Abstract
Hypervitaminosis A is increasingly a public health concern, and thus noninvasive quantitative methods merit exploration. In this study, we applied the 13C-retinol isotope dilution test to a nonhuman primate model with excessive liver stores. After baseline serum chemistries, rhesus macaques (Macaca mulatta; n = 16) were administered 3.5 μmol 13C2-retinyl acetate. Blood was drawn at baseline, 5 h, and 2, 4, 7, 14, 21, and 28 d following the dose. Liver biopsies were collected 7 d before and 2 d after dosing (n = 4) and at 7, 14, and 28 d (n = 4/time) after dosing. Serum and liver were analyzed by HPLC and GC-combustion-isotope ratio MS for retinol and its enrichment, respectively. Model-based compartmental analysis was applied to serum data. Lactate dehydrogenase was elevated in 50% of the monkeys. Total body reserves (TBR) of vitamin A (VA) were calculated at 28 d. Predicted TBR (3.52 ± 2.01 mmol VA) represented measured liver stores (4.56 ± 1.38 mmol VA; P = 0.124). Predicted liver VA concentrations (13.3 ± 9.7 μmol/g) were similar to measured liver VA concentrations (16.4 ± 5.3 μmol/g). The kinetic models predict that 27–52% of extravascular VA is exchanging with serum in hypervitaminotic A monkeys. The test correctly diagnosed hypervitaminosis A in all monkeys, i.e. 100% sensitivity. Stable isotope techniques have important public health potential for the classification of VA status, including hypervitaminosis, because no other technique besides invasive liver biopsies, correctly identifies excessive liver VA stores.
Introduction
The deuterated retinol dilution (DRD)7 test has been used to assess vitamin A (VA) status in populations with VA body stores ranging from deficient to normal [reviewed in (1)]. Using the DRD test, subtoxic hepatic VA concentrations, defined as >1 μmol/g liver, were reported in Nicaraguan schoolchildren 1 y after implementation of VA sugar fortification (2). Routine testing is unusual in countries with adequate VA, but hypervitaminosis A was reported in 1 U.S. child (3). Some commonly consumed foods provide preformed VA in excess of the recommended dietary allowance (4), which at times leads to health complications (5). Penniston et al. provided evidence for hypervitaminosis A in captive rhesus monkeys (6,7). Although rhesus monkeys are omnivores, they are predominantly frugivorous in the wild (8). Two wild-caught rhesus monkeys revealed liver VA concentrations significantly below (9) those reported. Taken together, it was concluded that captive monkeys are chronically overfed preformed VA (10).
Although 13C and deuterium (2H) were used to assess β-carotene bioavailability and bioefficacy and 2H to determine VA status (1), the current study applies 13C-dilution methodology to assess VA status and retinol kinetics during hypervitaminosis using a nonhuman primate model. The 13C technique, using 13C4-retinyl acetate and a GC-combustion-isotope ratio MS (GC/C/IRMS), was previously applied to rats (11) and 1 human (12). Because it is more sensitive to isotopic differences than other mass spectroscopic techniques, 13C-IRMS allows the use of smaller tracer doses and the possibility of greater tracer longevity (13). In rats with liver VA concentrations ranging from low to high, predicted total body reserves (TBR) across dietary groups were compared with measured TBR and were highly correlated (r = 0.98; P < 0.0001) (11). Researchers investigating more vulnerable populations, such as pregnant and lactating women and their infants, can adopt this technique (14) as well as the DRD test. The stable isotope techniques are more feasible in these groups than the use of 14C, the radioactive isotope that may be hazardous to human health. True 14C-tracer doses, which are not considered harmful, were used with accelerator MS to study β-carotene metabolism in adults (15,16).
Whereas hypervitaminosis A human case studies are readily found in the literature (17–19), there is little information (20) about the vitamin's metabolism under these conditions. The purposes of the present study were to validate the 13C-retinol isotope dilution test in a nonhuman primate model of hypervitaminosis A and demonstrate its use across the continuum of VA status compared with other studies (21). TBR of VA were calculated using a mass balance equation (22) and compared with HPLC VA analysis of liver biopsies, considered the gold standard for assessing VA status (23). Lastly, proposed kinetic models describe the vitamin's metabolism under dietary-induced hypervitaminosis, providing new information on how hypervitaminotic A monkeys metabolize a test dose.
Materials and Methods
Monkeys.
Rhesus monkeys (Macaca mulatta, n = 16) were housed from birth within the Wisconsin National Primate Research Center (WNPRC), which is accredited by the American Association for the Accreditation of Laboratory Animal Care-International and regulated by university committees and national agencies to ensure compliance with the Animal Welfare Act. All procedures were approved by the University of Wisconsin-Madison's Research Animal Resources Center. For most of the monkeys' lives, Diet A (Purina Mills) was fed and provided 10.5 μmol/d retinyl acetate (6). Fourteen months prior to this study, WNPRC switched the monkeys to Diet B (Harlan-Teklad) (24) providing ∼6 μmol/d retinyl acetate. Although lower than Diet A, it contained twice the NRC's recommendations for VA (10 nmol/g feed) (25). Screening blood samples were subjected to a comprehensive metabolic panel (Integra 800 automated analyzer; Roche Diagnostics).
Design.
Baseline characteristics were obtained before the isotopic dose (Table 1). After baseline blood draws, feed-deprived monkeys were returned to their cages and given one-half a hollowed-out banana that was injected with 3.5 μmol 14, 15-13C2-retinyl acetate in 98 μL corn oil (26) and sealed with 30 g peanut butter. All monkeys received a low-VA, high-fat meal [peanut butter, bread, apple, and banana]. Diet B was returned to the cages after the 5-h blood draw and continued throughout the study. Blood draws were at 5 h and 2, 4, 7, 14, 21, and 28 d following the dose. Brilliant Blue (1.67 mg/kg body weight) was dissolved in gelatin and fed as a fecal marker after the 5-h blood draw (27). Administering the fecal marker at 5 h after the dose allowed an estimation of intestinal transit time, because the marker could be seen in the feces. The dose was assumed to travel no slower than the marker; thus, the unabsorbed dose was excreted by the time the marker was observed. Feces were collected at baseline (n = 4) and up to 2 d following the marker (n = 12) and stored in glass jars at −80°C until 13C analysis by elemental analyzer-IRMS.
TABLE 1.
Baseline characteristics of captive rhesus monkeys (Macaca mulatta) in a VA status assessment study using 13C2-retinyl acetate12
| Characteristic | |
|---|---|
| Age, y | 11.8 ± 2.9 (7.5–15.9) |
| Body weight, kg | 13.4 ± 2.3 (8.6–16.4) |
| Liver VA3 | |
| μmol/g | 11.9 ± 5.39 (4.24–16.8) |
| μmol/liver | 3860 ± 1750 (1320–5280) |
All measurements were made before dose administration.
Values are mean ± SD (range), n = 16 or 4 (liver VA).
Mean liver VA is the sum of retinol and retinyl esters. Total liver VA is calculated based on an estimated liver size (6).
Liver biopsy.
WNPRC veterinarians collected liver biopsies (0.5 g; <1 cm3) 7 d before and 2 d after the dose in the same monkeys (n = 4) and at 7, 14, and 28 d after dosing in different monkeys (n = 4/time). The baseline liver biopsy was 1 wk prior to dose administration to ensure that the 4 monkeys undergoing the procedure twice had sufficient recovery time. Abdominal surgery (n = 12) and laparoscopic techniques (n = 4) were used.
Carbon isotopic composition.
The carbon isotopic composition of serum retinol was analyzed as described previously (28). Enrichments were generated as atom percent 13C (At% 13C).
For liver, 50 mg was ground with sodium sulfate (150 mg) and extracted into 25 mL dichloromethane (6). Extract (1 mL) was dried under argon, resuspended in ethanol (750 μL), and saponified (400 μL 50:50 potassium hydroxide:water; wt:v) at 45°C for 1 h. The reaction was quenched with water (500 μL) and extracted 3 times with hexanes (500 μL). The extract was dried under argon and the film was redissolved (100 μL methanol), centrifuged, and injected onto the first HPLC system with 92.5:7.5 acetonitrile:water (v:v) at 1 mL/min. Retinol was collected, dried, suspended in 100 μL methanol, and injected onto a second system using methanol at 1 mL/min. The retinol was collected into a GC vial and dried using vacuum centrifugation. The film was suspended in hexanes (20 μL) and injected (1.5 μL) into the GC/C/IRMS.
Samples were run on the GC (28) with minor modifications. The injector was run in splitless mode with a constant septum purge. The inlet and injector temperature was 50°C and increased at 1°C/s during the 0.9-min sample transfer. The oven was programmed with an initial temperature of 50°C for 1 min followed by 3 ramps: 30°C/min to 150°C, 15°C/min to 260°C, and 50°C/min to 300°C with a hold time of 3 min. Helium carrier was set to 1.2 mL/min.
Feces were lyophilized (Freezemobile 24; Virtis) for ∼40 h, ground in a mortar and pestle, and stored at −80°C. Using 10 replicates each time, 1.2–3.3 mg was weighed into tin cups and introduced into the EA-IRMS via an autosampler. Helium and oxygen were set to 100 and 25 mL/min, respectively. The sample setup included an 8-s delay, 9-s stop, 40- to 50-s oxygen stop, and 375-s run. The oxidation and reduction columns were heated to 1020°C and 650°C, respectively. The oven was 65°C.
Retinol and ester analysis.
Liver samples were extracted using C23 alcohol as an internal standard (29); 100 μL extract was dried under argon, redissolved [75:25 methanol:dichloromethane (v:v); 100 μL], and injected (50 μL) onto the HPLC [Sunfire C18 column, 4.6 × 250 mm, 5 μm, Waters; 70:15:15 acetonitrile:methanol:dichloroethane (v:v:v), 0.05% triethylamine].
Estimation of TBR.
VA TBR were calculated with the following mass balance equation, which equates the 13C of serum retinol after dose administration (Fc × c) with the sum of 13C absorbed and stored from the dose (Fa × a) and 13C in serum retinol at baseline (Fb × b) (22):
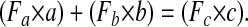 |
(Eq. 1) |
Equation 1 was used previously to calculate TBR of VA in rats (11). a describes the retinol absorbed and stored from the dose. This was calculated by multiplying the dose (3.5 μmol retinyl acetate) by a factor estimating absorption and storage. The retinol dilution test is typically applied in VA-deficient or -marginal populations for which a factor of 0.5 is considered appropriate (30). Because liver VA stores were known to be excessive in these monkeys (6), a factor of 0.8 (30) was used. Rats with increasing stores of liver VA (0.005–4.5 μmol/g) had increasing hepatic retention of a deuterated VA dose (15–77%) (30). In support of high dose retention, recovery of 3H-retinyl acetate from lymph duct-cannulated rats was 76 ± 5% (31). b represents the baseline TBR (μmol) of VA. c is the sum of a and b. Fa is the fraction of the dose that is labeled. In the present study, the 13C2-retinyl acetate dose is converted in vivo to 13C2-retinol with 2 13C out of 20 carbons. Because F = R/(R+1) and R is the ratio of 2 13C:18 12C (32), Fa = 0.10. Thus, the product of Fa and a is the 13C-fraction of the dose that was absorbed and stored. Fb and Fc are the decimal forms of At% 13C of serum retinol at baseline and 28 d after the dose, respectively.
System fractional catabolic rate.
The fractional catabolic rate is the daily rate of irreversible utilization of retinol as a fraction of the plasma retinol pool. It is calculated by dividing the disposal rate by the plasma retinol pool (33). In the compartmental analysis, a steady state is assumed (output equals input). For this calculation, disposal rate equals absorbed dietary VA. The system fractional catabolic rate (FCRs) was calculated by dividing the portion of dietary VA/d that was absorbed [assumed to be 80% (31)] by measured liver VA stores [or total liver reserves (TLR)]. This calculation is presented in Equation [2]. Finally, TLR are thought to represent the vast majority of TBR of VA in hypervitaminotic animals (33) and are estimated by multiplying measured liver VA concentration by the previously reported liver size (6).
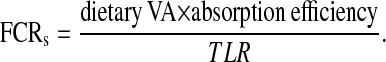 |
(Eq. 2) |
Model-based compartmental analysis.
The Windows version of the Simulation, Analysis and Modeling software (WinSAAM) (34) was used to mathematically model the kinetics of the fraction of oral dose in serum. Serum volume was estimated from baseline body weight assuming 41 mL/kg (35). Mean predose serum retinol concentration (1.93 ± 0.33 μmol/L; n = 3) was multiplied by estimated serum volume resulting in the estimated retinol pool in serum. This was multiplied by atom % excess yielding μmol 13C. This product was divided by 2 13C's/molecule, resulting in μmol 13C-retinol. Finally, this quotient was divided by the oral dose administered, 3.5 μmol, yielding the serum fraction of the oral dose at each time. A 4-parameter model was developed due to the availability of serum from 7 sampling times. Fractional transfer coefficients [L(I,J)]s (36) or the fraction of compartment J that is transferred to compartment I per unit time as well as other kinetic parameters were determined. A compartment, in this case, refers to a kinetically homogenous group of VA molecules typically associated with a physiological space such as serum or extravascular stores (36).
Statistical analysis.
Data are means ± SD. Student's t test (Minitab 13.32) was used to compare mean predicted TBR with measured liver stores. Regression analysis (SAS Institute, 2001) compared predicted TBR and measured liver reserves (μmol/g). The deviation of the slope of this line from 1 was formally tested. P < 0.05 was considered significant. For applicability of the isotope dilution test as a clinical diagnostic of hypervitaminosis A, the sensitivity (37) was determined in relation to excessive liver stores, i.e. 1.05 μmol retinol/g liver.
Results
Rhesus baseline characteristics.
At baseline, liver VA (11.9 ± 5.4 μmol/g) was 12 times the widely accepted upper-limit cutoff for the normal human liver retinol concentration of 1.05 μmol/g (38). In the serum chemistry profile (Table 2), generally accepted “normal” monkey values are extrapolated from apparently healthy monkeys held in captivity. Lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and serum albumin were elevated in 50, 19, and 44% of the monkeys, respectively. Three monkeys had abnormal serum iron, 2 below and 1 above the range.
TABLE 2.
Screening serum chemistry profiles for male rhesus monkeys (Macaca mulatta) enrolled in a VA status assessment study using 13C2-retinyl acetate
| Reference range2
|
||||
|---|---|---|---|---|
| Test | Rhesus1 | Rhesus | Humans | Out of range,3n |
| Glucose, mmol/L | 3.98 ± 0.99 (2.83–6.22) | 2.33–5.38 | 3.55–7.10 | 2 |
| Blood urea nitrogen, mmol/L | 13.3 ± 2.4 (9.29–18.6) | 7.14–18.6 | 5.0–14.3 | 0 |
| Creatinine, nmol/L | 88 ± 18 (62–115) | 71–141 | 71–124 | 1 |
| Cholesterol, mmol/L | 3.87 ± 0.71 (2.90–5.22) | 2.40–5.17 | 2.59–6.21 | 1 |
| Triglycerides,4mmol/L | 1.1 ± 0.9 (0.3–3.5) | 0–1.3 | <1.7 | 5 |
| Total bilirubin, nnol/L | 3.4 ± 1.7 (1.7–6.8) | 0–6.8 | 3.4–32.5 | 0 |
| AST, U/L | 38 ± 9.9 (26–60) | 16–50 | 10–34 | 3 |
| LDH, U/L | 574 ± 272 (269–1393) | 94–503 | 105–333 | 8 |
| γ-Glutamyl transferase, U/L | 53.9 ± 13.6 (40–93) | 24–81 | 0–51 | 1 |
| Alanine aminotransferase, U/L | 45.4 ± 19 (29–91) | 6–64 | 8–37 | 2 |
| Total protein, g/L | 79 ± 5 (68–88) | 65–83 | 63–79 | 2 |
| Albumin,5mmol/L | 0.72 ± 0.06 (0.64–0.82) | 0.55–0.73 | 0.58–0.75 | 7 |
| Alkaline phosphatase, U/L | 126.3 ± 42.7 (71–222) | 28–221 | 44–147 | 1 |
| Calcium, mmol/L | 2.52 ± 0.15 (2.24–2.72) | 2.29–2.69 | 2.12–2.72 | 4 |
| Phosphorous, mmol/L | 1.55 ± 0.29 (1.16–2.29) | 0.77–1.97 | 0.77–1.32 | 1 |
| Iron,6μmol/L | 27.0 ± 6.4 (13.8–38.1) | 16.3–37.4 | 10.7–30.4 | 3 |
| Sodium, mmol/L | 146.1 ± 2.8 (141–152) | 142–153 | 135–143 | 1 |
| Potassium, mmol/L | 4.3 ± 0.4 (3.3–5.2) | 3.4–5.1 | 3.7–5.2 | 1 |
| Chloride, mmol/L | 104.1 ± 2.3 (99–108) | 104–114 | 101–111 | 5 |
Values are mean ± SD (range), n = 16.
The rhesus reference range was determined by General Medical Laboratories in Madison, WI and based on a database of apparently healthy, captive rhesus monkey blood profiles. The human reference ranges are from MedlinePlus (39) unless noted otherwise.
Rhesus monkeys outside the rhesus reference range.
Normal human triglyceride values are from MedlinePlus (40). The molecular weight used for triglycerides was 909 g/mol.
The molecular weight used for albumin was 67,000 g/mol.
Normal human serum iron concentrations are from MedlinePlus (41).
Rhesus fecal enrichment.
Isotopic enrichment of the feces was 1.081 ± 0.0001 At% 13C for baseline samples (n = 3 pairs). The difference between baseline and up to 2 d postdose (1.081 ± 0.0003 At% 13C) was not within the diagnostic sensitivity of the instrument to detect unabsorbed dose.
Estimation of TBR.
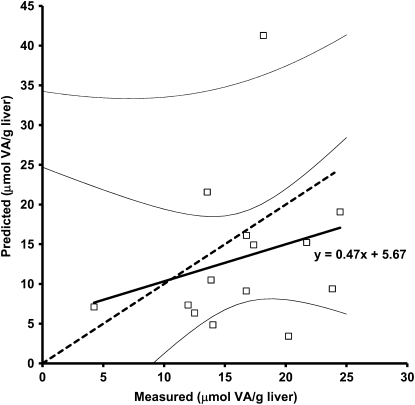
Predicted VA TBR in the monkeys using Eq. 1 and the serum data from d 28 were 3.52 ± 2.01 mmol [n = 14; 1 sample lost during preparation; 1 sample identified as an outlier by Dixon's criterion (42)] and ranged from 1.04 to 7.45 mmol. Measured TLR were 4.56 ± 1.38 mmol (n = 14; Student's t test P = 0.124) and ranged from 1.32 to 6.73 mmol. For most monkeys, predicted VA liver concentrations (13.3 ± 9.7 μmol/g) underestimated measured liver VA (16.4 ± 5.3 μmol/g; Student's t test, P = 0.311) (Fig. 1). To convert from predicted VA TBR to predicted liver VA concentration, the predicted TBR was divided by previously reported liver weights (6). From the regression analysis of d 28 serum enrichment prediction compared with measured stores (n = 14), the equation for the line is y = 0.47x+5.67 (R2 = 0.06; P = 0.38). The slope of this line did not differ from 1 (test of y = 1; P = 0.31). As a clinical diagnostic, the cutoff for excessive liver VA in humans was compared with predicted values. All predicted liver VA concentrations were >1.05 μmol/g liver (Fig. 1), corresponding to 100% sensitivity for the test to predict hypervitaminosis A and correctly diagnosing all monkeys as having hypervitaminosis A. When liver instead of serum enrichment (Table 3) was used in Eq. 1, the 14-d liver enrichment had a better prediction of measured liver VA than any of the serum enrichment values (R2 = 0.83; P = 0.087).
FIGURE 1 .
Predicted liver VA concentration plotted against measured liver VA (μmol/g) in 14 rhesus monkeys (Macaca mulatta). Liver VA concentration (retinol and retinyl esters) was measured by HPLC analysis of a 50-mg liver biopsy sample. Predicted liver VA concentration was calculated from the mass balance equation using serum 13C2-retinol enrichment at 28 d postdose and then divided by previously reported rhesus liver size. The thick line represents the regression line y = 0.47x + 5.67 (R2 = 0.06; P = 0.38) through the individual monkey data (□). The slope of this line is not different from 1 (test of y = 1; P = 0.31). The thick dashed line represents the 45-degree line. The uppermost thin line indicates the upper 95% confidence limit of individual rhesus (note: lower 95% confidence limit of individual rhesus is off scale), whereas the thin black lines represent the upper and lower 95% confidence limit of the fit line.
TABLE 3.
At% 13C in serum and liver following oral administration of 3.5 μmol 13C2-retinyl acetate in hypervitaminotic A rhesus monkeys (Macaca mulatta)12
| Time | Serum | Liver |
|---|---|---|
| d | At% 13C | |
| 23 | 1.406 ± 0.097a | 1.090 ± 0.002 |
| 7 | 1.110 ± 0.011b | 1.090 ± 0.002 |
| 14 | 1.094 ± 0.001b | 1.089 ± 0.001 |
| 28 | 1.085 ± 0.003b | 1.087 ± 0.002 |
Values are means ± SD; n = 4. Means in a column with superscripts without a common letter differ, P < 0.05.
Serum baseline At% 13C was 1.080 ± 0.003 (n = 16) and liver was 1.080 ± 0.000 (n = 4). Baseline At% 13C differed from d 2 in the same monkeys (paired t test: n = 4/tissue; serum, P = 0.0071; liver P = 0.0024).
Serum and liver At% 13C were different on d 2 in the same monkeys (paired t test: n = 4; P = 0.007).
Steady-state model and serum kinetics.
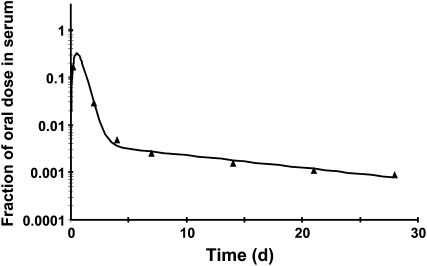
A steady-state model was developed (n = 15) as previously reported (43) and the serum tracer response profile plotted (Fig. 2). The bend by 4 d indicates that the dose was recycling into serum from tissues. The bend's relative sharpness is an indication of the total tissue retinol pool size relative to the plasma retinol pool size. In rats with liver stores ranging from 0.006 to 3.91 μmol, there was a significant negative correlation with liver total retinol and plasma fraction of dose at 5 d (33). By d 28 in the current study, serum kinetics were entering the terminal slope, which is indicative of the FCRs but had not yet entered a true terminal slope. For this reason, the model-predicted disposal rate represents a maximal value with a higher FCR than actual. The actual FCRs (Eq. 2) is closer to 0.12%.
FIGURE 2 .
Fraction of oral dose in serum from 1 representative rhesus monkey (Macaca mulatta). The observed serum data are represented by triangles and the solid line is generated by the compartmental model from the fraction of oral dose in serum for 1 rhesus dosed orally with 3.5 μmol 13C2-retinyl acetate.
Model-based compartmental analysis.
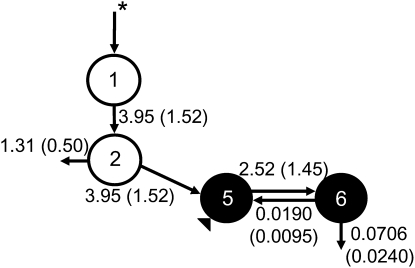
A visual representation of the model, its compartments, and fractional transfer coefficients was developed (Fig. 3). Compartments 1 and 2 represent the absorption and processing of the dose. Compartments 5 and 6 represent, respectively, serum and extravascular VA (primarily liver stores). The latter includes the larger, more slowly turning-over pool along with the smaller, more quickly turning-over pool. Compartment 0 represents loss at the level of the intestine, whereas compartment 10 represents irreversible loss from the extravascular pool. The model-predicted liver exchangeable VA pool [M(6)] equals 37.5 μmol (n = 15; Table 4). Kinetic parameters were estimated from the compartmental model.
FIGURE 3 .
Proposed compartmental model for VA turnover in hypervitaminotic A rhesus monkeys (Macaca mulatta). Compartments (5, serum; 6, extravascular) are represented by solid circles. Open circles indicate absorption and processing of VA (compartments 1 and 2). Compartment 0 represents loss at the level of the intestine whereas compartment 10 represents irreversible loss from the extravascular pool. Shown with each vector is its model-predicted fractional transfer coefficient [L(I,J) in pools/d with SD in parentheses; n = 15]. The asterisk denotes the oral delivery of the 13C2-retinyl acetate dose. The triangle denotes the sampling site.
TABLE 4.
Measured and model-predicted kinetic parameters for retinol in male rhesus monkeys (Macaca mulatta)1
| Parameter | |
|---|---|
| Compartment masses,2μmol | |
| M(5) | 1.06 ± 0.19 |
| M(6) | 37.5 ± 45.5 |
| T(I,J),3d | |
| T(5,5) | 0.59 ± 0.18 |
| T(6,5) | 15.7 ± 5.34 |
| Disposal rate,4μmol/d | |
| R(10,6) | 2.08 ± 1.37 |
| Fractional catabolic rate,5pools/d | |
| FCR(5,5) | 1.93 ± 0.99 |
Values are mean ± SD, n = 15. The model is shown in Figure 3.
M(5) was calculated from the product of mean predose serum retinol concentration and estimated serum volume for each monkey; M(6) is the model-predicted traced mass in extravascular compartment 6 generated from the compartmental model in WinSAAM.
T(I,J), mean residence time, is the mean of the distribution of times that retinol molecules spent in compartment I from the time of entering the system via compartment J (serum) until leaving compartment I irreversibly (36). These parameters were generated from the compartmental model in WinSAAM.
R(10,6) is the system disposal rate or the daily rate of irreversible utilization of VA: the amount of retinol (μmol) transferred out of the system from compartment 6/d; R(10,6) = L(10,6) · M(6) (36); since the model was solved in a steady-state solution, the VA disposal rate equals the VA input rate adjusted for loss at the level of the intestine.
Discussion
For humans, the tolerable upper intake level for VA is 10.5 μmol (3000 μg) retinol activity equivalents/d. Adjusting this value to the rhesus metabolic body weight (44) recorded in this study (13.4 kg) yielded 3.0 μmol; thus, their consumption of 6 μmol/d is 2 times higher than the adjusted upper level. As found in previous studies (6,45), captive rhesus monkeys' VA stores rival those of carnivorous arctic wildlife (46). The variability of rhesus liver VA concentration, regardless of when dosed (16.4 ± 5.3 μmol/g), reflects that previously reported (6).
One of the underlying principles of isotope dilution is that TBR are calculated once the dose equilibrates (30,47). Equilibrium is defined as serum and liver having the “same specific radioactivity” (30). For a technique whose ultimate goal is field-based assessment, however, this assumption is unrealistic due to incoming unlabeled dietary VA diluting the labeled dose in serum (1). In the current experiment, there could be no equilibration of the dose, because the rhesus consumed ∼6 μmol VA/d. When liver enrichment (Table 3) was used in Eq. 1, the 14-d liver enrichment compared with d 2, 7, or 28 had a better prediction of measured liver VA than d 28 serum enrichment. Discrepancies between predicted TBR and measured TLR using serum enrichment data were largely due to variability in the fraction of the dose in monkey plasma, suggesting that once tracer arrived in liver, exchange with plasma was compromised. Variability in the fraction of dose would necessarily lead to variability in the predicted values. Assumptions made in the conversion of predicted VA TBR to predicted liver VA concentration include homogeneous hepatic VA distribution as well as consistent liver:body weight ratio across animals. These assumptions introduce error in the comparison of predicted and measured liver VA concentrations. Direct comparison of TBR and TLR may not be ideal, because TBR relate to total body VA, whereas TLR capture the VA stored in the liver, which is typically the vast majority of the body's VA.
Model-based compartmental analysis was recently applied to serum turnover data from VA-sufficient U.S. subjects (n = 12) and a 2-compartment, postabsorption model was developed (43). The model-predicted compartment mass for extravascular stores in U.S. subjects was 892 μmol and did not differ from VA TBR calculated by isotope dilution. In the present study, the rhesus steady-state model predicts mean exchangeable liver VA stores (37.5 μmol) well below measured (4483 μmol), representing a very low fraction of liver VA (∼1%) exchanging with serum.
To investigate the effect of hypervitaminosis A on the ability of the kinetic model to trace the amount of extravascular VA measured by HPLC, more long-term models were developed by simulating a very shallow terminal slope starting with the 28-d serum fraction of dose for 1 monkey. Initially, serum fraction of dose data out to 178 d was calculated with exponential decay (y = y0 e−kt) representing 0.1% FCR, 10 times less than the model predicted. Although this model was characterized by an increase in the extravascular pool to 897 μmol VA and the presence of a second extravascular compartment, these only represent <30% of measured liver VA for this monkey (3276 μmol). This 178-d model suggests that had the experiment been carried out an additional 150 d, <30% of this rhesus' liver VA was exchangeable and, thus, traceable, within this prolonged time frame in a hypervitaminotic A model.
To test whether an even longer term and yet shallower terminal slope would better trace the measured liver VA, FCR was set to 0.019% [R(10,6)/measured liver stores (33)] from d 178 to 1128. To maximize the model-predicted extravascular stores, 100% dose absorption was assumed. Despite these changes to the model parameters, only 52% of the measured liver VA was traceable. Thus, this model predicts that a 3-y turnover study in hypervitaminotic A rhesus monkeys would still have underrepresented measured liver stores by one-half. These models suggest the monkeys' metabolism of an isotopic tracer diverges from what is reported in VA marginal and sufficient humans (47,48).
Previous investigations indicated that rhesus livers were free of fibrosis, yet the stellate cells were hypertrophic and hyperplasic (6), which is characteristic of the type II lipid droplets found within hepatic stellate cells of rats fed excessive VA (49,50). When type I lipid droplets accumulate significant VA, they transition to type II lipid droplets and undergo structural changes. These prevent the lipid droplets from being excreted and they remain stored in the cytoplasmic matrix (49,50). As such, these stores may be invisible to a tracer in a short-term experiment, because they are sequestered in a nonexchangeable pool, offering a physiologic basis for the underestimation of liver VA concentrations at the individual level.
Candidate sites for the dose's location other than serum and liver include kidney, lung, and adipose tissue, or other metabolic routes. Kidney and lung probably do not represent significant storage sites of VA based on reported values (45). The landmark study by Sauberlich et al. (51) provides information on other metabolic routes after a radioactive VA dose was administered: 1 human subject was dosed orally with 15-14C1-retinyl acetate (0.003–0.005 μmol/kg) and initial TBR (1438 μmol) were calculated. In the first week after the dose, 18, 13, and 7% of the dose was excreted in feces, urine, and the breath, respectively. Between 8 and 177 d postdose, additional cumulative fecal excretion decreased to 3% of radioactivity administered. Excretion may be higher during hypervitaminosis A (52). Detecting unabsorbed 13C-retinol is complicated by the sensitivity of 13C methods and purification of the dominant metabolites. This partly explains why none was detected in the current study.
The 3.5 μmol 13C2-retinyl acetate dose was 0.27 ± 0.05 μmol/kg body weight (0.21–0.41 μmol/kg body weight), which is less than levels administered in human stable isotope dilution tests (1). The ratio of predicted mass:isotopic label needs consideration during isotope dilution. In that regard, the ratio for this study was 503. More mass can be detected with this methodology while still maintaining sensitivity. In the DRD tests conducted in children using GC-MS and either electron ionization or electron capture negative chemical ionization detection, the predicted mass:label ratios were 1.73 and 1.55, respectively (53,54). In adults, the mass:label ratios were 0.191 and 4.38 for electron ionization and electron capture negative chemical ionization, respectively (55,56). Smaller doses, as used with GC/C/IRMS, perturb VA metabolism less and decrease costs (57).
“Abnormal” serum chemistry values were observed in most of the male rhesus monkeys, including elevated alkaline phosphatase, γ-glutamyl transferase, albumin, LDH, and AST. Elevations of these enzymes are all markers of liver disease or malfunction (58), which may be a direct outcome of VA toxicity (18,19). All 3 monkeys with elevated AST also had elevated LDH, which is consistent with functional liver impairment. None of the 16 animals tested had elevations of alanine aminotransferase and AST, which together indicate viral hepatitis. This suggests that none of the monkeys were infected with viral hepatitis, reinforcing the evidence that these monkeys may have suboptimal liver function due to excessive hepatic VA accumulation.
The present application of the 13C-retinol isotope dilution test in rhesus monkeys, reported previously to have excessive liver VA (6,45), allowed for the investigation of kinetic parameters and the prediction of TBR from serum enrichment as well as liver enrichment. The differences in serum kinetics between hypervitaminotic A rhesus monkeys and U.S. subjects suggest there may be a fundamental difference in the handling of orally ingested retinol. Whereas predictions of individual VA stores were poor, the test correctly assessed that each monkey had excessive liver VA stores considerably greater than the reference value of 1.05 μmol/g. None of the monkeys had actual values < 1.05 μmol/g; therefore, specificity could not be determined, which is a limitation of this study. This limitation highlights the reality of working with captive rhesus monkeys characterized by excessive liver VA concentrations (6,45). Given the current dietary VA fed at primate centers, long-term feeding trials or experiments that start during infancy would be required to work with monkeys that have normal liver VA stores. Long-term feeding trials in monkeys are not trivial when compared with other common laboratory animals, e.g. rodents.
The diagnostic capability of isotope dilution tests to predict hypervitaminosis A is increasingly relevant to global public health applications in countries with VA fortification programs of commonly consumed foodstuffs (2) or in groups where qualitative estimates of liver reserves would be useful. As observed with the DRD test (1,59), this study further demonstrates that stable isotope methodology provides a noninvasive, very good quantitative estimate of the mean TBR of a group. Governments can use these kinds of data to decide whether to invest in a VA fortification program. This methodology has a broader diagnostic range to predict hypervitaminosis A than previously reported (51,57).
Acknowledgments
We thank Kristina Penniston and Jordan Mills for coordinating the isotope dosing and collection of samples, Mandy Porter-Dosti for technical assistance on the IRMS, and Peter Crump for statistical analysis. A.L.E. conducted the research, analyzed the data, and wrote the paper. J.A.H. conducted the research. M.H.G. analyzed the data. S.A.T. designed the research and wrote the paper. A.L.E. and S.A.T. had primary responsibility for final content. All authors read and approved the final manuscript.
Supported by NIHNIDDK61973 and the Wisconsin National Primate Research Center grant 5P51RR000167 [NIH National Center for Research Resources (NCRR)]. This research was conducted at a facility constructed with support from Research Facilities Improvement Program grants RR15459-01 and RR020141-01. The content is the authors' responsibility and does not necessarily represent the views of NCRR or the NIH.
Author disclosures: A. L. Escaron, M. H. Green, J. A. Howe, S. A. Tanumihardho, no conflicts of interest.
This work was a finalist in ASN's Clinical Emerging Leaders Award Competition, Experimental Biology, Washington, DC, April 2007.
Abbreviations used: At% 13C, atom % 13C; AST, aspartate aminotransferase; DRD, deuterated retinol dilution; FCRs, system fractional catabolic rate; GC/C/IRMS, gas chromatograph-combustion-isotope ratio MS; LDH, lactate dehydrogenase; TBR, total body reserves; TLR, total liver reserves; VA, vitamin A; WinSAAM, Windows version of the Simulation, Analysis and Modeling software; WNPRC, Wisconsin National Primate Research Center.
References
- 1.Furr HC, Green MH, Haskell MJ, Mokhtar N, Nestel P, Newton S, Ribaya-Mercado JD, Tang G, Tanumihardjo S, et al. Stable isotope dilution techniques for assessing vitamin A status and bioefficacy of provitamin A carotenoids in humans. Public Health Nutr. 2005;8:596–607. [DOI] [PubMed] [Google Scholar]
- 2.Ribaya-Mercado JD, Solomons NW, Medrano Y, Bulux J, Dolnikowski GG, Russell RM, Wallace CB. Use of the deuterated-retinol-dilution technique to monitor the vitamin A status of Nicaraguan schoolchildren 1 y after initiation of the Nicaraguan national program of sugar fortification with vitamin A. Am J Clin Nutr. 2004;80:1291–8. [DOI] [PubMed] [Google Scholar]
- 3.Haskell MJ, Islam MA, Handelman GJ, Peerson JM, Jones AD, Wahed MA, Mahalanabis D, Brown KH. Plasma kinetics of an oral dose of [2H4]retinyl acetate in human subjects with estimated low or high total body stores of vitamin A. Am J Clin Nutr. 1998;68:90–5. [DOI] [PubMed] [Google Scholar]
- 4.Penniston KL, Tanumihardjo SA. Vitamin A in dietary supplements and fortified foods: too much of a good thing? J Am Diet Assoc. 2003;103:1185–7. [DOI] [PubMed] [Google Scholar]
- 5.Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. 2006;83:191–201. [DOI] [PubMed] [Google Scholar]
- 6.Penniston KL, Tanumihardjo SA. Subtoxic hepatic vitamin A concentrations in captive rhesus monkeys (Macaca mulatta). J Nutr. 2001;131:2904–9. [DOI] [PubMed] [Google Scholar]
- 7.Penniston KL, Thayer JC, Tanumihardjo SA. Serum vitamin A esters are high in captive rhesus (Macaca mulatta) and marmoset (Callithrix jacchus) monkeys. J Nutr. 2003;133:4202–6. [DOI] [PubMed] [Google Scholar]
- 8.Baskerville M. Old World monkeys. In: Poole T, editor. The UFAW handbook on the care and management of laboratory animals. Malden (MA): Blackwell Science; 1999. p. 611–28.
- 9.O'Toole BA, Fradkin R, Warkany J, Wilson JG, Mann GV. Vitamin A deficiency and reproduction in rhesus monkeys. J Nutr. 1974;104:1513–24. [DOI] [PubMed] [Google Scholar]
- 10.Penniston KL, Tanumihardjo SA. Vitamin A intake of captive rhesus monkeys exceeds National Research Council Recommendations. Am J Primatol. 2006;68:1114–9. [DOI] [PubMed] [Google Scholar]
- 11.Tanumihardjo SA. Vitamin A status assessment in rats with 13C4-retinyl acetate and gas chromatography/combustion/isotope ratio mass spectrometry. J Nutr. 2000;130:2844–9. [DOI] [PubMed] [Google Scholar]
- 12.Tanumihardjo SA. Application of 13C4-retinyl acetate and gas chromatography-combustion isotope ratio mass spectrometry to vitamin A assessment in the human. FASEB J 2001;15:A256. [Google Scholar]
- 13.Guo ZK, Luke AH, Lee WP, Schoeller D. Compound-specific carbon isotope ratio determination of enriched cholesterol. Anal Chem. 1993;65:1954–9. [DOI] [PubMed] [Google Scholar]
- 14.Bier DM. Stable isotopes in biosciences, their measurement and models for amino acid metabolism. Eur J Pediatr. 1997;156 Suppl 1:S2–8. [DOI] [PubMed] [Google Scholar]
- 15.Ho CC, de Moura FF, Kim S-H, Clifford AJ. Excentral cleavage of β-carotene in vivo in a healthy man. Am J Clin Nutr. 2007;85:770–7. [DOI] [PubMed] [Google Scholar]
- 16.Dueker SR, Lin Y, Buchholz BA, Schneider PD, Lamé MW, Segall HJ, Vogel JS, Clifford AJ. Long-term kinetic study of beta-carotene, using accelerator mass spectrometry in an adult volunteer. J Lipid Res. 2000;41:1790–800. [PubMed] [Google Scholar]
- 17.Krause RF. Liver lipids in a case of hypervitaminosis A. Am J Clin Nutr. 1965;16:455–7. [DOI] [PubMed] [Google Scholar]
- 18.Muenter MD, Perry HO, Ludwig J. Chronic vitamin A intoxication in adults. Hepatic, neurologic and dermatologic complications. Am J Med. 1971;50:129–36. [DOI] [PubMed] [Google Scholar]
- 19.Russell RM, Boyer JL, Bagheri SA, Hruban Z. Hepatic injury from chronic hypervitaminosis A resulting in portal hypertension and ascites. N Engl J Med. 1974;291:435–40. [DOI] [PubMed] [Google Scholar]
- 20.Mallia A, Smith J, Goodman D. Metabolism of retinol-binding protein and vitamin A during hypervitaminosis A in the rat. J Lipid Res. 1975;16:180–8. [PubMed] [Google Scholar]
- 21.Tanumihardjo SA. Assessing vitamin A status: past, present and future. J Nutr. 2004;134:S290–3. [DOI] [PubMed] [Google Scholar]
- 22.Goodman KJ, Brenna JT. High sensitivity tracer detection using high-precision gas chromatography-combustion isotope ratio mass spectrometry and highly enriched [U-13C]-labeled precursors. Anal Chem. 1992;64:1088–95. [DOI] [PubMed] [Google Scholar]
- 23.Amedee-Manesme O, Furr HC, Olson JA. The correlation between liver vitamin A concentrations in micro- (needle biopsy) and macrosamples of human liver specimens obtained at autopsy. Am J Clin Nutr. 1984;39:315–9. [DOI] [PubMed] [Google Scholar]
- 24.Mills JP, Terasawa E, Tanumihardjo SA. Excessive preformed vitamin A intake by mothers amplifies early fetal liver retinyl ester storage in captive Old World monkeys. Comp Med. 2007;57:505–11. [PubMed] [Google Scholar]
- 25.NRC. Nutrient requirements of nonhuman primates. 2nd ed. Washington, DC: National Academies Press; 2003.
- 26.Tanumihardjo SA. Synthesis of 10,11,14,15-13C4- and 14,15-13C2-retinyl acetate. J Labelled Comp Radiopharm. 2001;44:365–72. [Google Scholar]
- 27.Sabatier M, Arnaud MJ, Kastenmayer P, Rytz A, Barclay DV. Meal effect on magnesium bioavailability from mineral water in healthy women. Am J Clin Nutr. 2002;75:65–71. [DOI] [PubMed] [Google Scholar]
- 28.Howe JA, Valentine AR, Hull AK, Tanumihardjo SA. 13C natural abundance in serum retinol acts as a biomarker for increases in dietary provitamin A. Exp Biol Med. 2009;234:140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanumihardjo SA, Howe JA. Twice the amount of α-carotene isolated from carrots is as effective as β-carotene in maintaining the vitamin A status of Mongolian gerbils. J Nutr. 2005;135:2622–6. [DOI] [PubMed] [Google Scholar]
- 30.Bausch J, Rietz P. Method for the assessment of vitamin A liver stores. Acta Vitaminol Enzymol. 1977;31:99–112. [PubMed] [Google Scholar]
- 31.Allen LE, Green MH, Green JB. Correspondence re: SE Dew et al., Effects of pharmacological retinoids on several vitamin A-metabolizing enzymes. Cancer Res. 53:2965–9, 1993. Cancer Res. 1994;54:3319–20. [PubMed] [Google Scholar]
- 32.Brenna JT, Corso TN, Tobias HJ, Caimi RJ. High-precision continuous-flow isotope ratio mass spectrometry. Mass Spectrom Rev. 1997;16:227–58. [DOI] [PubMed] [Google Scholar]
- 33.Green MH, Green JB, Lewis KC. Variation in retinol utilization rate with vitamin A status in the rat. J Nutr. 1987;117:694–703. [DOI] [PubMed] [Google Scholar]
- 34.Cifelli CJ, Green JB, Green MH. Use of model-based compartmental analysis to study vitamin A kinetics and metabolism. Vitam Horm. 2007;75:161–95. [DOI] [PubMed] [Google Scholar]
- 35.Bourne GH, editor. The rhesus monkey. New York: Academic Press; 1975.
- 36.Green MH, Green JB. The application of compartmental analysis to research in nutrition. Annu Rev Nutr. 1990;10:41–61. [DOI] [PubMed] [Google Scholar]
- 37.Gibson RS. Principles of nutritional assessment. New York: Oxford University Press; 1990.
- 38.Olson JA. Serum levels of vitamin A and carotenoids as reflectors of nutritional status. J Natl Cancer Inst. 1984;73:1439–44. [PubMed] [Google Scholar]
- 39.MedlinePlus. Comprehensive metabolic panel. In: Medical encyclopedia [cited 2009 26 Jul]. Available from: http://www.nlm.nih.gov/medlineplus/ency/article/003468.htm).
- 40.MedlinePlus. Triglyceride level. In: Medical encyclopedia [cited 2009 26 Jul]. Available from http://www.nlm.nih.gov/medlineplus/ency/article/003493.htm.
- 41.MedlinePlus. Serum iron. In: Medical encyclopedia [cited 2009 26 Jul]. Available from http://www.nlm.nih.gov/medlineplus/ency/article/003488.htm.
- 42.Snedecor GW, Cochran WG. Statistical methods. 8th ed. Ames (IA): Iowa State University Press; 1989.
- 43.Cifelli CJ, Green JB, Wang Z, Yin S, Russell RM, Tang G, Green MH. Kinetic analysis shows that vitamin A disposal rate in humans is positively correlated with vitamin A stores. J Nutr. 2008;138:971–7. [DOI] [PubMed] [Google Scholar]
- 44.Kleiber M. Body size and metabolism. Hilgardia. 1932;6:315–53. [Google Scholar]
- 45.Mills JP, Penniston KL, Tanumihardjo SA. Extra-hepatic vitamin A concentrations in captive Rhesus (Macaca mulatta) and Marmoset (Callithrix jacchus) monkeys fed excess vitamin A. Int J Vitam Nutr Res. 2005;75:126–32. [DOI] [PubMed] [Google Scholar]
- 46.Higashi N, Senoo H. Distribution of vitamin A-storing lipid droplets in hepatic stellate cells in liver lobules–a comparative study. Anat Rec. 2003;271A:240–8. [DOI] [PubMed] [Google Scholar]
- 47.Furr HC, Amedee-Manesme O, Clifford AJ, Bergen HR III, Jones AD, Anderson DP, Olson JA. Vitamin A concentrations in liver determined by isotope dilution assay with tetradeuterated vitamin A and by biopsy in generally healthy adult humans. Am J Clin Nutr. 1989;49:713–6. [DOI] [PubMed] [Google Scholar]
- 48.Haskell MJ, Handelman GJ, Peerson JM, Jones AD, Rabbi MA, Awal MA, Wahed MA, Mahalanabis D, Brown KH. Assessment of vitamin A status by the deuterated-retinol-dilution technique and comparison with hepatic vitamin A concentration in Bangladeshi surgical patients. Am J Clin Nutr. 1997;66:67–74. [DOI] [PubMed] [Google Scholar]
- 49.Wake K. Development of vitamin A-rich lipid droplets in multivesicular bodies of rat liver stellate cells. J Cell Biol. 1974;63:683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wake K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. Int Rev Cytol. 1980;66:303–53. [DOI] [PubMed] [Google Scholar]
- 51.Sauberlich HE, Hodges RE, Wallace DL, Kolder H, Canham JE, Hood J, Raica N Jr, Lowry LK. Vitamin A metabolism and requirements in the human studied with the use of labeled retinol. Vitam Horm. 1974;32:251–75. [DOI] [PubMed] [Google Scholar]
- 52.Hicks VA, Gunning DB, Olson JA. Metabolism, plasma transport and biliary excretion of radioactive vitamin A and its metabolites as a function of liver reserves of vitamin A in the rat. J Nutr. 1984;114:1327–33. [DOI] [PubMed] [Google Scholar]
- 53.Haskell MJ, Lembcke JL, Salazar M, Green MH, Peerson JM, Brown KH. Population-based plasma kinetics of an oral dose of [2H4]retinyl acetate among preschool-aged, Peruvian children. Am J Clin Nutr. 2003;77:681–6. [DOI] [PubMed] [Google Scholar]
- 54.Tang G, Qin J, Hao LY, Yin SA, Russell RM. Use of a short-term isotope-dilution method for determining the vitamin A status of children. Am J Clin Nutr. 2002;76:413–8. [DOI] [PubMed] [Google Scholar]
- 55.Haskell MJ, Mazumder RN, Peerson JM, Jones AD, Wahed MA, Mahalanabis D, Brown KH. Use of the deuterated-retinol-dilution technique to assess total-body vitamin A stores of adult volunteers consuming different amounts of vitamin A. Am J Clin Nutr. 1999;70:874–80. [DOI] [PubMed] [Google Scholar]
- 56.Ribaya-Mercado JD, Solon FS, Fermin LS, Dolnikowski GG, Blumberg JB, Solon FS. Dietary vitamin A intakes of Filipino elders with adequate or low liver vitamin A concentrations as assessed by the deuterated-retinol-dilution method: implications for dietary requirements. Am J Clin Nutr. 2004;79:633–41. [DOI] [PubMed] [Google Scholar]
- 57.Vitamin A Tracer Task Force. Appropriate uses of vitamin A tracer (stable isotope) methodology. Washington, DC: ILSI Human Nutrition Institute; 2004.
- 58.Knight JA. Liver function tests: their role in the diagnosis of hepatobiliary diseases. J Infus Nurs. 2005;28:108–17. [DOI] [PubMed] [Google Scholar]
- 59.Haskell M, Ribaya-Mercado JD. Vitamin A Tracer Task Force. Handbook on vitamin A tracer dilution methods to assess status and evaluate intervention programs. Technical monograph 5. Washington, DC: HarvestPlus; 2005.