Abstract
Background: High-fiber diets have been associated with decreased breast cancer risk, likely mediated by the effect of fiber on lowering circulating estrogen concentrations. The influence of fiber on aspects of reproduction, which include ovulation, has not been well studied in premenopausal women.
Objective: The objective was to determine if fiber consumption is associated with hormone concentrations and incident anovulation in healthy, regularly menstruating women.
Design: The BioCycle Study was a prospective cohort study conducted from 2004 to 2006 that followed 250 women aged 18–44 y for 2 cycles. Dietary fiber consumption was assessed ≤4 times/cycle by using 24-h recall. Outcomes included concentrations of estradiol, progesterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH), which were measured ≤8 times/cycle, and incident anovulation.
Results: Dietary fiber consumption was inversely associated with hormone concentrations (estradiol, progesterone, LH, and FSH; P < 0.05) and positively associated with the risk of anovulation (P = 0.003) by using random-effects models with adjustment for total calories, age, race, and vitamin E intake. Each 5-g/d increase in total fiber intake was associated with a 1.78-fold increased risk (95% CI: 1.11, 2.84) of an anovulatory cycle. The adjusted odds ratio of 5 g fruit fiber/d was 3.05 (95% CI: 1.07, 8.71).
Conclusions: These findings suggest that a diet high in fiber is significantly associated with decreased hormone concentrations and a higher probability of anovulation. Further study of the effect of fiber on reproductive health and of the effect of these intakes in reproductive-aged women is warranted.
INTRODUCTION
Increased intake of fiber has been promoted due to fiber's favorable association with certain health outcomes. High-fiber diets have been associated with reduced risks of cardiovascular disease (1), stroke (2), diabetes (3), colon cancer (4), and breast cancer (5–8). Current recommendations from the American Heart Association (9), the US Department of Agriculture (10), and the Institute of Medicine (IOM) suggest that individuals should consume 20–35 g fiber/d depending on caloric intake (11). This is in contrast to the average fiber intake in the United States, which is substantially below these recommendations [13.8 g fiber/d for reproductive-aged women (12)]. Several studies have reported inverse associations between fiber intake and estrogen concentrations in older women (13–19), presumably because of a decrease of β-glucuronidase activity in feces that results from high fiber consumption and leads to a decreased reabsorption of estrogen in the colon (20).
Although certain beneficial effects of fiber on chronic diseases have been observed, the effect of intake on endogenous hormones and other reproductive factors (eg, anovulation) in younger women has had limited study (21). The influence of fiber intake on reproductive hormone concentrations and anovulation is of particular interest in reproductive-aged women, given the effect of these hormones on conception and pregnancy maintenance (22, 23). The objective of this study was to evaluate the association between dietary fiber consumption and reproductive hormone concentrations and risk of incident anovulation in the BioCycle Study. The hypothesis was that consumption of dietary fiber at or above the Dietary Reference Intake (DRI) would be associated with lower hormone concentrations and a higher risk of incident anovulation.
SUBJECTS AND METHODS
Study design
The BioCycle Study was a prospective cohort study of menstrual cycle function in 259 regularly menstruating, premenopausal, healthy female volunteers, aged 18–44 y, who were recruited from the Western New York region and followed for ≤2 menstrual cycles. Details of the study design are described elsewhere (24). Exclusion criteria included current use of oral contraceptives, vitamin and mineral supplements, or prescription medications; pregnancy or breastfeeding in the past 6 mo; and recent history of infections or diagnosis of chronic conditions, which included history of menstrual and ovulation disorders and gastrointestinal conditions (eg, Crohn's disease). Women with a self-reported body mass index (BMI; in kg/m2) of <18 or >35 at baseline were excluded as were women planning to restrict their diet for weight loss or medical reasons. The University at Buffalo Health Sciences Institutional Review Committee approved the study, and all of the participants provided written informed consent.
Participants were followed for 1 (n = 9) or 2 (n = 250) menstrual cycles with blood samples collected at the following times: on the second day of menstruation; at mid- and late follicular phase; at luteinizing hormone (LH)/follicle-stimulating hormone (FSH) surge and ovulation; and at early, mid-, and late luteal phase (approximately corresponding to days 2, 7, 12, 13, 14, 18, 22, and 27 of a 28-d cycle) in each cycle, with collection dates adjusted for cycle length. Fertility monitors (Clearblue Easy Fertility Monitor; Inverness Medical, Waltham, MA) assisted in the timing of specimen collection. Monitor indications of low, high, and peak fertility were used to time midcycle visits, with peak day and the following 2 d those that approximately represented late follicular, LH surge, and ovulation dates (standardized days 12, 13, and 14). Women began fertility testing on calendar day 6; if by day 14 there was no positive indication on the monitor, a visit was scheduled the following day while the participant continued daily monitor testing for 10 additional days. Women were highly compliant to the study protocol, with 94% of women completing ≥7 clinic visits/cycle.
Dietary assessment
Dietary intake was assessed on the same days as sample collection by using a 24-h dietary recall conducted 4 times/cycle (corresponding to standardized days 2, 7, 14, and 22), for a total of 8 recalls. Dietary intake data were collected and analyzed by using the Nutrition Data System for Research software version 2005 developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN. This program computed the nutrients (ie, dietary fiber), food components (ie, insoluble and soluble), and food sources (ie, vegetable, fruit, and grain) from the 24-h dietary recalls. The majority of women completed 4 dietary recalls/cycle (87%).
Hormone assessment
Reproductive hormone concentrations were measured in serum collected at each cycle visit (8 visits/cycle for 2 cycles), which included estradiol, progesterone, LH, and FSH. Fasting morning blood draws were collected at clinic visits and processed according to standardized protocols. Samples were processed and frozen at −80°C and sent as complete participant cycle batches for hormone analysis (Kaleida Health Center for Laboratory Medicine, Buffalo, NY). Estradiol concentrations were measured in all of the available serum samples by radioimmunoassay. FSH, LH, and progesterone were measured in all of the available serum samples by Specialty Laboratories Inc (Valencia, CA) by using solid-phase competitive chemiluminescent enzymatic immunoassays on the DPC Immulite 2000 analyzer (Siemens Medical Solutions Diagnostics, Deerfield, IL).
Classification of anovulation
Menstrual cycles were initially classified as anovulatory if the peak progesterone concentration across the cycle was ≤5 ng/mL (n = 65) (25, 26). To minimize misclassification, cycles with progesterone concentrations ≤5 ng/mL and an observed serum LH peak on days 22 or 27 of the standardized 28-d cycle were considered ovulatory cycles. On the basis of this algorithm, 42 of the 509 cycles (8.3%) in this study were classified as anovulatory.
Covariate assessment
Anthropometric measures were taken, which included height and weight by using standardized protocols, which were used to determine eligibility with respect to BMI. Participants were asked to complete questionnaires regarding physical activity [International Physical Activity Questionnaire (IPAQ) long form 2002], lifestyle, and health history (27). High, moderate, and low physical activity categories were formed on the basis of standard IPAQ cutoffs. Cycle length was defined as the number of days between menstrual bleeding. Day 1 of the cycle was defined as menstruating by 1600 on that day; the last day of the cycle was the last day before the next onset of bleeding. All of the covariates assessed had a ≥95% response rate.
Statistical analysis
Repeated-measures analysis of variance was used to compare dietary intake by visit for each participant to determine whether intake changed significantly over the cycle. Descriptive statistics were calculated for demographic characteristics, dietary intake, and hormone concentrations. Exact chi-square tests and analysis of variance were used to test for associations between demographic variables and fiber intake. No significant differences were shown in dietary fiber intakes across each cycle. As such, an average daily intake of fiber and other dietary variables was calculated per cycle. In categorizing fiber, consideration was given to levels relative to the DRI. The average daily calorie intake of women in this study was ≈1600 kcal, corresponding to a DRI of 22 g fiber/d in accordance with the recommendation of 14 g fiber/1000 kcal by the IOM (11). From this, we considered ≥22 g fiber/d intake to fulfill the DRI and based creation of the highest category on this cutoff. The additional cutoffs were chosen as 6 g fiber/d (equivalent to ≈1–2 servings of fiber) lower than the adjacent category. Alternative cutoffs were explored to assess sensitivity of results to this categorization, which included quartiles of fiber intake.
Linear mixed models on the log scale of the hormones were used to evaluate the association between hormone concentrations and average fiber intake per cycle (28). These random-intercept models were chosen to account for the variation between baseline concentrations of hormones in individual women and the correlation between cycles of the same women. The linear and nonlinear mixed models make use of all of the available observations and do not require balanced data. For estrogen, models included concentrations throughout the cycle, which included ≤8 measurements/cycle (each measurement was considered as a separate observation in the analysis). Similarly, for progesterone, only concentrations during the luteal phase (days 18, 22, and 27 of the standardized 28-d cycle) were included because there is minimal variation in progesterone concentrations during the follicular phase. For LH and FSH, models included concentrations around ovulation (days 12, 13, and 14 of the standardized 28-d cycle), because their midcycle peak is the most relevant period for these hormones. Nonlinear mixed models were used to model the association between average fiber intake and the probability of anovulation (29).
The presence of confounding was evaluated by using a hybrid approach that combined prior knowledge by using directed acyclic graphs and a statistical approach on the basis of change in point estimates (30). A set of variables was determined by a review of the prior literature, and a detailed directed acyclic graph was created that identified the variables that should be included in the models. Moreover, an exploratory confounding evaluation was used with covariates included in the model if they changed the exposure coefficient by >15% and were significant at P = 0.10. Factors that were shown to have an effect on the point estimates were energy intake (continuous), race (white, black, and other), age (continuous), and vitamin E intake (continuous). Highly correlated variables, such as magnesium, potassium, and folate, were analyzed as collinear variables and potential confounders. Sensitivity analyses were conducted to assess the effect of collinearity, which included the use of propensity score methods for confounding adjustment. SAS version 9.1 (SAS Institute, Cary, NC) was used for all of the statistical analyses.
RESULTS
Fiber consumption
Overall, this cohort of women was young (mean age: 27.5 y), of healthy weight (mean BMI: 24.1), had moderate to high physical activity (90.5%), and mostly comprised nonsmokers (82%; Table 1). Fiber intake, grouped by the DRI categories, varied significantly according to age and race-ethnicity, with younger and minority women tending to consume less fiber. BMI and physical activity were not significantly associated with fiber intake. Analysis of fiber intake according to quartiles of intake (as opposed to DRI categories) produced similar results for demographic and dietary characteristics (data not shown).
TABLE 1.
Characteristics of participants according to Dietary Reference Intake (DRI) categories of fiber consumption and ovulation status/cycle1
| DRI fiber groups (g/d) |
Ovulatory3 |
||||||||
| Total cohort | 1 (≤10) | 2 (10.01–16) | 3 (16.01– 21.99) | 4 (≥22) | P value2 | Yes | No | P value2 | |
| No. of cycles | 509 | 155 | 228 | 85 | 41 | 467 | 42 | ||
| Demographics | |||||||||
| Age (y) | 27.5 ± 8.24 | 25.9 ± 8.0 | 27.9 ± 8.4 | 29.5 ± 8.0 | 27.0 ± 8.0 | 0.009 | 27.9 ± 8.3 | 22.0 ± 5.3 | <0.001 |
| BMI (kg/m2) | 24.1 ± 3.9 | 24.5 ± 3.8 | 24.0 ± 3.9 | 24.0 ± 3.8 | 22.8 ± 4.3 | 0.08 | 24.2 ± 3.9 | 23.1 ± 3.6 | 0.08 |
| Physical activity [n (%)] | 0.15 | 0.93 | |||||||
| Low | 48 (9.5) | 17 (11.9) | 24 (10.5) | 3 (3.6) | 4 (9.8) | 44 (9.6) | 4 (8.7) | ||
| Moderate | 182 (36.0) | 51 (33.6) | 74 (32.5) | 38 (45.2) | 19 (46.3) | 168 (36.1) | 14 (33.3) | ||
| High | 275 (54.5) | 84 (55.3) | 130 (57.0) | 43 (51.2) | 18 (43.9) | 253 (54.4) | 24 (57.1) | ||
| Race [n (%)] | <0.001 | 0.93 | |||||||
| White | 300 (59.4) | 62 (40.8) | 142 (62.3) | 63 (75.0) | 33 (80.5) | 276 (59.4) | 25 (59.5) | ||
| African American | 100 (19.8) | 56 (36.8) | 34 (14.9) | 7 (8.3) | 3 (7.3) | 91 (19.6) | 9 (21.4) | ||
| Other | 105 (20.8) | 34 (22.4) | 52 (22.8) | 14 (16.7) | 5 (12.2) | 98 (21.1) | 8 (19.1) | ||
| Years of education [n (%)] | 0.06 | 0.73 | |||||||
| ≤High school | 65 (12.9) | 26 (17.1) | 31 (13.6) | 6 (7.1) | 2 (4.9) | 58 (12.5) | 6 (14.3) | ||
| Postsecondary | 440 (87.1) | 126 (82.9) | 197 (86.4) | 78 (92.9) | 39 (95.1) | 407 (87.5) | 36 (85.7) | ||
| History of smoking [n (%)] | 0.41 | 0.02 | |||||||
| No | 415 (81.9) | 133 (85.8) | 185 (81.1) | 65 (77.4) | 32 (80.5) | 375 (80.7) | 40 (95.2) | ||
| Yes | 92 (18.1) | 22 (14.2) | 43 (18.9) | 19 (22.6) | 8 (19.5) | 90 (19.3) | 2 (4.8) | ||
| Past oral contraceptive use [n (%)] | 0.11 | 0.02 | |||||||
| No | 228 (45.3) | 77 (51.3) | 105 (46.1) | 32 (38.1) | 14 (34.2) | 203 (43.8) | 25 (62.5) | ||
| Yes | 275 (54.7) | 73 (48.7) | 123 (54.0) | 52 (61.9) | 27 (65.9) | 260 (56.2) | 15 (37.5) | ||
| Cycle length (d) | 28.9 ± 4.1 | 28.7 ± 4.0 | 28.9 ± 4.4 | 29.9 ± 3.8 | 28.9 ± 3.2 | 0.95 | 28.9 ± 4.0 | 27.8 ± 5.1 | 0.13 |
| Menstrual hormones5 | |||||||||
| Average estradiol (pg/mL) | 112.3 ± 91.0 | 123.7 ± 97.2 | 109.8 ± 87.3 | 110.2 ± 94.8 | 87.4 ± 69.9 | <0.001 | 116.9 ± 92.5 | 64.9 ± 53.1 | <0.001 |
| Average luteal progesterone (ng/mL) | 7.3 ± 5.5 | 7.4 ± 5.5 | 7.5 ± 5.5 | 7.4 ± 5.8 | 5.7 ± 4.9 | 0.007 | 7.9 ± 5.4 | 1.2 ± 1.3 | <0.001 |
| LH (ng/mL) | 14.8 ± 14.9 | 14.8 ± 14.9 | 15.3 ± 15.2 | 14.6 ± 15.6 | 12.5 ± 12.1 | 0.22 | 15.2 ± 15.2 | 10.9 ± 10.5 | 0.003 |
| FSH (mIU/mL) | 7.8 ± 4.8 | 7.7 ± 4.8 | 7.8 ± 4.5 | 8.1 (5.4) | 7.5 ± 5.1 | 0.64 | 8.0 ± 4.9 | 6.2 ± 2.6 | <0.001 |
| Dietary variables | |||||||||
| Total energy (kcal) | 1608.1 ± 405.0 | 1381.7 ± 318.2 | 1615.2 ± 356.7 | 1840.2 ± 415.6 | 1943.3 ± 426.9 | <0.001 | 1610.3 ± 399.1 | 1583.3 ± 470.7 | 0.68 |
| Carbohydrate (%) | 50.9 ± 8.2 | 49.1 ± 7.8 | 50.5 ± 8.2 | 52.4 ± 8.2 | 56.8 ± 7.1 | <0.001 | 50.8 ± 8.3 | 52.2 ± 7.4 | 0.30 |
| Protein (%) | 15.7 ± 3.4 | 15.9 ± 3.6 | 16.0 ± 3.3 | 15.4 ± 3.7 | 14.6 ± 2.7 | 0.07 | 15.8 ± 3.5 | 15.5 ± 3.4 | 0.58 |
| Total fat (%) | 33.9 ± 6.3 | 35.1 ± 6.3 | 33.7 ± 6.1 | 33.4 ± 6.7 | 30.9 ± 5.6 | 0.001 | 33.8 ± 6.3 | 33.9 ± 5.8 | 0.94 |
| Cholesterol (mg/dL) | 209.4 ± 115.7 | 212.4 ± 102.7 | 210.2 ± 112.8 | 210.5 ± 147.7 | 191.2 ± 105.4 | 0.77 | 210.3 ± 115.0 | 199.9 ± 124.3 | 0.58 |
| Total fiber (g/d) | 13.6 ± 6.0 | 8.0 ± 1.4 | 12.9 ± 1.7 | 18.5 ± 1.6 | 28.1 ± 5.6 | <0.001 | 13.4 ± 5.5 | 16.0 ± 9.6 | 0.007 |
| Insoluble fiber (g/d) | 9.6 ± 4.7 | 5.4 ± 1.1 | 9.0 ± 1.5 | 13.4 ± 1.8 | 21.0 ± 4.6 | <0.001 | 9.4 ± 4.3 | 11.5 ± 7.7 | 0.006 |
| Soluble fiber (g/d) | 3.8 ± 1.4 | 2.5 ± 0.6 | 3.7 ± 0.7 | 4.9 ± 0.9 | 6.8 ± 1.4 | <0.001 | 3.7 ± 1.4 | 4.3 ± 2.0 | 0.02 |
| Vegetable fiber (g/d) | 4.9 ± 3.0 | 3.3 ± 1.5 | 4.5 ± 2.1 | 6.5 ± 2.5 | 9.9 ± 4.6 | <0.001 | 4.8 ± 2.8 | 5.5 ± 4.6 | 0.16 |
| Fruit fiber (g/d) | 2.3 ± 1.9 | 1.1 ± 1.0 | 2.4 ± 1.7 | 3.1 ± 2.1 | 4.6 ± 2.2 | <0.001 | 2.3 ± 1.9 | 3.1 ± 2.4 | 0.01 |
| Grain fiber (g/d) | 5.6 ± 3.1 | 3.4 ± 1.3 | 5.4 ± 2.0 | 7.5 ± 2.5 | 10.7 ± 5.1 | <0.001 | 5.5 ± 2.8 | 6.5 ± 5.5 | 0.04 |
| Magnesium (mg/d) | 222.1 ± 74.3 | 156.6 ± 34.9 | 218.8 ± 39.9 | 280.0 ± 47.4 | 367.7 ± 77.9 | <0.001 | 220.7 ± 70.9 | 237.5 ± 104.5 | 0.16 |
| Calcium (mg/d) | 697.9 ± 281.9 | 533.6 ± 214.8 | 712.3 ± 255.6 | 887.8 ± 306.5 | 845.8 ± 241.9 | <0.001 | 701.7 ± 283.5 | 656.5 ± 262.2 | 0.32 |
| Potassium (mg/d) | 1917.4 ± 607.0 | 1421.8 ± 357.4 | 1921.6 ± 435.2 | 2385.5 ± 502.4 | 2797.0 ± 610.2 | <0.001 | 1912.7 ± 600.2 | 1969.4 ± 684.1 | 0.56 |
| Caffeine (mg/d) | 92.1 ± 98.9 | 68.0 ± 81.5 | 98.2 ± 106 | 118.7 ± 102.0 | 93.5 ± 97.8 | 0.002 | 94.9 ± 100.0 | 61.0 ± 81.3 | 0.03 |
| Vitamin C (mg/d) | 70.0 ± 43.1 | 52.4 ± 36.1 | 70.0 ± 39.5 | 85.8 ± 46.2 | 104.0 ± 47.7 | <0.001 | 70.0 ± 42.5 | 70.4 ± 49.3 | 0.95 |
| Vitamin E (mg/d) | 9.8 ± 7.2 | 6.76 ± 3.4 | 9.16 ± 5.6 | 14.01 ± 11.4 | 15.8 ± 6.7 | <0.001 | 9.8 ± 7.3 | 9.7 ± 5.6 | 0.94 |
| Iron (mg/d) | 12.3 ± 5.1 | 9.03 ± 2.7 | 12.3 ± 4.0 | 16.1 ± 6.9 | 16.7 ± 4.4 | <0.001 | 12.3 ± 5.1 | 12.0 ± 4.6 | 0.69 |
LH, luteinizing hormone; FSH, follicle-stimulating hormone.
Calculated by using ANOVA for continuous variables and exact chi-square tests for categorical variables for associations between fiber intakes or ovulation status.
An ovulatory cycle is defined as progesterone >5 ng/mL and/or serum LH peak on day 22 or 27 of a standardized 28-d cycle.
Mean ± SD (all such values).
P values are based on the log(concentration).
Ovulation status (ovulatory compared with anovulatory) differed significantly according to age, history of smoking, and past use of oral contraceptives. Anovulatory women were on average younger, less likely to be past or current smokers, and less likely to have used oral contraceptives in comparison with ovulatory women. In the dietary assessment, all of the components and sources of dietary fiber were significantly different according to ovulation status except for vegetable fiber. Caffeine intake was also significantly lower in anovulatory women.
Menstrual hormones
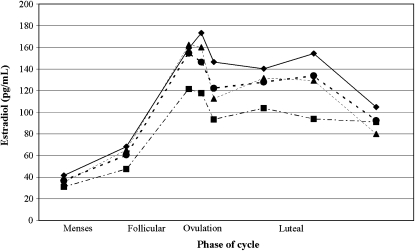
Fiber intake was inversely associated with estradiol concentrations, as shown in Figure 1, which displays the unadjusted means of estradiol over the menstrual cycle according to fiber intake categories of DRI. Increased fiber intake was also significantly associated with decreased concentrations of estradiol across the menstrual cycle in models adjusted for total calories and in models adjusted for total calories, age, race, and vitamin E (Table 2). In both models, for each additional 5 g of fiber, log(estradiol) decreased by ≈0.047 pg/mL over the menstrual cycle (P = 0.01). Luteal phase progesterone concentrations showed an inverse relation with dietary fiber intake after mixed-model analysis (β = −0.117, P = 0.004). LH and FSH concentrations around ovulation both showed significant inverse relations to increasing fiber intake in the fully adjusted models (β = −0.051, P = 0.04 for LH and β = −0.034, P = 0.05 for FSH).
FIGURE 1.
Crude concentrations of estradiol across the menstrual cycle according to Dietary Reference Intake categories of fiber consumption: ♦ : ≤10 g/d; •: 10.01–16 g/d; ▴: 16.01–21.99 g/d; ▪: ≥22 g/d.
TABLE 2.
Dietary fiber intake (5-g increments) and serum concentrations of menstrual hormones1
|
β (95% CI) |
||
| Model 12 | Model 23 | |
| Total fiber | ||
| Estradiol (pg/mL) | −0.047 (−0.083, −0.011) | −0.049 (−0.087, −0.011) |
| LH (ng/mL) | −0.035 (−0.080, 0.010) | −0.051 (−0.100, −0.002) |
| FSH (nIU/mL) | −0.034 (−0.068, −0.001) | −0.034 (−0.068, 0.005) |
| Progesterone (ng/mL), luteal phase | −0.091 (−0.167, −0.015) | −0.117 (−0.198, −0.037) |
| Soluble fiber | ||
| Estradiol (pg/mL) | −0.238 (−0.389, −0.087) | −0.222 (−0.377, −0.068) |
| LH (ng/mL) | −0.178 (−0.372, 0.0161) | −0.232 (−0.437, −0.027) |
| FSH (nIU/mL) | −0.127 (−0.270, 0.016) | −0.094 (−0.236, 0.049) |
| Progesterone, luteal phase (ng/mL) | −0.296 (−0.610, 0.017) | −0.328 (−0.650, −0.006) |
| Insoluble fiber | ||
| Estradiol (pg/mL) | −0.054 (−0.098, −0.009) | −0.057 (−0.104, −0.010) |
| LH (ng/mL) | −0.029 (−0.086, 0.027) | −0.049 (−0.111, 0.013) |
| FSH (nIU/mL) | −0.036 (−0.078, 0.006) | −0.035 (−0.078, 0.008) |
| Progesterone, luteal phase (ng/mL) | −0.111 (−0.205, −0.016) | −0.146 (−0.245, −0.047) |
| Vegetable fiber | ||
| Estradiol (pg/mL) | −0.024 (−0.087, 0.040) | −0.027 (−0.090, 0.036) |
| LH (ng/mL) | −0.002 (−0.085, 0.081) | −0.010 (−0.094, 0.074) |
| FSH (nIU/mL) | 0.016 (−0.043, 0.074) | 0.008 (−0.049, 0.065) |
| Progesterone, luteal phase (ng/mL) | 0.027 (−0.102, 0.156) | −0.023 (−0.152, 0.107) |
| Grain fiber | ||
| Estradiol (pg/mL) | −0.068 (−0.133, −0.004) | −0.073 (−0.139, −0.007) |
| LH (ng/mL) | −0.016 (−0.099, 0.067) | −0.029 (−0.117, 0.058) |
| FSH (nIU/mL) | −0.059 (−0.119, 0.002) | −0.048 (−0.109, 0.012) |
| Progesterone (ng/mL), luteal phase | −0.154 (−0.287, −0.021) | −0.163 (−0.300, −0.027) |
| Fruit fiber | ||
| Estradiol (pg/mL) | −0.128 (−0.225, −0.031) | −0.104 (−0.200, −0.008) |
| LH (ng/mL) | −0.046 (−0.173, 0.080) | −0.068 (−0.198, 0.061) |
| FSH (nIU/mL) | −0.019 (−0.110, 0.072) | −0.005 (−0.093, 0.084) |
| Progesterone, luteal phase (ng/mL) | −0.288 (−0.484, −0.092) | −0.242 (−0.439, −0.045) |
LH, luteinizing hormone; FSH, follicle-stimulating hormone. Analyses were performed by using linear mixed models on the log scale of hormones.
Adjusted for energy intake (continuous).
Adjusted for energy intake (continuous), race (white, black, other), age (continuous), and vitamin E (continuous).
Dietary fiber was stratified into insoluble and soluble components, yielding results similar to those of total dietary fiber. Soluble fiber had a stronger inverse relation with estradiol concentrations (β = −0.222, P = 0.01) than did insoluble fiber (β = −0.057, P = 0.02). Of the 3 sources of fiber, fruit fiber had the strongest association with concentrations of estradiol (β = −0.104, P = 0.03), followed by grain fiber (β = −0.073, P = 0.03), whereas vegetable fiber was not associated (β = −0.027, P = 0.40) with estradiol concentration. Fruit and grain fiber were also associated with statistically significant decreases in concentrations of progesterone (β = −0.242, P = 0.02, for fruit fiber, and β = −0.163, P = 0.02, for grain fiber).
Fiber-rich foods are also typically rich in magnesium, potassium, iron, vitamin C, and vitamin E. Models of fiber intake adjusted for these strongly correlated dietary intake variables by using propensity scores yielded similar results (data not shown). Data from fully adjusted models of magnesium and potassium showed strong associations with estradiol but not with other menstrual hormones (data not shown). The significant associations between estradiol and magnesium and potassium intake, along with strong correlations between fiber and magnesium (r = 0.87) and between fiber and potassium (r = 0.70), confirmed the possibility of collinearity and limited the ability to discern whether the association of fiber with estradiol is independent of magnesium or potassium.
Anovulation
Results from a nonlinear mixed model adjusting for energy intake, race, age, and vitamin E intake showed that for each 5-g increase in dietary fiber, the adjusted odds ratio (aOR) of anovulation was 1.78 (95% CI: 1.11, 2.84; Table 3). Thus, a 5-g/d increase in dietary fiber intake, equivalent to ≈2 slices whole-grain bread (4.6 g/d) or one large apple (4.7 g/d), would result in a 78% elevation in the risk of anovulation. Analysis of continuous soluble and insoluble fiber intake as linear exposures yielded similar results. Soluble fiber had a stronger, positive association with an elevated risk of anovulation (aOR: 6.73; 95% CI: 1.18, 38.26) than did insoluble fiber (aOR: 2.15; 95% CI: 1.22, 3.77). When analyzed by fiber source, a 5-g/d increase in fruit fiber had the strongest association with probability of anovulation (aOR: 3.05; 95% CI: 1.07, 8.71). Grain and vegetable fiber were not significantly associated with anovulation [5-g grain fiber/d increase (aOR: 1.84; 95% CI: 0.89, 3.78); 5-g/d vegetable fiber increase (aOR: 1.74; 95% CI: 0.87, 3.47)].
TABLE 3.
Dietary fiber consumption (5-g increments) and the risk of anovulation1
| Total fiber | Soluble | Insoluble | Vegetable | Fruit | Grain | |
| Range (g/d) | 3.46–48.97 | 1.93–37.91 | 0.73–9.89 | 0.18–28.8 | 0.0–12.54 | 0.51–27.08 |
| Intake (g/d) | 13.57 ± 6.02 | 9.61 ± 4.71 | 3.76 ± 1.45 | 4.90 ± 2.95 | 2.33 ± 1.94 | 5.57 ± 3.09 |
| aOR3 | 1.65 (1.08, 2.52) | 6.03 (1.11, 32.67) | 1.85 (1.12, 3.08) | 1.59 (0.79, 3.19) | 3.33 (1.08, 10.31) | 1.72 (0.85, 3.49) |
| aOR4 | 1.78 (1.11, 2.84) | 6.73 (1.18, 38.26) | 2.15 (1.22, 3.77) | 1.74 (0.87, 3.47) | 3.05 (1.07, 8.71) | 1.84 (0.89, 3.78) |
aOR, adjusted odds ratio. Analyses were performed by using nonlinear mixed models.
Mean ± SD (all such values).
Adjusted for energy intake (continuous); 95% CIs in parentheses.
Adjusted for energy intake (continuous), race (white, black, other), age (continuous), and vitamin E (continuous); 95% CIs in parentheses.
Of the 509 menstrual cycles included in this study, 42 (8.3%) were anovulatory. Of the cycles in which women consumed at or above the DRI (≥22 g fiber/d; n = 41), 22% of cycles were anovulatory compared with 7.1% of cycles in which women consumed ≤10 g fiber/d (n = 155). Although CIs are wide, after adjustment for energy intake, race, age, and vitamin E intake, category of dietary fiber consumption was positively associated with incident anovulation (P = 0.004), with an aOR of 10.98 (95% CI: 1.5, 80.5) for women at or above the DRI compared with the lowest DRI grouping (Table 4). Further adjustment for a wide variety of other demographic and dietary characteristics (shown in Table 1) had little effect on these results.
TABLE 4.
Risk of anovulation according to Dietary Reference Intake (DRI) categories1
| DRI categories |
||||
| 1 | 2 | 3 | 4 | |
| Fiber range (g/d) | ≤10 | 10.01–16 | 16.01–21.99 | ≥22 |
| Mean fiber (g/d) | 8.0 ± 1.42 | 12.9 ± 1.7 | 18.5 ± 1.6 | 28.1 ± 5.6 |
| Anovulatory/total [n (%)] | 11/155 (7.10) | 16/228 (7.02) | 6/85 (7.06) | 9/41 (21.95) |
| aOR3 | 1.00 | 1.32 (0.42, 4.29) | 1.51 (0.32, 7.12) | 11.00 (1.40, 86.69) |
| aOR4 | 1.00 | 1.62 (0.54, 4.88) | 2.56 (0.56, 11.75) | 10.98 (1.50, 80.46) |
Analyses were run with nonlinear mixed models. aOR, adjusted odds ratio.
Mean ± SD (all such values).
Adjusted for energy intake (continuous); 95% CIs in parentheses.
Adjusted for energy intake (continuous), race (white, black, other), age (continuous), and vitamin E (continuous); 95% CIs in parentheses.
DISCUSSION
Higher consumption of dietary fiber was significantly associated with lower concentrations of reproductive hormones and an increased risk of incident anovulation in this cohort of young, healthy women. The significant association persisted and remained strong, whether fiber intake was considered as a continuous variable or categorized according to groupings on the basis of the DRI. The observed associations between high fiber intake, decreased hormone concentrations, and increased risk of anovulation highlights the potential for reproductive health implications related to fiber intake in young women that to date may not have been recognized.
The role of fiber in lowering estrogen concentrations has previously been shown predominantly in older women. High-fiber diets cause a decrease of β-glucuronidase activity in feces that leads to decreased reabsorption of estrogen in the colon (20). In addition, fiber binds to estrogen in the intestine, increasing its fecal excretion (31, 32). Through fiber's influence on estrogen, fiber subsequently influences other menstrual hormones due to the strict feedback mechanisms, which dictate hormonal fluctuations in the menstrual cycle (33–36). However, fiber also seems to decrease LH and FSH concentrations independent of estradiol. Prior research has confirmed that episodic gonadotropin release and a specific range of concentrations of FSH and LH are required for follicular development and ovulation (22, 37). The biological mechanisms behind the influence of fiber on LH and FSH have yet to be elucidated and require more research.
In women with normal reproductive function, one might anticipate that in response to lower concentrations of estradiol, an intact hypothalamic-pituitary-ovarian axis would respond by increasing FSH and estradiol production from the follicles followed by ovulation. Although this response could explain why some of the women who consumed higher intakes of dietary fiber were ovulatory, the anovulatory women did not exhibit this response; rather, they had consistently lower concentrations of reproductive hormones. Thus, we concluded that the decreased hormone concentrations associated with higher fiber intakes could result in anovulatory cycles due to the close relation between diet and the hypothalamic-pituitary axis (38). However, more research is needed to understand the exact biological mechanisms.
It is also possible that dietary fiber intake is associated with other lifestyle factors related to increased menstrual irregularity in this population, such as intense physical activity, low or high BMI, or low-fat/low-calorie diets. However, the associations in this study were not greatly altered by adjustment for a wide variety of demographic, lifestyle, and dietary characteristics. Due to the homogeneous and healthy nature of the cohort, factors such as BMI and physical activity were not shown to have a significant effect on the findings. Although this may limit generalizability of these findings to all women, it increased the ability to reduce potential confounding and improved the study's internal validity.
The observed association between fiber intake and estradiol concentration is consistent with several observational studies and randomized trials (13–19) and at odds with several studies that did not observe a significant effect (39–42). In addition, previous studies failed to observe associations with other reproductive hormones. The past research studies were limited by sample size (15, 42), only taking measurements during the follicular phase (14, 16, 19, 39), issues with serum collection timing (13, 14, 16, 17, 19, 39, 40, 42), and low ranges of fiber consumption (13–19, 39–42).
A large percentage of prior studies based the timing of visits on an ≈28-d cycle measured post menses (13, 18, 39, 41, 42) despite the evident difficulty in timing visits during critical windows of the menstrual cycle on the basis of participant-reported cycle length or a standardized cycle length (43). Several studies also relied on only one serum measurement in either the follicular or luteal phase of the menstrual cycle and allowed several days for women to come into the clinic for serum collection despite the extreme variability of hormones by day (13, 18, 39, 41, 42). Furthermore, compared with the fiber intakes in this cohort, most of the previous studies had a much higher average fiber intake in the experimental groups (13, 14, 16) and in the control groups and overall population (15, 18, 39, 41). The narrower range in other studies lessened their ability to perceive the dose-response relation that our results suggested. Importantly, the high fiber intakes evaluated in those studies (22.9 ± 8.9 g/d) were substantially higher than the average intake of most Americans (12), calling into question the applicability of their findings. Our study had a wider range of intake (3.46–48.97 g/d), which increased the ability to observe an effect according to dose.
Although the observed association with estradiol concentrations was consistent with previous research, the effect of fiber on anovulation has not been observed. Only 4 studies to date have evaluated this association, but none showed an effect (15, 18, 40, 42). In previous studies, ovulation was not assessed on an individual cycle basis but rather by whether there was a significant change in mean progesterone concentrations between the high-fiber and low-fiber groups. In addition to the crude methods for defining ovulation, these studies suffered from the methodologic problems discussed previously, which limited their ability to detect an association with ovulation.
Intensive monitoring of a large number of young, ethnically diverse women throughout 2 menstrual cycles, with multiple clinic visits timed with fertility monitors, was a significant improvement and helped distinguish the BioCycle Study from previous studies (43). Individual cycle assessment of ovulation on the basis of multiple hormone measurements was an important advancement over past studies on anovulation. The prospective design and exclusion criteria at baseline of the BioCycle Study strengthen the ability to draw inference, having reduced the potential for bias from known risk factors for anovulation. In addition, standardized assessment of a wide variety of participant and dietary characteristics increased the ability to adjust for confounding. Collectively, these unique aspects of the study design allowed us to improve and expand on previous studies of fiber and hormonal outcomes, which included anovulation.
Nevertheless, the study faced several limitations, which included the small number of women consuming at or above the DRI (≥22 g dietary fiber/d; n = 41 cycles) and the small number of anovulatory cycles (n = 42), which limited the power of the findings and resulted in wide CIs due to imprecision. In addition, women were only followed for 2 menstrual cycles. Because anovulation occurs commonly in many women, long-term findings may be different. In absence of a daily transvaginal ultrasound or daily first morning urine measurements, the direct detection of ovulation has some degree of misclassification. We assessed the effect of misclassification through a sensitivity analysis by comparing the results of a commonly used classification for anovulation (≤5 ng progesterone/mL; n = 65 cycles) with a more conservative classification (n = 22 cycles). The effect of fiber on anovulation was strong and consistent regardless of the definition used for classification (data not shown). Although the study included use of a fertility monitor to help time visits, bias could have been introduced through mistimed sample collection. However, various indicators of successfully timed visits were shown to be unrelated to fiber consumptions; thus, any misclassification is likely nondifferential (44). Last, due to the strong correlations between fiber and magnesium and other nutrients, it is difficult to discern the independent effects of these nutrients on hormone concentrations and anovulation. However, the biological mechanism linking dietary fiber to estradiol concentrations is the most plausible, and models that adjusted for highly correlated dietary variables produced similar results.
In conclusion, we observed that fiber consumption at or above the recommended intakes was significantly associated with decreased reproductive hormone concentrations and a substantially elevated probability of anovulatory cycles in women of reproductive age. Although this is a single study, these findings call into question whether current DRIs are applicable to women of reproductive age who are trying to conceive. Further studies are needed to confirm these findings and elucidate the role of fiber intake on reproductive health to inform current recommendations for adequate fiber intake in young women.
Acknowledgments
We thank the editor and reviewers for their valuable comments. In addition, we are indebted to all of the investigators and staff at the University at Buffalo and the Eunice Kennedy Shriver National Institute of Child Health and Human Development for their respective roles in the study and their dedication and effort; to Jennifer Reschke, Andrea Hughes, Michael Bloom, Maurizio Trevisan, Richard Browne, Karen Falkner, Carole Rudra, Mary Hediger, Audra Gollenberg, Aiyi Liu, and Liwei Chen for their assistance in study implementation; and to the BioCycle Study participants for their extraordinary commitment to the study.
The authors' responsibilities were as follows—AJG, SLM, JW-W, and EFS: full access to all of the data in the study and responsibility for the integrity of the data and the accuracy of the data analysis; EFS and JW-W: study concept and design; EFS, KMH, and JW-W: acquisition of data; EFS, AJG, SLM, and KMH: analysis and interpretation of data; AJG, SLM, and EFS: drafting of the manuscript; EFS, BWW, CZ, EY, PPH, and JW-W: critical revision of manuscript for important intellectual content; AJG, SLM, EFS, and NJP: statistical analysis; and EFS and JW-W: study supervision. None of the authors had a conflict of interest.
REFERENCES
- 1.Mozaffarian D, Kumanyika SK, Lemaitre RN, Olson JL, Burke GL, Siscovick DS. Cereal, fruit, and vegetable fiber intake and the risk of cardiovascular disease in elderly individuals. JAMA 2003;289:1659–66 [DOI] [PubMed] [Google Scholar]
- 2.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000;343:16–22 [DOI] [PubMed] [Google Scholar]
- 3.Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr 2004;80:348–56 [DOI] [PubMed] [Google Scholar]
- 4.Reddy BS. Role of dietary fiber in colon cancer: an overview. Am J Med 1999;106:16S–19S [DOI] [PubMed] [Google Scholar]
- 5.Cho E, Spiegelman D, Hunter DJ, Chen WY, Colditz GA, Willett WC. Premenopausal dietary carbohydrate, glycemic index, glycemic load, and fiber in relation to risk of breast cancer. Cancer Epidemiol Biomarkers Prev 2003;12:1153–8 [PubMed] [Google Scholar]
- 6.Gerber M. Fibre and breast cancer. Eur J Cancer Prev 1998;7(Suppl 2):S63–7 [DOI] [PubMed] [Google Scholar]
- 7.Holmes MD, Liu S, Hankinson SE, Colditz GA, Hunter DJ, Willett WC. Dietary carbohydrates, fiber, and breast cancer risk. Am J Epidemiol 2004;159:732–9 [DOI] [PubMed] [Google Scholar]
- 8.Edefonti V, Decarli A, La VC, et al. Nutrient dietary patterns and the risk of breast and ovarian cancers. Int J Cancer 2008;122:609–13 [DOI] [PubMed] [Google Scholar]
- 9.Krauss RM, Eckel RH, Howard B, et al. AHA Dietary Guidelines: revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Stroke 2000;31:2751–66 [DOI] [PubMed] [Google Scholar]
- 10.US Department of Agriculture Nutrition and your health: dietary guidelines for Americans. 5th ed Washington, DC: Department of Agriculture and Department of Health and Human Services, 2000 [Google Scholar]
- 11.US Department of Health and Human Services, US Department of Agriculture Dietary guidelines for Americans, 2005. 6th ed Washington, DC: US Government Printing Office, 2005 [Google Scholar]
- 12.Food and Nutrition Board Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington, DC: Institute of Medicine, 2005 [Google Scholar]
- 13.Rose DP, Goldman M, Connolly JM, Strong LE. High-fiber diet reduces serum estrogen concentrations in premenopausal women. Am J Clin Nutr 1991;54:520–5 [DOI] [PubMed] [Google Scholar]
- 14.Goldin BR, Woods MN, Spiegelman DL, et al. The effect of dietary fat and fiber on serum estrogen concentrations in premenopausal women under controlled dietary conditions. Cancer 1994;74:1125–31 [DOI] [PubMed] [Google Scholar]
- 15.Bagga D, Ashley JM, Geffrey SP, et al. Effects of a very low fat, high fiber diet on serum hormones and menstrual function: implications for breast cancer prevention. Cancer 1995;76:2491–6 [DOI] [PubMed] [Google Scholar]
- 16.Woods MN, Barnett JB, Spiegelman D, et al. Hormone levels during dietary changes in premenopausal African-American women. J Natl Cancer Inst 1996;88:1369–74 [DOI] [PubMed] [Google Scholar]
- 17.Kaneda N, Nagata C, Kabuto M, Shimizu H. Fat and fiber intakes in relation to serum estrogen concentration in premenopausal Japanese women. Nutr Cancer 1997;27:279–83 [DOI] [PubMed] [Google Scholar]
- 18.Gann PH, Chatterton RT, Gapstur SM, et al. The effects of a low-fat/high-fiber diet on sex hormone levels and menstrual cycling in premenopausal women: a 12-month randomized trial (the diet and hormone study). Cancer 2003;98:1870–9 [DOI] [PubMed] [Google Scholar]
- 19.Aubertin-Leheudre M, Gorbach S, Woods M, Dwyer JT, Goldin B, Adlercreutz H. Fat/fiber intakes and sex hormones in healthy premenopausal women in the USA. J Steroid Biochem Mol Biol 2008;112:32–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldin BR, Adlercreutz H, Gorbach SL, et al. Estrogen excretion patterns and plasma levels in vegetarian and omnivorous women. N Engl J Med 1982;307:1542–7 [DOI] [PubMed] [Google Scholar]
- 21.Springhouse Amenorrhea: professional guide to signs & symptoms. Ambler, PA: Lippincott Williams & Wilkins, 2006 [Google Scholar]
- 22.Mason P, Adams J, Morris DV, et al. Induction of ovulation with pulsatile luteinising hormone releasing hormone. Br Med J (Clin Res Ed) 1984;288:181–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bussen S, Sutterlin M, Steck T. Endocrine abnormalities during the follicular phase in women with recurrent spontaneous abortion. Hum Reprod 1999;14:18–20 [DOI] [PubMed] [Google Scholar]
- 24.Wactawski-Wende J, Schisterman EF, Hovey KM, et al. BioCycle Study: design of the logitudinal study of the oxidative stress and hormone variating during the menstrual cycle. Paediatr Perinat Epidemiol 2009;23:171–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malcolm CE, Cumming DC. Does anovulation exist in eumenorrheic women? Obstet Gynecol 2003;102:317–8 [DOI] [PubMed] [Google Scholar]
- 26.Abdulla U, Diver MJ, Hipkin LJ, Davis JC. Plasma progesterone levels as an index of ovulation. Br J Obstet Gynaecol 1983;90:543–8 [DOI] [PubMed] [Google Scholar]
- 27.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95 [DOI] [PubMed] [Google Scholar]
- 28.Littell RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci 1998;76:1216–31 [DOI] [PubMed] [Google Scholar]
- 29.McMahon JM, Pouget ER, Tortu S. A guide for multilevel modeling of dyadic data with binary outcomes using SAS PROC NLMIXED. Comput Stat Data Anal 2006;50:3663–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng HY, Hsueh YH, Messam LL, Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol 2009;169:1182–90 [DOI] [PubMed] [Google Scholar]
- 31.Lewis SJ, Heaton KW, Oakey RE, McGarrigle HH. Lower serum oestrogen concentrations associated with faster intestinal transit. Br J Cancer 1997;76:395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shultz TD, Howie BJ. In vitro binding of steroid hormones by natural and purified fibers. Nutr Cancer 1986;8:141–7 [DOI] [PubMed] [Google Scholar]
- 33.Micevych PE, Chaban V, Ogi J, Dewing P, Lu JK, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology 2007;148:782–9 [DOI] [PubMed] [Google Scholar]
- 34.Filicori M, Santoro N, Merriam GR, Crowley WF., Jr Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab 1986;62:1136–44 [DOI] [PubMed] [Google Scholar]
- 35.Tsai CC, Yen SS. Acute effects of intravenous infusion of 17-beta-estradiol on gonadotropin release in pre- and post-menopausal women. J Clin Endocrinol Metab 1971;32:766–71 [DOI] [PubMed] [Google Scholar]
- 36.Liu JH, Yen SS. Induction of midcycle gonadotropin surge by ovarian steroids in women: a critical evaluation. J Clin Endocrinol Metab 1983;57:797–802 [DOI] [PubMed] [Google Scholar]
- 37.diZerega GS, Hodgen GD. Folliculogenesis in the primate ovarian cycle. Endocr Rev 1981;2:27–49 [DOI] [PubMed] [Google Scholar]
- 38.Hill P, Garbaczewski L, Haley N, Wynder EL. Diet and follicular development. Am J Clin Nutr 1984;39:771–7 [DOI] [PubMed] [Google Scholar]
- 39.London S, Willett W, Longcope C, McKinlay S. Alcohol and other dietary factors in relation to serum hormone concentrations in women at climacteric. Am J Clin Nutr 1991;53:166–71 [DOI] [PubMed] [Google Scholar]
- 40.Dorgan JF, Reichman ME, Judd JT, et al. Relation of energy, fat, and fiber intakes to plasma concentrations of estrogens and androgens in premenopausal women. Am J Clin Nutr 1996;64:25–31 [DOI] [PubMed] [Google Scholar]
- 41.Maskarinec G, Morimoto Y, Takata Y, Murphy SP, Stanczyk FZ. Alcohol and dietary fibre intakes affect circulating sex hormones among premenopausal women. Public Health Nutr 2006;9:875–81 [DOI] [PubMed] [Google Scholar]
- 42.Chearskul S, Supingklud N, Nitithamyong A, Sirichakwal P. Assessment of hormonal and metabolic effects of dietary fiber in young Thai women. J Med Assoc Thai 2006;89:997–1003 [PubMed] [Google Scholar]
- 43.Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol 2009;169:105–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flegal KM, Keyl PM, Nieto FJ. Differential misclassification arising from nondifferential errors in exposure measurement. Am J Epidemiol 1991;134:1233–44 [DOI] [PubMed] [Google Scholar]