Abstract
Soy isoflavones and their metabolites, with estrogenic activity, have been considered candidates for reducing postmenopausal bone loss. In this study, we examined the effect of dietary equol, a bioactive metabolite of the soy isoflavone daidzein, on equol tissue distribution, bone parameters, and reproductive tissue activity using an adult ovariectomized (OVX) rat model. An 8-wk feeding study was conducted to compare 4 dietary treatments of equol (0, 50, 100, 200 mg/kg diet) in 6-mo-old OVX female Sprague-Dawley rats. A dose response increase in tissue equol concentrations was observed for serum, liver, kidney, and heart, and a plateau occurred at 100 mg equol/kg diet for intestine. In OVX rats receiving 200 mg equol/kg diet, femoral calcium concentration was greater than those receiving lower doses but was still less than SHAM (P < 0.05), and other bone measures were not improved. Tibia calcium concentrations were lower in OVX rats receiving 100 and 200 mg equol/kg diet compared with the OVX control rats. Trabecular bone mineral density of tibia was also lower in equol-fed OVX rats. At this dietary equol intake, uterine weight was higher (P < 0.05) than in other OVX groups but lower than the SHAM-operated intact rats. The 200 mg/kg diet dose of dietary equol significantly increased proliferative index in the uterine epithelium. Dietary equol had no stimulatory effect on mammary gland epithelium. We conclude that in OVX rats, a dietary equol dose that had modest effect on bone also exerts mild uterotropic effects.
Introduction
In 2002, results of the Women';s Health Initiative Study revealed adverse effects of estrogen therapy, including increased risk for cardiovascular disease and breast cancer, despite positive benefits of reduced bone loss (1). This created interest in alternative choices with bone protective effects such as soy isoflavones and their metabolites (2). Soy isoflavones are commonly referred to as phytoestrogens due to their chemical structure, which is similar to estrogen. It has been postulated that soy isoflavones exert their actions through binding with estrogen receptor (ER)9-β (2) in osteoblast cells. This leads to suppression of osteoclast differentiation via osteoprotegerin (OPG) and subsequent inhibition of bone resorption (3). Specifically, OPG competes with receptor-activated nuclear factor k receptor (RANK) for binding to RANK ligand, which is essential for osteoclast differentiation (3). A recent study showed that administering soy isoflavone genistein to osteopenic postmenopausal women for 2 y resulted in higher OPG: RANK ligand ratios and femoral neck bone mineral density (BMD) compared with a placebo group (4).
Some evidence suggests that soy isoflavone metabolites exert more potent effects on bone than their parent precursors (5). In particular, equol [7-hydroxy-3-(4-hydroxyphenyl)-chroman], a metabolite derived from the soy isoflavone daidzein, has 80 times more ERβ binding affinity than its parent compound (6). Equol is produced by intestinal bacterial metabolism of daidzein. After consumption of soy, the glycoside form of daidzein, daidzin, is converted to the aglycone form, daidzein, by enzymatic hydrolysis of the sugar group and daidzein is further metabolized to equol. Thus, equol production is exclusively dependent on the bacterial composition of intestinal microbiota. Several bacteria (bifidobacteria and lactobacilli) have been speculated to influence equol-producing ability (7). It is estimated that only 30–50% of humans are equol producers (8–10). The difference in equol-producing ability could explain the inconsistencies among clinical studies of isoflavones (2,9). Although rodents are equol producers, variability exists among various strains. Ward et al. (11) examined the equol production of 4 mouse strains, 2 inbred and 2 outbred, by providing the same amount of daidzein (200 mg/kg diet) in the diet for 3 wk and assessing serum equol levels. The outbred strains had an almost 3- to 4-fold increase in serum equol levels and concurrent lower serum daidzein levels compared with inbred strains supplemented with daidzein (11).
There have been only a few studies that assessed the biological effects of equol, due to its limited commercial availability. Most studies have examined the effect of dietary equol on reproductive tissues (12–14), and few have assessed the impact on bone health (15,16). All studies had to use high doses of equol (≥400 mg/kg diet) to inhibit bone loss, which also caused adverse effects on reproductive tissues. However, there is currently no direct evidence supporting a positive action of dietary equol on bone metabolism at physiological doses (0–250 mg/kg diet).
Our aim was to determine the effect of dietary equol on bone metabolism in ovariectomized (OVX) rats. Such a study became feasible only with the recent capability to synthesize equol (6). A dietary dose ranging study allowed insight into the potential benefits of dietary equol supplement for those unable to produce equol naturally.
Materials and Methods
Rats.
Seventy-eight virgin female Sprague Dawley rats (6 mo old) were purchased from Harlan, of which 62 were OVX and 16 were SHAM operated 2 d before shipment. Rats were housed in individual cages in a temperature- and humidity-controlled room with lights on a 12:12-h on:off cycle. An AIN-93M diet (17) and distilled water were provided ad libitum for an acclimation period of 2 d. All procedures were approved by and performed in compliance with the rules of Purdue University';s Animal Care and Use Committee.
Diet and equol supplement.
Equol powder (50% R-equol, 50% S-equol; Supplemental Fig. 1) was produced as previously described (6) and mixed evenly with AIN-93M base diet (17) ingredients (Dyets) at the following concentrations: 0, 50, 100, and 200 mg/kg diet. Doses were selected based on a pilot study in rats aimed to achieve serum human equol levels, 0–1, 3–39, and 5–49 μmol/L, observed in postmenopausal women in response to dietary soy isoflavones (0, 56, and 90 mg/d), respectively (18).
Study design.
All OVX rats were weighed and assigned to 1 of the following dietary treatments: OVX-0, OVX-50, OVX-100, and OVX-200 to ensure similar mean body weight across OVX groups. Dietary treatments consisted of an AIN-93M diet with 0 (n = 15), 50 (n = 16), 100 (n = 15), and 200 (n = 16) mg equol/kg diet, respectively. They were pair-fed the mean intake of SHAM controls (n = 16; 0 mg/kg diet equol) to avoid OVX-induced hyperphagia. After 8 wk of dietary treatment, rats were killed by excess CO2. Blood was drawn via the portal vein and serum was stored at −80°C for isoflavone analysis. Various tissues, including kidney, liver, whole intestine, and heart (flushed with 50 mL saline), were excised and stored at −80°C for isoflavone analysis. The uterus and caudal mammary glands were collected and stored in 10% buffered formalin for histopathologic examination and assessment of epithelial proliferation. Right femurs and tibiae were wrapped in saline-soaked gauze and stored at −20°C for evaluation of bone mechanical properties. Left femurs and tibiae were collected and placed in 70% ethanol and stored at 4°C for scanning by peripheral quantitative computed tomography (pQCT).
Serum and tissue equol measurement.
Serum and tissue concentrations of equol were quantified using a highly sensitive and specific electrospray ionization liquid chromatography-multiple reaction ion monitoring MS method as described previously (19). There were modifications in conditions of chromatography as listed below. Chromatography was carried out on a 30- × 2.0-mm i.d. Phenomenex Phenyl-Hexyl column using mobile phase consisting of a gradient of 10–80% acetonitrile in 10 mmol/L ammonium acetate over 5 min with a flow rate of 0.4 mL/min. Equol was detected using the transition of its m/z 241 molecular ion and its m/z 119 fragment ion. Tissue samples were prepared by removing 0.2–0.250 g of tissue from sample and homogenization in 2 mL of an 80% methanol, 0.01% (wt:v) ascorbic acid solution. Extraction techniques were described previously (19).
Bone density, size, and mechanical properties.
Right femurs and tibiae were measured for length and width at the midshaft using an ABS digimatic solar caliper (Tri-State Instrument Service). Bone density was measured by underwater weighing using a density determination kit and Mettler Toledo analytical balance. Bone strength was assessed using a 3-point bending test on a TA-XT2 Texture analyzer (Texture Technologies).
pQCT measurements.
pQCT analysis was performed with the Stratec peripheral quantitative computed tomograph (model Research SA+ of Norland Stratec XCT, Stratec Electronics). Three cross-sectional sites (1.0 mm thick) from distal, midshaft, and proximal sites of bones, determined at 12, 50, and 88% of the length of from distal, were scanned using a 0.46-mm collimation (4 × 105 counts/s) and a 0.08-mm voxel size. Thresholds for segmentation of trabecular and cortical bone were set at 300 mg/cm3 and 900 mg/cm3, respectively.
Micro computed tomography measurements.
Bone architecture was assessed by micro computed tomography (μCT) analysis using a Scanco microCT tomograph (μCT 40, Scanco Medical). Eight femurs were randomly selected from each of the following groups: SHAM, OVX-0, and OVX-200. Cancellous and cortical bone of femurs was evaluated using methods and parameters described previously (20). Cancellous bone at the distal femur was scanned with an isotropic resolution of 16 μm obtained from 0.2 down the growth plate extended for 62 slices. In the femur, 62 contiguous slices were contoured and imaging proceeded proximally covering a distance of 1 mm.
Bone calcium content.
After mechanical testing, right femurs and tibias were dissolved in concentrated nitric acid overnight and analyzed for total calcium content using atomic absorption spectrometry (PE S100, Perkin Elmer).
Reproductive tissue analysis.
The extent of epithelial proliferation in mammary glands and uterine epithelium was determined by visually scoring formalin-fixed tissue sections. Each specimen was scored on scale from 0 to 3, with 0 and 2 equivalent to the extent of epithelial proliferation in OVX and SHAM controls, respectively. In addition to visual scoring, the proliferative index in these tissues was determined by proliferative cell nuclear antibody (PCNA) immunohistochemistry using a technique previously reported (12). The proliferative index was defined as the percentage of cells with unequivocal nuclear immunostaining.
Statistical analysis.
Data were analyzed using SAS (version 9.1, SAS Institute). The effect of equol on bone parameters was determined by 1-way ANOVA and multiple comparisons using Tukey';s test after checking the normal distribution and constant variance assumptions of ANOVA. In the case of nonnormal distribution (reproductive tissue data), data underwent log transformation before analysis by 1-way ANOVA and Tukey';s test. Significance was accepted at P < 0.05.
Results
Body and uterine weight.
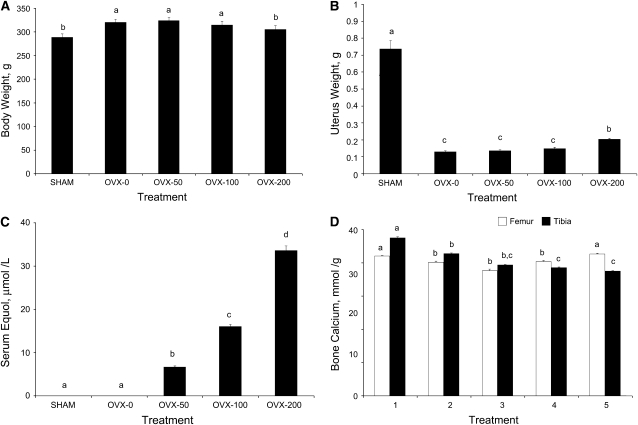
All OVX groups except OVX-200 had significantly higher body weight than the SHAM despite pair feeding. However, there was no significant effect of equol supplementation on body weight of OVX rats (Fig. 1A). Dietary intake was similar among all OVX groups, which were pair-fed to the mean intake of SHAM rats. Feed efficiency ratios were similar among OVX-0 (0.036 ± 0.023), OVX-50 (0.046 ± 0.020), and OVX-100 (0.035 ± 0.023) but significantly higher than for SHAM (0.090 ± 0.019) and OVX-200 (0.210 ± 0.024) rats. Uterine weight in all OVX groups was significantly lower than in SHAM rats (Fig. 1B). In OVX rats, the uterine weight was greater only in the OVX-200 group (55% higher than OVX-0; P ≤ 0.05) but was still substantially less than in SHAM (72% lower; P ≤ 0.05).
FIGURE 1 .
Body weight (A), uterine weight (B), serum equol concentrations (C), and right femoral and tibia calcium concentration (D) in sham-operated and OVX rats fed diets containing various levels of equol (n = 15–16/group). Means without a common letter differ, P < 0.05.
Equol analysis.
Equol levels of 50, 100, and 200 mg/kg diet of OVX rats resulted in total serum (S/R) equol concentrations increasing with increasing dose of dietary equol (Fig. 1C). Liver, kidney, and heart tissue concentrations also increased with increasing dose of dietary equol. Equol levels in the intestine increased in all equol groups compared with OVX-0, but the increase was not dose dependent (data not shown).
Bone calcium concentration, size, and mechanical properties.
Femoral density measured by underwater weighing was higher (P < 0.05) in the SHAM group than in the OVX groups but was not affected by equol in the diet (Table 1). There were no group differences in femoral length, width, or breaking force. SHAM rats had higher (P < 0.05) femoral calcium concentration than OVX-0 (Fig. 1D). Among all OVX groups, only the OVX-200 group maintained a femoral calcium concentration similar to SHAM rats and higher (P < 0.05) femoral calcium concentration than OVX-0 rats.
TABLE 1.
Bone characteristics of SHAM-operated and OVX rats fed diets containing various levels of equol1
| Treatments
|
|||||
|---|---|---|---|---|---|
| SHAM | OVX-0 | OVX-50 | OVX-100 | OVX-200 | |
| Femur | |||||
| Bone density,2g/cm3 | 1.56 ± 0.030a | 1.51 ± 0.030b | 1.50 ± 0.040b | 1.50 ± 0.030b | 1.52 ± 0.030b |
| Tibia | |||||
| Bone density,2g/cm3 | 1.50 ± 0.040a | 1.48 ± 0.030ab | 1.46 ± 0.030b | 1.46 ± 0.050ab | 1.48 ± 0.030ab |
| Distal femur, pQCT | |||||
| Total BMC, mmol Ca/g body wt | 0.414 ± 0.082a | 0.337 ± 0.051b | 0.307 ± 0.025b | 0.319 ± 0.042b | 0.321 ± 0.041b |
| Trabecular BMD, mg/cm3 | 558 ± 51.0a | 448 ± 60.0b | 410 ± 43.0b | 414 ± 36.0b | 425 ± 55.0b |
| Distal femur,3μCT | |||||
| Bone volume/tissue volume | 0.250 ± 1.00a | 0.080 ± 0.030b | 0.090 ± 0.050b | ||
| Trabecular connectivity density, 1/mm3 | 78.0 ± 19.0a | 19.0 ± 11.0b | 24.0 ± 16.0b | ||
| Trabecular number, 1/mm | 4.28 ± 0.550a | 2.39 ± 0.390b | 2.61 ± 0.520b | ||
| Proximal femur, pQCT | |||||
| Trabecular BMD, mg/cm3 | 543 ± 65.0a | 481 ± 36.0b | 454 ± 46.0b | 466 ± 54.0b | 486 ± 45.0b |
| Proximal tibia, pQCT | |||||
| Total BMC, mmol Ca/g body wt | 0.270 ± 0.033a | 0.255 ± 0.035b | 0.255 ± 0.038b | 0.263 ± 0.087b | 0.230 ± 0.025b |
| Trabecular BMD, mg/cm3 | 652 ± 48.0a | 609 ± 43.0b | 554 ± 30.0c | 559 ± 31.0c | 578 ± 49.0c |
Values are means ± SD, n = 15–16. Means without a common letter differ, P < 0.05.
Bone density was calculated by underwater weighing.
μCT measurements were only performed on SHAM, OVX-0 ppm, and OVX-200 ppm equol groups, n = 8.
There was no significant decrease in tibial density in response to ovariectomy, but the OVX-50 group had a lower tibia density from SHAM. There were no group differences in tibia length, width, or breaking force. SHAM rats had a significantly higher tibia calcium concentration than all OVX rats. Among OVX rats, OVX-100 and OVX-200 had significantly lower tibia calcium concentrations than the OVX-0 and OVX-50 groups (Fig. 1D).
pQCT measurements.
In the midshaft femur and tibia, SHAM rats had significantly higher total BMC than all OVX groups. There were no differences among treatment groups for total BMD, cortical BMD, and cortical thickness at the midshaft femur and tibia.
For the distal femur, the SHAM group had significantly higher total BMC, total BMD, trabecular BMD, and cortical thickness than OVX-0 rats. There were no differences among OVX rats at the distal femur.
At the proximal femur, the SHAM group had significantly higher trabecular BMD than all OVX groups (Table 1). Treatment groups did not differ for total BMD, cortical BMD, and cortical thickness at the proximal femur.
At the proximal tibia, SHAM rats had significantly higher total BMC than all OVX groups (Table 1). Among OVX rats, all those fed dietary equol had significantly lower trabecular BMD compared with OVX-0 rats. Treatment groups did not differ for total BMD, cortical BMD, and cortical thickness at the proximal tibia.
μCT measurements.
At the distal femur, the SHAM group had significantly higher trabecular connectivity and significantly lower trabecular spacing than the OVX-0 and OVX-200 groups (Table 1). Treatment groups did not differ in cortical thickness, porosity, and cortical BMD at the midshaft of the femur.
Evaluation of mammotropic and uterotropic effects of equol.
The SHAM group had significantly higher mammary gland epithelial mass on visual scoring than all OVX groups (Table 2). Among OVX groups, equol did not affect mammary epithelial proliferation as evident by PCNA immunostaining (Table 2).
TABLE 2.
Reproductive tissue proliferation in SHAM-operated and OVX rats fed diets containing various levels of equol1
| Treatments
|
|||||
|---|---|---|---|---|---|
| SHAM | OVX-0 | OVX-50 | OVX-100 | OVX-200 | |
| Mammary tissue | |||||
| Gland proliferation | 1.94 ± 0.570a | 0.53 ± 0.740b | 0.38 ± 0.50b | 0.47 ± 0.510b | 0.94 ± 0.570b |
| PCNA, % | 14.8 ± 15.3a | 1.98 ± 2.11b | 1.14 ± 1.66b | 2.32 ± 3.72b | 2.59 ± 4.70b |
| Uterine tissue | |||||
| Epithelial proliferation | 1.94 ± 0.770a | 0.000 ± 0.000c | 0.130 ± 0.340c | 0.330 ± 0.490bc | 0.750 ± 0.580b |
| Epithelial PCNA, % | 37.6 ± 21.8a | 1.14 ± 2.09b | 0.670 ± 1.15b | 8.95 ± 12.4a | 15.3 ± 18.0a |
| Stroma PCNA, % | 19.2 ± 21.1a | 0.650 ± 1.45b | 0.920 ± 1.92b | 2.67 ± 2.99ab | 4.18 ± 3.98a |
Values are means ± SD, n = 15–16. Means without a common letter differ, P < 0.05.
Based on visual scoring of histologic sections of the uterus, the SHAM group had significantly greater uterine epithelial mass than all OVX groups. OVX-200 rats had significantly greater epithelial mass than OVX-0 and OVX-50 but not OVX-100 rats. PCNA immunostaining showed SHAM control, OVX-100, and OVX-200 rats had significantly higher uterine epithelial proliferative index compared with the OVX-0 and OVX-50 rats (Table 2). SHAM rats and OVX-200 rats had significantly higher uterine stroma cell proliferative index than OVX-0 and OVX-50 but not OVX-100 rats.
Discussion
Because soy is the most abundant source of isoflavones in the human diet, the 2 major soy isoflavones, daidzein and genistein, have been extensively evaluated for their impact on mitigating estrogen-related bone loss (11,21–24). Investigators have also started to examine soy isoflavone metabolites, such as equol, as potentially more biologically potent than their endogenous precursors (6,9,25). Here, we show in OVX rats that dietary intake of equol at a level of 200 mg/kg diet induces few changes in bone calcium concentration, specifically a beneficial increase in femoral calcium concentration but a negative decrease in tibia calcium concentration. Furthermore, this level of dietary equol intake exerted adverse effects in uterine, but not mammary, tissue in OVX animals.
Few studies have characterized humans by their ability to produce equol. Wu et al. (26) classified 68 study participants out of 122 as equol producers based on conversion rate of daidzein to equol present in fecal bacteria. They found that equol producers had a significantly lower percent change in total hip BMD after 24 wk of soy isoflavone treatment compared with nonproducers. Similarly, Setchell et al. (2) observed a 2.4% increase in lumbar spine BMD of equol producers (45% of participants) after a 2-y intervention with soy isoflavones compared with the 0.6% increase of nonproducers. Studies on the direct effect of dietary equol on bone health have been limited because of the high cost and limited availability of commercial sources. Consequently, the few studies that examined the effects of equol in vivo have administered it by subcutaneous injection or osmotic pump to conserve the compound rather than via the more physiological route of dietary intake (14,15). This may reflect the relationship between a metabolite produced in vivo and its health effects but does not address bioavailability of equol as a dietary supplement. Administration of 0.5 mg/μL equol, but not 0.1 mg in 1 μL, by osmotic pump inhibited OVX-induced bone loss of the whole body, femur, and lumbar spine in ddY mice (27). In our study, equol was fed to rats as a dietary supplement, so that serum levels reflected digestion and absorption, relevant for designing a dietary supplement.
Serum and most tissue concentrations of equol increased with increasing dietary equol. Serum concentrations corresponded to levels seen in previous studies with rodents receiving medium to high doses of dietary daidzein (28–30). Serum concentrations are also similar to levels in clinical participants (postmenopausal women) from our laboratory, classified as equol producers, who consumed between 120–150 mg isoflavones/d (20).
In this dose response study on the effect of dietary equol on bone health, we found a modest effect on femoral calcium concentration. In contrast, dietary equol appeared to have a detrimental effect on tibia calcium concentration and trabecular BMD at the proximal tibia. Whole bone measures of the femur and tibia are largely cortical bone. Calcium absorption efficiency tended to be greater (P = 0.07) in the OVX-100 (0.338 ± 0.088) and OVX-200 (0.352 ± 0.124) groups than in the SHAM (0.300 ± 0.121), OVX-0 (0.280 ± 0.103), and OVX-50 (0.020 ± 0.037) groups. Skeletal site-specific results could be attributed to a differential response between cortical and trabecular bone. Alternatively, skeletal site-specific results could be due to differences in mechanical loading for the animal model used in this investigation. In this animal model, the tibia undergoes more mechanical stress than the femur (31). Saxon et al. (32) demonstrated that estrogen-like actions mediated through ERβ could inhibit bone formation under mechanical loading. Investigators applied daily loading to 16-wk-old mice with ERβ null mutations (ERβ−/−) and found that ERβ−/− mice had a 3.6-fold increase in bone formation after mechanical loading compared with wild type, ERβ+/+, mice. Additional research should examine the effect of estrogenic-like actions on bone mechanical signaling and the subsequent impact on bone health.
Our results also showed that despite pair feeding, only the highest dietary equol dose (200 mg/kg diet) helped maintain body weight at SHAM levels, probably by exerting estrogen-like effects on general metabolism. The highest dietary equol level was also associated with increased uterine weight. The weight differential between SHAM and OVX despite pair feeding (21) and uterotropic effects of dietary equol (13) have been observed previously. A risk factor associated with estrogen therapy or compounds exerting estrogen-like effects is the growth stimulation of reproductive tissue, especially uterus. Increased uterine weight is a classical estrogen effect mediated by ERα (33–35). At equol levels of 0.1 or 0.5 mg/d, no effect on uterine weight was observed in OVX mice (27), whereas weight was increased in mice receiving equol injections at 12 and 20 mg/kg body weight per day (14). Similarly, Rachon et al. (15) observed increased uterine weight as well as higher proliferation in uterine epithelial cells after administrating 2 doses of racemic equol (50 and 400 mg/kg unpurified diet) for 3 mo to OVX Sprague Dawley rats. In our study, only the highest dose of dietary equol, 200 ppm, caused a significant increase (55%) in uterine weight compared with the OVX control group as a reference as well as increased epithelial proliferation. Findings of uterotropic effects from dietary equol could be due to the fact that the only form of synthesized equol available is the racemic mixture and the R-equol isomer has an affinity for ERα, which is abundant in reproductive tissue. Moreover, S-equol has a greater affinity for ERβ than R-equol (6). Recently, Heemstra et al. (36) developed a method for total synthesis of S-equol. Future studies should determine whether greater efficacy without uterotropic effects will occur with dietary administration of S-equol.
Our findings demonstrate that a 200-mg equol/kg diet dose in OVX rats has modest bone health benefits. Results also showed that a 200-mg equol/kg diet dose did not induce changes in the mammary epithelium but mildly stimulated the uterine epithelium. Our study suggests that the benefit:risk ratio of racemic equol has low promise as a commercial supplement. Given that S-equol is the natural bacterial product with a higher binding affinity for ERβ and not ERα compared with R-equol, future research should establish whether S-equol would have greater bone protective properties without reproductive tissue stimulation.
Supplementary Material
Acknowledgments
We thank Pamela Lachcik for technical assistance. L.L., B.M., and C.W. designed the research; L.L., M.S., W.L., J.W., A.A., J.Q., and D.B. conducted the research; L.L. and M.S. analyzed the data. W.H., D.W., and S.B. provided essential materials. L.L. and C.W. wrote the paper and had primary responsibility for final content. All authors read and approved the final manuscript.
Supported by Purdue University, University of Alabama Botanical Center for Age Related Diseases, and NIH grants P50 AT00477-01 and P50 AT000477-07S1.
Author disclosures: L. L. Legette, B. R. Martin, M. Shahnazari, W-H. Lee, W. G. Helfreich, J. Qian, D. J. Waters, A. Arabshahi, J. Welch, and D. G. Bostwick, no conflicts of interest. C. M. Weaver is on the board of Pharmavite; S. Barnes has a U.S. patent on the use of conjugated isoflavones for prevention of osteoporosis.
Supplemental Figure 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: BMC, bone mineral content; BMD, bone mineral density; ER, estrogen receptor; OPG, osteoprotegerin; OVX, ovariectomized; OVX-0, ovariectomized rats receiving 0 mg equol/kg diet in an AIN-93M diet; OVX-50, ovariectomized rats receiving 50 mg equol/kg diet in an AIN-93M diet; OVX-100, ovariectomized rats receiving 100 mg equol/kg diet in an AIN-93M diet; OVX-200, ovariectomized rats receiving 200 mg equol/kg diet in an AIN-93M diet; pQCT, peripheral quantitative computed tomography; RANK, receptor activated nuclear factor k receptor; μCT, micro computed tomography.
References
- 1.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. [DOI] [PubMed] [Google Scholar]
- 2.Setchell KD, Lydeking-Olsen E. Dietary phytoestrogens and their effect on bone: evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am J Clin Nutr. 2003; 78 Suppl 3:S593–609. [DOI] [PubMed] [Google Scholar]
- 3.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. [DOI] [PubMed] [Google Scholar]
- 4.Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, Gaudio A, Mazzaferro S, Frisina A, et al. OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J Bone Miner Res. 2008;23:715–20. [DOI] [PubMed] [Google Scholar]
- 5.Phrakonkham P, Chevalier J, Desmetz C, Pinnert MF, Berges R, Jover E, Davicco MJ, Bennetau-Pelissero C, Coxam V, et al. Isoflavonoid-based bone-sparing treatments exert a low activity on reproductive organs and on hepatic metabolism of estradiol in ovariectomized rats. Toxicol Appl Pharmacol. 2007;224:105–15. [DOI] [PubMed] [Google Scholar]
- 6.Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559–67. [DOI] [PubMed] [Google Scholar]
- 7.Bolca S, Possemiers S, Herregat A, Huybrechts I, Heyerick A, De Vriese S, Verbruggen M, Depypere H, De Keukeleire D, et al. Microbial and dietary factors are associated with the equol producer phenotype in healthy postmenopausal women. J Nutr. 2007;137:2242–6. [DOI] [PubMed] [Google Scholar]
- 8.Frankenfeld CL, McTiernan A, Thomas WK, LaCroix K, McVarish L, Holt VL, Schwartz SM, Lampe JW. Postmenopausal bone mineral density in relation to soy isoflavone-metabolizing phenotypes. Maturitas. 2006;53:315–24. [DOI] [PubMed] [Google Scholar]
- 9.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–84. [DOI] [PubMed] [Google Scholar]
- 10.Song KB, Atkinson C, Frankenfeld CL, Jokela T, Wahala K, Thomas WK, Lampe JW. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J Nutr. 2006;136:1347–51. [DOI] [PubMed] [Google Scholar]
- 11.Ward WE, Kim S, Chan D, Fonseca D. Serum equol, bone mineral density and biomechanical bone strength differ among four mouse strains. J Nutr Biochem. 2005;16:743–9. [DOI] [PubMed] [Google Scholar]
- 12.Rachon D, Menche A, Vortherms T, Seidlova-Wuttke D, Wuttke W. Effects of dietary equol administration on the mammary gland in ovariectomized Sprague-Dawley rats. Menopause. 2008;15:340–5. [DOI] [PubMed] [Google Scholar]
- 13.Rachon D, Vortherms T, Seidlova-Wuttke D, Menche A, Wuttke W. Uterotropic effects of dietary equol administration in ovariectomized Sprague-Dawley rats. Climacteric. 2007;10:416–26. [DOI] [PubMed] [Google Scholar]
- 14.Selvaraj V, Zakroczymski MA, Naaz A, Mukai M, Ju YH, Doerge DR, Katzenellenbogen JA, Helferich WG, Cooke PS. Estrogenicity of the isoflavone metabolite equol on reproductive and non-reproductive organs in mice. Biol Reprod. 2004;71:966–72. [DOI] [PubMed] [Google Scholar]
- 15.Rachon D, Seidlova-Wuttke D, Vortherms T, Wuttke W. Effects of dietary equol administration on ovariectomy induced bone loss in Sprague-Dawley rats. Maturitas. 2007;58:308–15. [DOI] [PubMed] [Google Scholar]
- 16.Mathey J, Mardon J, Fokialakis N, Puel C, Kati-Coulibaly S, Mitakou S, Bennetau-Pelissero C, Lamothe V, Davicco MJ, et al. Modulation of soy isoflavones bioavailability and subsequent effects on bone health in ovariectomized rats: the case for equol. Osteoporos Int. 2007;18:671–9. [DOI] [PubMed] [Google Scholar]
- 17.Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 18.Persky VW, Turyk ME, Wang L, Freels S, Chatterton R Jr, Barnes S, Erdman J Jr, Sepkovic DW, Bradlow HL, et al. Effect of soy protein on endogenous hormones in postmenopausal women. Am J Clin Nutr. 2002;75:145–53. [DOI] [PubMed] [Google Scholar]
- 19.Sites CK, Cooper BC, Toth MJ, Gastaldelli A, Arabshahi A, Barnes S. Effect of a daily supplement of soy protein on body composition and insulin secretion in postmenopausal women. Fertil Steril. 2007;88:1609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver C, Janle E, Martin B, Browne S, Guiden H, Lachcik P, Lee WH. Dairy vs. calcium carbonate in promoting peak bone mass and bone maintenance during subsequent calcium deficiency. J Bone Miner Res. 2009;24:1411–9. [DOI] [PubMed]
- 21.Cai DJ, Zhao Y, Glasier J, Cullen D, Barnes S, Turner CH, Wastney M, Weaver CM. Comparative effect of soy protein, soy isoflavones, and 17beta-estradiol on bone metabolism in adult ovariectomized rats. J Bone Miner Res. 2005;20:828–39. [DOI] [PubMed] [Google Scholar]
- 22.Arjmandi BH, Getlinger MJ, Goyal NV, Alekel L, Hasler CM, Juma S, Drum ML, Hollis BW, Kukreja SC. Role of soy protein with normal or reduced isoflavone content in reversing bone loss induced by ovarian hormone deficiency in rats. Am J Clin Nutr. 1998; 68 Suppl 6:S1358–63. [DOI] [PubMed] [Google Scholar]
- 23.Bahr JM, Nakai M, Rivera A, Walsh J, Evans GL, Lotinun S, Turner RT, Black M, Jeffery EH. Dietary soy protein and isoflavones: minimal beneficial effects on bone and no effect on the reproductive tract of sexually mature ovariectomized Sprague-Dawley rats. Menopause. 2005;12:165–73. [DOI] [PubMed] [Google Scholar]
- 24.Wu J, Wang X, Chiba H, Higuchi M, Nakatani T, Ezaki O, Cui H, Yamada K, Ishimi Y. Combined intervention of soy isoflavone and moderate exercise prevents body fat elevation and bone loss in ovariectomized mice. Metabolism. 2004;53:942–8. [DOI] [PubMed] [Google Scholar]
- 25.Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–9. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Oka J, Higuchi M, Tabata I, Toda T, Fujioka M, Fuku N, Teramoto T, Okuhira T, et al. Cooperative effects of isoflavones and exercise on bone and lipid metabolism in postmenopausal Japanese women: a randomized placebo-controlled trial. Metabolism. 2006;55:423–33. [DOI] [PubMed] [Google Scholar]
- 27.Fujioka M, Uehara M, Wu J, Adlercreutz H, Suzuki K, Kanazawa K, Takeda K, Yamada K, Ishimi Y. Equol, a metabolite of daidzein, inhibits bone loss in ovariectomized mice. J Nutr. 2004;134:2623–7. [DOI] [PubMed] [Google Scholar]
- 28.Lamartiniere CA, Wang J, Smith-Johnson M, Eltoum IE. Daidzein: bioavailability, potential for reproductive toxicity, and breast cancer chemoprevention in female rats. Toxicol Sci. 2002;65:228–38. [DOI] [PubMed] [Google Scholar]
- 29.Ohta A, Uehara M, Sakai K, Takasaki M, Adlercreutz H, Morohashi T, Ishimi Y. A combination of dietary fructooligosaccharides and isoflavone conjugates increases femoral bone mineral density and equol production in ovariectomized mice. J Nutr. 2002;132:2048–54. [DOI] [PubMed] [Google Scholar]
- 30.Zafar TA, Weaver CM, Jones K, Moore DR II, Barnes S. Inulin effects on bioavailability of soy isoflavones and their calcium absorption enhancing ability. J Agric Food Chem. 2004;52:2827–31. [DOI] [PubMed] [Google Scholar]
- 31.Drapeau MS, Streeter MA. Modeling and remodeling responses to normal loading in the human lower limb. Am J Phys Anthropol. 2006;129:403–9. [DOI] [PubMed] [Google Scholar]
- 32.Saxon LK, Robling AG, Castillo AB, Mohan S, Turner CH. The skeletal responsiveness to mechanical loading is enhanced in mice with a null mutation in estrogen receptor-beta. Am J Physiol Endocrinol Metab. 2007;293:E484–91. [DOI] [PubMed] [Google Scholar]
- 33.Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA. 1997;94:6535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jefferson WN, Padilla-Banks E, Clark G, Newbold RR. Assessing estrogenic activity of phytochemicals using transcriptional activation and immature mouse uterotrophic responses. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:179–89. [DOI] [PubMed] [Google Scholar]
- 35.Padilla-Banks E, Jefferson WN, Newbold RR. The immature mouse is a suitable model for detection of estrogenicity in the uterotropic bioassay. Environ Health Perspect. 2001;109:821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heemstra JM, Kerrigan SA, Doerge DR, Helferich WG, Boulanger WA. Total synthesis of (S)-equol. Org Lett. 2006;8:5441–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.