paraformaldehyde (PFA) at room temperature for 45 minutes. The sections were then washed with 0.1M phosphate buffered saline (PBS) (Gibco) to remove the fixative. Following the washes, the brain slices were blocked with 10% Normal Goat Serum (NGS) (Gibco) and 1% Triton X-100 (Gibco) in PBS for 2 hours. The explants were then incubated for 2 days at 4°C with primary antibody solution consisting of mouse anti-glial fibrillary acidic protein (GFAP) (1:500, Chemicon, Temecula, CA) or rabbit anti-GFAP (1:500, Sigma). GFAP is an astrocyte marker that identifies astrocyte intermediate filaments.
Following primary antibody staining, the organotypic cultures were thoroughly washed with PBS and incubated with Alexa secondary antibodies (Gibco) diluted in PBS (1:500) overnight at 4°C. The next day, the explants were washed and then stained with the nuclear marker 4′,6-Diamidino-2-phenylindole (DAPI) (Gibco) diluted in deionized distilled water (1:500) for 5 minutes. The explants were once again washed and then mounted onto subbed slides (Fisher, Auburn, AL) with the use of Prolong Gold (Gibco) and glass cover slips (Gibco). The slides were viewed and photographed with a Nikon C1si confocal microscope from the surface to the center of the explant. The slices were analyzed for their glial cytoarchitecture, cellular composition, and cell morphology. An intact cytoarchitecture is defined as an explant with a well-preserved (displaying clear astrocyte morphology) and uniform composition of glial cells from the surface to its core.
2.3. Tissue Preparation for Electron Microscopy Analysis
The medium in the wells was removed, and the tissue sections were submerged and fixed with 2% PFA/2.5% glutaraldehyde in PBS overnight at room temperature. The next day, the fixative was removed and the sections were thoroughly washed with 0.1M phosphate buffer (PB) solution (Sigma). The explants were stored in 0.05% sodium azide (Sigma) diluted in PB solution.
Prior to inclusion, the explants were sectioned in half in order to analyze each explant in both horizontal and vertical planes. This was done to thoroughly evaluate the entire explant, especially those surfaces farthest from the nutrient supply. For inclusion, the PB solution was removed and post-fixed with phosphate-buffered 2% osmium oxide (EMS, Hatfield, PA) for two hours in the dark. The tissue slices were then rinsed with distilled water and dehydrated by sequential washes with 25%, 50%, and then 70% ethanol (EMS). The sections were then incubated in 2% uranyl acetate (EMS) for 3 hours at 4°C and further dehydrated by sequential washes with 70%, 95%, 100% ethanol (EMS), and then propylene oxide (EMS). Following dehydration, the explants were embedded in araldite (Durcupan, Fluka, Buchs, Switzerland). Serial 1 μm semi-thin and 0.05 μm ultra-thin sections were cut with a diamond knife, stained with lead citrate, and examined under a FEI Tecnai Spirit electron microscope from the surface to the center of the explant. The sections were analyzed for glial cytoarchitecture, cellular composition, cellular integrity, and presence and/or absence of cytoplasmic organelles. Once again, an intact cytoarchitecture is defined as an explant with a well-preserved (displaying clear astrocyte morphology) and uniform composition of glial cells from the surface of to its core.
2.4. Samples Analyzed
Five normal cortical (3 adult, 2 pediatric) and five tumor (3 glioblastoma, 1 oligodendrogliomas, and 1 low grade glioma) specimens were used for analysis. For normal cortical tissue, we analyzed a total of 24 adult and 16 pediatric organotypic cultures. For tumors, we analyzed a total of 24 glioblastomas, 24 oligodendrogiomas, and 8 low-grade organotypic cultures.
2.5 Statistical Analysis
The Mann-Whitney U test was used to perform inter-group comparisons in order to determine if there was a significant difference in cellular composition and numbers among different culturing times for both non-tumor and tumor tissue (SigmaStat 3.0, Systat Software Inc.). Values of p <0.05 were considered significant.
3. Results
We report the establishment of tumor and non-tumor human organotypic cultures from tissues obtained intraoperatively from both adult and pediatric patients. This method involves taking tissue samples directly from the operating room and maintaining them on porous membranes at an interface between the culture medium below and the carbon dioxide-enriched environment above. In preliminary studies, the methods previously described for maintaining rodent cortical explants were used (Stoppini et al. 1991; Tanaka et al. 1994), and did not work as well for human brain tissue (Fig. 5). Using a modified method, we have been able to sustain these explants for a period of nearly two weeks without any significant ultrastructural changes, as evaluated by IHC and EM analyses.
Figure 5.
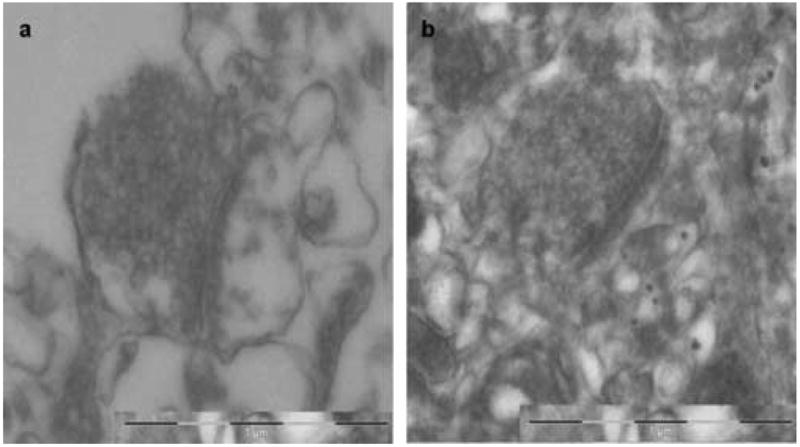
EM analysis of normal human cortex under the standard protocol used for mice (Stoppini et al. 1991; Tanaka et al. 1994). A, 7 days in culture. B, 14 days in culture. Under these conditions, the cytoarchitecture of the explants were not preserved even at 7 days. With continued culturing, less cell bodies were observed, membranes appeared more diffuse and irregular, and synaptic contacts and vesicles were less infrequently observed as compared with the revised protocol.
3.1. Immunohistochemistry (IHC)
The glial cytoarchitecture of these sections was uniform by confocal microscopy from the air-exposed surface to the surface abutting the membrane throughout for both tumor and non-tumor tissue at all time intervals, indicating that the media sufficiently penetrated the explants. A summary of the IHC analysis for both tumor and non-tumor tissue is presented in Table I.
Table I.
Summary of Tissue Preservation by Immunohistochemistry. The quality of different structures by +‘s: + = infrequent; +++ = occasional, +++++= frequent.
| Non-Tumor | Tumor | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Days | 0 | 3 | 7 | 11 | 14 | 0 | 4 | 6 | 10 | 14 |
| DAPI+ cells | +++ | +++ | +++ | +++ | + | +++ | ++++ | +++++ | +++++ | +++ |
| GFAP+ astrocytes | +++ | +++ | +++ | +++ | + | +++ | ++++ | +++++ | +++++ | ++ |
| Intermediate filaments | +++++ | +++++ | +++++ | +++ | + | ++++ | ++++ | +++++ | +++++ | ++ |
| No. of astrocytic processes | +++++ | ++++ | +++ | +++ | + | +++ | +++ | +++ | +++ | + |
| Length of astrocytic processes | +++ | +++++ | ++++ | +++++ | ++ | ++++ | +++++ | ++++ | +++++ | ++ |
| Cytoarchitecture | +++++ | +++++ | ++++ | ++++ | + | ++++ | ++++ | +++++ | +++++ | ++ |
3.1.1. Immunohistochemical Analysis of Normal Human Cortical Organotypic Explants
For normal cortical tissue, all analyzed specimens preserved their overall glial cytoarchitecture through 11 days of culturing (Fig. 1). This was characterized by a uniformly dense distribution of astrocytes with interdigitating astrocytic processes from the surface to the center of the explants (Fig. 1a-1d). However, by day 14, most samples possessed few astrocytes and no interdigitating processes (Fig. 2a).
Figure 1.
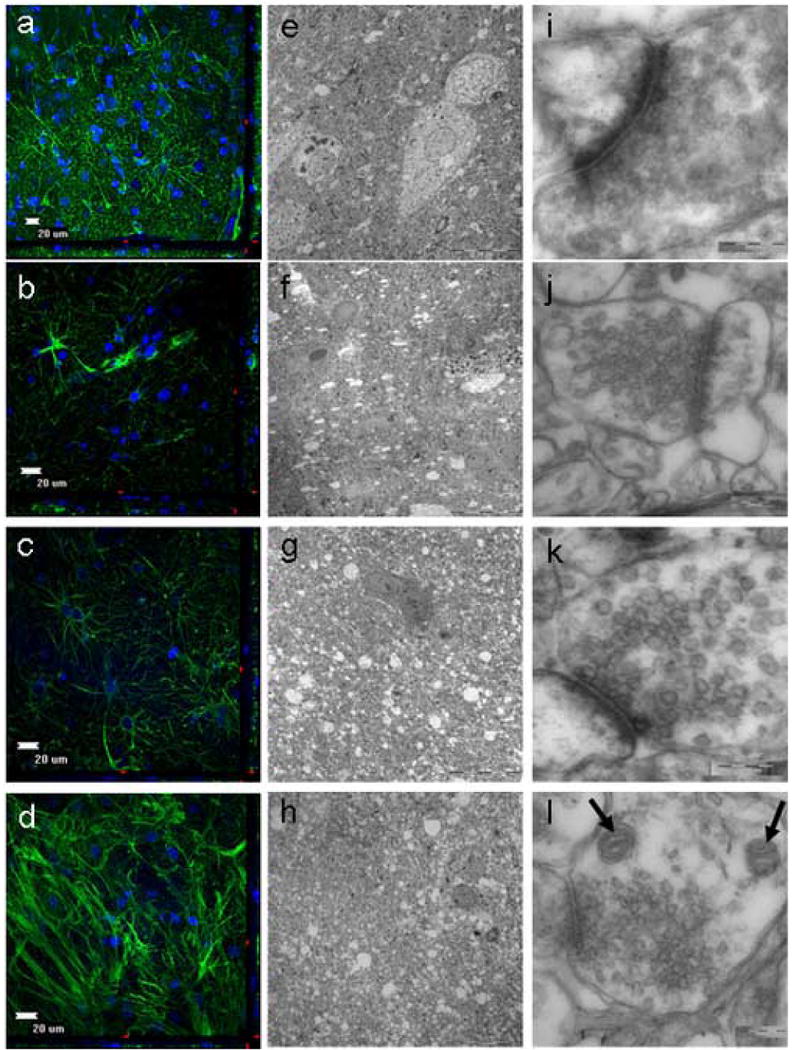
Evaluation of normal adult human cortex obtained from a 46 year old female using electron microscopy (EM) and immuohistochemistry (IHC). a,e,i, 0 days in culture. b,f,i, 3 days in culture. c,g,k, 7 days in culture. d,h,l, 11 days in culture. The explants retained their cytoarchitecture for at least 11 days while in culture as seen by both EM and IHC. With the use of IHC analysis, the tissue cytoarchitecture remained intact through 11 days of culturing. This was evident by the numerous astrocytes and intact astrocytic processes. (a-d; GFAP-green, DAPI-blue). With the use of EM analysis, glial bodies appeared to persist through 11 days of culturing, and large vacuoles were notably absent, signifying surviving cells (e-h). At higher magnification, the cell membranes are maintained without distortion and the synaptic contacts are highly preserved (i-l). Moreover, both the presynaptic and postsynaptic vesicles can be clearly seen, as well as mitochondria (l: arrows) without altered morphology (e-h).
Figure 2.
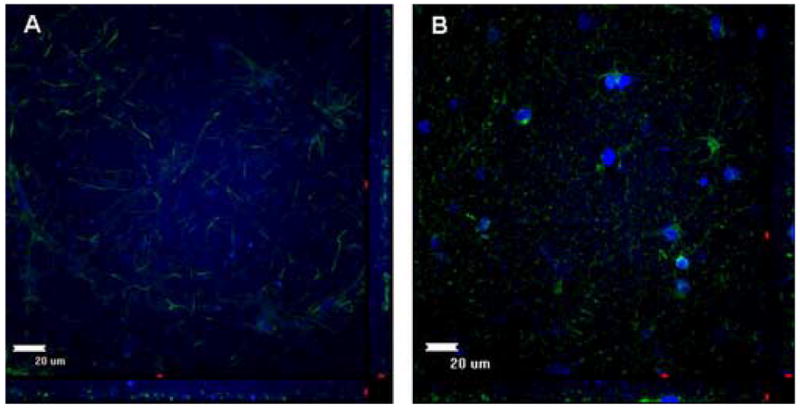
Non-tumor and Tumor Organotypic Explants at 14 days. A, Non-tumor cortical explant at 14 days displaying very little DAPI (nuclear marker) staining and sparse astrocytic processes. B, Glioblastoma tumor explant at 14 days displaying very few DAPI staining and infrequent astrocytic processes.
The cellular composition of the explants was analyzed by DAPI nuclear staining, which stains all cells, and GFAP staining, which stains the intermediate filaments of astrocytes. Total cell counts remained fairly constant from day 0 through day 11, during which there were approximately 25-35 DAPI-stained cells per high powered field (HPF). By day 14, however, this number had significantly declined to fewer than 5 cells per HPF (p<0.05, Mann-Whitney U test) (Supplementary Fig.1). In a similar trend, the number of GFAP-positive astrocytes remained stable through 11 days of culturing, with 5-7 astrocytes per HPF; by day 14, most samples contained only 0-2 astrocytes per HPF (Supplementary Fig.1).
The astrocyte morphology in each explant was also examined for changes that occurred with culturing time. The density of intermediate filaments, as evidenced by the intensity of GFAP+ staining (that is the number of processes emanating from the center of the cell), was robust and constant from day 0 to day 7 (Fig. 1a-1c). However, by day 11 this distribution was slightly decreased (Fig. 1d), and by day 14 significantly decreased (Fig. 2a). The average number of astrocytic processes also changed with culturing time. On day 0, each astrocyte possessed an average of 8 processes. This was reduced to 7 processes on day 3, 6 processes on days 7 and 11, and 2 processes by day 14 (Supplementary Fig.1). The average length of these astrocytic processes changed as well. On day 0, the average length was approximately 30 microns. Between days 3 and 11, the average length increased to between 40-50 microns. By day 14, the average length was approximately 15 microns (Supplementary Fig.1).
3.1.2. Immunohistochemical Analysis of Human Brain Tumor Organotypic Explants
For the tumor explants, the cytoarchitecture remained intact through at least 10 days of culturing for all samples (Fig. 3). Unlike the normal cortical explants, tumor-derived tissues were characterized by closely packed, disorganized clusters of cells (Fig. 3a-3d). These were comprised primarily of astrocytes with densely tangled processes (Fig. 3a-3c). This was seen from the center of the explants to the core. As seen with the normal cortical explants, however, the density of cells and processes was substantially decreased by day 14 (Fig. 2b).
Figure 3.
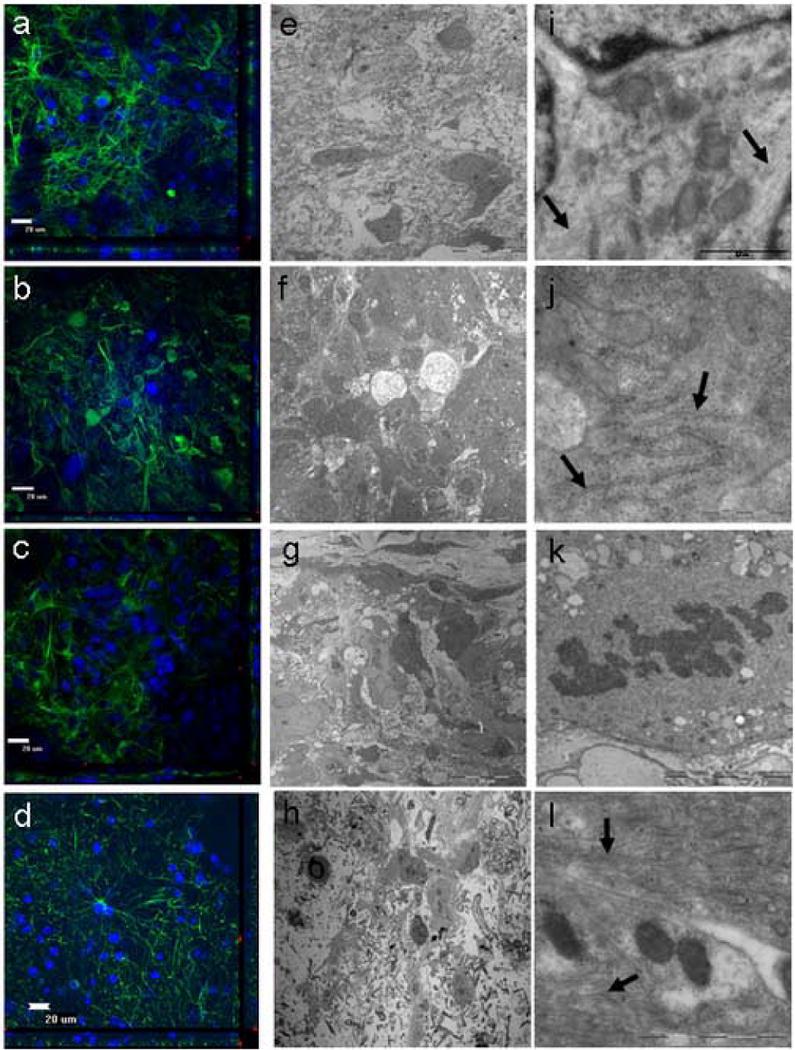
Evaluation of human glioblastoma multiforme obtained from a 55 year old male using electron microscopy (EM) and immuohistochemistry (IHC). a,e,i, 0 days in culture. b,f,j, 4 days in culture. c,g,k, 6 days in culture. d,h,l, 10 days in culture. Unlike the normal cortex, the tumor cytoarchitecture appeared less organized and thus more characteristic of tumors (a-d; GFAP-green, DAPI-blue). The tumor explants retained their cytoarchitecture for at least 10 days while in culture as seen by both EM and IHC. With the use of EM analysis, the organization of the tumor cells in culture did not show any significant differences compared to tumor cells observed in vivo (e-h). At higher magnification, the characteristic organelles within the tumor astrocytes were preserved. This included intermediate filaments (i, l: arrows) and rough endoplasmic reticulum (j: arrows). Mitotic bodies were also seen in the explants, supporting the notion that the tumor explants were still proliferating and were remarkably preserved (k) (e-h). With the use of IHC analysis, the tissue cytoarchitecture from the tumor explants remained intact through 10 days of culturing.
The cellular composition of the explants was also analyzed with DAPI nuclear and GFAP astrocyte staining. The average number of DAPI-stained cells in the tumor explants, as compared to the normal cortical explants, was increased (p<0.05, Mann-Whitney U test). The average number of cells per HPF was 30 on day 0, 45 on day 4, 50 on day 6, and 70 on day 10. By day 14, there were a significant decrease with only 25 DAPI-stained cells per HPF (p<0.05, Mann-Whitney U test) (Supplementary Fig.2). The average number of GFAP-positive astrocytes was likewise increased compared to the cortical explants (p<0.05). There were approximately 7 astrocytes per HPF on day 0, 10 on day 4, 15 on day 6, and 11 on day 10. By day 14, this number significantly decreased to 4 astrocytes per HPF (p<0.05) (Supplementary Fig.2).
Astrocyte morphology was also examined. As with the normal cortical explants, the density of intermediate filaments, as evidenced by the density of GFAP+ staining (that is the number of processes emanating from the center of the cell), was robust (Fig. 3a-3d). The abundance of intermediate filaments was greatest at days 6 and 10 (Fig. 3c and 3d), but dramatically decreased by day 14 (Fig. 2b). The number of astrocytic processes stayed constant from day 0 to day 10, averaging 5-6 processes per astrocyte. By day 14, this number significantly decreased to 3 processes per astrocyte (p<0.05) (Supplementary Fig.2). The length of these processes remained relatively constant between 30-40 microns from day 0 to day 6; however, by day 10, the processes were 60 microns in length. This decreased to 20 microns by day 14 (Supplementary Fig.2).
3.2. Electron Microscopy
As for the IHC analysis, the same five normal cortical and five tumor specimens were used for analysis. The tissue sections were analyzed from the surface of the explant to the center, in both the horizontal and vertical planes, using one micron-thick semi-thin sections. The cytoarchitecture of these sections was uniform in all planes, at all times studied, for both tumor and non-tumor tissue, signifying that the media sufficiently permeated the explants. Analysis was not done at 14 days for both cortical and tumor explants because the majority of the explants deteriorated between day 11 and 14 (Fig. 2). The IHC revealed little intact architecture, rendering EM analysis unnecessary. A summary of the EM analysis for both tumor and non-tumor tissue is presented in Table II.
Table II.
Summary of Tissue Preservation by Electron Microscopy. The quality of different structures by +‘s: + = infrequent; +++ = occasional, +++++= frequent. n.d. = no data.
| Non-Tumor | Tumor | |||||||
|---|---|---|---|---|---|---|---|---|
| Days | 0 | 3 | 7 | 11 | 0 | 4 | 6 | 10 |
| Mitochondria | +++++ | ++++ | +++ | +++ | +++++ | +++/++++ | +++/++ | +++/++ |
| Myelin | +++++ | ++++ | +++ | +++ | ++++ | +++ | +++ | +++ |
| Non-myelinated Axons or Cellular expansions | ++++ | ++++ | +++ | +++ | +++ | +++ | + | +++ |
| Synaptic Contacts | ++++ | +++++ | +++ | +++ | n.d. | n.d. | n.d. | n.d. |
| Synaptic Vesicles | +++ | +++++ | ++++/+++ | ++++/+++ | n.d. | n.d. | n.d. | n.d. |
| Microtubules | ++++ | ++++ | +++ | +++ | Not studied | |||
| Intermediate Filaments | ++++ | ++++ | ++ | + | +++++ | +++++ | ++++ | ++++ |
| Basal Lamina | +++++ | +++ | +++ | +++ | +++++ | n.d. | ++++ | n.d. |
3.2.1. Electron Microscopy Analysis of Normal Human Cortical Organotypic Explants
For normal cortical tissue, the tissue architecture was uniform and intact for all studied times (0-11 days). The astrocytes and their processes, as well as the neuropil (dendrites, axons, and synaptic buttons), were preserved through 11 days (Fig. 1e-1h). In addition, cytoplasmic organelles associated with these structures were also well preserved. These included mitochondria, rough endoplasmic reticulum, dictosomes, microtubules, synapses, and synaptic vesicles. However, the soma of astrocytes started deteriorating along with the perisomal intermediate filaments so that by day 10 they were hard to identify. Although, the quality of the astrocytic filaments had decreased on ultrastructural analysis from day 7, their intermediate filament proteins (GFAP) were still present and easy to detect by IHC analysis.
As with the astrocytes, the neuropil, endothelial cells, basal lamina, myelinating and non-myelinating axons were also well preserved without substantial changes through 11 days of culturing. Remarkably, the morphology of the synaptic contacts was maintained at all culture times and both synaptic vesicles and mitochondria were clearly identified (Fig. 1i-1l). As expected, the neurons degraded with increased culturing time (Fig. 4). Neuronal degradation was most important between day 0 and day 3 (Fig. 1e, 1f, 4) and several empty spaces were observed in the brain sections (Fig. 1).
Figure 4.
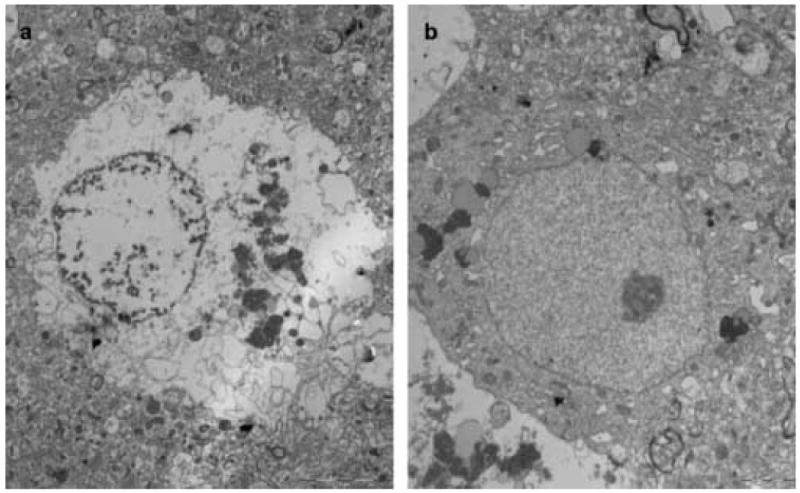
EM analysis of neurons at 3 days. In contrast with glial cells that were well conserved with prolonged culturing, the neurons degenerate rapidly (A). Intact neurons were infrequently observed (B).
3.2.2. Electron Microscopy Analysis of Human Brain Tumor Organotypic Explants
For tumor tissue, the tissue architecture was uniform and intact for all studied times (0-10 days). The tumor cells in the explants remained intact through 10 days, indicating their ex vivo viability (Fig. 3e-3h). In fact, mitoses were commonly observed, indicating that, under these cell culture conditions, cells in the explants continue proliferating (Fig. 3k). In addition, some of the glioblastoma explants displayed abundant collagen formation presumably in culture, which has been hypothesized to facilitate tumor cell invasion and infiltration (Han and Daniel 1995; Han et al. 1995). The tumor cells in these explants were heterogeneous and displayed abundant cytoplasmic organelles. Interestingly, unlike with the normal cortical astrocytes, the astrocytic intermediate filaments were well-preserved through 10 days of culturing. Synaptic contacts and/or vesicles were not detected, as expected, because the majority of the cells in the tumor were glia. It was not possible to analyze microtubules because the tumor explants we examined did not contain neurons.
4. Discussion
4.1. Cytoarchitecture Preservation of Normal Cortical Explants and Tumor Explants
The cytoarchitecture of the normal cortical explants was visibly intact through 11 days of culturing. The astrocytes, neuropil, endothelial cells, basal lamina, myelinating and non-myelinating axons were well preserved without substantial changes. In addition, the organelles associated with these cells including mitochondria, rough endoplasmic reticulum, dictosomes, microtubules, synapses, and synaptic vesicles were intact. The main changes were the expected loss of neurons by day three and the degradation of the astrocytic soma and perisomal intermediate filaments through day 11. In the majority of explants studied, by day 14, few DAPI-stained cells and GFAP-positive astrocytes remained.
The brain tumor explants can be maintained for at least 10 days without any significant changes to the cytoarchitecture. As with the normal cortical tissue, the tumor-derived tissue had intact cell populations and their respective cellular organelles. In fact, the explants were proliferating, where the number of DAPI-stained cells and GFAP-positive astrocytes increased with time, and mitotic bodies were readily detected. However, by day 14, many of these explants, as with the cortical tissue, deteriorated in terms of their cytoarchitecture and cellular composition.
4.2. Establishment of an Ex Vivo Human Model to Study Human Disease
The interface method for organotypic cultures, where tissue explants are placed on a membrane at the interface between air and medium, was initially described in rodent hippocampal slices by Stoppini et al (Stoppini et al. 1991). Since this initial study, there have been several more that have used these methods to evaluate rodent-based brain tissue models (Cavaliere et al. 2006; Holopainen 2005; Holtkamp et al. 2005; Tanaka et al. 1994; Valster et al. 2005). Human-based brain tissue models have been primarily limited to fetal tissue (Jakovcevski and Zecevic 2005; Lyman et al. 1992; Tenenbaum et al. 2004) and cadaveric tissue (Bsibsi et al. 2006; Verwer et al. 2002). Brain slices obtained from living human patients have been described infrequently, and the preservation of the cytoarchitecture in these explants is not known (Jung et al. 1999; Jung et al. 2002). Additionally, no studies to our knowledge have used this technique and/or model to maintain brain tumor tissue.
4.3. Applications of Human Ex Vivo Models and Technique Modifications
The ability to establish and reliably predict the cytoarchitectural changes that occur with time in an organotypic model of tumor and non-tumor human brain tissue has several potential applications. One of these is that this model offers a more realistic in vitro model to study the human brain. This model is the closest representation of in vivo conditions since explants are obtained directly from living human patients (Gahwiler et al. 1997; Tanaka et al. 1994). Therefore, the explants can be used as a substrate to more accurately study cell migration (Jung et al. 2002; Valster et al. 2005), drug toxicity (Eyupoglu et al. 2006; Oest et al. 2006), and other functions as they occur primarily in humans. In addition, the tumor explants provide new avenues to study human brain cancer. These include tumor cell migration, pharmacological therapies, and tumor cellular composition.
The organotypic techniques that have been previously described in rodent models (Stoppini et al. 1991; Tanaka et al. 1994) do not seem to work as well for human brain tissue (Fig. 5). This technique for maintaining human brain explants has undergone several modifications from previous organotypic interface methods. The first is to limit the thickness of the tissue slices to 350 microns. Tissue slices in past studies were greater than 500 microns (Jung et al. 1999; Jung et al. 2002; Plenz and Kitai 1996; Stoppini et al. 1991; Tanaka et al. 1994). We found that tissue slices greater than 350 microns did not survive as long, most likely because the nutrients supplied by the culture media did not effectively permeate the tissue. Another modification was to increase the concentration of glucose in the culture media. The glucose concentration in past studies was less than 10.0 mg/ml (Plenz and Kitai 1996; Stoppini et al. 1991; Tanaka et al. 1994). We used a glucose concentration of 25.8 mg/ml to provide the brain slices with adequate nutrients, which is especially crucial for rapidly metabolizing tumor tissue. Furthermore, we increased the frequency of culture media changes from past studies (Plenz and Kitai 1996; Stoppini et al. 1991; Tanaka et al. 1994). Tumor tissue survived best when the media was changed daily to ensure adequate nutrient supply and waste removal, presumably due to the rapid metabolism associated with tumor tissue (Utriainen et al. 2003). Non-tumor tissue, which presumably has lower metabolic demands, required less frequent changes. The explants are minimally disturbed by the media changes because they are not in direct contact with the media. We also used a limited number of media components, and did not find it necessary to include progesterone (Jung et al. 2002; Tanaka et al. 1994; Tenenbaum et al. 2004), medications including antibiotics and antimycotics (Cavaliere et al. 2006; Jakovcevski and Zecevic 2005; Tanaka et al. 1994), transferrin (Jung et al. 2002; Tenenbaum et al. 2004), sodium selenite (Tanaka et al. 1994; Tenenbaum et al. 2004), or sodium pyruvate (Tanaka et al. 1994) to maintain tissue cytoarchitecture. Additionally, we used MEM because it is often used to maintain astrocyte cell cultures, and may thus promote preservation of astrocytes in our organotypic explants (Kornyei et al. 2000).
4.4. Advantages and Limitations of Our Ex Vivo Model
This technique has several advantages. One of these advantages is that human tumor and non-tumor brain tissue can be maintained for a prolonged period of time. This amount of time is necessary for conducting long-term experiments such as pharmacological interventions (Eyupoglu et al. 2006; Oest et al. 2006; Sundstrom et al. 2005), cytoarchitecture evaluation (Michelini et al. 2005), migration assays (Jung et al. 2002; Valster et al. 2005), and disease model evaluation (Cimarosti et al. 2006; Duveau et al. 2005; Shahani et al. 2006; Stein et al. 2004). Another benefit is that this model utilizes otherwise discarded tissue to create an experimental model that closely represents what actually occurs in humans, in vivo. Finally, this technique is relatively simple, and requires minimal media components. Limiting extraneous media components decreases the chance of confounding variables, and strengthens experimental analysis.
However, this technique also has some limitations. One limitation is that both the tumor and non-tumor explants start to deteriorate around 14 days. This model is therefore ideal for week-long studies, which correspond to the time that the glial architecture is best preserved. This is not as long as prior studies using postmortem brain tissue, but it should be realized that the tissue in our study is obtained intra-operatively and thus subjected to surgical manipulation (Verwer et al. 2002). Also, because these tissues are obtained intra-operatively from human patients, it makes comparative analysis difficult because these tissues are obtained from various cortical regions and from different patients with different underlying pathologies. We tried to control for the heterogeneous origin by only studying frontal and anterior temporal cortical tissue in the non-tumor studies. It also remains to be studied whether the neurons from normal cortical explants can be maintained for longer periods of time. Prior studies using rodent explants used serum-free media and media supplemented with neurotrophic factors to increase the viability of neurons, which may also be applicable in human cortical explants (Benninger et al. 2003; Gates et al. 1996; Kearns et al. 2006).
Another limitation is that it does not perfectly recapitulate an in vivo model. This model removes the brain tissue from its natural environment, to one devoid of an innate immune system, vascular supply, and innervation. Nevertheless, this is still the closest representation to an in vivo human model (Gahwiler et al. 1997; Tanaka et al. 1994). Another consideration is the supply of human tissue. While the strength of this model is its use of human rather than rodent-derived tissue, it is also limited by access to these specimens. It is therefore crucial to establish collaborations with a neurological surgeon to appropriately take advantage of this tissue that otherwise would be discarded (Quinones-Hinojosa et al. 2007; Quinones-Hinojosa et al. 2006; Sanai et al. 2004). Another potential disadvantage is that the tissue undergoes morphological changes with time. The explants initially have a thickness of 350 microns, but over a three week period decrease to approximately 200 microns (Stoppini et al. 1991). This is expected since the tissue tends to spread along the membrane with time. However, based on our analysis, the tissue cytoarchitecture remains relatively well preserved from the air-exposed surface to the surface abutting the membrane.
5. Conclusion
The present study demonstrates the feasibility and effectiveness of an interface method to maintain human tumor and non-tumor explants for a prolonged period of time. Prior to this study, this model was predominantly limited to rodent-based models. This study extends the applicability of this model to human tissue. Based on thorough IHC and EM analyses, these explants maintain their cytoarchitecture for a period of almost two weeks. This model therefore has important applications to the fields of neuroscience, neuro-oncology, and pharmacotherapy, among others.
Supplementary Material
Supplementary Fig.1. Cortical explants from non-cancer cortical tissue. a, the number of DAPI+ cells per high powered field (HPF); b, the number of GFAP+ cells per HPF; c, the number of processes per astrocyte; and d, the length of the astrocyte processes were all significantly decreased at day 14 compared to all previous days, *p<0.05 (Mann-Whitney U test). Values are the mean±SEM of 5 cortical samples per group.
Supplementary Fig. 2. Tumor explants from patients with brain tumors. a, the number of DAPI+ cells per high powered field (HPF); b, the number of GFAP+ cells per HPF; c, the number of processes per astrocyte; and d, the length of the astrocyte processes were all significantly decreased at day 14 compared to all previous days [*p<0.05 (Mann-Whitney U test)] except for the length of the astrocytic processes at day 0 vs. day 14 [†p=0.20 (Mann-Whitney U test)]. This exception may possibly be due to the trauma incurred during surgical resection procedures, and requires a few days for the astrocytes to recover as has been noted previously. (Verwer 2002). Values are the mean±SEM of 5 tumor samples per group.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benninger F, Beck H, Wernig M, Tucker KL, Brustle O, Scheffler B. Functional integration of embryonic stem cell-derived neurons in hippocampal slice cultures. J Neurosci. 2003;23(18):7075–7083. doi: 10.1523/JNEUROSCI.23-18-07075.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53(7):688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- Cavaliere F, Dinkel K, Reymann K. The subventricular zone releases factors which can be protective in oxygen/glucose deprivation-induced cortical damage: An organotypic study. Exp Neurol. 2006 doi: 10.1016/j.expneurol.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Cimarosti H, O'Shea RD, Jones NM, Horn AP, Simao F, Zamin LL, Nassif M, Frozza R, Netto CA, Beart PM, Salbego C. The effects of estradiol on estrogen receptor and glutamate transporter expression in organotypic hippocampal cultures exposed to oxygen--glucose deprivation. Neurochem Res. 2006;31(4):483–490. doi: 10.1007/s11064-006-9043-9. [DOI] [PubMed] [Google Scholar]
- Duveau V, Arthaud S, Serre H, Rougier A, Le Gal La Salle G. Transient hyperthermia protects against subsequent seizures and epilepsy-induced cell damage in the rat. Neurobiol Dis. 2005;19(12):142–149. doi: 10.1016/j.nbd.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Eyupoglu IY, Hahnen E, Trankle C, Savaskan NE, Siebzehnrubl FA, Buslei R, Lemke D, Wick W, Fahlbusch R, Blumcke I. Experimental therapy of malignant gliomas using the inhibitor of histone deacetylase MS-275. Mol Cancer Ther. 2006;5(5):1248–1255. doi: 10.1158/1535-7163.MCT-05-0533. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20(10):471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Gates MA, Fillmore H, Steindler DA. Chondroitin sulfate proteoglycan and tenascin in the wounded adult mouse neostriatum in vitro: dopamine neuron attachment and process outgrowth. J Neurosci. 1996;16(24):8005–8018. doi: 10.1523/JNEUROSCI.16-24-08005.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Daniel JC. Biosynthesis of type VI collagen by glioblastoma cells and possible function in cell invasion of three-dimensional matrices. Connect Tissue Res. 1995;31(2):161–170. doi: 10.3109/03008209509028404. [DOI] [PubMed] [Google Scholar]
- Han J, Daniel JC, Pappas GD. Expression of type VI collagen during glioblastoma cell invasion in brain tissue cultures. Cancer Lett. 1995;88(2):127–132. doi: 10.1016/0304-3835(94)03627-u. [DOI] [PubMed] [Google Scholar]
- Hanson JE, Emond MR, Madison DV. Blocking polysynaptic inhibition via opioid receptor activation isolates excitatory synaptic currents without triggering epileptiform activity in organotypic hippocampal slices. J Neurosci Methods. 2006;150(1):8–15. doi: 10.1016/j.jneumeth.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Holopainen IE. Organotypic hippocampal slice cultures: a model system to study basic cellular and molecular mechanisms of neuronal cell death, neuroprotection, and synaptic plasticity. Neurochem Res. 2005;30(12):1521–1528. doi: 10.1007/s11064-005-8829-5. [DOI] [PubMed] [Google Scholar]
- Holtkamp N, Afanasieva A, Elstner A, van Landeghem FK, Konneker M, Kuhn SA, Kettenmann H, von Deimling A. Brain slice invasion model reveals genes differentially regulated in glioma invasion. Biochem Biophys Res Commun. 2005;336(4):1227–1233. doi: 10.1016/j.bbrc.2005.08.253. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Zecevic N. Sequence of oligodendrocyte development in the human fetal telencephalon. Glia. 2005;49(4):480–491. doi: 10.1002/glia.20134. [DOI] [PubMed] [Google Scholar]
- Jung S, Hinek A, Tsugu A, Hubbard SL, Ackerley C, Becker LE, Rutka JT. Astrocytoma cell interaction with elastin substrates: implications for astrocytoma invasive potential. Glia. 1999;25(2):179–189. doi: 10.1002/(sici)1098-1136(19990115)25:2<179::aid-glia8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Jung S, Kim HW, Lee JH, Kang SS, Rhu HH, Jeong YI, Yang SY, Chung HY, Bae CS, Choi C, Shin BA, Kim KK, Ahn KY. Brain tumor invasion model system using organotypic brain-slice culture as an alternative to in vivo model. J Cancer Res Clin Oncol. 2002;128(9):469–476. doi: 10.1007/s00432-002-0366-x. [DOI] [PubMed] [Google Scholar]
- Kearns SM, Scheffler B, Goetz AK, Lin DD, Baker HD, Roper SN, Mandel RJ, Steindler DA. A method for a more complete in vitro Parkinson's model: slice culture bioassay for modeling maintenance and repair of the nigrostriatal circuit. J Neurosci Methods. 2006;157(1):1–9. doi: 10.1016/j.jneumeth.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Kornyei Z, Czirok A, Vicsek T, Madarasz E. Proliferative and migratory responses of astrocytes to in vitro injury. J Neurosci Res. 2000;61(4):421–429. doi: 10.1002/1097-4547(20000815)61:4<421::AID-JNR8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Lyman WD, Hatch WC, Pousada E, Stephney G, Rashbaum WK, Weidenheim KM. Human fetal myelinated organotypic cultures. Brain Res. 1992;599(1):34–44. doi: 10.1016/0006-8993(92)90849-5. [DOI] [PubMed] [Google Scholar]
- Michelini M, Rosellini A, Mandys V, Simoncini T, Revoltella RP. Cytoarchitecture modifications of the human uterine endocervical mucosa in long-term three-dimensional organotypic culture. Pathol Res Pract. 2005;201(10):679–689. doi: 10.1016/j.prp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Oest TM, Dehghani F, Korf HW, Hailer NP. The immunosuppressant mycophenolate mofetil improves preservation of the perforant path in organotypic hippocampal slice cultures: a retrograde tracing study. Hippocampus. 2006;16(5):437–442. doi: 10.1002/hipo.20182. [DOI] [PubMed] [Google Scholar]
- Plenz D, Kitai ST. Organotypic cortex-striatum-mesencephalon cultures: the nigrostriatal pathway. Neurosci Lett. 1996;209(3):177–180. doi: 10.1016/0304-3940(96)12644-6. [DOI] [PubMed] [Google Scholar]
- Quinones-Hinojosa A, Sanai N, Gonzalez-Perez O, Garcia-Verdugo JM. The human brain subventricular zone: stem cells in this niche and its organization. Neurosurg Clin N Am. 2007;18(1):15–20. doi: 10.1016/j.nec.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494(3):415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427(6976):740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Shahani N, Subramaniam S, Wolf T, Tackenberg C, Brandt R. Tau aggregation and progressive neuronal degeneration in the absence of changes in spine density and morphology after targeted expression of Alzheimer's disease-relevant tau constructs in organotypic hippocampal slices. J Neurosci. 2006;26(22):6103–6114. doi: 10.1523/JNEUROSCI.4245-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa H, Katsuki H, Kume T, Kaneko S, Akaike A. Aminoglutethimide prevents excitotoxic and ischemic injuries in cortical neurons. Br J Pharmacol. 2006;147(7):729–736. doi: 10.1038/sj.bjp.0706636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein TD, Anders NJ, DeCarli C, Chan SL, Mattson MP, Johnson JA. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPSW mice resulting in tau phosphorylation and loss of hippocampal neurons: support for the amyloid hypothesis. J Neurosci. 2004;24(35):7707–7717. doi: 10.1523/JNEUROSCI.2211-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37(2):173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Sundstrom L, Morrison B, 3rd, Bradley M, Pringle A. Organotypic cultures as tools for functional screening in the CNS. Drug Discov Today. 2005;10(14):993–1000. doi: 10.1016/S1359-6446(05)03502-6. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Tomita A, Yoshida S, Yano M, Shimizu H. Observation of the highly organized development of granule cells in rat cerebellar organotypic cultures. Brain Res. 1994;641(2):319–327. doi: 10.1016/0006-8993(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Tenenbaum L, Peschanski M, Melas C, Rodesh F, Lehtonen E, Stathopoulos A, Velu T, Brotchi J, Levivier M. Efficient early and sustained transduction of human fetal mesencephalon using adeno-associated virus type 2 vectors. Cell Transplant. 2004;13(5):565–571. doi: 10.3727/000000004783983684. [DOI] [PubMed] [Google Scholar]
- Utriainen M, Komu M, Vuorinen V, Lehikoinen P, Sonninen P, Kurki T, Utriainen T, Roivainen A, Kalimo H, Minn H. Evaluation of brain tumor metabolism with [11C]choline PET and 1H-MRS. J Neurooncol. 2003;62(3):329–338. doi: 10.1023/a:1023342516925. [DOI] [PubMed] [Google Scholar]
- Valster A, Tran NL, Nakada M, Berens ME, Chan AY, Symons M. Cell migration and invasion assays. Methods. 2005;37(2):208–215. doi: 10.1016/j.ymeth.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Verwer RW, Hermens WT, Dijkhuizen P, Ter Brake O, Baker RE, Salehi A, Sluiter AA, Kok MJ, Muller LJ, Verhaagen J, Swaab DF. Cells in human postmortem brain tissue slices remain alive for several weeks in culture. Faseb J. 2002;16(1):54–60. doi: 10.1096/fj.01-0504com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig.1. Cortical explants from non-cancer cortical tissue. a, the number of DAPI+ cells per high powered field (HPF); b, the number of GFAP+ cells per HPF; c, the number of processes per astrocyte; and d, the length of the astrocyte processes were all significantly decreased at day 14 compared to all previous days, *p<0.05 (Mann-Whitney U test). Values are the mean±SEM of 5 cortical samples per group.
Supplementary Fig. 2. Tumor explants from patients with brain tumors. a, the number of DAPI+ cells per high powered field (HPF); b, the number of GFAP+ cells per HPF; c, the number of processes per astrocyte; and d, the length of the astrocyte processes were all significantly decreased at day 14 compared to all previous days [*p<0.05 (Mann-Whitney U test)] except for the length of the astrocytic processes at day 0 vs. day 14 [†p=0.20 (Mann-Whitney U test)]. This exception may possibly be due to the trauma incurred during surgical resection procedures, and requires a few days for the astrocytes to recover as has been noted previously. (Verwer 2002). Values are the mean±SEM of 5 tumor samples per group.


