Abstract
Background: The nonsteroidal estrogen equol occurs as diastereoisomers, S-(−)equol and R-(+)equol, both of which have significant biological actions. S-(−)equol, the naturally occurring enantiomer produced by 20–30% of adults consuming soy foods, has selective affinity for estrogen receptor-β, whereas both enantiomers modulate androgen action. Little is known about the pharmacokinetics of the diastereoisomers, despite current interest in developing equol as a nutraceutical or pharmaceutical agent.
Objective: The objective was to compare the pharmacokinetics of S-(−)equol and R-(+)equol by using [13C] stable-isotope-labeled tracers to facilitate the optimization of clinical studies aimed at evaluating the potential of these diastereoisomers in the prevention and treatment of estrogen- and androgen-dependent conditions.
Design: A randomized, crossover, open-label study in 12 healthy adults (6 men and 6 women) compared the plasma and urinary pharmacokinetics of orally administered enantiomeric pure forms of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and the racemic mixture. Plasma and urinary [13C]R-equol and [13C]S-equol concentrations were measured by tandem mass spectrometry.
Results: Plasma [13C]equol concentration appearance and disappearance curves showed that both enantiomers were rapidly absorbed, attained high circulating concentrations, and had a similar terminal elimination half-life of 7–8 h. The systemic bioavailability and fractional absorption of R-(+)[2-13C]equol were higher than those of S-(−)[2-13C]equol or the racemate. The pharmacokinetics of racemic (±)[2-13C]equol were different from those of the individual enantiomers: slower absorption, lower peak plasma concentrations, and lower systemic bioavailability.
Conclusions: The high bioavailability of both diastereoisomers contrasts with previous findings for the soy isoflavones daidzein and genistein, both of which have relatively poor bioavailability, and suggests that low doses of equol taken twice daily may be sufficient to achieve biological effects.
INTRODUCTION
Equol, [7-hydroxy-3-(4′-hydroxyphenyl)-chroman], a nonsteroidal estrogen, is found in high concentrations in the urine of many adults consuming soy foods (1, 2). It is a metabolite of daidzin, one of the principal isoflavones found in most soy foods, and is formed after initial intestinal hydrolysis of this soy isoflavone glycoside to yield the aglycone daidzein and then colonic bacterial biotransformation (1–3). Most animal species exclusively and efficiently produce equol when fed diets containing soy protein, particularly rodents, because commercial rodent diets are generally formulated with soy protein (4–7). However, only 20–30% of humans living in Western countries produce equol after ingesting soy foods or isoflavone supplements containing either daidzin or daidzein, whereas the frequency of equol producers in Asian populations that consume soy foods is typically 50–60% (8–10). The reason for these differences remains unclear (2, 11, 12), and factors involved in equol production are now being extensively investigated after a proposal that those who are capable of producing equol may have more favorable responses to soy isoflavone–containing diets—the so called “equol hypothesis” (8). Whereas several studies appear to indicate that dietary factors may influence the ability of humans to produce equol when ingesting soy foods (12–14), strategies to convert equol nonproducers to equol producers with either pre- or probiotics to enhance the effects of a soy-based diet have thus far proven elusive (15–19). What is apparent is that equol production appears to be a relatively stable phenomenon (8) and that it may be difficult to convert an equol nonproducer to an equol producer because its production is primarily dependent on the presence of specific equol-producing bacteria in the intestine, of which several strains have been identified (2, 20–26). One approach to overcome this problem would be to administer equol as either a pharmaceutical or nutraceutical agent (27) or to use it as a food additive.
Interest in equol is stimulated by the finding that it has unique biological properties in exhibiting affinity for estrogen receptors (28–34) while also being able to antagonize the actions of the potent androgen dihydrotestosterone (35). It is also an excellent antioxidant, having greater antioxidant capacity than vitamin C or E in several in vitro tests (36, 37). Furthermore, equol, unlike the soy isoflavones daidzein and genistein, has a unique chemical structure by having a chiral carbon atom at position C-3 of the furan ring. It therefore occurs in 2 distinct enantiomeric forms, S-(−)equol and R-(+)equol, and these differ significantly in conformational structure (8, 34). We showed in humans and rats that intestinal bacteria produce exclusively the S-(−)equol enantiomer when fed soy-containing diets (34), which is coincidental because S-(−)equol, unlike R-(+)equol, has selective and significant affinity for the estrogen receptor-β (ERβ) (33, 34), which makes it more similar to a selective estrogen receptor modulator (SERM) rather than an estrogen. Additionally, both enantiomers have been found to antagonize the action of dihydrotestosterone (35), which suggests a potential clinical or therapeutic role for equol in androgen-mediated conditions such as prostate cancer or skin diseases. For this reason, there is considerable interest in using equol as a dietary, nutraceutical, or pharmacologic agent (27), but little is known about its pharmacokinetic behavior (8, 34). Such information is fundamental to optimizing the design of future clinical studies that propose to explore the action of both enantiomers in humans. We developed a method for the bulk synthesis of S-(−)equol and R-(+)equol in high enantiomeric purity using chiral chemistry (38), which permits detailed studies of both enantiomers to be conducted in humans. We now report, for the first time, a comparison of the pharmacokinetics of S-(−)equol, R-(+)equol, and the racemic mixture (± equol) in healthy human adults with the use of the [13C]-labeled analogs of both enantiomers as tracers.
SUBJECTS AND METHODS
Study design
Twelve healthy adults (6 women and 6 men) aged 18–51 y with a body mass index (BMI; in kg/m2) of 18–30 were enrolled and randomly assigned to treatment according to a Latin-square design, crossover, open-label study to compare the pharmacokinetics of orally administered S-(−)[2-13C]equol, R-(+)[2-13C]equol, and a racemic mixture of both isotopes, (±)[2-13C]equol. Subjects were asked to refrain from eating foods containing soy protein for 1 wk before and during the study. Subjects with preexisting chronic renal, liver, pulmonary, or cardiovascular disease; who had been administered antibiotics within the preceding 3 mo; or who were taking oral contraceptives or hormone replacements or any over-the-counter medications that influence gastrointestinal function, such as antacids or laxatives, were excluded. The study protocol was approved by the Human Investigations Review Board of the Children's Hospital Medical Center (protocol CCHMC no. 05-08-29; approval date: 29 August 2005) and conducted under Food and Drug Administration–approved IND no. 73,012. Signed informed consent was obtained from each subject. The study was performed at the General Clinical Research Center of the Children's Hospital Medical Center, Cincinnati, OH, and the first subject was enrolled on 11 September 2007.
After an overnight fast, blood samples (10 mL) were obtained before (baseline) and then 1, 2, 4, 6, 8, 12, 24, 30, 36, and 48 h after administration as a single-bolus 20-mg dose of S-(−)[13C]equol, R-(+)[13C]equol, or (±)[2-13C]equol according to the randomization schedule. Each enantiomer was taken with a glass of water after an overnight fast and before breakfast. Subjects were given a standardized meal consisting of cereal with milk, eggs (scrambled or as an omelet), and bread, accompanied by fruit juice or coffee that was prepared at the General Clinical Research Center; the meal was consumed within 1 h of administration of equol. Blood was obtained via an indwelling catheter in the antecubital vein for the more frequent samplings and/or by venipuncture for the later sample times, depending on the choice of the individual and collected into potassium (K3) EDTA–containing tubes. The blood sampling times were selected on the basis of previous data for the pharmacokinetics of soy isoflavones and from preliminary studies of equol (34). The blood samples were centrifuged at 3000 rpm for 10 min, and the plasma removed and immediately frozen at −20°C.
Two 12-h pooled urine collections were obtained on the day immediately before administration of the [13C]-labeled enantiomers; thereafter, urine was collected and pooled every 12 h for 3 consecutive days. After collection, the total 12-h volumes were recorded and a 20-mL volume of each collection was retained and frozen at −20°C. Each study subject was then allowed a minimum of a 21-d washout period before returning to the GCRC to repeat the identical procedure but after ingestion of the 2 further assigned [13C]equol tracers according to the randomization schedule.
Synthesis of equol enantiomers for pharmacokinetic studies
(±)[2-13C]Daidzein was synthesized by a reaction of [13C]diethoxydimethylamino-methane with 2,4-dihydroxybenzoin, as previously described (39). The stable-isotope-labeled tracers S-(−)[2-13C]equol and R-(+)[2-13C]equol were then synthesized from (±)[2-13C]daidzein after protection of the hydroxyl groups by formation of a methoxymethoxy (MOM) derivative, oxidoreduction to generate the 3–4 chromene structure, stereoselective hydrogenation of the 3–4 chromene by using specific iridium-based chiral catalysts, and finally hydrolysis of the protecting groups. The method used to prepare these enantiomers is described in detail in US patent 2007/0027329A1 and the PCT WO 2007/016423A2 (38). The final products, S-(−)[2-13C]equol and R-(+)[2-13C]equol, were recrystallized to >98.5% purity and were confirmed by mass spectrometry and nuclear magnetic resonance spectroscopy to be >99.8% isotopically pure and to have an enantioselective purity >99.5%. Capsules containing accurately weighed 20-mg quantities of S-(−)[2-13C]equol or R-(+)[2-13C]equol and 20 mg of the racemic mixture (±)[2-13C]equol (10 mg of each enantiomer) were formulated for administration to healthy adults.
Analytic methods
Chemicals and reagents
Methanol, acetone, isopropanol, and all other solvents (HPLC grade) were purchased from Fisher Scientific (Hampton, NH). Dansyl chloride (95% TLC) was purchased from Sigma-Aldrich Co (St Louis, MO). Sodium acetate, sodium hydroxide, acetic acid, and sodium bicarbonate were also purchased from Fisher Scientific. Helix pomatia digestive juice (β-glucuronidase 96,000 units/mL and sulfatase 390 units/mL solution) was purchased from Sigma-Aldrich Co. All reagents were of analytic grade. The triple stable-isotope-labeled equol analog (±)[2,3,4-13C3]equol was purchased from Nigel Botting at the University of Edinburgh, United Kingdom, and used as the internal standard for the liquid chromatography–mass spectrometry (LC-MS) analysis of S-(−)[2-13C]equol and R-(+)[2-13C]equol.
Determination of S-(−)[2-13C]equol and R-(+)[2-13C]equol in plasma and urine
The concentrations of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol were measured by electrospray ionization LC-MS with stable-isotope dilution analysis and multiple reaction ion monitoring (MRM). Plasma concentrations of S-(−)[2-13C]equol, R-(+)[2-13C]equol and the racemate were measured after solid-phase extraction and enzymatic hydrolysis of the conjugates with a combined sulfatase and glucuronidase enzyme preparation, reextraction by using solid-phase extraction (10), and preparation of the dansylated derivative (40). Specifically, plasma (0.2 mL) samples were equilibrated with 40 ng of the internal standard [(±)[2,3,4-13C3]equol], diluted with 10 vol 0.5 mol triethylamine sulfate/L (pH 5.0), and heated to 64°C. The heated diluted sample was passed through a solid-phase C18-Bond Elut cartridge (Varian Inc, Palo Alto, CA) to absorb isoflavones, and, after being washed with distilled water, [13C]equol enantiomers and their conjugates were recovered by elution with methanol (5 mL). The methanolic extract was evaporated to dryness under nitrogen and reconstituted in 2 mL of 0.5 mol acetate buffer/L (pH 4.5), and then the sample was subjected to hydrolysis at 37°C overnight with a solution of 10,000 Fishman Units of a mixed β-glucuronidase/sulfatase (H. pomatia; Sigma Chemicals Inc) that had been previously filtered through a C18-Bond Elut cartridge to remove any naturally occurring isoflavones present in this enzyme preparation. After hydrolysis, S-(−)[2-13C]equol and R-(+)[2-13C]equol were isolated by solid-phase extraction on a C18-Bond Elut cartridge as described above. The methanolic extract was taken to dryness under a stream of nitrogen gas, and S-(−)[2-13C]equol and R-(+)[2-13C]equol were converted to the dansylated derivative (40) by adding sodium bicarbonate buffer, pH 10.5 (0.1 mL), and by reaction with dansyl chloride (0.1 mL of a 1 mg/mL solution in acetone). The sample was injected directly on column, and S-(−)[2-13C]equol and R-(+)[2-13C]equol were analyzed by LC-MS.
For the analysis of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol in urine samples, the same method was used except that the initial dilution with triethylamine sulfate was not necessary. Urine was diluted 1–25-fold with water, depending on the anticipated concentrations, and 0.1 mL of the diluted sample was then collected for analysis. After the internal standard [(±)[2,3,4-13C]equol (40 ng)] was added, the sample was hydrolyzed at 37°C overnight with a mixture of β-glucuronidase sulfatase as described above. After hydrolysis, the sample was passed through a C18-Bond Elut cartridge to extract the isoflavones, including the equol enantiomers, and was processed as described for the plasma samples.
Quantification by electrospray LC-MS analysis
After derivatization, S-(−)[2-13C]equol, R-(+)[2-13C]equol and, (±)[2-13C]equol were quantified by using HPLC coupled with electrospray tandem mass spectrometry (ESI-MS/MS) on an Acquity Quattro Micro (Waters Corp, Milford, MA) with MRM in positive ion mode. The [13C]equol enantiomers were retained and eluted from a reversed-phase Hypersil Gold C18 LC column (150 cm × 2.1 mm internal diameter, 3-μm particle size; Thermo Fisher Scientific, Waltham, MA). Chromatography was performed with an isocratic mobile phase of solvent A/solvent B (12/88, vol:vol) at a flow rate 200 μL/min at 25°C. Solvent A consisted of methanol/water (5/95, vol:vol) with 2 mmol ammonium acetate/L and 0.1% formic acid, and solvent B consisted of methanol/water (95/5, vol:vol) with 2 mmol ammonium acetate/L and 0.1% formic acid. The dansylated derivatives were injected (10/200 μL) directly on column.
S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol were detected and quantified by monitoring the positive ion MRM transition m/z 710→170 generated by fragmentation and loss of the dansyl group, and the internal triply labeled standard was detected from the corresponding positive ion MRM transition m/z 712→170. The concentrations of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol were determined from the peak area ratio of each ion relative to the internal standard and by interpolation of this area ratio against calibration curves plotted for known concentrations of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol. The assay was performed under good laboratory practice and with quality assurance. The intraassay and interassay precisions of the plasma [2-13C]equol assays were 2.2–14% and 2.6–9.0%, respectively, expressed as CV for samples containing S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol at concentrations ranging from 20 to 200 ng/mL. The lower limit of quantification of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol was 1 ng/mL for each enantiomer; at this level, the precision was <15% (expressed as %CV). For the urinary [2-13C]equol assays, the intraassay and interassay precisions ranged from 0.8% to 7.2% and from 6.1% to 9.9%, respectively, and were expressed as the CV for samples containing S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol at concentrations ranging from 40 to 200 ng/mL. The lower limit of quantification of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol in urine was 1 ng/mL for each enantiomer; at this level, the precision was <15% (expressed as %CV). The limit of detection of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol for both the plasma and urine assays was 0.1 ng/mL.
Chiral-phase HPLC separation of S-(−)[2-13C]equol and R-(+)[2-13C]equol
To determine whether any racemization of S-(−)[2-13C]equol or R-(+)[2-13C]equol had occurred after administration of these enantiomers, plasma samples were processed as described above but without conversion to the dansyl derivatives. The underivatized sample was dissolved in 100 μL isopropanol/water (10/90, vol:vol), and a 10-μL was sample injected on column. The identity of the enantiomeric form of equol in the human plasma was based on the retention time of the eluting peak compared with the mass chromatograms obtained under identical conditions for baseline samples of plasma to which pure S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol were added, respectively. Separation and identification of each enantiomer was achieved by HPLC-ESI-MS/MS, essentially as described previously (34), by using a Chiral-AGP LC analytic column (100 mm × 2.0 mm internal diameter, 5-μm particle size; Supelco, Bellefonte, PA). The mobile phase consisted of isopropanol/water (5/95, vol:vol), and the flow rate was 200 μL/min. S-(−)[2-13C]equol and R-(+)[2-13C]equol were well separated and identified by monitoring the negative ion MRM transition m/z 242→121 generated by fragmentation of the charged molecular ion.
Determination of plasma and urinary pharmacokinetics
The plasma (±)[2-13C]equol concentration-time profile for each study subject was determined by using a noncompartmental approach. The WinNonlin (Pharsight Corporation, Cary, NC) computer program was used for the analysis. The total area under the plasma concentration-time curve (AUC 0→∞; AUCinf) was computed by using the following equation:
where t is the last time point for blood sampling (which in this study was 48 h.), Ct is the plasma concentration at the last blood sampling time point, λz is the apparent elimination rate constant, and λz was determined from the slope of the best fitting regression line of the plasma samples in the terminal phase. At least 4 time points were included in the estimation of λz. When required, appropriate weighting schemes (usually 1/y or 1/y2, where y is the observed plasma concentration) were used to improve the goodness of fit. The choice of the number of points included in the terminal phase of the plasma concentration-time curves was based on the weighted residual (difference between model-predicted and observed concentrations) values, dispersion of the residual values, and regression coefficient. In all cases the regression lines were drawn without exclusion of any time points, and the R2 values were >0.91. The terminal half-life was calculated as ln(2)/λz; the systemic clearance after oral administration (CL/F) was determined as dose/AUCinf, and the apparent volume of distribution after oral administration (Vz/F) was determined as dose/(λz.AUCinf). Note that “F” refers to the bioavailable fraction after oral administration, which in this case is unknown.
S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol concentrations in urine were converted to daily outputs by multiplying by the total urine volume, and values were expressed as mg/12 h collection. The fraction of the isoflavone excreted in urine was determined as the ratio of the cumulative amount excreted in urine to the administered dose.
Statistical analysis
All analyses were conducted by using SPSS 17.0 for Windows (SPSS Inc, Chicago, IL). Repeated-measures ANOVA was used for each test; significance was set at 0.0055. Tests that showed significance were selected for further review, and matched-pairs t tests were conducted with Bonferroni correction for multiple comparisons.
RESULTS
Demographics of study subjects
The mean (±SD) age of the 12 healthy adults enrolled was 35.3 ± 13.7 y. The 6 women ranged in age from 20 to 46 y, and the 6 men ranged in age from 30 to 51 y; no significant age difference was observed between the 2 sexes. The mean (±SD) BMI for the group was 25.2 ± 3.1, and no significant difference was observed between the mean BMI of the women (24.5 ± 3.5) and that of the men (25.8 ± 2.7). No significant adverse events were reported during or after administration of S-(−)[2-13C]equol, R-(+)[2-13C]equol, or (±)[2-13C]equol. One female subject reported a headache after administration of S-(−)[2-13C]equol and R-(+)[2-13C]equol but not after (±)[2-13C]equol.
Plasma pharmacokinetics
Typical ion current chromatograms obtained after analysis of the plasma samples obtained 2 h after administration of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol to the same healthy adult are shown in Figure 1. For comparison, a sample of the baseline plasma and plasma to which (±)[13C]equol was added is shown. These ion current chromatograms confirmed the expected lack of either [13C]equol isotopic enantiomer in the baseline samples furthermore established that no interconversion by racemization of either S-(−)[2-13C]equol or R-(+)[2-13C]equol enantiomers had occurred after administration or during absorption. Plasma collected after administration of S-(−)[2-13C]equol contained S-(−)[2-13C]equol exclusively, whereas the plasma contained R-(+)[2-13C]equol exclusively after administration of R-(+)[2-13C]equol. When both enantiomers were administered in equal proportions, as was the case for (±)[2-13C]equol, the relative intensity of the ion current response for R-(+)[2-13C]equol was consistently greater than that of S-(−)[2-13C]equol, which suggested that the fractional absorption of R-(+)[2-13C]equol was greater than that of S-(−)[2-13C]equol.
FIGURE 1.
Typical chiral phase liquid chromatography tandem mass spectrometry profiles of the negative ion multiple reaction ion monitoring (MRM) transition m/z 242→121 for plasma samples collected 2 h after administration of 20 mg S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol to the same healthy adult. Shown for comparison is a sample of plasma taken at baseline and the same sample after adding the enantiomeric pure standards of S-(−)[2-13C]equol and R-(+)[2-13C]equol. These analyses established a lack of biotransformation of the individual enantiomers after oral administration.
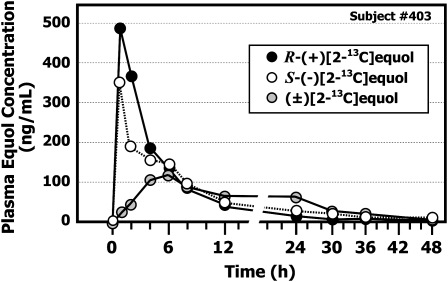
A typical plasma concentration appearance and disappearance curve obtained after administration of the same dose of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol to the same subject is illustrated in Figure 2. The plots confirm rapid absorption of both isotopic enantiomers. The peak plasma concentration occurred 2–3 h after oral administration of S-(−)[2-13C]equol and R-(+)[2-13C]equol; however, when (±)[2-13C]equol was administered, the uptake was consistently slower [mean time to reach maximum concentration (tmax): 5.75 ± 0.87 h]; consequently, the peak plasma concentrations attained were significantly lower for the racemate than for the individual enantiomers.
FIGURE 2.
Typical plasma equol concentration appearance and disappearance curves obtained in a healthy adult after administration of a single-bolus, 20-mg dose of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol.
The shape of the plasma concentration appearance and disappearance curve for S-(−)[2-13C]equol and R-(+)[2-13C]equol was similar, but the maximum plasma concentration (Cmax) was consistently higher in all subjects after administration of R-(+)[2-13C]equol when compared with the same dose of S-(−)[2-13C]equol. This was also evident for comparisons of the peak area responses for the transition m/z 710→170 in the MRM mass chromatograms (Figure 1); R-(+)[2-13C]equol yielded a higher peak area response than did S-(−)[2-13C]equol, and this difference was particularly evident after administration of (±)[2-13C]equol. The plasma pharmacokinetic profiles of S-(−)[2-13C]equol and R-(+)[2-13C]equol were similar, and both enantiomers showed first-order kinetics as indicated from the log/linear plots for the mean (± SD) plasma concentration appearance and disappearance curves for all 12 subjects (Figure 3). On average, the difference in maximum plasma concentration attained after administration of 20 mg R-(+)[2-13C]equol was 211 ng/mL (871 nmol/L) greater than the concentration attained after administration of the same dose of S-(−)[2-13C]equol. With the racemate, this difference was less apparent because of the lower dose of each enantiomer (10 mg each) administered (data not shown).
FIGURE 3.
Log linear plots of appearance and disappearance curves for mean (±SD) plasma S-(−)[2-13C]equol and R-(+)[2-13C]equol concentrations obtained in the same 12 healthy adults after administration of a single-bolus, 20-mg dose of S-(−)[2-13C]equol and R-(+)[2-13C]equol administered in a randomized crossover design study; data for (±)[2-13C]equol are not included so that the comparison of the characteristics of the 2 enantiomers is clear. Cmax, maximum plasma concentration.
The group data obtained when these plasma concentrations were computed by using a noncompartmental pharmacokinetic approach to determine the apparent bioavailability—expressed as AUCinf, tmax, Cmax, elimination half-life (t1/2), Cl/F, and Vz/F—are summarized in Table 1. Pairwise comparisons of these data showed significantly higher values for plasma Cmax and AUCinf for R-(+)[2-13C]equol than for (±)[2-13C]equol and correspondingly higher values than for S-(−)[2-13C]equol, although the latter comparison was not statistically significant. A t1/2 of ≈7–8 h was observed for S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol. A subanalysis of these data failed to find any significant sex differences in any of the pharmacokinetic measures.
TABLE 1.
Comparison of the plasma pharmacokinetics determined after a single-bolus, 20-mg dose of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol in the same 12 healthy adults1
| R-(+)[2-13C]equol | S-(−)[2-13C]equol | (±)[2-13C]equol | |
| tmax (h) | 2.92 ± 0.66 | 3.17 ± 0.63 | 5.75 ± 0.87 |
| Cmax (nmol/L · h) | 1202 ± 1482 | 991 ± 1292 | 567 ± 66 |
| AUCinf (nmol/L · h) | 12,230 ± 7702 | 11,048 ± 1276 | 9047 ± 1001 |
| Vz/F (L) | 71.3 ± 8.2 | 96.2 ± 12.4 | 132 ± 23.8 |
| t1/2 (h) | 6.90 ± 0.52 | 7.89 ± 0.69 | 8.15 ± 0.53 |
| Cl/F (L/h) | 7.08 ± 0.423 | 8.73 ± 1.23 | 10.73 ± 1.44 |
All values are means ± SEMs. tmax, time to reach maximum concentration; Cmax, maximum plasma concentration; AUCinf, total area under the plasma concentration-time curve; Vz/F, apparent volume of distribution; t1/2, terminal elimination half-life; Cl/F, systemic clearance. Repeated-measures ANOVA were calculated for each test, and matched-pairs t tests were conducted with Bonferroni correction for multiple comparisons.
Significantly different from (±)[2-13C]equol, P < 0.05.
Significantly different from S-(−)[2-13C]equol and (±)[2-13C]equol, P < 0.05.
Urinary excretion of S-(−)[2-13C]equol and R-(+)[2-13C]equol
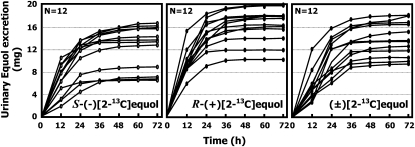
The cumulative urinary excretion of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol—expressed as the mass of isotope excreted for all 12 subjects over 72 h—is shown in Figure 4. Although there was some interindividual variation, the recoveries of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol were relatively high. For S-(−)[2-13C]equol and R-(+)[2-13C]equol, most of the isotope was excreted in the first 12 h after administration (Figure 5), consistent with its rapid appearance in plasma. For (±)[2-13C]equol, most of the isotope excreted was distributed between the first two 12-h collections, again consistent with the slower absorption as evident from the plasma concentration curves. In some individuals the fraction of the administered dose recovered in urine, expressed as the percentage of the dose administered, approached 100%, especially for R-(+)[2-13C]equol. The greater bioavailability of R-(+)[2-13C]equol than of S-(−)[2-13C]equol or (±)[2-13C]equol observed from the plasma pharmacokinetics was also reflected by its higher mean fractional recovery in urine over the 72-h period. The overall fractional recoveries of R-(+)[2-13C]equol, S-(−)[2-13C]equol, and (±)2-13C]equol were 83.2 ± 11.2%, 61.3 ± 19.5%, and 69.3 ± 15.4%, respectively (Figure 6).
FIGURE 4.
Cumulative urinary excretion of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol over 72 h in 12 healthy adults after administration of a single-bolus, 20-mg dose of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol.
FIGURE 5.
Mean (±SD) urinary excretion of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol in 12-h pooled collections over 72 h in 12 healthy adults after administration of a single-bolus, 20-mg dose of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol.
FIGURE 6.
Mean (±SD) total recovery of S-(−)[2-13C]equol [S-(−)], R-(+)[2-13C]equol [R-(+)], and (±)[2-13C]equol (±) over 72 h in 12 healthy adults after administration of a single-bolus, 20-mg dose of S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol. Urinary recovery of R-(+)[2-13C]equol was higher than that of either S-(−)[2-13C]equol or (±)[2-13C], but the differences were not statistically significant when calculated by repeated-measures ANOVA.
DISCUSSION
It was recently suggested that the metabolism of soy isoflavones, specifically the ability of intestinal bacteria to convert one of the principal soy isoflavones, daidzin, into equol, could provide a clue as to why data from recent dietary or clinical intervention studies have been so discouraging and at variance with the seemingly beneficial properties of soy foods in the Asian diet (8). Clearly, few studies have stratified for equol-producer status (10) and those that have are generally not sufficiently powered to be able to reflect whether equol production is truly important in enhancing the efficacy of a soy diet. This problem could be overcome by direct investigation of equol, which is now possible because of the ability to chemically synthesize the pure compound in quantities sufficient for clinical testing (38).
Equol can occur in 2 distinct diastereoisomeric forms, S-(−)equol and R-(+)equol, but S-(−)equol is the natural enantiomer and the exclusive product of intestinal bacterial metabolism of the precursor daidzein (34). Nevertheless, R-(+)equol is of considerable interest because of its ability to antagonize the action of dihydrotestosterone in vivo (35). Furthermore, our recent studies have shown that R-(+)equol is potently chemopreventive in the DMBA (dimethylbenz [a] anthracene) animal model of breast cancer (Brown et al, unpublished data, 2009). In most instances, when equol is chemically synthesized from daidzein, it is the racemate that is obtained, and this has been the form that has been commercially available and mostly used in older research studies. Chiral chromatography, described here and previously, can be used to resolve the 2 diastereoisomers (34), and this approach was applied in previous studies of equol (34); however, it is unsuitable for the bulk production of the enantiomers. Equol can now be produced by patented processes that use either a specific equol-producing bacterium (Lactococcus garvieae) (21, 27) to produce the natural S-(−)equol enantiomer or by chiral chemistry to separately produce S-(−)equol and R-(+)equol (38). In this study, we took advantage of the use of an iridium-based chiral catalyst to effect an enantioselective reduction of a 2,3-chromene intermediate (38) to produce multigram quantities of either S-(−)equol or R-(+)equol with high enantiomeric and chemical purities, starting from daidzein. Applying this same chemistry, but starting instead with (±)[2-13C]daidzein that was synthesized by a previously described method (39), we were able to produce enantiomeric pure stable-isotope-labeled S-(−)[2-13C]equol and R-(+)[2-13C]equol for use in these pharmacokinetic studies. The advantage of using stable-isotope-labeled analogs is that the pharmacokinetics of both equol enantiomers can be accurately determined even in the presence of a background of natural equol. LC-MS/MS analysis of the baseline samples from all subjects taken at the start of the study, and after a washout period between the dosing of the individual enantiomers, confirmed that, expectedly, there was no S-(−)[2-13C]equol or R-(+)[2-13C]equol present in plasma (Figure 1) and served to show that a 21-d washout period was more than sufficient to ensure complete elimination of each isotope. Furthermore, qualitative analysis of the plasma samples by chiral phase chromatography ESI-LC-MS (34) confirmed that no interconversion by racemization of the individual enantiomers took place during or after intestinal uptake (Figure 1). Equol, therefore, is a highly stable molecule that undergoes essentially no further metabolism, save phase II metabolism, to form predominantly the glucuronide, and to a minor extent sulfate conjugates, as was shown >2 decades ago (1, 41). In this regard, its metabolic handling is similar to that of daidzein and genistein, which circulate predominantly as glucuronide and sulfate conjugates (42–45). This lack of biotransformation accounts for the very high bioavailability of equol (Table 1 and Figure 6) when compared with its precursor daidzin/daidzein and the other principal soy isoflavone genistin/genistein (46, 47). The plasma concentration appearance and disappearance curves resulting from single-bolus oral administration of S-(−)[2-13C]equol, R-(+)[2-13C]equol, or the racemic mixture to the same 12 healthy adults established that the apparent systemic exposure to R-(+)[2-13C]equol was higher than that of S-(−)[2-13C]equol or (±)[2-13C]equol. This was clearly evident from data for AUCinf and from the overall 72-h recovery of the isotopic tracers in urine. In a previous exploratory study of 3 healthy adults, we reported that the pharmacokinetic behavior of S-(−)equol and R-(+)equol, based only on plasma kinetics, was similar (34); however, given the individual variability in the pharmacokinetics of equol, this earlier study was underpowered to identify differences in the pharmacokinetics of the 2 enantiomers. Nevertheless, the computed mean values for the Cl/F, Vd/F, t1/2, and AUCinf in this previous study were of a similar magnitude to those found in this larger randomized crossover-designed study using the [2-13C]stable-isotope-labeled tracers, which, because of its greater power (n = 12), was able to define differences in the pharmacokinetics of the 2 enantiomers. The greater bioavailability of R-(+)[2-13C]equol compared with S-(−)[2-13C]equol, and especially of the racemate, as determined from the systemic exposure, was further supported by its much higher fractional absorption. An average of 83.2 ± 11.2% of the administered 20-mg dose of R-(+)[2-13C]equol was recovered in urine over a 72-h period compared with 61.3 ± 19.5% when S-(−)[2-13C]equol (P = 0.051) was administered (Figure 5). Indeed, in 5 subjects, the recovery of R-(+)[2-13C]equol exceeded 90% of the orally administered dose, and in one it was almost complete (Figure 4). R-(+)[2-13C]equol, therefore, has exceptionally high bioavailability.
In agreement with our earlier observations for the natural enantiomers (34), both S-(−)[2-13C]equol and R-(+)[2-13C]equol rapidly appeared in plasma with peak plasma concentrations occurring 2–3 h after oral intake. This was not the case when (±)[2-13C]equol was administered at a comparable dose and indicates that, pharmacologically, the racemate seems to behave differently from the individual diastereoisomers. The most notable difference was a significantly slower rate of absorption, lower peak plasma concentration, and reduced systemic bioavailability as assessed from plasma AUCinf. The reason for this difference is unclear, but it may be that there is competitive interference between the diastereoisomers for intestinal uptake. It may also reflect an effective lower dose of the 2 enantiomers (10 mg of each diastereoisomer in the 20-mg mixture). The rapid appearance of S-(−)[2-13C]equol or R-(+)[2-13C]equol in plasma is consistent with the behavior of the isoflavone aglycones daidzein and genistein, which also appear rapidly in the plasma after oral administration (48–50). In contrast, the tmax for isoflavone glycosides, either from soy foods or as pure compounds, is generally between 4 and 8 h after intake (48, 51–53), because hydrolysis to the aglycone forms by intestinal brush border and bacterial glucosidases (54) is a necessary prerequisite because conjugated isoflavones do not cross the enterocyte and are not bioavailable (3). The clinical implication of this difference, as for drugs, is that maintenance of higher peak plasma concentrations translate to greater efficacy but also to a greater risk of potential adverse events.
The mean plasma t1/2 for S-(−)[2-13C]equol, R-(+)[2-13C]equol, and (±)[2-13C]equol was not significantly different (Table 1), which is consistent with our earlier preliminary findings in 3 adults given the natural isotopic forms of the 2 enantiomers (34). The range of t1/2 values was similar to that of daidzein and genistein, ie, 6–10 h (46, 48, 49, 51, 52, 55). The mean plasma clearance normalized to the bioavailable dose (Cl/F) for both equol enantiomers was consistently slower for R-(+)[2-13C]equol (6.92 ± 0.57 L/h) than for S-(−)[2-13C]equol or the racemate, which accounted for its higher plasma Cmax value (Table 1). The plasma clearance rates of both equol enantiomers was markedly slower than the clearance rates previously reported for daidzein (17.5 ± 1.4 L/h) or genistein (18.3 ± 5.7 L/h) (46), and this relatively slower clearance of equol contributes to the maintenance of high circulating concentrations at relatively low doses of equol—an important consideration when designing future clinical trials of equol. No sex differences were apparent in any of the pharmacokinetic measures; however, the sample size was perhaps too small to discern any difference between men and women. If there is a need to examine sex differences in the pharmacokinetics of equol enantiomers, then these data provide a basis for assessing the required power of such a study.
Our data provide the most comprehensive information to date on the pharmacokinetics of equol's enantiomers obtained by [13C]equol as tracers. The dose of S-(−)[2-13C]equol and R-(+)[2-13C]equol used in this study was chosen on the basis of the dose we anticipated would be sufficient to evoke clinical effects in future trials. It was also within the estimated physiologic range for adults that produce equol when consuming soy foods (10), given that the typical average intake of total soy isoflavones is ≈25–50 mg (56–59), of which ≈50% usually represents daidzin or daidzein—the precursors to equol. Dose-response relations were not examined in this study; however, with a single 20-mg dose, perhaps not surprisingly, none of the subjects reported any significant adverse events considered related to its administration, except for one female who reported a headache after taking both enantiomers but not the racemate. Chronic administration of a supplement containing S-(−)equol, produced by incubation of soy germ with the equol-producing bacterium Lactococcus garvieae, was reported to show acceptable tolerance in a group of postmenopausal Japanese women over the dose range 10–30 mg/d (27). The issue of whether higher doses of either enantiomer of equol may have adverse effects on the uterus remains an important question to address as it undergoes development as a potential pharmacologic or nutraceutical agent. This is because equol gained its notoriety as a metabolite of formononetin—a methoxylated isoflavone found in species of clover—that caused endometriosis and infertility in sheep and devastated the sheep breeding industry in regions of Southwest Australia >60 y ago, referred to as Clover disease (60, 61).
In conclusion, we defined in healthy adults the pharmacokinetics and disposition of the diastereoisomers of equol by uniquely using tracer stable-isotope-labeled analogs of S-(−)[2-13C]equol and R-(+)[2-13C]equol produced by novel chiral chemistry (38). Our findings showed that, in contrast with published data for the soy isoflavones daidzein or genistein (46, 47), both equol enantiomers have high systemic bioavailability, although R-(+)[2-13C]equol exhibits greater bioavailability than its diastereoisomer, S-(−)[2-13C]equol or a racemic mixture. The latter behaves differently, ie, has a slower uptake and lower peak plasma concentrations. The rapid appearance and high plasma concentrations resulting from the slow clearance of both enantiomers suggest that only modest doses (20–30 mg) of either diastereoisomer may be needed for potential biological effects. These studies provide fundamental data to permit the appropriate design of clinical trials aimed at investigating the action of equol as a selective estrogen receptor modulator (33, 34) or of its effect on androgen-mediated diseases because of its ability to antagonize the action on dihydrotestosterone (35).
Acknowledgments
We appreciate the support of Tracy Glauser, Cincinnati Children's Hospital Medical Center, who monitored the safety data for these studies.
The authors' responsibilities were as follows—KDRS: Principal Investigator; NMB: screened, recruited, and enrolled the study subjects; JEH: oversaw and monitored the studies conducted at the GCRC; and XZ and PJ: conducted the analytic methods using tandem mass spectrometry. All authors provided input about the manuscript. KDRS is an inventor of several patents related to equol that were licensed by Cincinnati Children's Hospital Medical Center for pharmaceutical development. None of the authors had a conflict of interest.
REFERENCES
- 1.Axelson M, Kirk DN, Farrant RD, Cooley G, Lawson AM, Setchell KDR. The identification of the weak oestrogen equol [7-hydroxy-3-(4'-hydroxyphenyl)chroman] in human urine. Biochem J 1982;201:353–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Setchell KDR, Borriello SP, Hulme P, Kirk DN, Axelson M. Nonsteroidal estrogens of dietary origin: possible roles in hormone-dependent disease. Am J Clin Nutr 1984;40:569–78 [DOI] [PubMed] [Google Scholar]
- 3.Setchell KDR, Brown NM, Zimmer-Nechemias L, et al. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr 2002;76:447–53 [DOI] [PubMed] [Google Scholar]
- 4.Brown NM, Setchell KDR. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest 2001;81:735–47 [DOI] [PubMed] [Google Scholar]
- 5.Thigpen JE, Haseman JK, Saunders HE, Setchell KDR, Grant MG, Forsythe DB. Dietary phytoestrogens accelerate the time of vaginal opening in immature CD-1 mice. Comp Med 2003;53:607–15 [PubMed] [Google Scholar]
- 6.Thigpen JE, Setchell KDR, Padilla-Banks E, et al. Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD-1 mice and F344 rats but not in CD Sprague-Dawley rats. Environ Health Perspect 2007;115:1717–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu L, House SE, Prior RL, et al. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J Nutr 2006;136:1215–21 [DOI] [PubMed] [Google Scholar]
- 8.Setchell KDR, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol—a clue to the effectiveness of soy and its isoflavones. J Nutr 2002;132:3577–84 [DOI] [PubMed] [Google Scholar]
- 9.Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood) 2005;230:155–70 [DOI] [PubMed] [Google Scholar]
- 10.Setchell KDR, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr 2006;136:2188–93 [DOI] [PubMed] [Google Scholar]
- 11.Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med 1998;217:335–9 [DOI] [PubMed] [Google Scholar]
- 12.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut flora. Nutr Cancer 2000;36:27–32 [DOI] [PubMed] [Google Scholar]
- 13.Lydeking-Olsen E, Beck-Jensen JE, Setchell KDR, Holm-Jensen T. Soymilk or progesterone for prevention of bone loss—a 2 year randomized, placebo-controlled trial. Eur J Nutr 2004;43:246–57 [DOI] [PubMed] [Google Scholar]
- 14.Bolca S, Possemiers S, Herregat A, et al. Microbial and dietary factors are associated with the equol producer phenotype in healthy postmenopausal women. J Nutr 2007;137:2242–6 [DOI] [PubMed] [Google Scholar]
- 15.Lampe JW, Skor HE, Li S, Wahala K, Howald WN, Chen C. Wheat bran and soy protein feeding do not alter urinary excretion of the isoflavone equol in premenopausal women. J Nutr 2001;131:740–4 [DOI] [PubMed] [Google Scholar]
- 16.Steer TE, Johnson IT, Gee JM, Gibson GR. Metabolism of the soybean isoflavone glycoside genistin in vitro by human gut bacteria and the effect of prebiotics. Br J Nutr 2003;90:635–42 [DOI] [PubMed] [Google Scholar]
- 17.Bonorden MJ, Greany KA, Wangen KE, et al. Consumption of Lactobacillus acidophilus and Bifidobacterium longum do not alter urinary equol excretion and plasma reproductive hormones in premenopausal women. Eur J Clin Nutr 2004;58:1635–42 [DOI] [PubMed] [Google Scholar]
- 18.Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. Plasma phytoestrogens are not altered by probiotic consumption in postmenopausal women with and without a history of breast cancer. J Nutr 2004;134:1998–2003 [DOI] [PubMed] [Google Scholar]
- 19.Larkin TA, Price WE, Astheimer LB. Increased probiotic yogurt or resistant starch intake does not affect isoflavone bioavailability in subjects consuming a high soy diet. Nutrition 2007;23:709–18 [DOI] [PubMed] [Google Scholar]
- 20.Ueno T, Uchiyama S. Identification of the specific intestinal bacteria capable of metabolising soy isoflavone to equol. Ann Nutr Metab 2001;45:114 (abstr) [Google Scholar]
- 21.Uchiyama S, Ueno T, Kumemura M, Imaizumi K, Masaki K, Shimizu S, inventors; Otsuka Pharmaceutical Co, Ltd, assignee. Streptococcus and isoflavone-containing composition. US patent 6,716,424 B1. 2004 [Google Scholar]
- 22.Wang XL, Hur HG, Lee JH, Kim KT, Kim SI. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl Environ Microbiol 2005;71:214–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decroos K, Eeckhaut E, Possemiers S, Verstraete W. Administration of equol-producing bacteria alters the equol production status in the Simulator of the Gastrointestinal Microbial Ecosystem (SHIME). J Nutr 2006;136:946–52 [DOI] [PubMed] [Google Scholar]
- 24.Wang XL, Kim HJ, Kang SI, Kim SI, Hur HG. Production of phytoestrogen S-equol from daidzein in mixed culture of two anaerobic bacteria. Arch Microbiol 2007;187:155–60 [DOI] [PubMed] [Google Scholar]
- 25.Tamura M, Ohnishi-Kameyama M, Shinohara K. Lactobacillus gasseri: effects on mouse intestinal flora enzyme activity and isoflavonoids in the caecum and plasma. Br J Nutr 2004;92:771–6 [DOI] [PubMed] [Google Scholar]
- 26.Zhou-Teng Y, Wen Y, Wei-Yun Z. Isolation and identification of equol-producing bacterial strains from cultures of pig faeces. FEMS Microbiol Lett 2008;282:73–80 [DOI] [PubMed] [Google Scholar]
- 27.Yee S, Burdock GA, Kurata Y, et al. Acute and subchronic toxicity and genotoxicity of SE5-OH, an equol-rich product produced by Lactococcus garvieae. Food Chem Toxicol 2008;46:2713–20 [DOI] [PubMed] [Google Scholar]
- 28.Cheng E, Yoder L, Story C, Burrough W. Estrogenic activity of some isoflavone derivatives. Science 1954;120:575–6 [DOI] [PubMed] [Google Scholar]
- 29.Shemesh M, Lindner H, Ayalon N. Affinity of rabbit uterine oestradiol receptor for phytoestrogens and its use in a competitive protein binding radioassay for plasma coumestrol. J Reprod Fertil 1972;29:1–9 [DOI] [PubMed] [Google Scholar]
- 30.Shutt DA, Cox RI. Steroid and phyto-oestrogen binding to sheep uterine receptors in vitro. J Endocrinol 1972;52:299–310 [DOI] [PubMed] [Google Scholar]
- 31.Morito K, Hirose T, Kinjo J, et al. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull 2001;24:351–6 [DOI] [PubMed] [Google Scholar]
- 32.Kostelac D, Rechkemmer G, Briviba K. Phytoestrogens modulate binding response of estrogen receptors a and b to the estrogen response element. J Agric Food Chem 2003;51:7632–5 [DOI] [PubMed] [Google Scholar]
- 33.Muthyala RS, Ju YH, Sheng S, et al. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem 2004;12:1559–67 [DOI] [PubMed] [Google Scholar]
- 34.Setchell KDR, Clerici C, Lephart ED, et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr 2005;81:1072–9 [DOI] [PubMed] [Google Scholar]
- 35.Lund TD, Munson DJ, Haldy ME, Setchell KDR, Lephart ED, Handa RJ. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol Reprod 2004;70:1188–95 [DOI] [PubMed] [Google Scholar]
- 36.Mitchell JH, Gardner P, McPhail D, Morrice P, Collins A, Duthie G. Antioxidant efficacy of phytoestrogens in chemical and biological model systems. Arch Biochem Biophys 1998;360:142–8 [DOI] [PubMed] [Google Scholar]
- 37.Arora A, Nair MG, Strasburg GM. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch Biochem Biophys 1998;356:133–41 [DOI] [PubMed] [Google Scholar]
- 38.Setchell KDR, Sirokin V, inventors. Method for the enantioselective hydrogenation of chromenes. US patent 2007/0027329A1 and PCT WO 2007/016423A2. 2005 [Google Scholar]
- 39.Baraldi PG, Spalluto G, Cacciari B, Romangnoli R, Setchell KDR. Chemical synthesis of [13C]daidzein. J Med Food 1999;2:99–102 [DOI] [PubMed] [Google Scholar]
- 40.Nelson RE, Grebe SK, Okane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem 2004;50:373–84 [DOI] [PubMed] [Google Scholar]
- 41.Axelson M, Setchell KDR. Conjugation of lignans in human urine. FEBS Lett 1980;122:49–53 [DOI] [PubMed] [Google Scholar]
- 42.Axelson M, Sjovall J, Gustafsson BE, Setchell KDR. Soya—a dietary source of the non-steroidal oestrogen equol in man and animals. J Endocrinol 1984;102:49–56 [DOI] [PubMed] [Google Scholar]
- 43.Doerge DR, Chang HC, Churchwell MI, Holder CL. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab Dispos 2000;28:298–307 [PubMed] [Google Scholar]
- 44.Ronis MJ, Little JM, Barone GW, Chen G, Radominska-Pandya A, Badger TM. Sulfation of the isoflavones genistein and daidzein in human and rat liver and gastrointestinal tract. J Med Food 2006;9:348–55 [DOI] [PubMed] [Google Scholar]
- 45.Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJ, Badger TM. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am J Clin Nutr 2002;76:588–94 [DOI] [PubMed] [Google Scholar]
- 46.Setchell KDR, Brown NM, Desai P, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr 2001;131:1362S–75S [DOI] [PubMed] [Google Scholar]
- 47.Setchell KDR, Faughnan MS, Avades T, et al. Comparing the pharmacokinetics of daidzein and genistein using [13C]labeled tracers in premenopausal women. Am J Clin Nutr 2003;77:411–9 [DOI] [PubMed] [Google Scholar]
- 48.Izumi T, Piskula M, Osawa S, et al. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr 2000;130:1695–9 [DOI] [PubMed] [Google Scholar]
- 49.Busby MG, Jeffcoat AR, Bloedon LT, et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr 2002;75:126–36 [DOI] [PubMed] [Google Scholar]
- 50.Zubik L, Meydani M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am J Clin Nutr 2003;77:1459–65 [DOI] [PubMed] [Google Scholar]
- 51.King RA, Bursill D. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am J Clin Nutr 1998;67:867–72 [DOI] [PubMed] [Google Scholar]
- 52.Setchell KDR, Brown NM, Desai PB, et al. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J Nutr 2003;133:1027–35 [DOI] [PubMed] [Google Scholar]
- 53.Piazza C, Privitera MG, Melilli B, et al. Influence of inulin on plasma isoflavone concentrations in healthy postmenopausal women. Am J Clin Nutr 2007;86:775–80 [DOI] [PubMed] [Google Scholar]
- 54.Day AJ, DuPont MS, Ridley S, et al. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett 1998;436:71–5 [DOI] [PubMed] [Google Scholar]
- 55.Bloedon LT, Jeffcoat AR, Lopaczynski W, et al. Safety and pharmacokinetics of purified soy isoflavones: single-dose administration to postmenopausal women. Am J Clin Nutr 2002;76:1126–37 [DOI] [PubMed] [Google Scholar]
- 56.Nagata C, Takatsuka N, Kurisu Y, Shimizu H. Decreased serum total cholesterol concentration is associated with high intake of soy products in Japanese men and women. J Nutr 1998;128:209–13 [DOI] [PubMed] [Google Scholar]
- 57.Chen Z, Zheng W, Custer LJ, et al. Usual dietary consumption of soy foods and its correlation with the excretion rate of isoflavonoids in overnight urine samples among Chinese women in Shanghai. Nutr Cancer 1999;33:82–7 [DOI] [PubMed] [Google Scholar]
- 58.Wakai K, Egami I, Kato K, et al. Dietary intake and sources of isoflavones among Japanese. Nutr Cancer 1999;33:139–45 [DOI] [PubMed] [Google Scholar]
- 59.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer 2006;55:1–12 [DOI] [PubMed] [Google Scholar]
- 60.Bennetts HW, Underwood EJ, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust J Agric Res 1946;22:131–8 [DOI] [PubMed] [Google Scholar]
- 61.Shutt DA, Braden AWH. The significance of equol in relation to the oestrogenic responses in sheep ingesting clover with a high formononetin content. Aust J Agric Res 1968;19:545–53 [Google Scholar]