Abstract
Beta-catenin can be cleaved by caspase-3 or degraded by activated glycogen synthase kinase-3β via phosphorylating β-catenin. We tested the hypothesis that β-catenin undergoes degradation after stroke, and its degradation is dependent on caspase activity. Stroke was generated by permanent middle cerebral artery occlusion and 1h of transient bilateral common carotid artery occlusion in rats. Active caspase-3 was expressed in the ischemic cortex from 5 to 48 h after stroke, whereas β-catenin markedly degraded at 24 and 48 h after stroke. The caspase 3-specific inhibitor, Z-DQMD-FMK, attenuated β-catenin degradation, but it did not affect phosphorylation of both β-catenin and glycogen synthase kinase-3β. In conclusion, β-catenin degraded after stroke, and its degradation was caspase-3 dependent.
Keywords: β-catenin, caspase-3, focal ischemia, glycogen synthase kinase-3β, stroke
Introduction
Beta-catenin as a transcription factor plays a critical role in cell survival [1]. It is phosphorylated by activated glycogen synthase kinase (GSK)-3β. When GSK-3β is dephosphorylated, β-catenin then degrades in the proteosome [1]. Conversely, β-catenin is stabilized by GSK-3β phosphorylation and translocates into the nuclei regulating gene expression, therefore supporting cell survival [2]. β-catenin has other functions in neurons, such as forming synapses by associating with multiple cell adhesion proteins, including α-catenin, p-catenin, and cadherin [3]. Therefore, stabilizing β-catenin is essential to maintain neuronal functions.
Both signal pathways of the GSK-3β/β-catenin [4] and caspase-3 [5] are known to mediate ischemic injury after stroke. GSK-3β is dephosphorylated, thus activated after stroke, and β-catenin translocates into the nuclei in the ischemic penumbra after stroke [6]. In addition, inhibiting GSK-3β activity reduces ischemic injury [4]. In contrast, caspase-3 activity increases after stroke contributing to neuronal apoptosis [5]. We and others have shown that inhibiting caspase-3 activity reduces ischemic injury [5]. Beta-catenin has been reported to be cleaved by activities of caspases in non-neuronal cells [7]. Nevertheless, whether β-catenin is degraded after stroke and whether its degradation is regulated by caspase-3 activity have not been studied.
In this study, we tested the hypothesis that β-catenin undergoes degradation after stroke, and studied whether its degradation is dependent on caspase-3 activity in a focal ischemia model in rats.
Methods and materials
Experimental protocols were approved by the Stanford University Administrative Panel on Laboratory Animal Care.
Focal cerebral ischemia
Focal ischemia was generated as described previously [8]. Anesthesia was induced by 5% isoflurane and maintained with 2–3% isoflurane during surgery in male Sprague—Dawley rats (350–390 g). Core body temperatures were monitored with a rectal probe and were maintained at 37°C throughout the experiment. The distal middle cerebral artery was exposed and cauterized permanently above the rhinal fissure. The bilateral common carotid arteries (CCA) were occluded for 1 h and then released.
Drug delivery
A cell-permeable caspase-3-specific inhibitor, Z-DQMD-FMK, was dissolved in dimethylsulfoxide and PBS as described [9]. Briefly, the drug solution (1.5 μg) or the vehicle was injected into the ventricular space (from bregma: anteroposterior=−0.92 mm, mediolateral=1.5 mm, dorsoventral=3.5 mm) ipsilateral to the ischemia at 2 h after CCA release. At 24 h after CCA release, the brains were removed and whole cell extracts were prepared for western blots.
Immunofluorescence staining and confocal microscopy
Rats were perfused transcardially with normal saline followed by fixation with 4% paraformaldehyde for 24 h. Brains were cut on a vibratome into slices of 50 μm, and stored in anti-freeze solution at −20°C. Free-floating immunostaining was performed as described [8]. The primary antibody of active caspase-3 (1;200, Cat #557035, BD Pharmingen, San Diego, California, USA) and the secondary antibody of Alexra Fluor donkey anti-rabbit IgG (1 : 500, Cat #A21026, Invitrogen, Carlsbad, California, USA) were used. Sections were double stained with DAPI (Vector Laboratories, Inc., Burlingame, California, USA), coverslipped and examined under a LSM510 confocal laser scanning microscope (Carl Zeiss, Thornwood, New York, USA). Negative controls, in which the primary antibodies were omitted, were run in parallel.
Western blots
To determine protein levels of phosphorylated and total protein of β-catenin, at 5, 24, and 48 h after stroke onset, brain tissues corresponding with the ischemic penumbra were dissected for western blot (Fig. 1). Whole cell protein was extracted from the fresh brain tissue, and western blot was performed as described with modification [8]. In each lane 20 μg of protein was subjected to sodium dodecyl sulfate—polyacrylamide gel electrophoresis using 4–15% Ready Gel (BIO-RAD Laboratories, Cat #L050505A2, Hercules, California, USA) for 1.5 h. Protein bands were transferred from the gel to polyvinylidinene fluoride (Millipore, Bedford, Massachusetts, USA) membranes for 1 h.
Fig. 1.

A diagram indicating the penumbral and core areas of the ischemic cortex, and the corresponding areas from sham animals that were dissected for western blot.Regions I and II represent the ischemic penumbra and core, respectively.
To determine phosphorylated GSK-3β or total protein levels of GSK-3β, and phosphorylated β-catenin, primary antibodies of rabbit antiphosphorylated GSK-3β (Ser9) (1 : 1000, Cat #9336, Cell signaling, MA), rabbit anti-GSK3β (1 : 1000, Cat #9332, Cell signaling, Massachusetts, USA), and rabbit antiphosphorylated β-catenin (1 : 1000, Cat #9561, Cell signaling) were incubated overnight and followed by a horseradish peroxidase-conjugated secondary anti-rabbit antibody (1 : 1000, Cell Signaling Technology, Massachusetts, USA) for 1 h, incubated with ECL plus solution (GE Healthcare, Sunnyvale, California, USA) for 5 min. The membranes were scanned using Typhoon trio (GE Health, Sunnyvale, California, USA).
To detect β-catenin and β-actin, the membranes were incubated overnight at 4°C with gentle agitation in a mixture of primary antibodies consisting of anti-β-catenin and β-actin (1 : 20000, rabbit, Bethyl, cat. A300–491A, Texas, USA) then incubated with secondary antibodies of Alexa Fluor647 donkey anti-mouse (1 : 1500) for detecting β-catenin and Alexa Fluor488 donkey anti-rabbit (cat.A21206, Invitrogen, Eugene, Oregon, USA) at 1 : 5000 for detecting β-actin, for 1 h at room temperature and shielded from light. Protein bands of β-catenin and β-actin were scanned simultaneously by the Typhoon trio.
Statistics
For western blot, t-test was used to compare optical densities of protein bands between vehicle and the caspase inhibitor-treated rats. Data were considered statistically significant for P values of ≤0.05. Data are presented as mean±SEM.
Results
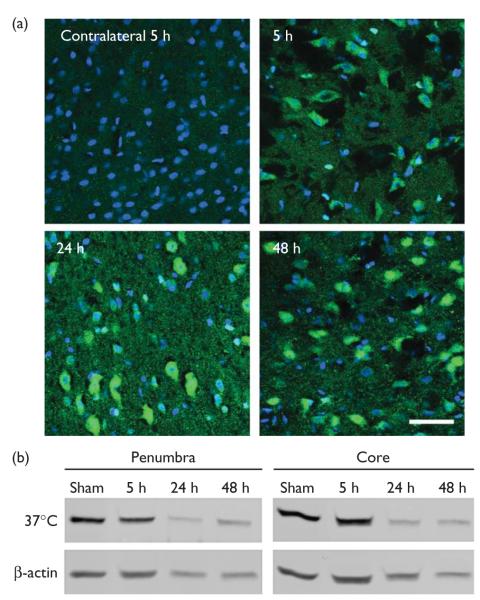
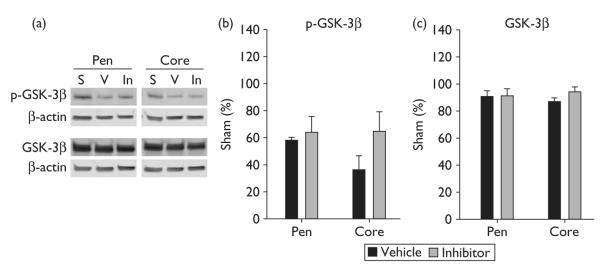
Active caspase-3 was expressed in the ischemic cortex from 5 to 48 h after stroke (Fig. 2a), whereas protein levels of β-catenin markedly decreased at 24 and 48 h after stroke (Fig. 2b), which has been reported in our earlier study [8]. We then studied the effect of caspase-3 inhibitor on β-catenin levels at 24 h. Inhibiting caspase-3 activity attenuated reduction in protein levels of β-catenin at 24 h while it had no significant effect on β-catenin phosphorylation (Fig. 3). We further examined the effect of the caspase inhibitor on GSK-3β phosphorylation and total protein of GSK-3β, molecules upstream of β-catenin. GSK-3β phosphorylation decreased at 24 h but caspase inhibition did not significantly affect its levels; total protein of GSK-3β did not change at 24 h, nor did caspase inhibition affect its level (Fig. 4).
Fig. 2.
(a) Active caspase-3 was expressed in the ischemic cortex after stroke. Double staining of DAPI (blue) and active caspase-3 (green) was conducted. Active caspase-3 was not detected in the nonischemic contralateral cortex, whereas it was expressed in the ischemic cortex 5, 24, and 48 h after stroke. Scale bar, 20 μm. (b) Beta-catenin degraded after stroke.Representative protein bands of β-catenin from western blot were shown; samples of both the ischemic penumbra and core were prepared from rat brains harvested at 5, 24, and 48 h after stroke. Beta-actin was probed to show even loading of protein.
Fig. 3.
The caspase-3 inhibitor blocked degradation of β-catenin but did not affect protein levels of phosphorylated β-catenin at 24 h after stroke. (a) Representative protein bands of β-catenin and bar graphs for optical densities of β-catenin bands. Samples from rats subjected to sham surgery and ischemia plus vehicle or the caspase inhibitor were compared.The optical densities of protein bands after stroke were normalized to that of the bands of sham group and expressed as a percentage; the optical density of sham is 100%. S, sham; V, vehicle; In, caspase inhibitor. *P<0.05, versus inhibitor. (b) Representative protein bands of phosphorylated β-catenin and bar graphs for its optical densities.
Fig. 4.
Caspase-3 inhibitor did not affect phosphorylation of glycogen synthase kinase (GSK)-3β and protein levels of total GSK-3β at 24 h after stroke. (a) Representative protein bands of phosphorylated GSK-3β and GSK-3β, β-actin from western blot. S, sham; V, vehicle; In, caspase inhibitor. (b) Optical densities of the protein bands of phosphorylated GSK-3β. (c) Optical densities of the protein bands of total GSK-3β.
Discussion
To the best of our knowledge, we are the first to demonstrate that inhibiting caspase-3 activity blocks degradation of β-catenin 24 h after stroke. Beta-catenin is known to be a substrate of caspase-3, and several reports have shown that caspase activity cleaves β-catenin and some cleaved bands are detected by western blots [10]. We have shown previously that caspase-3 activity was increased in the ischemic brain using this ischemic model [11]. We did not detect cleaved bands of β-catenin in this study. The lack of cleavage band in this study is probably because of the choice of antibody, as one earlier study has reported that only a C-terminal antibody, but not the N-terminal antibody which was used in our study, can detect cleavage bands of β-catenin [12]. Nevertheless, active caspase-3 was expressed in the ischemic cortex from 5 to 48 h after stroke, and inhibiting caspase-3 activity did block the reduction in total β-catenin after stroke, suggesting that degradation of β-catenin is caspase-3 activity dependent.
Although the protein level of β-catenin is decreased by GSK-3β activity causing its proteosome degradation, the effect of the caspase-3 inhibition blocking β-catenin degradation may not be achieved via its effect on GSK-3β in this study. This conclusion is on the fact that the caspase-3 inhibitor did not affect phosphorylation of both GSK-3β and β-catenin. Therefore, the effect of the caspase inhibitor attenuating reduction in protein levels of β-catenin may be achieved by inhibiting the ability of caspase-3 to cleave β-catenin. In conclusion, we demonstrated that β-catenin was degraded after stroke, and that the degradation is caspase dependent.
Acknowledgements
The authors thank Elizabeth Hoyte for figure preparation and Felicia Beppu for manuscript editing. This study was supported by AHA National Scientist Development Grant 0730113N (HZ), NINDS grants R01 NS27292 (G.K.S.) and P01 NS37520 (G.K.S.).
References
- 1.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shukla S, MacLennan GT, Flask CA, Fu P, Mishra A, Resnick MI, et al. Blockade of beta-catenin signaling by plant flavonoid apigenin suppresses prostate carcinogenesis in TRAMP mice. Cancer Res. 2007;67:6925–6935. doi: 10.1158/0008-5472.CAN-07-0717. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly S, Zhao H, Hua Sun G, Cheng D, Qian Y, Luo J. Glycogen synthase kinase 3beta inhibitor Chir025 reduces neuronal death resulting from oxygen-glucose deprivation, glutamate excitotoxicity, and cerebral ischemia. Exp Neurol. 2004;188:378–386. doi: 10.1016/j.expneurol.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Biphasic cytochrome c release after transient global ischemia and its inhibition by hypothermia. J Cereb Blood Flow Metab. 2005;25:1119–1129. doi: 10.1038/sj.jcbfm.9600111. [DOI] [PubMed] [Google Scholar]
- 6.Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, et al. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naito M, Katayama R, Ishioka T, Suga A, Takubo K, Nanjo M, et al. Cellular FLIP inhibits beta-catenin ubiquitylation and enhances Wnt signaling. Mol Cell Biol. 2004;24:8418–8427. doi: 10.1128/MCB.24.19.8418-8427.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Ren C, Gao X, Takahashi T, Sapolsky RM, Steinberg GK, et al. Hypothermia blocks beta-catenin degradation after focal ischemia in rats. Brain Res. 2008;1198:182–187. doi: 10.1016/j.brainres.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimohata T, Zhao H, Sung JH, Sun G, Mochly-Rosen D, Steinberg GK. Suppression of deltaPKC activation after focal cerebral ischemia contributes to the protective effect of hypothermia. J Cereb Blood Flow Metab. 2007;27:1463–1475. doi: 10.1038/sj.jcbfm.9600450. [DOI] [PubMed] [Google Scholar]
- 10.Hwang SG, Lee HC, Trepel JB, Jeon BH. Anticancer-drug-induced apoptotic cell death in leukemia cells is associated with proteolysis of beta-catenin. Leuk Res. 2002;26:863–871. doi: 10.1016/s0145-2126(02)00018-8. [DOI] [PubMed] [Google Scholar]
- 11.Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003;85:1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- 12.Abe K, Takeichi M. NMDA-receptor activation induces calpain-mediated beta-catenin cleavages for triggering gene expression. Neuron. 2007;53:387–397. doi: 10.1016/j.neuron.2007.01.016. [DOI] [PubMed] [Google Scholar]