Abstract
Background: Prior research indicates that successful weight-loss maintainers (SWLs) work harder than people of normal weight to maintain their weight loss, including greater dietary restriction of fat and higher physical activity levels. However, little work to date has examined how SWLs differ biologically from normal-weight (NW) and obese controls.
Objective: The objective was to compare the brain responses of SWLs to food pictures with those of NW and obese controls.
Design: Blood oxygen level–dependent responses to high- and low-energy food pictures were measured in 18 NW controls, 16 obese controls, and 17 SWLs.
Results: Group differences were identified in 4 regions, which indicated significant change in activation in response to the food pictures. SWLs showed greater activation in the left superior frontal region and right middle temporal region than did NW and obese controls—a pattern of results confirmed in exploratory voxel-wise analyses. Obese controls also showed greater activation in a bilateral precentral region.
Conclusions: These results suggest that SWLs show greater activation in frontal regions and primary and secondary visual cortices—a pattern consistent with greater inhibitory control in response to food cues and greater visual attention to the food cues. A greater engagement of inhibitory control regions in response to food cues as well as a greater monitoring of foods may promote control of food intake and successful weight-loss maintenance.
See corresponding editorial on page 908.
INTRODUCTION
Maintenance of weight loss remains a major problem in the treatment of obesity. On average, participants in behavioral weight-loss programs will lose 9 kg (8–10% of their weight) during the first 6 mo of treatment and will maintain approximately two-thirds of this initial weight loss (5–6 kg) at the 1-y follow-up (1). Despite intensive efforts, weight regain appears to continue for the next several years, with most patients returning back to their baseline weight after 5 y (2). As a result, efforts to better understand the factors that contribute to successful weight-loss maintenance are of particular interest.
The National Weight Control Registry (NWCR) was established in 1993 to study individuals who have been successful at long-term weight loss. To be eligible, individuals must have maintained a weight loss of ≥13 kg (30 lb) for ≥1 y. On average, the participants have lost >27 kg (60 lb) before enrolling in the NWCR and have maintained the minimum 13 kg (30 lb) weight loss for an average of 6.5 ± 8.1 y. Prior research from the NWCR indicates that successful weight-loss maintainers (SWLs) work harder to maintain their achieved weight than do normal-weight (NW) controls. For example, SWLs report a greater reduction in dietary fat, more frequent self-weighing, and more physical activity than do weight-stable individuals with no history of obesity and weight regainers (3). However, little work to date has characterized how SWLs differ biologically from NW and overweight individuals.
One potential biological indicator of vulnerability to obesity is the pattern of brain response to food images. Previously, it has been proposed that individuals who are obese may have heightened arousal to foods cues (4). This arousal may be due to a learning process in which sensory cues that have previously been paired with consumption (ie, the sight or smell of palatable food) begin to elicit anticipatory arousal in preparation for consumption (5). Thus, the visual properties of food can become conditioned stimuli and influence subsequent food consumption (6). Because food is found to be more reinforcing in obese than in NW individuals (7), it might be expected that obese individuals may have greater activation of reward-related regions in the brain in response to food images than do NW individuals. Indeed, neuroimaging studies have identified differences between obese and lean individuals in response to food images in these regions (8, 9).
Although no studies have examined the brain response to food cues in SWLs, 2 prior studies have examined brain response to food consumption among SWLs. These appear to support differences in both primary gustatory and memory regions as well as regions associated with executive control. For example, SWLs show brain responses similar to those of obese participants but different from those of NW controls in the middle insula and posterior hippocampus (10). SWLs also exhibit greater activation in frontal regions involved in inhibitory control relative to nondieters, which suggests that SWLs may exhibit greater central regulation of eating (11). In the present study, we examined brain responses to food pictures among SWL, obese, and NW participants by using functional magnetic resonance imaging (fMRI).
SUBJECTS AND METHODS
Sample
The sample included 3 groups defined by lifetime weight history. The NW group reported a current and lifetime maximum body mass index (BMI; in kg/m2) of >18.5 and ≤24.9. The obese group reported a current BMI ≥30.0. The SWL group reported having lost ≥13.6 kg (30 lb) and having maintained a weight loss of ≥13.6 kg (30 lb) from their maximum weight for a minimum of 3 y, a lifetime maximum BMI ≥30.0, and a current BMI >18.5 and ≤24.9. All subjects reported being weight stable: within ± 4.5 kg (10 lb) for the 2 previous years for the NW and SWL groups and within ± 6.8 kg (15 lb) for the 2 previous years for the obese group. Additional exclusion criteria included use of weight-loss medications, use of medications that affect salivation (eg, antihistamines or antidepressants), binge eating, standard MRI contraindications (eg, metal implants, claustrophobia, and pregnancy), left-handedness, food allergies, and neurologic or psychiatric conditions, including but not limited to schizophrenia, bipolar disorder, epilepsy, stroke, and traumatic brain injury with loss of consciousness.
Study recruitment was conducted from April 2006 to December 2007. NW and obese participants were recruited by using advertisements, and SWLs were recruited through the NWCR and advertisements. Nineteen NW, 17 obese, and 17 SWL participants completed the study. One participant in the NW group was excluded because of a technical error during data acquisition, and one participant in the obese group was excluded because of excessive head motion (displacement: 3 mm; rotation: 4°), which resulted in a final sample of 18 NW, 16 obese, and 17 SWL participants. The protocol was approved by the Miriam Hospital Institutional Review Board.
Visual food cue paradigm
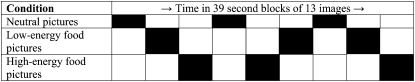
The food cue paradigm was adapted from Killgore et al (12) by using the images provided by the authors. Three conditions were presented in 9 blocks in one 8-min imaging run: 1) low-energy foods (eg, whole-grain cereals, salads, fresh vegetables, and fruit), 2) high-energy foods (eg, cheeseburgers, hot dogs, French fries, ice cream, cake, and cookies), and 3) nonfood objects with similar visual complexity, texture, and color (eg, rocks, shrubs, bricks, trees, and flowers). Each condition included three 39-s blocks of 13 images that were presented for 3 s each. The blocks were presented 3 times each, in alternating order (Figure 1).
FIGURE 1.
The food cue reactivity paradigm.
Procedures
All participants refrained from food and beverages other than water for 4 h before scanning and were limited to consuming ≤2 alcoholic beverages [29 mL (1 oz) hard liquor equivalent] or 2 equivalents of 236 mL (8 oz) caffeinated coffee in the previous 24 h. Immediately preceding the scans, the participants applied earplugs and MR-compatible vision correction, if applicable, and lay supine on the MR scanner table. Visual stimuli were presented by using E-Prime software (Psychology Software Tools Inc, Pittsburgh, PA) back-projected onto a screen positioned at the participant's head and viewed through a mirror attached to the head coil.
MRI data acquisition
Neuroimaging was conducted in a single session on a 3T Siemens Tim Trio MRI scanner (Siemens, New York, NY) equipped with a standard head coil. Functional imaging was performed by using a whole-brain echo-planar imaging sequence (repetition time = 2500 ms, time to echo = 28 ms, field of view = 192 mm2, 642 matrix, 42 axial slices, and 3-mm slice thickness). High-resolution T1-weighted magnetization prepared rapid gradient echo scans of the entire brain (256 × 256 matrix, field of view = 256 mm2, 1-mm slice thickness) were acquired in the sagittal plane for anatomical reference.
fMRI data processing and analysis
All images were processed by using Analysis of Functional NeuroImages software (13). Each time series was adjusted for differences in adjacent slice timing due to interleaved slice acquisition and spatially registered to the 10th volume of the session to reduce the effects of head movement. This Analysis of Functional NeuroImages 3-dimensional registration program also yields information on displacement and rotation for each volume that was used later as a covariate when determining task-related activity. Data preprocessing also included temporal smoothing and spatial filtering. Task-related brain activation relative to neutral baseline was determined by using voxel-wise multiple regression analyses with the following parameters: neutral, low-energy, and high-energy reference waveforms convolved with a gamma function, and covariates accounting for instruction screens, head movement, and linear trends.
Because the primary aim was to determine whether the brain responses to food cues vary between the NW, obese, and SWL groups and because no prior studies have examined brain responses to food cues across these 3 groups, a region of interest (ROI) analysis was conducted in brain regions specifically related to food reactivity in our sample. Therefore, an empirically defined set of ROIs was created. The first step in these analyses was the identification of clusters of voxels showing changes in activation in response to food cues compared with neutral cues for each of our groups. To accomplish this, the results from individual multiple regression analyses were transformed to standard stereotaxic space (14), resampled to 1-mm3 voxel size, and a 6-mm Gaussian kernel was applied. For each group, individual activation maps of food cue-related effects relative to the neutral cues (from the voxel-wise multiple regression analyses) were compared voxel-wise to a hypothetical mean of zero (ie, no different from neutral cues) by using a Student's one-sample t test. Voxels with significant task-related activation were determined separately for each group and condition by using an α level of P < 0.001 and a cluster threshold of 300 mm3. A single set of task-related ROIs was constructed by combining ROI sets from all 3 groups and both conditions. All regions of activity were combined by using an equally weighted conjunctive “or” logic to avoid bias toward any group or condition. The resulting ROI mask was applied to individual activation maps for each condition to determine mean task-related activity for each individual within each ROI, which was the dependent variable in subsequent group analyses. This “or” masking procedure was used to spatially define regions of task-related activity within which a priori hypotheses were tested. This procedure improves the validity of construct measurement by including only clusters showing significant change in activity in at least one of the groups in response to the low-energy or high-energy stimuli.
Statistical analyses (hypothesis testing)
Group comparisons were performed by using mean cue-related brain response as the dependent variable for each ROI. Between-group differences were tested by using repeated-measures analysis of variance procedures with one between-subjects group factor (NW, obese, and SWL groups) and condition (low-energy, high-energy image condition) as the time factor by using SPSS version 14.0 (SPSS Inc, Chicago, IL). Age and sex were included as covariates in all models. The inclusion of race and ethnicity as covariates did not substantially alter the results (data not shown).
Exploratory voxel-wise group contrasts were also performed to identify differences in brain activity outside of regions significantly engaged by the task. The rationale for these analyses was to avoid the possibility of missing important group- or condition-related effects on brain activity in regions in which statistical significance was not observed. This is an important step, given the stringent corrections for type I error used in mask generation and the loss of continuous variable information imposed by thresholding. It also avoids missing potential group differences that may be washed out in the creation of the “or” mask. Therefore voxel-wise contrasts are an important follow-up to hypothesis testing. To accomplish this, groups were contrasted by using 3 voxel-wise paired t tests to examine the effects of high-energy food cues on brain response.
RESULTS
Demographic characteristics and weight history
The demographic characteristics and current BMI and lifetime maximum BMI for the NW, obese, and SWL groups are presented in Table 1. No statistically significant group differences were observed for age, sex, or race. The current BMI of the NW and SWL groups differed significantly from that of the obese group. A trend was observed for a greater current BMI among SWLs than in the NW group (P = 0.06). Both the obese and SWL groups reported lifetime maximum BMI in the obese range, which differed significantly from the NW group, who reported a lifetime maximum BMI in the normal range. The obese participants, on average, reported a lifetime maximum BMI that was significantly greater than that reported by the SWL group.
TABLE 1.
Demographic and weight characteristics in the 3 groups of subjects1
| NW (n = 18) | Obese (n = 16) | SWL (n = 17) | Overall P value2 | |
| Age (y) | 43.72 ± 8.383 | 49.12 ± 6.99 | 48.47 ± 11.37 | 0.17 |
| Men (%) | 11 | 13 | 12 | 0.93 |
| White (%) | 100 | 84 | 94 | 0.35 |
| Current BMI (kg/m2) | 21.70 ± 1.98a | 34.52 ± 3.72b | 23.71 ± 1.55a | <0.001 |
| Lifetime maximum BMI (kg/m2) | 22.59 ± 2.19a | 35.81 ± 3.76c | 33.01 ± 3.00b | <0.001 |
NW, normal-weight; SWL, successful weight-loss maintainers. Values with different superscript letters are significantly different, P < 0.05 (Tukey's test).
The analyses were conducted by using chi-square analyses for categorical data and a one-factor ANOVA for continuous data.
Mean ± SD (all such values).
Clusters of significant activation in response to food images
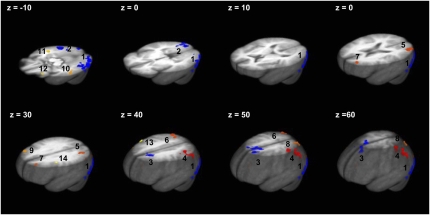
Clusters of significant activation in ≥1 of the 3 groups during either low-energy stimuli or high-energy stimuli are presented in Figure 2. Their mass center coordinates and cluster sizes are listed in Table 2. The largest clusters of cue-related effects across conditions were observed in occipito-temporal (ROI nos. 1, 2, 11, and 12) and occipito-parietal regions (ROI nos. 4, 5, 6, and 8), followed by several clusters in the frontal lobe.
FIGURE 2.
Clusters of significant activation in response to low-energy or high-energy food (t test, P < 0.001). Pictures in numerical order from the largest cluster of activation: 1, left middle occipital/Brodmann area (BA) 18; 2, right inferior occipital/lingual gyrus; 3, superior frontal/bilateral cingulate; 4, left inferior parietal; 5, left middle occipital; 6, right inferior and superior parietal/precuneus; 7, left superior frontal; 8, left precuneus; 9, right middle frontal; 10, left middle temporal; 11, right middle temporal; 12, left inferior frontal; 13, right precentral/BA 6; and 14, left precentral/inferior frontal/BA 6.
TABLE 2.
Clusters of significant activation in response to the low-energy or high-energy food pictures (t test, P < 0.001) and the significance of group differences in these regions
| ROI no. | Region | x | y | z | Size | Group P value1 |
| 1 | Left middle occipital/BA 18 | 40 | −77 | −9 | 10,039 | 0.59 |
| 2 | Right inferior occipital/lingual gyrus | 27 | −86 | −2 | 7109 | 0.72 |
| 3 | Superior frontal/bilateral cingulate | 1 | 12 | 49 | 5248 | 0.30 |
| 17 | 16 | 38 | ||||
| 4 | Left inferior parietal | 39 | −42 | 50 | 3253 | 0.20 |
| 5 | Left middle occipital | 31 | −81 | 16 | 3031 | 0.41 |
| 6 | Right inferior and superior parietal/precuneus | 35 | −68 | 45 | 1826 | 0.58 |
| 7 | Left superior frontal | 24 | 45 | 23 | 768 | 0.02 |
| 8 | Left precuneus | 20 | −70 | 46 | 767 | 0.25 |
| 9 | Right middle frontal | 40 | 34 | 30 | 706 | 0.11 |
| 10 | Left middle temporal | 53 | −25 | −9 | 611 | 0.18 |
| 11 | Right middle temporal | 47 | −30 | −13 | 492 | 0.04 |
| 12 | Left inferior frontal | 29 | 29 | −13 | 387 | 0.06 |
| 13 | Right precentral/BA 6 | 55 | 3 | 36 | 358 | 0.04 |
| 14 | Left precentral/inferior frontal/BA 6 | 35 | 1 | 30 | 330 | 0.05 |
The analysis was conducting by using repeated-measures ANOVA. No significant group × conditions were observed. ROI, region of interest; BA, Brodmann area.
Group differences
The statistical significance of group differences in brain responses to food cues is reported in the final column of Table 2. Group differences were identified in the left superior frontal and right middle temporal regions, and a bilateral effect was observed in the right and left precentral regions. No other significant group differences were observed. No group × condition interactions were identified.
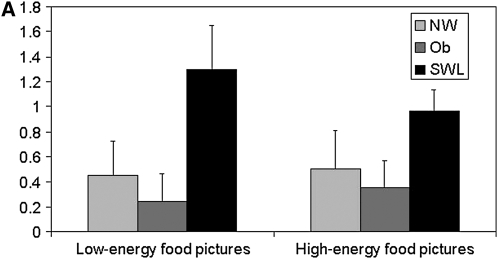
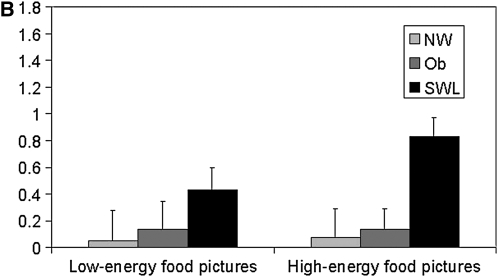
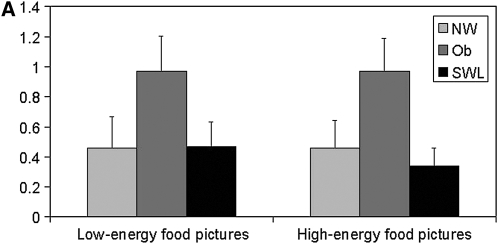
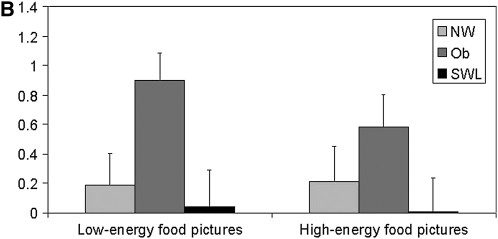
Changes in the blood oxygen level–dependent (BOLD) signal in response to the low- and high-energy food item pictures, relative to the neutral pictures, for the 4 regions showing significant group differences are depicted in Figure 3, A and B and Figure 4, A and B. In the left superior frontal region (Figure 3A), SWLs showed a greater degree of activation relative to the NW (P = 0.07) and obese (P = 0.04) groups. SWLs also showed a greater degree of activation in the right middle temporal region (Figure 3B) than did the NW group (P = 0.07). Bilateral activation in the precentral region was greater in the obese group than in the SWL group (right: P = 0.07; left: P = 0.04), who showed little change in activation in this ROI .
FIGURE 3.
Mean (±SEM) changes in the blood oxygen level–dependent signal during blocks of low-energy and high-energy food picture cues relative to blocks of neutral pictures in the significant clusters of activation in the left superior frontal region [A: n = 18 normal-weight (NW) subjects, 16 obese (Ob) subjects, and 17 successful weight-loss maintainers (SWL); repeated-measures ANOVA: group P = 0.02; Bonferroni-adjusted post hoc comparison: P = 0.04 between Ob and SWL, P = 1.0 between Ob and NW, and P = 0.07 between NW and SWL; no significant group × task interaction] and in the right middle temporal region (B: n = 18 NW, 16 Ob, and 17 SWL; repeated-measures ANOVA: group P = 0.04; Bonferroni-adjusted post hoc comparison: P = 0.12 between Ob and SWL, P = 1.0 between Ob and NW, and P = 0.07 between NW and SWL; no significant group × task interaction).
FIGURE 4.
Mean (±SEM) changes in the blood oxygen level–dependent signal during blocks of low-energy and high-energy food picture cues relative to blocks of neutral pictures in the significant clusters of activation in the right precentral region [A: n = 18 normal-weight (NW) subjects, 16 obese (Ob) subjects, and 17 successful weight-loss maintainers (SWL); repeated-measures ANOVA: group P = 0.05; Bonferroni-adjusted post hoc comparison: P = 0.07 between Ob and SWL, P = 0.16 between Ob and NW, and P = 1.0 between NW and SWL; no significant group × task interaction] and in the left precentral region (B: n = 18 NW, 16 Ob, and 17 SWL; repeated-measures ANOVA: group P = 0.04; Bonferroni-adjusted post hoc comparison: P = 0.04 between Ob and SWL, P = 0.25 between Ob and NW, and P = 1.0 between NW and SWL; no significant group × task interaction).
Exploratory analyses
Exploratory voxel-wise group comparisons are presented in Table 3. The most striking result was the number of regions of the brain that appeared to be more active in the SWL group than in the obese group. For example, compared with the obese group, SWLs showed greater activation in prefrontal regions, including the bilateral superior frontal and bilateral middle frontal gyri, occipital, lingual, and fusiform regions and in the precentral and postcentral gyri in response to food images relative to neutral images. The only region of greater activation in the obese than in the SWL group was the left anterior cingulate. Compared with the obese group, the NW group showed greater activation in 3 frontal regions, including clusters within the right and left superior frontal gyri. Compared with the NW group, the obese group did not show greater activity in clusters >200 mm3 at the P < 0.01 level in addition to the bilateral precentral sites identified in the ROI analyses.
TABLE 3.
Regions that exhibit significant group differences in high-energy cue effects (P < 0.01, clusters >200 mm3)1
| ROI no. and groups | Region | x | y | z | Size |
| NW > Obese | |||||
| 1 | Right superior frontal | 36 | 48 | 16 | 559 |
| 2 | Left superior frontal | 9 | 29 | 54 | 495 |
| 3 | Right precuneus | 12 | −62 | 60 | 444 |
| 4 | Right superior parietal | 36 | −58 | 54 | 383 |
| 5 | Right superior frontal | 12 | −15 | 65 | 282 |
| NW > SWL | |||||
| 1 | Right cingulate | 18 | −6 | 42 | 324 |
| 2 | Right superior parietal | 36 | −60 | 51 | 285 |
| SWL > Obese | |||||
| 1 | Right middle occipital | 29 | −89 | 7 | 6645 |
| 2 | Left middle occipital | 35 | −87 | 3 | 5054 |
| 3 | Right medial frontal/right anterior cingulate | 16 | 47 | −5 | 4157 |
| 10 | 36 | −5 | |||
| 4 | Bilateral superior frontal | 16 | 56 | 24 | 1838 |
| 5 | Left fusiform/left parahippocampal | 31 | −39 | −10 | 904 |
| 6 | Left lingual | 8 | −100 | −5 | 320 |
| 7 | Right fusiform/right parahippocampal | 26 | −49 | −9 | 446 |
| 8 | Left Postcentral | 44 | −22 | 47 | 356 |
| 9 | Left middle frontal | 32 | 35 | −10 | 331 |
| 10 | Right precentral/BA 4 | 50 | −10 | 47 | 295 |
| 11 | Right middle frontal | 41 | 35 | −11 | 254 |
| 12 | Left paracentral | 8 | −32 | 69 | 249 |
| 13 | Left insula | 37 | −10 | 5 | 232 |
| 14 | Left cuneus | 5 | −91 | 24 | 223 |
| Obese > SWL | |||||
| 1 | Left anterior cingulate | 22 | 37 | 5 | 389 |
ROI, region of interest; NW, normal-weight; SWL, successful weight-loss maintainers; BA, Brodmann area.
DISCUSSION
The brain mechanisms known to be involved in the control of hunger and eating behavior are complex, because past studies of laboratory animals have suggested interactions of multiple brain circuits. Neuroimaging research on human obesity has pointed to abnormalities in regions associated with reward and inhibitory control of behavior. The current results provide evidence for the input of inhibitory neural systems of the frontal cortex, particularly among people who were formerly obese and successfully lost weight.
SWLs showed greater activation in the left superior frontal region in response to food cues than did the NW and obese subjects. Additional frontal regions were also identified as distinguishing SWL from obese participants in follow-up analyses. The finding of greater inhibitory control in the SWLs is consistent with several findings from the behavioral literature. SWLs have reported maintaining control over their food intake, including consuming a reduced-calorie, low-fat diet (15, 16). SWLs also exhibit high dietary restraint (15, 16), which indicates a high degree of conscious control exerted over eating behavior. SWLs also report low scores on dietary disinhibition, which suggests infrequent loss of control over eating and an ability to refrain from eating in response to emotional, cognitive, or social food cues (17). The present findings extend prior observations describing how SWLs successfully maintain their weight loss by suggesting that they may exhibit patterns of brain activity consistent with restraining their response to the presentation of food cues.
Of note, one of the frontal regions showing greater activation in SWLs than in obese participants in exploratory analyses, a bilateral superior frontal region (MNI x = 16, y = 56, z = 24), replicates one of the few prior imaging studies to include SWLs. DelParigi et al (11) reported that SWLs showed greater activation than nondieters in an overlapping region (with peak activation at MNI x = 13, y = 63, z = 28) in response to a satiating meal. Taken together, these results suggest a consistent pattern of frontal activation in response to food stimuli as a characteristic neurofunctional feature of persons who have successfully lost weight.
SWLs also showed significantly greater activation in the right middle temporal gyrus relative to both the NW and obese groups. Indeed, although the group × task interaction was not significant, our results suggest that SWLs showed differential activation in this region in response to the high-energy food images—a pattern not seen in the NW or obese group. The location of this region is consistent with greater visual processing or visual attention to food images in SWLs than in the obese and NW group. This result is supported by voxel-wise group contrast analyses, in which SWLs showed greater activation in many primary and secondary visual processing regions than did the obese. It is notable that the comparison condition for all contrasts was a series of pictures of nonfood objects. Thus, this differential visual attention in SWLs does not simply reflect a greater attention to pictures but specifically to food pictures.
This greater visual attention to food cues may reflect effective monitoring of food, which may lead to preventive or corrective behaviors that promote long-term weight control. Behavioral research has shown that SWLs frequently engage in food monitoring (18, 19). Monitoring of food intake is considered a cornerstone to successful weight control; it helps reduce passive overconsumption and to promote control over intake (19–21).
In addition to identifying differences in brain response to food images in SWLs, we also identified a novel region showing greater bilateral activation in the obese group than in the NW and SWL groups in response to the food cues. Specifically, the obese group showed the greatest activation in the left and right precentral region, whereas little response was shown by the NW and SWL groups. This suggests the potential for a greater motor readiness (22) to respond to food cues in the obese group than in the NW and SWL groups and offers a novel addition to the list of brain regions associated with obesity.
Prior reports examining obese-lean differences in response to food pictures have identified ROIs in regions thought to be associated with reward or motivation processes (8, 9). Our study differed from these prior studies in the methodologic approach. Because we were primarily interested in how the groups differed in response to food images, we specifically limited our hypothesis testing to regions showing task-related activation. This improves the validity of our results because our identified group differences occur in clusters of documented cue-related reactivity. In prior studies, group comparisons were conducted without evidence of significant task-related effects in ROIs (8, 9). However, in the present study, food images did not elicit significant activation in reward or motivation-related regions. Stoeckel et al (9) also limited their analyses to reward and motivational regions to the exclusion of other regions. On this basis, they would have been unable to detect obese-lean differences, such as the ones observed in the precentral region in our study. Finally, Rothemund et al (8) focused on peak activation within a cluster as opposed to mean activation across a cluster, which suggested that the group differences reported may not have been representative of the clusters reported.
It is important to note some limitations of the present study. The NCWR, from which the SWLs were recruited, is a self-selected sample and is predominantly composed of white and female subjects. Participants recruited through advertising were also predominantly white and female. Thus, the generalizability of the present results to men and other racial groups remains to be determined. In addition, we are among the first groups to incorporate SWLs into imaging studies. Although we did show some evidence of replication of prior imaging results with SWLs, the experimental contexts, including recording techniques and stimuli, were different and further replication of these results is important. Finally, our analyses focused on the change in BOLD response in response to high- and low-energy food pictures relative to the response to nonfood pictures. It is plausible that the groups may also have differed in response to the nonfood pictures or in blood flow, which may have affected the change in BOLD response during viewing of the nonfood images and food images.
In summary, the results of this neuroimaging study indicate that SWLs show greater activation in frontal regions and primary and secondary visual cortices—a pattern consistent with greater inhibitory control in response to food cues and greater visual attention to the food cues. This greater engagement of inhibitory control regions in response to food cues and greater monitoring of foods may mediate control of food intake and successful weight-loss maintenance.
Acknowledgments
The authors' responsibilities were as follows—JMM, APH, and LHS: participated in the data analysis; ADP: contributed to the design of the study before his appointment with Pfizer Inc and contributed to the writing of the manuscript in his free time; and all authors: contributed to the writing and/or critical revision of the manuscript. Pfizer Inc did not provide financial support for the research reported in this article. None of the authors declared any conflicts of interest.
REFERENCES
- 1.Wing RR. Behavioral approaches to the treatment of obesity. Bray GA, Bouchard C, James WPT, eds. Handbook of obesity New York, NY: Marcel Dekker Inc, 1998:855–73 [Google Scholar]
- 2.Wadden TA, Sternberg JA, Letizia KA, Stunkard AJ, Foster GD. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five-year perspective. Int J Obes 1989;13(suppl 2):39–46 [PubMed] [Google Scholar]
- 3.McGuire MT, Wing RR, Klem ML, Hill JO. Behavioral strategies of individuals who have maintained long-term weight losses. Obes Res 1999;7:334–41 [DOI] [PubMed] [Google Scholar]
- 4.Rodin J, Schank D, Striegel-Moore R. Psychological features of obesity. Med Clin North Am 1989;73:47–66 [DOI] [PubMed] [Google Scholar]
- 5.Craeynest M, Crombez G, Koster EH, Haerens L, De Bourdeaudhuij I. Cognitive-motivational determinants of fat food consumption in overweight and obese youngsters: the implicit association between fat food and arousal. J Behav Ther Exp Psychiatry 2008;39:354–68 [DOI] [PubMed] [Google Scholar]
- 6.Wardle J. Conditioning processes and cue exposure in the modification of excessive eating. Addict Behav 1990;15:387–93 [DOI] [PubMed] [Google Scholar]
- 7.Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite 1996;27:41–50 [DOI] [PubMed] [Google Scholar]
- 8.Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 2007;37:410–21 [DOI] [PubMed] [Google Scholar]
- 9.Stoeckel LE, Weller RE, Cook EW, III, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008;41:636–47 [DOI] [PubMed] [Google Scholar]
- 10.DelParigi A, Chen K, Salbe AD, et al. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord 2004;28:370–7 [DOI] [PubMed] [Google Scholar]
- 11.DelParigi A, Chen K, Salbe AD, et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes 2007;31:440–8 [DOI] [PubMed] [Google Scholar]
- 12.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage 2003;19:1381–94 [DOI] [PubMed] [Google Scholar]
- 13.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–73 [DOI] [PubMed] [Google Scholar]
- 14.Talairach J, Tournoux P. Co-planar sterotaxic atlas of the human brain: 3-dimensional proportional system—an approach to cerebral imaging. New York, NY: Thieme Medical Publishers, 1988 [Google Scholar]
- 15.Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr 1997;66:239–46 [DOI] [PubMed] [Google Scholar]
- 16.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 2005;82:222S–5S [DOI] [PubMed] [Google Scholar]
- 17.Niemeier HM, Phelan S, Fava JL, Wing RR. Internal disinhibition predicts weight regain following weight loss and weight loss maintenance. Obesity (Silver Spring) 2007;15:2485–94 [DOI] [PubMed] [Google Scholar]
- 18.Phelan S, Wing RR, Raynor HA, Dibello J, Nedeau K, Peng W. Holiday weight management by successful weight losers and normal weight individuals. J Consult Clin Psychol 2008;76:442–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med 2006;355:1563–71 [DOI] [PubMed] [Google Scholar]
- 20.Marlatt GA, Gordon JR. Relapse prevention: maintenance strategies in additive behavior change. New York, NY: Guilford, 1985 [Google Scholar]
- 21.Boutelle KN, Kirschenbaum DS. Further support for consistent self-monitoring as a vital component of successful weight control. Obes Res 1998;6:219–24 [DOI] [PubMed] [Google Scholar]
- 22.Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and readiness for voluntary movement: a high-field event-related fMRI study of the Bereitschafts-BOLD response. Neuroimage 2003;20:404–12 [DOI] [PubMed] [Google Scholar]