Abstract
Food consumption increases protein synthesis in most tissues by promoting translation initiation, and in the neonate, this increase is greatest in skeletal muscle. In this study, we aimed to identify the currently unknown time course of changes in the rate of protein synthesis and the activation of factors involved in translation in neonatal muscle after a meal. After overnight food deprivation, 36 5- to 7-d-old piglets were administered a nutritionally complete bolus i.g. meal and were killed immediately before or 30, 60, 90, 120, or 240 min later. The increase in skeletal muscle protein synthesis peaked 30 min after the meal and this was sustained through 120 min, returning to baseline thereafter. The relative proportion of polysomes to nonpolysomes was higher only after 30 min. Protein kinase B phosphorylation peaked 30 min after feeding and returned to baseline by 90 min. The phosphorylation of mammalian target of rapamycin, eukaryotic initiation factor (eIF) 4E binding protein (4E-BP1), ribosomal protein S6, and eIF4G was increased within 30 min of feeding and persisted through 120 min, but all had returned to baseline by 240 min. The association of 4E-BP1·eIF4E was reduced and eIF4E·eIF4G increased 30 min after receiving a meal, remaining so for 120 min, before returning to baseline at 240 min. Thus, in neonates, food consumption rapidly increased skeletal muscle protein synthesis by enhancing translation initiation and this increase was sustained for at least 120 min after the meal but returned to baseline by 240 min after the feeding.
Introduction
During the neonatal period of development, when rates of growth and protein turnover are at their highest, food intake stimulates protein synthesis in most tissues (1–3). The most profound postprandial increase in protein synthesis occurs in skeletal muscle. The rise in protein synthesis in neonatal muscle can be mimicked by independently raising circulating insulin or amino acid concentrations to levels normally associated with the fed state, while maintaining the other anabolic agents and glucose at baseline concentrations (4–6). Previous studies that investigated the effect of food intake on skeletal muscle protein synthesis have focused on 1 specific time point, usually 90–120 min after the meal. Consequently, little is known about the postprandial time course of the changes in muscle protein synthesis.
The postprandial stimulation of skeletal muscle protein synthesis is the result of the activation of the translation initiation factors involved in regulating mRNA binding to the ribosome (7–10). A complex of 3 eukaryotic initiation factors (eIF),6 the RNA helicase eIF4A, the scaffolding protein eIF4G, and the cap binding protein eIF4E, comprise eIF4F and regulate mRNA binding to the ribosome (11,12). The rate-limiting step in eIF4F formation is eIF4E availability, which can be sequestered by the eIF4E binding protein (4E-BP1), preventing its association with eIF4G. Phosphorylation of 4E-BP1 by the mammalian target of rapamycin complex 1 (mTORC1) releases eIF4E, allowing eIF4F formation (11,12). mTORC1, a complex comprised of mTOR, regulatory associated protein of mTOR (raptor), G-protein β subunit-like protein, and ras homolog enriched in brain (Rheb) (13,14), is sensitive to treatment with rapamycin (15). Although the activation of mTORC1 is regulated by both insulin and amino acids, only the insulin-induced activation is well characterized. Rheb (a ras-like small guanosine triphosphatase), a positive regulator of mTORC1, has been identified as a key player in both insulin and amino acid-induced mTORC1 activation (16). Insulin enhances Rheb GTP charging through the ability of activated protein kinase B (PKB; also known as Akt) to inhibit the Rheb-GTPase–activating function of the tuberous sclerosis heretodimer (TSC1/TSC2), allowing activation of mTORC1 (16). Amino acids activate mTORC1 independently of PKB and the insulin pathway (16); however, the exact mechanism by which amino acids promote mTORC1 has not been elucidated. Additionally, mTORC1 phosphorylates ribosomal protein S6 kinase (S6K1), an activator of S6 (11), a protein that enhances translation initiation (17,18). A second mTOR complex, mTORC2, is rapamycin insensitive and is comprised of mTOR, rictor, and G-protein β subunit-like protein (13,14,19) and has been shown to activate PKB, allowing feedback activation of mTORC1 (20).
In addition to mRNA binding to the ribosome, translation initiation requires initiator methionyl-tRNA (met-tRNAi) binding to the start codon, a step mediated by eIF2. In its GTP form, eIF2 binds the ribosome and locates the mRNA start site, causing eIF2 hydrolysis and disassociation; eIF2B then recharges eIF2 with a GTP (21). Phosphorylation of the α subunit of eIF2 transforms it into a competitive inhibitor of eIF2B, preventing activation of eIF2 and, consequently, reducing met-tRNAi binding to the ribosome (21). Translation is also dependent upon rates of elongation. Whereas it has been suggested that the eukaryotic elongation factor 2 (eEF2) is a substrate of mTORC1, rapamycin treatment does not alter the phosphorylation of this factor (22).
Our focus in this study was to characterize the changes in protein synthesis rates in skeletal muscle of neonatal pigs for a 4-h time period after they had consumed one-sixth of their daily nutrient requirements as a bolus i.g. meal. Piglets were food deprived overnight to ensure that there would be no residual effects of a previous meal. Additionally, this study aimed to determine the associated changes in the activation of the factors that mediate translation initiation and elongation.
Materials and Methods
Animals.
Four multiparous crossbred (Yorkshire × Landrace × Hampshire × Duroc) pregnant sows obtained from the Agricultural Headquarters of the Texas Department of Criminal Justice (Huntsville, TX) were brought to the animal facility of the Children's Nutrition Research Center prior to their due date. Sows and piglets were housed and managed as previously described (23). Piglets were studied at 5–7 d of age. The Animal Care and Use Committee of Baylor College of Medicine approved all experimental procedures. This study was conducted in accordance with the NRC's Guide for the Care and Use of Laboratory Animals.
Three to 5 d prior to infusion, piglets weighing 1.59 ± 0.52 kg underwent surgery when catheters were placed in their left external jugular vein and left common carotid artery. All surgical procedures were conducted using sterile techniques under general anesthesia (Aerrane, Anaquest) as described previously (3). After recovering from anesthesia, piglets were returned to their respective sows until studied.
Treatments and infusions.
Overnight food-deprived piglets weighing 1.77 ± 0.07 kg were randomly assigned to 1 of 6 treatment groups (n = 6 · treatment group−1): 1) overnight food deprivation (0 min controls); 2) 30 min postprandial; 3) 60 min postprandial; 4) 90 min postprandial; 5) 120 min postprandial; and 6) 40 min postprandial. Piglets assigned to a fed group were gavage fed a gastric bolus meal (40 mL·kg body weight−1 delivered by syringe pump over a 15-min period) of Soweena Litter Life (Merricks),7 a sow milk replacer. Soweena is a highly digestible semipurified diet that derives 20% of it composition from whey protein and provides 11.882 MJ·kg−1. Because the effects of the meal were assessed over one-sixth of 1 d, the amount of Soweena administered was calculated to provide one-sixth of the piglets' daily requirement. The 15-min blood sample was collected immediately after the meal was delivered. Piglets were killed immediately before the feeding (0 min) or 30, 60, 90, 120, or 240 min later. Blood samples were collected every 15 min and immediately analyzed for branched-chain amino acid (BCAA) and glucose concentrations, as previously described (24). Additionally, plasma samples for insulin and glucose measurements were collected every 15 min for the first 2 h and every 30 min for the remaining 2 h of infusion. Plasma immunoreactive insulin concentrations were measured using a porcine insulin RIA kit (Linco). The concentrations of individual amino acids from frozen plasma samples collected every 30 min were measured using an HPLC method (PICO-TAG reverse-phase column; Waters) as previously described (2).
Tissue protein synthesis in vivo.
The fractional rate of protein synthesis was measured with a flooding dose of l-[4-3H] phenylalanine (25). For all groups, except the 30 min fed group, piglets received l-[4-3H] phenylalanine (1.5 mmol·kg body weight−1, 0.5 mCi·kg body weight−1; Amersham Bioscience) injected 30 min before they were killed. Piglets in the 30 min fed group received l-[4-3H] phenylalanine (1.5 mmol·kg body weight−1, 1.0 mCi·kg body weight−1) injected 15 min prior to being killed. Piglets were killed and samples were obtained from the longissimus dorsi muscle and immediately frozen in liquid nitrogen and stored at −70°C until analyzed as previously described (1). Previous studies have demonstrated that, after a flooding dose of 3H-phenylalanine is administered, the specific radioactivity of tissue free phenylalanine is in equilibrium with the aminoacyl-tRNA specific radioactivity and therefore the tissue free phenylalanine is a valid measure of the tissue precursor pool specific radioactivity (26).
Polysome profiles.
Analysis of ribosome distribution between polysomal and nonpolysomal fractions was assessed by sucrose density gradient centrifugation as previously described (27).
Protein immunoblot analysis.
Proteins from longissimus dorsi muscle homogenates were separated by electrophoresis on PAGE. For each assay, all samples were analyzed at the same time on triple-wide gels (C.B.S Scientific) to eliminate interassay variation. Proteins were electrophoretically transferred to polyvinylidene difluoride transfer membranes (Pall) and subsequently incubated with appropriate primary antibodies, washed, and exposed to an appropriate secondary antibody as previously described (7).
For normalization, immunoblots performed with antiphospho-specific antibodies were stripped in stripping buffer (Pierce Biotechnology) and reprobed with corresponding nonphospho-specific antibodies. Blots were developed using an enhanced chemiluminescence kit (GE health Sciences), visualized, and analyzed using a ChemiDoc-It Imaging system (UVP). Primary antibodies that were used in the immunoblotting were PKB (total and Ser473, Cell Signaling), AMP-activated kinase (AMPK) (total and Thr172, Cell Signaling), mTOR (total and Ser2448, Cell Signaling), raptor (total and Ser792, Cell Signaling), 4E-BP1 (total, Bethyl Laboratories, and Thr70, Cell Signaling), eIF4G (total and Ser1180, Cell Signaling), S6 (total, Ser235/236, and Ser240/244, Cell Signaling), eIF2α (total and Ser51, Cell Signaling), and eEF2 (total and Thr56, Cell Signaling).
Quantification of eIF4E·4E-BP1 and eIF4E·eIF4G complexes.
The eIF4E·4EBP1 and eIF4E·eIF4G complexes were immunoprecipitated using an anti-eIF4E monoclonal antibody from aliquots of fresh tissue homogenates (28). Briefly, samples were homogenized in 7 volumes of buffer (in mmol·L−1: 20 HEPES, 2 EGTA, 50 NaF, 100 KCl, and 0.2 EDTA, pH 7.4) containing Sigma P3840 Protease Inhibitor Cocktail (Sigma Chemical) and centrifuged at 10,000 × g for 10 min at 4°C. Supernatants were incubated overnight at 4°C, with constant rocking, with an anti-eIF4E antibody. Immunoprecipitates were recovered with goat anti-mouse IgG magnetic beads (Polysciences), washed, and resuspended in sample buffer as described elsewhere (28) and immediately subjected to protein immunoblot analysis using rabbit anti-4E-BP1 (Bethyl Laboratories) antibody or rabbit anti-eIF4G (Novus Biologicals). Amounts of 4E-BP1 and eIF4G were corrected for the amount of eIF4E recovered in the immunoprecipitate.
Calculations and statistics.
The fractional rate of protein synthesis (Ks, percentage of protein mass synthesized in a day) was calculated as Ks (%·d−1) = [(Eb/Ea) × (1440/t)] × 100, where Eb (in dpm·nmol−1) is the specific radioactivity of the protein-bound phenylalanine, Ea (in dpm·nmol−1) is the specific radioactivity of the tissue free phenylalanine at the time of tissue collection corrected by the linear regression of the blood specific radioactivity of the animal against time, t is the time of labeling in minutes, and 1440 is the minutes-to-day conversion.
ANOVA analysis was carried out in Minitab (version 13.31) using a general linear model to determine the main statistical differences effects. Where main effects were significant, between-group analysis was performed using a Fisher's least significant difference test. Where variances were unequal, ANOVA was performed assuming unequal variances. P-values < 0.05 were considered significant for all comparisons and data are presented as means ± SEM.
Results
Circulating substrate concentrations.
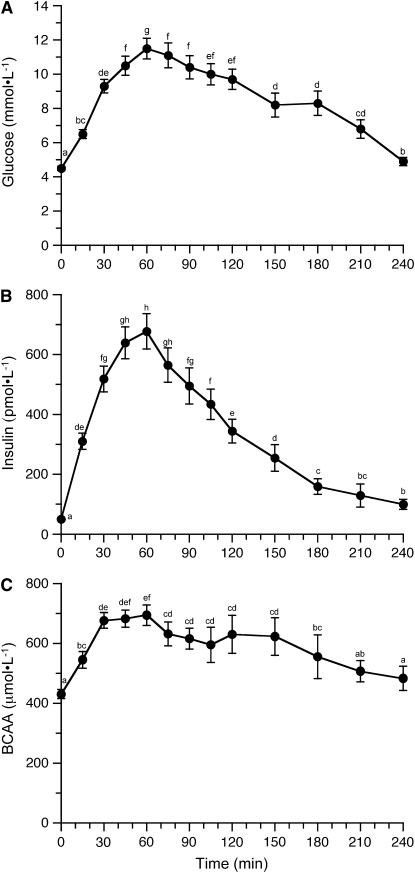
Circulating concentrations of glucose increased ∼1.5 fold in response to a bolus meal delivered over a 15-min period and reached a maximum around 60 min postprandially (Fig. 1A; P < 0.001). Glucose concentrations declined substantially 240 min after the feeding but remained above baseline values (P = 0.016). Circulating insulin concentrations increased postprandially (Fig. 1B; P < 0.001), reached a maximum concentration by 45 min that was maintained through 75 min, and declined thereafter, although it had not attained the baseline concentration by 240 min (P = 0.004). BCAA almost doubled in response to a meal (Fig. 1C; P < 0.001); maximum levels were attained 30 min after the feeding and were maintained to 60 min. BCAA concentrations returned to baseline (time 0 group) levels (∼400 μmol·L−1) by 240 min postfeeding. Circulating levels of individual amino acids were generally increased within 30 min of receiving a meal and returned to baseline by 240 min after the initiation of feeding (P < 0.05; Supplemental Table 1). The only exceptions to this were histidine and glutamic acid in which no increases occurred, whereas changes in glycine (P = 0.055), serine (P = 0.058), and taurine (P = 0.056) concentrations were marginal.
FIGURE 1 .
Plasma glucose (A), insulin (B), and BCAA (C) concentrations in neonatal pigs for the 240-min period after a meal. Data from all groups were pooled at each time point. ANOVA showed a time effect of each substrate (P < 0.001). Values are means ± SEM, n = 6–36. Means without a common letter differ, P < 0.05.
Protein synthesis and polysome profiles.
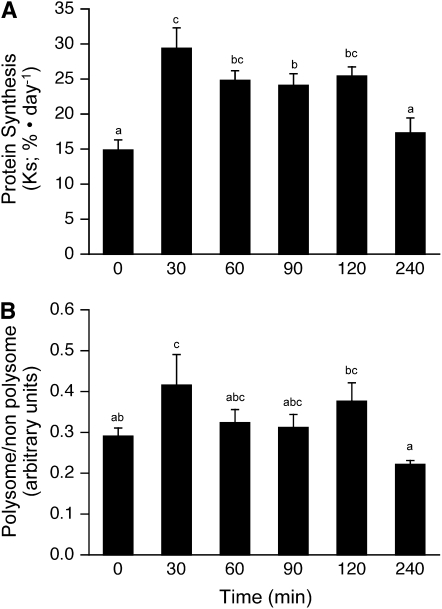
There was a rapid postprandial increase in the fractional rates of protein synthesis in skeletal muscle (Fig. 2A; P < 0.001) that reached a maximum by 30 min (P < 0.005). Fractional synthesis rates remained elevated through 120 min postfeeding and tended to be lower at 60 (P = 0.057) and 120 min (P = 0.130) compared with those at 30 min. However, by 240 min, protein synthesis rates had returned to baseline levels.
FIGURE 2 .
Time course of the changes in the fractional rate of protein synthesis (A), proportion of ribosomes in polysomes (B) in skeletal muscle of neonatal pigs after a meal. ANOVA showed an effect of treatment for the rate of protein synthesis (P < 0.001) and a trend for the polysome profile (P = 0.063). Values are means ± SEM, n = 4–6. Means without a common letter differ, P < 0.05.
Analysis of the fraction of the ribosomes in polysomes compared with those either in monosomes or not associated with mRNA is a measure of the ratio of the rate of translation initiation to elongation. Compared with baseline (time 0 group) controls, the number of ribosomes associated with mRNA increased 30 min after the feeding (P < 0.05; Fig. 2B), indicating a rapid increase in the rate of initiation compared with elongation during the immediate postprandial stages. However, by 60 min postfeeding, the polysomal to nonpolysomal distribution of ribosomes did not differ from the 0-min value, suggesting that the rate of elongation increased, such that the ratio of initiation:elongation was similar to that in food-deprived control animals. Combined, the protein synthesis and polysome analyses suggest that both elongation and initiation had returned to baseline 240 min after a meal.
Biomarkers of translation initiation.
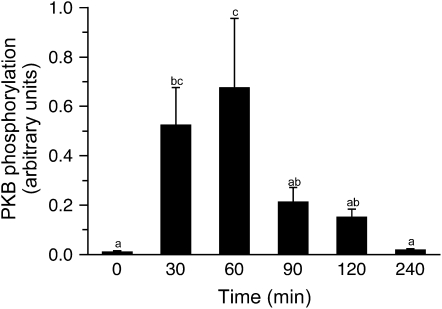
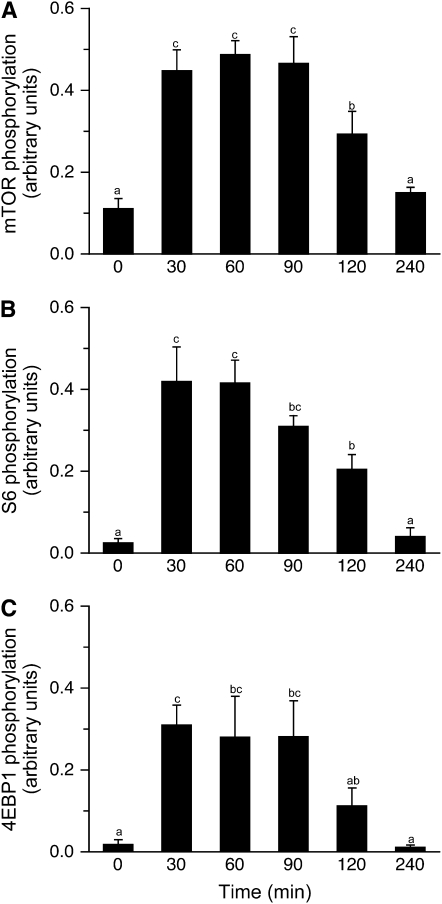
Food consumption increases translation initiation by increasing the activation of the mTORC1 signaling pathway (7,10). PKB mediates the insulin-associated activation of mTOR (16). Phosphorylation of PKB on Ser473 (Fig. 3) increased only higher than baseline at 30 and 60 min posteating (P = 0.004). The meal did not affect the phosphorylation status of the negative mTORC1 regulator, AMPK (Supplemental Fig. 1A). The phosphorylation of mTOR on Ser2448 increased in response to the feed (Fig. 4A; P < 0.001), with maximum levels of stimulation achieved by 30 min postprandially. Phosphorylation of mTOR was sustained for at least 120 min after the meal, although the levels of phosphorylation were ∼70% of maximum at this time point and activation had returned to baseline by 240 min. However, no effect was observed for the phosphorylation of raptor (Supplemental Fig. 1B). The phosphorylation of S6 mirrored that of mTOR. Maximal S6 phosphorylation was achieved by 30 min postfeeding (Fig. 4B; P < 0.001), with stimulation being sustained until at least 120 min, but at a lower level (P < 0.001).
FIGURE 3 .
Time course of the changes in PKB phosphorylation in skeletal muscle of neonatal pigs after a meal. All results are corrected for total protein. ANOVA showed an effect of treatment (P = 0.005). Values are means ± SEM, n = 6. Means without a common letter differ, P < 0.05.
FIGURE 4 .
Time course of the changes in mTOR (A), S6 (B), and 4EBP1 (C) phosphorylation in skeletal muscle of neonatal pigs after a meal. All results are corrected for total protein. ANOVA showed an effect of treatment (P < 0.005) for each factor. Values are means ± SEM, n = 6. Means without a common letter differ, P < 0.05.
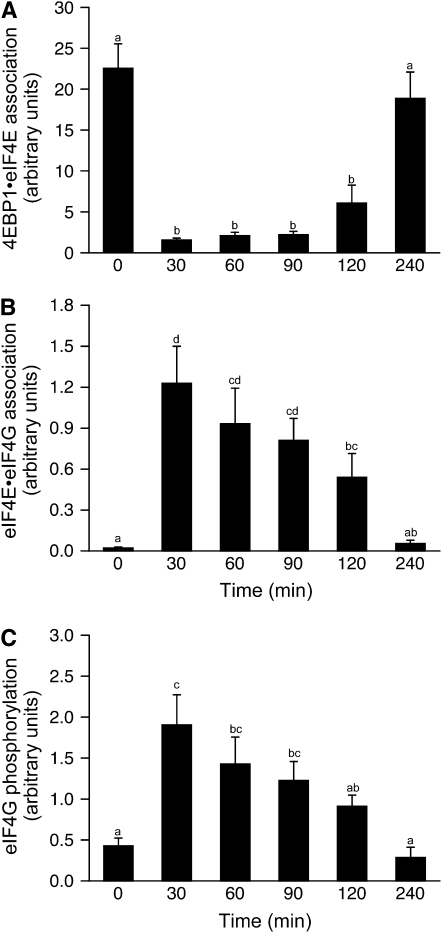
The phosphorylation of 4E-BP1 (P = 0.001) increased to a maximum by 30 min postfeeding and remained high until at least 90 min postfeeding (Fig. 4C). By 120 min, 4E-BP1 phosphorylation tended to be greater than at baseline (P = 0.067), however, and returned to baseline by 240 min. The phosphorylation of 4E-BP1 reduces its association with eIF4E, an effect previously observed in response to feeding (7,10). The association of 4E-BP1·eIF4E was reduced by 30 min postfeeding and remained suppressed at this level to 120 min postfeeding (Fig. 5A; P < 0.05). The formation of the active eIF4E·eIF4G complex increased to a maximum by 30 min after the feeding (Fig. 5B; P < 0.001). As with the proteins downstream of mTORC1, meal consumption increased the phosphorylation of eIF4G, reaching a maximum 30 min after the meal (Fig. 5C; P = 0.004). Phosphorylation of eIF4G decreased over time and returned to baseline after 240 min. Although the factors associated with mRNA binding to the ribosome increased in response to feeding, the phosphorylation status of eIF2α did not change (Supplemental Fig. 1C), nor eEF2, a factor associated with elongation (Supplemental Fig. 1D).
FIGURE 5 .
Time course of the changes in the 4E-BP1·eIF4E complex (A) and eIF4E·eIF4G complex (B) and the phosphorylation of eIF4G (C) in skeletal muscle of neonatal pigs after a meal. All results are corrected for total protein. ANOVA showed an effect of treatment (P < 0.001) for each complex and eIF4G phosphorylation (P < 0.005). Values are means ± SEM, n = 6. Means without a common letter differ, P < 0.05.
Discussion
To allow protein deposition and rapid growth during the neonatal period, feeding a meal increases skeletal muscle protein synthesis in neonatal pigs by enhancing translation initiation (7–9). However, the postprandial time course for the changes in muscle protein synthesis and the regulatory factors involved have not been defined. Ascertaining these changes is important to further our understanding of the detailed regulation of protein synthesis in response to a meal and also to provide a mechanistic underpinning for devising strategies to optimize protein utilization for growth. Furthermore, the increase in protein synthesis in skeletal muscle is of high metabolic significance, because skeletal muscle comprises a large proportion of the body mass in neonatal pigs. Previous reports on the effect of food consumption on muscle protein synthesis have focused on time points at least 90 min after a meal (29,30) or in response to a continuous feeding regimen (31). In this study, a gastric bolus meal of a sow milk replacer increased skeletal muscle protein synthesis within 30 min of meal commencement and returned to baseline by 240 min. This response was largely paralleled by changes in mTORC1 signaling, eIF4F complex formation, and circulating insulin, amino acid, and glucose concentrations.
Feeding raises circulating insulin, amino acid, and glucose concentrations, metabolites known to independently promote skeletal muscle protein synthesis (6,32), largely through activation of mTORC1 and, hence, translation initiation (16,33,34). In the current study, skeletal muscle protein synthesis was maximal within 30 min and remained elevated for 120 min postprandially, indicating a rapid and sustained induction of translation. However, circulating insulin and glucose levels did not peak until after 45–75 min and declined sharply thereafter. Maximum circulating amino acids levels were attained 30–60 min after the meal commenced, with a slow decline thereafter, suggesting that in the presence of fed levels of amino acids, protein synthesis is maximally stimulated at insulin levels lower than the peak values achieved following a meal. These results support our previous studies using pancreatic-substrate clamps that showed a dose-response effect of both amino acids and insulin on muscle protein synthesis that is additive until maximal rates of protein synthesis are achieved (5,6). Of the amino acids, leucine has been shown to be of particular importance in mediating the increase in translation initiation and protein synthesis. Indeed, infusion of leucine alone acutely increases protein synthesis in skeletal muscle of neonatal pigs by an mTORC1-mediated process (22,23,28). In the current study, circulating leucine levels increased by 30 min after the initiation of eating and had returned to baseline by 240 min, mirroring the increase in rates of protein synthesis. We cannot exclude the possibility, however, that amino acids other than leucine may also be involved.
The rapid induction of protein synthesis in this study may reflect the dietary composition of Soweena, which derives its protein content from whey. Whey is a highly digestible protein that is quickly absorbed and increases circulating amino acids rapidly, whereas casein protein is less easily digested and results in a slower release of amino acids (35). Beaufrere et al. (36) have shown that the consumption of a liquid protein meal comprised of whey protein produced a higher, faster, and more transient appearance of circulating leucine and a more rapid increase in whole-body protein synthesis than when the meal contained casein. The high carbohydrate content of Soweena also could result in a rapid increase in protein synthesis. However, in previous studies, piglets fed diets containing different levels of carbohydrate did not differ in muscle protein synthesis rates, likely because the insulin concentration in the low lactose-fed pigs was elevated sufficiently to not limit muscle protein synthesis (29). As newborns consume multiple meals daily, we designed the current study to reflect a common feeding regimen in which a meal that contained one-sixth of the daily nutrient requirement was administered and the response over a 4-h-period was followed. Ingestion of a meal of this size was able to maintain elevated rates of muscle protein synthesis between 0.5 and 2 h postprandially. This response pattern indicates that to maintain rapid and sustained muscle growth, neonates require frequent meals. This study differs from that of Vary and Lynch (37) who recently examined the time course of the changes in biomarkers of translation initiation in cardiac muscle during and after meal feeding in adult rats, in that the rats were trained to eat their daily allotment of food during a 3-h period (38).
In this study, changes in the rate of initiation relative to elongation were assessed by the changes in ribosome distribution in sucrose density gradients. In this analysis, the increase in protein synthesis in skeletal muscle was concomitant with an increase in the number of ribosomes associated with polysomes (i.e. an increase in the polysome:nonpolysome ratio) at 30 min, suggesting that the rate of translation initiation is upregulated compared with elongation early after a meal, thus supporting the observed increase in mTORC1 signaling at this time point (39). However, by 60 min, the ratio of initiation relative to elongation, as assessed by polysome aggregation status, did not differ from baseline (time 0 group). The reduction in the polysome:nonpolysome ratio could be a consequence of either decreased initiation or increased elongation rates. However, because protein synthesis remained elevated at 60 min after the start of feeding, we assume that the change in the polysome:nonpolysome ratio reflects a corresponding increase in elongation. This finding suggests that elongation also increased after meal consumption but that the increase was delayed relative to the change in initiation. Interestingly, increased rates of elongation did not further increase muscle protein synthesis, supporting the idea that initiation is the rate-limiting step in translation. Phosphorylation of eEF2, an elongation factor that has previously been reported as an mTORC1 substrate (34), was unaltered by feeding. However, we previously demonstrated that rapamycin treatment did not alter eEF2 phosphorylation (22). Taken together, however, this data suggests that following the consumption of a meal, increased rates of initiation, and not elongation, are responsible for the increase in muscle protein synthesis in the neonate.
The postprandial increase in muscle protein synthesis is mediated by enhanced mTORC1 activation (8,16,33,34), which in turn promotes mRNA translation (7,10). Whereas the feeding-associated increase in insulin promotes mTORC1 activation via PKB, the amino acid involvement is less well defined but is PKB independent, providing 2 separate signaling pathways for mTORC1 activation in response to a meal (8,16,33,34,40,41). Activation of PKB was rapid and peaked at submaximal insulin concentrations, returning to baseline by 90 min (mirroring changes in circulating insulin concentrations), prior to the reduction in mTORC1 activation, and indicative of feedback inhibition on the insulin pathway (42). The discrepancy between PKB and mTOR activation reflects the ability of both amino acids (which attained maximal circulating levels for a more rapid and prolonged period than insulin) and insulin (16,33), but not glucose (32), to stimulate mTORC1, but not PKB (41). However, amino acids may be capable of eliciting a more rapid and prolonged activation of mTORC1 than insulin, or, alternatively, PKB may stimulate maximal mTORC1 activity before its full phosphorylation potential is achieved. Further investigation of the time course of the independent effects of insulin and amino acids on the factors involved in translation initiation clearly is warranted. The energy sensor, AMPK, negatively regulates mTORC1 activity by promoting TSC2-induced inhibition of mTORC1 (43). Although we previously found no change in AMPK phosphorylation in muscle of neonatal pigs 90 min after a meal (44), whether a change in AMPK phosphorylation occurred at an earlier time point had not been investigated previously. We found that AMPK phosphorylation was unaltered over the 4-h postprandial time course, suggesting that AMPK is not involved in the regulation of mTOR under physiological feeding conditions.
The phosphorylation of mTOR peaked within 30 min of starting the feeding and paralleled the stimulation of skeletal muscle protein synthesis. However, food consumption did not affect the mTORC1-associated protein, raptor. Knockdown of raptor has previously been shown to prevent the leucine-induced phosphorylation of S6K1 (16) and suggests that raptor is essential for mTOR signaling. However, cell culture studies have shown that raptor is phosphorylated on both Ser772 and Ser792 by AMPK (45) and because AMPK activation was not altered in the current study, no change in the phosphorylation of raptor on Ser792 would be anticipated. We observed phosphorylation of the downstream targets of mTORC1, 4E-BP1 and S6, following the postprandial activation of mTOR. In rats trained to eat their daily requirement in a 180-min meal, mTOR, S6K1, and 4E-BP1 phosphorylation remained elevated from 30 to 180 min of the feeding period (46). Additionally, when C2C12 muscle myoblast in culture were treated with AICAR to inhibit mTOR activation, S6K1 and 4E-BP1 phosphorylation was reduced and eIF4E association altered within 15 min (47), suggesting that reassignment of the complex association is rapid. Treatment of H4IIE hepatoma cells in culture with growth hormone, a known protein synthesis promoter, stimulates S6 phosphorylation within 30 to 45 min and 4E-BP1 phosphorylation within 10–20 min posttreatment (48). In the current in vivo study, formation of the eIF4E·4E-BP1 and eIF4E·eIF4G complexes showed a gradual return to baseline by 4 h, which was slower than the decline in protein synthesis, suggesting that assembly of eIF4E·eIF4G is not the rate-limiting step in the feeding-induced stimulation of skeletal muscle protein synthesis. The eIF4E·eIF4G complex is also regulated by eIF4G phosphorylation (12), and in the current study, eIF4G phosphorylation status was similar to that of other translation initiation factors. Taken together, these studies suggest that modulation of activation of mTORC1 and its downstream targets to regulate the binding of the mRNA with the 43S preinitiation complex occurs within minutes in response to numerous stimuli both in vivo and in vitro and lead to enhanced rates of protein synthesis. We previously showed that the activity of a regulator of the binding of met-tRNAi to the 40S ribosomal subunit, eIF2B, is unchanged in neonatal muscle 90 min after a meal (7). In the current study, phosphorylation of eIF2α was unaltered over the postprandial time course, suggesting that this step in mRNA translation is not limiting for the stimulation of protein synthesis in neonatal muscle after a meal.
In summary, consumption of a milk replacer high in whey protein at one-sixth of the daily requirement increased muscle protein synthesis in neonatal pigs to a maximum rate within 30 min of the feeding. This increase in muscle protein synthesis was modulated by an increase in translation initiation, with mirrored activation of the mTORC1 signaling pathway. The rise and maintenance of muscle protein synthesis rates followed the rapid and sustained increase in circulating insulin and amino acids levels, which peaked by 30 min after the feeding as a consequence of the rapid digestion of whey protein (36). The feeding-induced stimulation of protein synthesis returned to baseline by 4 h after the meal and preceded a reduction in eIF4E·eIF4G association, suggesting that other factors, such as amino acid availability, may have limited protein synthesis. The results further suggest that rates of protein synthesis in skeletal muscle are increased for only a limited period of time following a feeding. Therefore, frequent meals are essential for sustaining optimal protein synthesis rates and, hence, muscle growth in the neonate.
Supplementary Material
Acknowledgments
We thank Rosemarie Almonaci and Sharon Rannels for technical assistance, Jerome Stubblefield for care of animals, E. O'Brian Smith for statistical assistance, Adam Gillum for graphics, and Linda Weiser for secretarial assistance.
Supported in part by NIH grants R01 AR-44474, R01 DK15658, and K08 AR-51563 and the USDA/Agricultural Research Service (ARS) under Cooperative Agreement number 6250510000-33. This work is a publication of the USDA, ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX. The contents of this publication do not necessarily reflect the views or policies of the USDA, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Author disclosures: F. A. Wilson, A. Suryawan, R. A. Orellana, S. R. Kimball, M. C. Gazzaneo, H. V. Nguyen, M. L. Fiorotto, and T. A. Davis, no conflicts of interest.
Supplemental Figure 1 and Table 1 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AMPK, AMP-activated kinase; BCAA, branched-chain amino acid; 4E-BP1, eIF 4E binding protein 1; eEF2, eukaryotic elongation factor 2; eIF, eukaryotic initiation factor; methionyl-tRNA, initiator methionyl-tRNA; mTORC1, mammalian target of rapamycin complex 1; PKB, protein kinase B; raptor, regulatory associated protein of mammalian target of rapamycin; Rheb, ras homolog enriched in brain; S6, ribosomal protein S6; TSC1/2, tuberous sclerosis heterodimer.
Soweena contains: 25 g·100 g−1crude protein; 49 g·100 g−1 lactose; 10 g·100 g−1 crude fat; 0.04 g·100 g−1 crude fiber; 1.1 g·100 g−1 calcium; 0.79 g·100 g−1 phosphorus; 0.16 g·100 g−1 magnesium; 1.5 g·100 g−1 potassium; 12 g·100 g−1 iron; 1.9 g·100 g−1 manganese; 2.9 μg·100 g−1 selenium; 12 mg·100 g−1 zinc; 275 ng cholecalciferol ·g−1; 19.8 μg retinol·g−1; 0.22 μg α-tocopherol·g−1; 0.90 μg·g−1 menadione; 0.05 μg·g−1 biotin; 0.34 mg·g−1 choline; 0.41 μg·g−1 folic acid; 0.03 mg·g−1 niacin; 0.05 mg·g−1 pantothenic acid; 8.1 μ·g−1 pyridoxine; 0.03 mg·g−1 riboflavin; 5.9 μg·g−1 thiamin; 0.03 μg·g−1 cyanocobalamin; 0.27 mg·g−1 ascorbic acid; 2.6 g·100 g−1 leucine; and 2.3 g·100 g−1 lysine.
References
- 1.Davis TA, Fiorotto MF, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of suckling rats. Am J Physiol. 1989;257:R1141–6. [DOI] [PubMed] [Google Scholar]
- 2.Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Enhanced response of muscle protein synthesis and plasma insulin to food intake in suckled rats. Am J Physiol. 1993;265:R334–40. [DOI] [PubMed] [Google Scholar]
- 3.Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than in 26-day-old pigs. Am J Physiol Endocrinol Metab. 1996;270:E802–9. [DOI] [PubMed] [Google Scholar]
- 4.Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O'Connor PMJ. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab. 2002;282:E880–90. [DOI] [PubMed] [Google Scholar]
- 5.O'Connor PMJ, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2003;285:E40–53. [DOI] [PubMed] [Google Scholar]
- 6.O'Connor PMJ, Bush JA, Suryawan A, Nguyen HV, Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2003;284:E110–9. [DOI] [PubMed] [Google Scholar]
- 7.Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR. Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2000;279:E1226–34. [DOI] [PubMed] [Google Scholar]
- 8.Kimball SR, Jefferson LS, Nguyen HV, Suryawan A, Bush JA, Davis TA. Feeding stimulates protein synthesis in muscle and liver of neonatal pigs through an mTOR-dependent process. Am J Physiol Endocrinol Metab. 2000;279:E1080–7. [DOI] [PubMed] [Google Scholar]
- 9.Suryawan A, Nguyen HV, Bush JA, Davis TA. Developmental changes in the feeding-induced activation of the insulin-signaling pathway in neonatal pigs. Am J Physiol Endocrinol Metab. 2001;281:E908–15. [DOI] [PubMed] [Google Scholar]
- 10.Yoshizawa F, Endo M, Ide H, Yagasaki K, Funabiki R. Translational regulation of protein synthesis in the liver and skeletal muscle of mice in response to refeeding. J Nutr Biochem. 1995;6:130–6. [Google Scholar]
- 11.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–26. [DOI] [PubMed] [Google Scholar]
- 12.Mamane Y, Petroulaskis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–22. [DOI] [PubMed] [Google Scholar]
- 13.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. [DOI] [PubMed] [Google Scholar]
- 14.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. [DOI] [PubMed] [Google Scholar]
- 15.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. [DOI] [PubMed] [Google Scholar]
- 16.Avruch J, Hara K, Lin Y, Liu M, Long X, Oritz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–72. [DOI] [PubMed] [Google Scholar]
- 17.Jefferies HB, Reinhard C, Kozma SC, Thomas G. Rapamycin selectively represses translation of the 'polyprimidine tract' mRNA family. Proc Natl Acad Sci. 1994;91:4441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–8. [DOI] [PubMed] [Google Scholar]
- 19.Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR. Oncogene. 2006;25:6347–60. [DOI] [PubMed] [Google Scholar]
- 20.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. [DOI] [PubMed] [Google Scholar]
- 21.Proud CG. eIF2 and the control of cell physiology. Semin Cell Dev. 2004;16:3–12. [DOI] [PubMed] [Google Scholar]
- 22.Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab. 2008;295:E868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab. 2005;288:E914–21. [DOI] [PubMed] [Google Scholar]
- 24.Wray-Cahen D, Nguyen HV, Burrin DG, Beckett PR, Fiorotto ML, Reeds PJ, Wester TJ, Davis TA. Response of skeletal muscle protein synthesis to insulin in suckling pigs decreases with development. Am J Physiol Endocrinol Metab. 1998;275:E602–9. [DOI] [PubMed] [Google Scholar]
- 25.Garlick PJ, Mcnurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980;192:719–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis TA, Fiorotto ML, Nguyen HV, Burrin DG. Aminoacyl-tRNA and tissue free amino acid pools are equilibrated after a flooding dose of phenylalanine. Am J Physiol. 1999;277:E103–9. [DOI] [PubMed] [Google Scholar]
- 27.Kubica N, Bolster DR, Farrell PA, Kimball SR, Jefferson LS. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2B{epsilon} mRNA in a mammalian target of Rapamycin-dependent manner. J Biol Chem. 2005;280:7570–80. [DOI] [PubMed] [Google Scholar]
- 28.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab. 2006;290:E612–21. [DOI] [PubMed] [Google Scholar]
- 29.Frank JW, Escobar J, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Dietary protein and lactose increase translation initiation factor activation and tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2006;290:E225–33. [DOI] [PubMed] [Google Scholar]
- 30.Frank JW, Escobar J, Suryawan A, Kimball SR, Nguyen HV, Jefferson LS, Davis TA. Protein synthesis and translation initiation factor activation in neonatal pigs fed increasing levels of dietary protein. J Nutr. 2005;135:1374–81. [DOI] [PubMed] [Google Scholar]
- 31.Thivierge MC, Bush JA, Suryawan A, Nguyen HV, Orellana RA, Burrin DG, Jahoor F, Davis TA. Whole-body and hindlimb protein breakdown are differentially altered by feeding in neonatal piglets. J Nutr. 2005;135:1430–7. [DOI] [PubMed] [Google Scholar]
- 32.Jeyapalan AS, Orellana R, Suryawan A, O'Connor PMJ, Nguyen HV, Escobar J, Frank J, Davis TA. Glucose stimulates protein synthesis in skeletal muscle of neonatal pigs through AMPK and mTOR independent process. Am J Physiol Endocrinol Metab. 2007;293:E595–603. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Rhodes CJ, Lawrence JC. Activation of mammalian target of rapamycin (mTOR) by insulin is associated with stimulation of 4EBP1 binding to dimeric mTOR complex 1. J Biol Chem. 2006;281:24293–303. [DOI] [PubMed] [Google Scholar]
- 34.Proud CG. mTOR-mediated regulation of translation factors by amino acids. Biochem Biophys Res Commun. 2004;313:429–36. [DOI] [PubMed] [Google Scholar]
- 35.Boirie Y, Dangin M, Gachon P, Vasson M, Maubois J, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA. 1997;94:14930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dangin M, Boirie Y, Guillet C, Beaufrere B. Influence of the protein digestion rate on protein turnover in young and elderly subjects. J Nutr. 2002;132:S3228–33. [DOI] [PubMed] [Google Scholar]
- 37.Vary TC, Lynch CJ. Meal feeding stimulates phosphorylation of multiple effector proteins regulating protein synthetic processes in rat hearts. J Nutr. 2006;136:2284–90. [DOI] [PubMed] [Google Scholar]
- 38.Vary TC, Deiter G, Lynch CJ. Rapamycin limits formation of active eukaryotic initiation factor 4F complex following meal feeding in rat hearts. J Nutr. 2007;137:1857–62. [DOI] [PubMed] [Google Scholar]
- 39.Kelly FJ, Jefferson LS. Control of peptide-chain initiation in rat skeletal muscle. J Biol Chem. 1985;260:6677–83. [PubMed] [Google Scholar]
- 40.Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–8. [DOI] [PubMed] [Google Scholar]
- 41.Suryawan A, Orellana RA, Nguyen HV, Jeyapalan AS, Fleming JR, Davis TA. Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated. Am J Physiol Endocrinol Metab. 2007;293:E1597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tremblay F, Gagnon A, Veilleux A, Sorisky A, Marette A. Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3–L1 and human adipocytes. Endocrinology. 2005;146:1328–37. [DOI] [PubMed] [Google Scholar]
- 43.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:S227–31. [DOI] [PubMed] [Google Scholar]
- 44.Suryawan A, Escobar J, Frank JW, Nguyen HV, Davis TA. Developmental regulation of the activation of signaling components leading to translation initiation in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2006;291:E849–59. [DOI] [PubMed] [Google Scholar]
- 45.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vary TC, Anthony JC, Jefferson LS, Kimball SR, Lynch CJ. Rapamycin blunts nutrient stimulation of eIF4G, but not PKC epsilon phosphorylation, in skeletal muscle. Am J Physiol Endocrinol Metab. 2007;293:E188–96. [DOI] [PubMed] [Google Scholar]
- 47.Williamson DL, Bolster DR, Kimball SR, Jefferson LS. Time course changes in signaling pathways and protein synthesis in C2C12 myotubes following AMPK activation by AICAR. Am J Physiol Endocrinol Metab. 2006;291:E80–9. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi AA, Proud CG. The rapid activation of protein synthesis by growth hormone requires signaling through mTOR. Am J Physiol Endocrinol Metab. 2007;292:E1647–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.