Abstract
Increased intestinal permeability (IP) has emerged recently as a common underlying mechanism in the pathogenesis of allergic, inflammatory, and autoimmune diseases. The characterization of zonulin, the only physiological mediator known to regulate IP reversibly, has remained elusive. Through proteomic analysis of human sera, we have now identified human zonulin as the precursor for haptoglobin-2 (pre-HP2). Although mature HP is known to scavenge free hemoglobin (Hb) to inhibit its oxidative activity, no function has ever been ascribed to its uncleaved precursor form. We found that the single-chain zonulin contains an EGF-like motif that leads to transactivation of EGF receptor (EGFR) via proteinase-activated receptor 2 (PAR2) activation. Activation of these 2 receptors was coupled to increased IP. The siRNA-induced silencing of PAR2 or the use of PAR2−/− mice prevented loss of barrier integrity. Proteolytic cleavage of zonulin into its α2- and β-subunits neutralized its ability to both activate EGFR and increase IP. Quantitative gene expression revealed that zonulin is overexpressed in the intestinal mucosa of subjects with celiac disease. To our knowledge, this is the initial example of a molecule that exerts a biological activity in its precursor form that is distinct from the function of its mature form. Our results therefore characterize zonulin as a previously undescribed ligand that engages a key signalosome involved in the pathogenesis of human immune-mediated diseases that can be targeted for therapeutic interventions.
Keywords: autoimmune diseases, epidermal growth factor receptor, gut permeability, proteinase-activated receptor 2, celiac disease
Increased hygiene leading to a reduced exposure to various microorganisms has been implicated as a cause for the “epidemic” of allergic, inflammatory, and autoimmune diseases recorded in industrialized countries during the past 3–4 decades (1). Apart from genetic makeup and exposure to environmental triggers, a third key element [i.e., increased intestinal permeability (IP)] has been proposed in the pathogenesis of these diseases (2–4). IP, together with antigen sampling by enterocytes and luminal dendritic cells, regulates molecular trafficking between the intestinal lumen and the submucosa, leading to either tolerance or immunity to non–self-antigens (5). However, the dimensions of the paracellular space (10–15 Å) suggest that solutes with a molecular radius exceeding 15 Å (≈3.5 kDa) (including proteins) are normally excluded from this uptake route. The intercellular tight junctions (TJs) tightly regulate this paracellular antigen trafficking. TJs are now appreciated to be extremely dynamic structures operative in several key functions of the intestinal epithelium under both physiological and pathological circumstances (3). However, despite major progress in our knowledge regarding the composition and function of intercellular TJs, the mechanism(s) by which they are regulated is(are) still incompletely understood. The discovery of Vibrio cholerae zonula occludens toxin (Zot), a toxin that increases TJ permeability, led us to the identification of its eukaryotic counterpart, zonulin, as the only physiological mediator known to regulate IP reversibly by modulating intercellular TJs (6, 7). Human zonulin is a ≈47-kDa protein that increases IP in nonhuman primate intestinal epithelia (7), participates in intestinal innate immunity (8), and is overexpressed in autoimmune disorders in which TJ dysfunction is central, including celiac disease (CD) (9, 10) and type 1 diabetes (T1D) (11). Although zonulin's role as an intestinal permeating modulator in health and disease has been described functionally, its biochemical characterization has remained elusive. Through proteomic analysis of human sera, we report herein that zonulin is identical to the precursor of haptoglobin-2 (pre-HP2), a molecule that, to date, has only been regarded as the inactive precursor for HP2, one of the two genetic variants (together with HP1) of human HPs (see Fig. S1). Our studies demonstrate the previously undescribed functional characterization of zonulin as pre-HP2, a multifunctional protein that, in its intact single-chain precursor form, appears to regulate IP by transactivating the epidermal growth factor receptor (EGFR) via proteinase-activating receptor 2 (PAR2) activation, whereas in its cleaved 2-chain form, it acts as an Hb scavenger.
Results
Characterization of Zonulin from CD Human Sera.
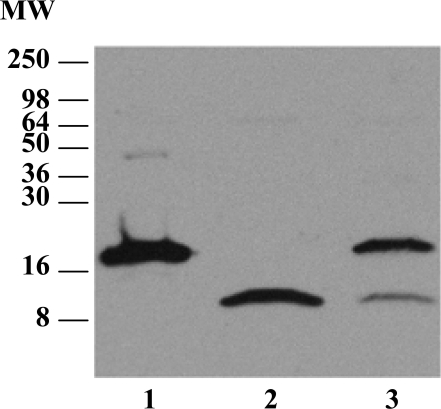
Because zonulin is detected in human sera by a zonulin cross-reacting anti-Zot Ab-based ELISA (7–10) and is increased in patients with CD compared with normal controls (10), we initially used Western blot (WB) analysis to detect zonulin immunoreactivity of proteins in albumin- and IgG-depleted sera from CD subjects. These sera displayed 2 major protein bands with apparent molecular weights (MWs) of 18 and 9 kDa (Fig. 1). Three distinct patterns of reactivity were identified in CD sera: an 18-kDa protein band (Fig. 1, lane 1), a 9-kDa protein band (Fig. 1, lane 2), and both 9- and 18-kDa protein bands (Fig. 1, lane 3). Of note, a ≈45-kDa band was detected only in sera that displayed the single 18-kDa band (Fig. 1, lane 1) but was not detected in sera with either the 9-kDa band or both bands (Fig. 1, lanes 2 and 3). Two-dimensional gel electrophoresis (2-DE) of sera from CD patients who expressed the 18-kDa band revealed 2 zonulin immunoreactive spots [see supporting information (SI) Text and Fig. S1 A and B] that were subjected to MS/MS analysis. The 18-kDa spot was identified as the α2-chain of HP2 (accession no. GI:223976) and the 9-kDa spot as the α1-chain of HP1 (accession no. GI:3337390). A diagram showing the structure of 2-chain HP1 and HP2 and their precursors is presented in Fig. S1C. A random screening of 14 sera from CD patients revealed that 7% were HP1 homozygous, 57% were HP1/HP2 heterozygous, and 36% were HP2 homozygous (Fig. S1D).
Fig. 1.
WB analysis using zonulin cross-reacting anti-Zot polyclonal Ab on CD patient sera samples that were depleted of albumin and immunoglobulins. Three main patterns were detected: sera showing an 18-kDa immunoreactive band and a fainter ≈45-kDa band (lane 1), sera showing only a 9-kDa band (lane 2), and sera showing both the 18- and 9-kDa bands (lane 3).
Characterization of Zonulin from Human HP Preparations.
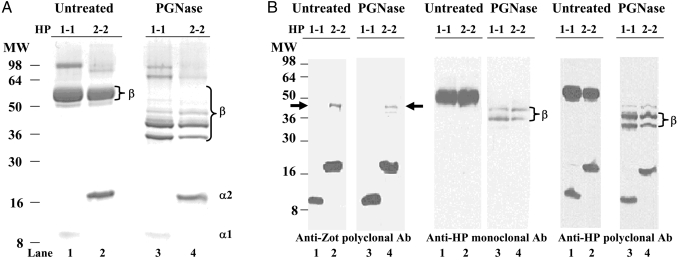
To confirm the identity of the immunoreactive bands recognized by the polyclonal zonulin cross-reacting anti-Zot IgG Ab in human CD sera, commercially purified preparations of human HP from subjects homozygous for either HP1 (HP1–1) or HP2 (HP2–2) were simultaneously resolved on a single gel by SDS/PAGE and analyzed by Coomassie staining (Fig. 2A). As expected, the α1-chain of HP1–1 exhibited a MW of ≈9 kDa (Fig. 2A, lane 1), whereas the α2-chain of HP2–2 had a MW of ≈18 kDa (Fig. 2A, lane 2). Because of its glycosylation, the β-chain exhibited a MW of ≈52 kDa in both HP1–1 and HP2–2 preparations (Fig. 2A, lanes 1 and 2). After a 3-h deglycosylation reaction with N-glycosidase F (PGNase F), the β-chain of both HP1–1 and HP2–2 ran as multiple bands below 52 kDa, presumably attributable to varying degrees of deglycosylation (Fig. 2A, lanes 3 and 4). As anticipated, after glycosidase treatment, no changes in gel mobility for either the α1-chain of HP1–1 (Fig. 2A, compare lanes 1 and 3) or the α2-chain of HP2–2 (Fig. 2A, compare lanes 2 and 4) were evident.
Fig. 2.
Coomassie and Western immunoblotting of purified human homozygote HP1–1 and HP2–2 both untreated and after deglycosylation with PGNase. (A) Coomassie staining of untreated HPs showed a shared glycosylated β-chain migrating at a MW of ≈52 kDa, whereas the α of HP1–1 (α1) and of HP2–2 (α2) migrated at the predicted MWs of 9 and 18 kDa, respectively. Deglycosylation with PGNase caused a shift of the β-chain to a MW of ≈36 kDa (complete deglycosylation) or higher (incomplete deglycosylation). As expected, no shifts were observed in the nonglycosylated α1- and α2-chains. (B) WB of purified human homozygote HP1–1 and HP2–2 both untreated and after deglycosylation with PGNase run in triplicate on a single gel, transferred, and then separately subjected to WB analysis using polyclonal anti-Zot (Left), monoclonal anti-HP (Center), or polyclonal anti-HP (Right) Ab. The polyclonal Ab tested recognized both the α1- and α2-chains (lanes 1 and 2), whose pattern of reactivity did not change after deglycosylation of both HP1–1 and HP2–2 protein preparations (lanes 3 and 4). Conversely, deglycosylation caused the expected gel mobility shift of the β-chain in both HP1–1 and HP2–2 detected by either the anti-HP monoclonal (Center, lanes 3 and 4) or anti-HP polyclonal (Right, lanes 3 and 4) Ab. The zonulin cross-reacting anti-Zot Ab recognized an extra ≈45-kDa band in HP2–2 but not in HP1–1 that did not shift after deglycosylation (arrows). MS/MS analysis and N-terminal sequencing identified this ≈47-kDa band as pre-HP2.
Fig. 2B presents immunoblots of commercially available purified homozygous HP1–1 and HP2–2 proteins both before and after deglycosylation. Proteins were run simultaneously on a single gel and immunoblotted with polyclonal zonulin cross-reacting anti-Zot Ab (Fig. 2B Left), monoclonal antiglycosylated β-chain HP (Fig. 2B Center), or polyclonal anti-HP Ab (Fig. 2B Right). Anti-Zot Ab reacted strongly with both the HP1–1 α1-chain and the HP2–2 α2-chain (Fig. 2B Left, lanes 1 and 2, respectively) and revealed an additional band at ≈45 kDa present in the HP2–2 but not the HP1–1 preparations (Fig 2B Left, arrows). As expected, the monoclonal anti-HP Ab, raised against the ≈52-kDa HP β-glycosylated subunit, recognized only the β-chain of either HP1–1 or HP2–2 (Fig. 2B Center, lanes 1 and 2, respectively), whereas the polyclonal anti-HP Ab recognized epitopes of the α1-, α2-, and β-chains of both HP1–1 and HP2–2 (Fig. 2B Right, lanes 1 and 2, respectively). Fig. 2B also shows immunoblotted HP1–1 and HP2–2 preparations after deglycosylation using the same 3 Ab. The pattern of reactivity of the 3 Ab tested for the nonglycosylated 9-kDa α1-subunit and the 18-kDa α2-subunit did not change after deglycosylation (Fig. 2B, lanes 3 and 4, respectively). However, deglycosylation caused the expected gel mobility shift of the β-chain in both HP1–1 and HP2–2. The monoclonal anti-HP Ab (Fig. 2B Center, lanes 3 and 4) recognized only 2 incomplete deglycosylated β-chain bands, whereas the polyclonal anti-HP Ab also recognized the completely deglycosylated ≈36-kDa β-chain (Fig. 2B Right, lanes 3 and 4). The 45-kDa band that was present only in the HP2–2 preparation and recognized by anti-Zot Ab did not show any change in gel mobility on deglycosylation, but it appeared less intense (Fig. 2B Left, lane 4). MS/MS analysis and NH2-terminal sequencing of this 45-kDa protein band performed on 2 distinct samples analyzed at different times identified this protein as the human pre-HP2 (accession no. P00738). The combined MS/MS analyses covered a total of 49.8% of nonoverlapping protein and 13 unique peptides spanning the entire protein sequence. Therefore, in addition to α1- and α2-chains, the anti-Zot Ab recognized the uncleaved single-chain pre-HP2 but not the β-chain. These results suggest that the anti-Zot Ab used to measure serum zonulin by ELISA should supposedly detect the highly abundant HP1 and HP2 proteins as well as pre-HP2. However, the amount of serum zonulin detected by ELISA is in the ng/mL range (11), whereas the entire HP pool in serum is in the mg/mL range (12). To address this apparent discrepancy, we repeated the WB analysis of both human sera and purified HPs under nondenaturing conditions using anti-Zot Ab (see SI Text). The WB showed a series of bands in HP2–2 phenotype sera (Fig. S2A) and in commercially purified HP2–2 (Fig. S2B), although no bands were detected in either HP1–1 phenotype sera (Fig. S2A) or in commercial purified HP1–1 (Fig. S2B). Conversely, the anti-HP polyclonal Ab, which did not recognize the uncleaved pre-HP2 (Fig. 2B), detected bands in both commercially purified HP1–1 and HP2–2 preparations. Combined, these data suggest that under nondenaturing conditions, the anti-Zot Ab detect only the single-chain pre-HP2 but not the 2-chain mature HPs, further supporting the notion that the single-chain pre-HP2, but not its cleaved 2-chain mature form, corresponds to the zonulin molecule.
Functional Analysis of Recombinant Zonulin.
The primary translation product of the mammalian HP2 mRNA transcript is a polypeptide that dimerizes cotranslationally and is proteolytically cleaved while still in the endoplasmic reticulum by the serine Cr1-like protease (Cr1LP) (13). Conversely, zonulin is detectable in human serum as uncleaved pre-HP2 (Fig. 2 and Fig. S2). To confirm the identification of zonulin as the single-chain pre-HP2 and not the cleaved mature 2-chain HP2, we expressed recombinant pre-HP2 by inserting the pre-HP2 cDNA into an insect cell vector and expressed it using a baculovirus expression system. We obtained highly purified recombinant pre-HP2 that was recognized by the anti-Zot polyclonal Ab similar to Fig. 2B and that migrated at an apparent MW of ≈53 kDa because of the 6xHis tag attached at the C-terminus (Fig. S3A). The single-chain pre-HP2 was then subjected to proteolytic cleavage using a series of serine proteases. Matriptase, urokinase, thrombin, and plasma kallikrein did not cleave pre-HP2, whereas plasmin caused complete degradation of the protein (Fig. S3B). In contrast, treatment with the intestinal serine protease trypsin led to the appearance of 2 major bands that migrated with MWs compatible with the α2- and β-subunits of zonulin (Fig. S3B). NH2-terminal sequencing of these 2 bands showed the 2 proteins to be identical to the pre-H2 α2- and β-chains cleaved at the predicted 161Arg cleavage site. The intact single-chain pre-HP2 and the cleaved 2-chain mature HP2 obtained after trypsin digestion were both tested for their biological activities in the studies below.
Ex Vivo Effect of Recombinant Zonulin on TEER in Mouse Small Intestine Mounted in the Microsnapwell System.
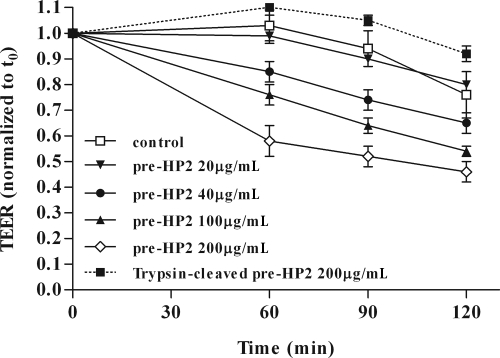
Recombinant pre-HP2 (henceforth defined as zonulin) was applied to WT C57BL/6 murine small intestine segments mounted in microsnapwells. Recombinant single-chain zonulin added to the mucosal (luminal) aspect of mouse intestinal segments decreased transepithelial electrical resistance (TEER) (i.e., increased permeability) when applied at concentrations ≥40 μg/mL (Fig. 3). In contrast, no consistent TEER changes were detected when the trypsin-cleaved 2-chain HP2 was tested (Fig. 3).
Fig. 3.
Zonulin increased IP in C57BL/6 WT mice in a dose- and time-dependent manner. Zonulin was applied to the luminal side of C57BL/6 WT intestinal segments at increasing concentrations. Trypsin-cleaved pre-HP2 was applied at a rate of 200 μg/mL. Starting at 60 min postexposure, zonulin induced a significant drop in TEER when applied at concentrations ≥40 μg/mL (P value ranging from 0.03–0.036). Data are mean values ± SEM from 4 independent experiments.
In Vivo Effect of Recombinant Zonulin on Mouse Gastrointestinal Permeability.
To establish whether zonulin might alter IP in vivo, mice were gavaged with zonulin (170 μg per mouse), and gastroduodenal permeability and small intestine permeability were tested using specific sugar probes (sucrose and lactulose/mannitol, respectively) as described (14). Zonulin increased both small intestinal and gastroduodenal permeability compared with BSA-treated controls (Table 1). Gastroduodenal permeability and small intestine permeability each returned to baseline within 48 h following exposure to zonulin (Table 1).
Table 1.
Effect of zonulin on mouse gastroduodenal (sucrose) and small intestinal (lacman) permeability in vivo
| Treatment | Challenge |
Recovery After 48 h |
||
|---|---|---|---|---|
| Change in sucrose, % | Change in lacman, % | Change in sucrose, % | Change in lacman, % | |
| Zonulin | 68.44 ± 17.52* | 22.91 ± 5.4† | 1.33 ± 3.59 | 0.94 ± 2.19 |
| 2-Chain HP2 | 8.75 ± 6.67 | 0.09 ± 4.40 | 0.60 ± 6.14 | 0.48 ± 1.78 |
| BSA | −0.40 ± 2.91 | 0.03 ± 1.54 | — | — |
*Sucrose P = 0.0049 compared with both BSA control and 2-chain HP2 (n = 10 for each group of treatment).
†Lacman P = 0.0024 compared with both BSA control and 2-chain HP2.
To determine whether the 2-chain mature HP2 affected IP, the in vivo experiments described previously were repeated by administering 2-chain proteolytically cleaved protein. In contrast to the single-chain zonulin, 2-chain HP2 (170 μg per mouse) failed to alter either gastroduodenal or small intestine permeability compared with BSA-treated controls (Table 1). Combined, these data indicate that the single-chain zonulin, but not its 2-chain mature HP2 form generated by proteolytic cleavage, retains the reversible permeating activity previously reported for zonulin.
Transcriptional Expression of Zonulin in Human Duodenal Tissues.
Zonulin mRNA expression and quantification in human intestinal mucosae.
Using specific primers and the cDNA of human intestinal biopsies from zonulin-positive subjects, we amplified a 686-bp fragment, of which 144 bp belong to the α-chain and 542 bp belong to the β-chain of both HP1 and HP2 genes. Sequencing of this fragment confirmed its identity as HP, but HP1 could not be distinguished from HP2 because of the common sequence in the amplified region. To overcome this and specifically to quantify the expression of the zonulin gene in the human intestine, cDNA obtained from the intestinal mucosae of healthy individuals (n = 10), CD patients with acute-phase disease (n = 7), and CD patients with disease in remission following a gluten-free diet (GFD) (n = 3) was analyzed by real-time PCR using primers and probes specific for the α2-chain. Compared with healthy individuals, zonulin mRNA expression was increased in the intestinal mucosae of CD subjects with active disease (3-fold increase; P < 0.05). Intestinal mucosae of 3 CD subjects adhering to a GFD showed only a 1.5-fold increase in zonulin expression compared with controls (Fig. S4).
Recombinant Zonulin Activates EGFR and Causes TEER Changes Through PAR2.
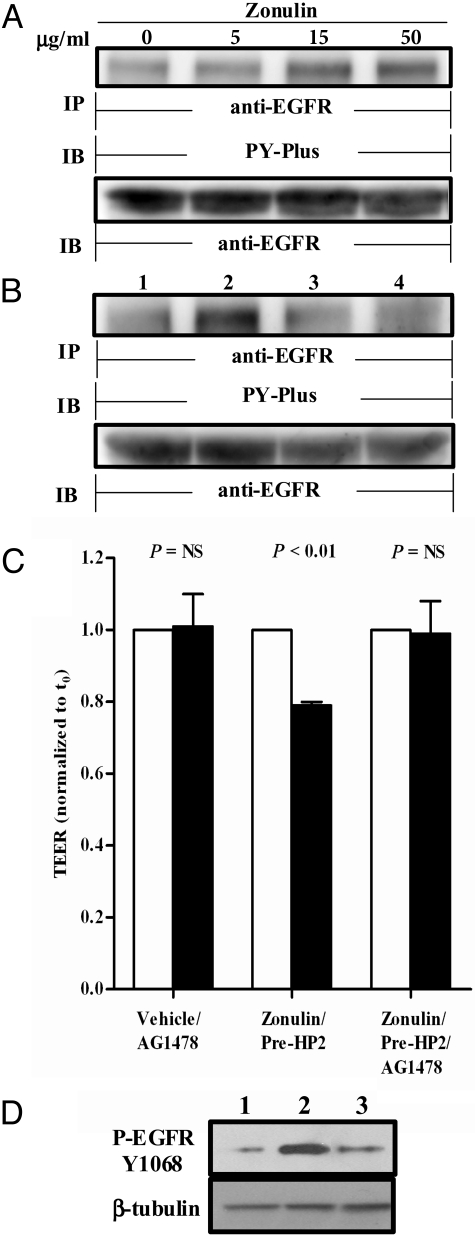
It has recently been reported that gliadin, a glycoprotein present in wheat and several other cereals and identified as the environmental trigger responsible for the autoimmune damage of the small intestine typical of CD (15), fully reproduces the effects of EGF on the actin cytoskeleton (16), effects that are very similar to those previously reported for zonulin (7, 10, 16). Furthermore, structural analysis revealed that the pre-HP2 β-chain includes an EGF motif that contains 6 spatially conserved cysteine residues that form 3 intramolecular disulfide bonds (Fig. S1C) necessary for EGF-like activity. To determine whether zonulin can activate EGFR, increasing concentrations of baculovirus-derived recombinant zonulin were added to Caco-2 intestinal epithelial cells. The cells were lysed, immunoprecipitated with anti-EGFR Ab, and processed for phosphotyrosine immunoblotting (PY-Plus). At concentrations ≥15 μg/mL, zonulin increased tyrosine phosphorylation of EGFR (Fig. 4A and Fig. S5A). To establish the role of EGFR in zonulin-induced alterations in TEER further, we also performed both the in vitro and ex vivo experiments described previously in the presence of the EGFR-selective protein tyrosine kinase (PTK) inhibitor AG1478. Preincubation of Caco-2 cells for 2 h with AG1478 (5 μM) prevented zonulin-induced EGFR phosphorylation on Y1068 (Fig. 4B and Fig. S5B). Similarly, pretreatment with AG1478 abolished the reduction in TEER in response to zonulin (Fig. 4C). Finally, trypsin digestion of zonulin dramatically reduced its ability to activate EGFR (Fig. 4D). Combined, these data suggest that the single-chain zonulin activates EGFR and induces an EGFR-driven decrease in TEER, whereas the cleaved 2-chain HP2 fails both to activate EGFR and to increase IP.
Fig. 4.
(A) Zonulin at increasing concentrations was incubated on serum-starved Caco-2 cells. The cells were lysed, immunoprecipitated using anti-EGFR Ab, and processed for WB using anti-phospho EGFR (PY-Plus) Ab. To ensure equal loading, the blots were stripped and reprobed for EGFR. Zonulin caused a dose-dependent increase in EGFR phosphorylation that reached a plateau at 15 μg/mL. (B) Zonulin at 50 μg/mL was incubated either alone (lane 2) or in the presence of 5 μM of the EGFR-selective PTK inhibitor AG1478 (lane 3) on serum-starved Caco-2 cells. Cells exposed to media (lane 1) or AG1478 alone (lane 4) were used as additional controls. Zonulin caused an increase in EGFR phosphorylation that was completely abolished by the PTK inhibitor AG1478 (n = 3 experiments). (C) Zonulin, either alone or in the presence of 5 μM AG1478, was applied to the luminal side of C57BL/6 WT intestinal segments at a concentration of 50 μg/mL, and TEER was measured at baseline (open bars) and 90 min postincubation (closed bars). Zonulin caused a significant drop in TEER that was prevented by the presence of AG1478 (n = 4 mice for each group). t0, time point t = 0. (D) The zonulin-induced EGFR phosphorylation was significantly reduced following treatment with 2-chain mature HP2 (50 μg/mL; lane 3) compared with single-chain zonulin (lane 2). Lane 1 shows EGFR phosphorylation in cells treated with media alone.
Several G protein-coupled receptors, including PAR2 (17), transactivate EGFR (18). Because Zot and zonulin share a similar mechanism of action (6) and the zonulin protein sequence contains a Zot-like and PAR2-activating peptide (AP)–like motif in its β-chain (FCAGMS), we asked whether zonulin-induced EGFR activation might be dependent on PAR2. Experiments in Caco-2 in which PAR2 was silenced and experiments in PAR2−/− mice demonstrated that zonulin induced PAR2-dependent transactivation of EGFR, which, in turn, caused TEER changes (see SI Text, Figs. S6 and S7).
Discussion
In the current study, we have identified zonulin as the precursor of HP2. Mature human HPs are heterodimeric plasma glycoproteins composed of α- and β-polypeptide chains that are covalently associated by disulfide bonds and in which only the β-chain is glycosylated (19). Unlike the β-chain (36 kDa), the α-chain exists in 2 forms [i.e., α1 (≈9 kDa) and α2 (≈18 kDa)]. The presence of one or both of the α-chains results in the 3 phenotypes HP1–1, HP2–1, and HP2–2. These HP variants evolved from a mannose-binding lectin-associated serine protease (MASP) (12, 20), with the α-chain containing a complement control protein and the β-chain a catalytically dead chymotrypsin-like serine protease domain (21–24). Other members of the MASP family include a series of plasminogen-related growth factors [e.g., EGF, hepatocyte growth factor (HGF)] involved in cell growth, proliferation, differentiation, migration, and disruption of intercellular junctions. Despite this multidomain structure, the only function assigned to HPs, to date, is to bind Hb to form stable HP-Hb complexes, thereby preventing Hb-induced oxidative tissue damage (25). No function has ever been described for their precursor forms. HPs are unusual secretory proteins in that their precursor proteins, instead of being cleaved in the trans-Golgi complex, are proteolytically processed by complement Cr1LP in the endoplasmic reticulum (13). Of interest, the endoplasmic reticulum fraction was the cellular fraction in which the highest zonulin concentrations were detected (9).
Because the key biological effect of zonulin is to regulate intercellular TJ function (7, 9–11), we studied recombinant pre-HP2 in IP assays. In a dose- and time-dependent manner, pre-HP2 reduced TEER across murine small intestinal mucosa both ex vivo and in vivo. The observation that zonulin lost its permeating activity after cleavage into its 2 α2- and β-subunits further supports the notion that zonulin and mature 2-chain HP2 exert distinct biological functions. Whether this functional divergence relates to conformational differences between the uncleaved precursor form versus the cleaved mature protein is under study. The importance of protein conformation in dictating HP protein function is further supported by the finding that zonulin cross-reactive anti-Zot Ab recognized the HP1 α1-chain under denaturing conditions (Figs. 1A and 2B) but failed to recognize nondenatured HP1 (Fig. S2 A and B). Combined, these data confirm the identity of zonulin as pre-HP2.
We previously reported that the NH2-terminal amino-acid sequence of zonulin has striking similarities to the light chain of human γ-globulins (7), a similarity also noted for HP (26). Clearance of the HP-Hb complex can be mediated by the monocyte/macrophage scavenger receptor CD163 (25). Clustal W dendrogram analysis showed a region in the zonulin β-chain just upstream of the CD163 binding site with the following γ-globulin–like consensus motif: QLVE—V—P. Whether discrepancies between our previously reported zonulin sequence and the pre-HP2 sequence as related to this consensus motif are attributable to intraspecies variability associated with a high zonulin mutation rate or to our sequence error at that time remains to be established.
Zonulin contains growth factor-like repeats. Like zonulin, growth factors affect intercellular TJ integrity (27, 28). We now show that the single-chain zonulin, but not its cleaved mature form, transactivates EGFR via PAR2 and that its effect on TEER is prevented by pharmacological inhibition of EGFR or siRNA-induced PAR2 silencing. This suggests that the growth factor motif in the single-chain zonulin, but not in the mature 2-chain HP2, has the molecular conformation required to induce TJ disassembly by indirect transactivation via PAR2. Whether the EGF-like repeat in zonulin might also directly engage the EGFR ectodomain remains to be established.
Gliadin, the environmental trigger of CD, reportedly reproduces the effects of EGF on the actin cytoskeleton (16). These effects are very similar to the effects we reported for zonulin (7). Gliadin binds to the CXCR3 chemokine receptor (29), and this interaction is coupled to zonulin release from both intestinal cells (9) and whole intestinal tissues (10). Hence, it is likely that the gliadin-related EGF effects are mediated through zonulin release. We also have identified intestinal bacterial colonization as a stimulus for zonulin release (8). Gliadin and microorganisms both cause polarized luminal secretion of zonulin (8). Therefore, we focused our studies on early zonulin action (i.e., its activity at intestinal luminal side). This approach may appear counterintuitive, given the observation that both EGFR and PAR2 are expressed basolaterally (3, 30). However, evidence exists that they also are apically expressed (31). The fact that we have demonstrated that zonulin exerts a permeating effect, both in ex vivo and in vivo, when applied to the luminal aspect of the intestinal mucosa does not dispute the possibility that the protein acts basolaterally as well. When environmental triggers (i.e., bacteria, gluten) are present in the intestinal lumen, zonulin is released from enterocytes, a process that is mediated, at least for gliadin, by CXCR3 (29). Following zonulin release and the subsequent increase in IP, these triggers can reach the submucosa, where zonulin-expressing immune cells can secrete zonulin to the basolateral side. A similar bilateral action has been reported for mucosal mast cell protease II, another serine protease that controls IP acting from both luminal and serosal sides (32).
The role of both EGFR and PAR2 in regulating epithelial permeability has been previously reported (33, 34). However, our study provides previously undescribed evidence that the 2 receptors work cooperatively to regulate small intestine permeability.
We have previously reported that zonulin is up-regulated during the acute phase of CD (9, 10). Using HP-specific primers, we now report the previously undescribed expression of zonulin mRNA in human intestine. Furthermore, real-time PCR experiments showed that zonulin expression was increased in CD patients compared with normal controls. The enhanced expression of zonulin correlated with disease activity, because CD patients who were on a GFD showed mean values for zonulin expression that were intermediate to those of patients with active CD and normal controls. Interestingly, Papp et al. (35) recently reported that a polymorphism in the HP gene represents a previously undescribed genetic risk factor for CD development and its clinical manifestations.
The human plasma levels of HPs are between 100 and 300 mg per 100 mL, with HP2–2 ranging between 100 and 260 mg per 100 mL. Almost 8% of HPs are secreted in their proform (36), suggesting that under physiological circumstances, 80–208 μg/mL pre-HP2 is present in human plasma. Therefore, the concentrations of zonulin used in this study are within physiological range and are most likely indicative of the signaling pathways activated when zonulin is up-regulated during pathological processes. Besides CD, increased IP has been reported in other autoimmune diseases, including T1D (11), systemic lupus erythematosus (37), and ankylosing spondylitis (38), further delineating the importance of the paracellular pathway in the pathogenesis of autoimmune diseases. These findings, together with the observation that zonulin is overexpressed during the acute phase of several immune-mediated diseases and its blockage prevents the onset of the autoimmune response, suggest that zonulin contributes to the pathogenesis of these conditions, opening previously undescribed paradigms in the pathobiology and treatment options of immune-mediated diseases.
Methods
Human Serum Samples.
Human sera from both healthy volunteers and patients with CD were obtained from the Center for Celiac Research serum bank. All samples were depleted of albumin and IgG using commercially available kits (Enchant Life Science kit; Pall Corporation and IgG ImmunoPure immobilized protein G plus; PIERCE, respectively). The albumin- and IgG-depleted sera were analyzed by SDS/PAGE, 2-DE, and WB analysis.
Human HPs.
HP1–1 and HP2–2 extracted from human plasma were purchased from Sigma. HP SDS/PAGE, both monodimensional gel electrophoresis and 2-DE, WB, and MS analyses are described in detail in (SI Text). HP deglycosylation was performed by addition of PNGase F according to the manufacturer's instructions (Sigma).
Human Zonulin/Pre-HP2 Cloning and Expression in a Baculovirus Expression System and Its Cleavage by Proteases.
Recombinant zonulin/preHP2 protein production using a baculovirus system and its purification are described in SI Text. Purified single-chain zonulin was subjected to proteolytic cleavage using the serine proteases indicated, resolved by SDS/PAGE, and then stained with SimplyBlue SafeStain solution (Invitrogen). For generation of 2-chain HP2, single-chain zonulin was exposed to trypsin-agarose beads (T-1763; Sigma) for 20 min at 25 °C. The beads were removed by centrifugation, and the effectiveness of the removal of trypsin was confirmed by assay of trypsin peptidase activity against the substrate Glu-Gly-Arg-pNA (Bachem BioScience).
Ex Vivo and In Vivo IP Studies.
The effects of zonulin on ex vivo and in vivo IP were determined as previously described (8, 14) and are reported in detail in SI Text.
Zonulin Activation of EGFR.
To determine whether zonulin can activate EGFR, increasing concentrations of either zonulin or 2-chain mature HP2 were added for increasing exposure times to serum-starved high EGFR-expressing Caco-2 cells. The cells were lysed and processed for WB analysis with anti-phospho EGFR (Y1068) Ab (Cell Signaling Technology, Inc.) as previously reported (39). Experiments were repeated in the presence of 5 μM of the EGFR-selective PTK inhibitor AG1478 (Calbiochem).
Knockdown of PAR2 Through RNA Interference.
The methods used to silence PAR2 are reported in detail in SI Text.
Zonulin Gene Sequencing and Quantification from Intestinal Tissue from Patients With and Without CD.
Samples of small-intestine mucosae were obtained from the second or third portion of the duodenum from subjects undergoing a diagnostic upper gastrointestinal endoscopy. Subjects included were 10 healthy controls, 7 patients with active CD at diagnosis, and 3 patients with CD on treatment with a GFD for at least 6 months. All patients had clinical indications for the procedure and gave their informed consent to undergo an additional biopsy for the purpose of this study. The study protocol was approved by the Ethics Committee of the University of Maryland. The small-intestine biopsies were immediately collected in RNAlater RNA Stabilization Reagent (Qiagen) and stored at −20 °C until processed. Total RNA extraction, cDNA synthesis, and real-time PCR are described in SI Text.
Statistical Analysis.
All values are expressed as mean ± SE. The analysis of differences was performed by 2-tailed Student's t tests to test differences between 2 groups for either paired or unpaired varieties. Multivariate analysis was performed where appropriate. Values of P ≤ 0.05 were regarded as significant.
Supplementary Material
Acknowledgments.
This manuscript was partially supported by National Institutes of Health grant DK048373 (to A.F.).
Footnotes
Conflict of interest statement: A.F. and S.N.V. have financial interest in Alba Therapeutics, a company involved in the development of treatments of CD alternative to the GFD.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906773106/DCSupplemental.
References
- 1.Rook GA, Stanford JL. Give us this day our daily germs. Immunol Today. 1998;19:113–116. doi: 10.1016/s0167-5699(97)01204-8. [DOI] [PubMed] [Google Scholar]
- 2.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–1520. doi: 10.1136/gut.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fasano A, Shea-Donohue T. Mechanisms of disease: The role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:416–422. doi: 10.1038/ncpgasthep0259. [DOI] [PubMed] [Google Scholar]
- 4.Wapenaar MC, et al. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis. Gut. 2008;57:463–467. doi: 10.1136/gut.2007.133132. [DOI] [PubMed] [Google Scholar]
- 5.Rescigno M, Lopatin U, Chieppa M. Interactions among dendritic cells, macrophages, and epithelial cells in the gut: Implications for immune tolerance. Curr Opin Immunol. 2008;20:669–675. doi: 10.1016/j.coi.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Fasano A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann N Y Acad Sci. 2000;915:214–222. doi: 10.1111/j.1749-6632.2000.tb05244.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113(Pt 24):4435–4440. doi: 10.1242/jcs.113.24.4435. [DOI] [PubMed] [Google Scholar]
- 8.El Asmar R, et al. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123:1607–1615. doi: 10.1053/gast.2002.36578. [DOI] [PubMed] [Google Scholar]
- 9.Drago S, et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006;41:408–419. doi: 10.1080/00365520500235334. [DOI] [PubMed] [Google Scholar]
- 10.Fasano A, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;355:1518–1519. doi: 10.1016/S0140-6736(00)02169-3. [DOI] [PubMed] [Google Scholar]
- 11.Sapone A, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–1449. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 12.Bowman BH, Kurosky A. Haptoglobin: The evolutionary product of duplication, unequal crossing over, and point mutation. Adv Hum Genet. 1982;12:189–194. doi: 10.1007/978-1-4615-8315-8_3. [DOI] [PubMed] [Google Scholar]
- 13.Wicher KB, Fries E. Prohaptoglobin is proteolytically cleaved in the endoplasmic reticulum by the complement C1r-like protein. Proc Natl Acad Sci USA. 2004;101:14390–14395. doi: 10.1073/pnas.0405692101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meddings JB, Swain MG. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology. 2000;119:1019–1028. doi: 10.1053/gast.2000.18152. [DOI] [PubMed] [Google Scholar]
- 15.Sollid LM. Coeliac disease: Dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2:647–655. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- 16.Barone MV, et al. Growth factor-like activity of gliadin, an alimentary protein: Implications for coeliac disease. Gut. 2007;56:480–488. doi: 10.1136/gut.2005.086637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cenac N, et al. PAR2 activation alters colonic paracellular permeability in mice via IFN-gamma-dependent and -independent pathways. J Physiol. 2004;558:913–925. doi: 10.1113/jphysiol.2004.061721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Merwe JQ, Hollenberg MD, MacNaughton WK. EGF receptor transactivation and MAP kinase mediate proteinase-activated receptor-2-induced chloride secretion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G441–G451. doi: 10.1152/ajpgi.00303.2007. [DOI] [PubMed] [Google Scholar]
- 19.Haugen TH, Hanley JM, Heath EC. Haptoglobin. A novel mode of biosynthesis of a liver secretory glycoprotein. J Biol Chem. 1981;256:1055–1057. [PubMed] [Google Scholar]
- 20.Maeda N, Yang F, Barnett DR, Bowman BH, Smithies O. Duplication within the haptoglobin Hp2 gene. Nature. 1984;309:131–135. doi: 10.1038/309131a0. [DOI] [PubMed] [Google Scholar]
- 21.Kurosky A, et al. Covalent structure of human haptoglobin: A serine protease homolog. Proc Natl Acad Sci USA. 1980;77:3388–3392. doi: 10.1073/pnas.77.6.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen MJ, et al. A unique loop extension in the serine protease domain of haptoglobin is essential for CD163 recognition of the haptoglobin-hemoglobin complex. J Biol Chem. 2007;282:1072–1079. doi: 10.1074/jbc.M605684200. [DOI] [PubMed] [Google Scholar]
- 23.Polticelli F, Bocedi A, Minervini G, Ascenzi P. Human haptoglobin structure and function—A molecular modelling study. FEBS J. 2008;275:5648–5656. doi: 10.1111/j.1742-4658.2008.06690.x. [DOI] [PubMed] [Google Scholar]
- 24.Wicher KB, Fries E. Haptoglobin, a hemoglobin-binding plasma protein, is present in bony fish and mammals but not in frog and chicken. Proc Natl Acad Sci USA. 2006;103:4168–4173. doi: 10.1073/pnas.0508723103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asleh R, et al. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res. 2003;92:1193–1200. doi: 10.1161/01.RES.0000076889.23082.F1. [DOI] [PubMed] [Google Scholar]
- 26.Hunt LT, Dayhoff MO. The origin of the genetic material in the abnormally long human hemoglobin and chains. Biochem Biophys Res Commun. 1972;47:699–704. doi: 10.1016/0006-291x(72)90548-7. [DOI] [PubMed] [Google Scholar]
- 27.Hollande F, et al. HGF regulates tight junctions in new nontumorigenic gastric epithelial cell line. Am J Physiol Gastrointest Liver Physiol. 2001;280:G910–G921. doi: 10.1152/ajpgi.2001.280.5.G910. [DOI] [PubMed] [Google Scholar]
- 28.Jin M, Barron E, He S, Ryan SJ, Hinton DR. Regulation of RPE intercellular junction integrity and function by hepatocyte growth factor. Invest Ophthalmol Visual Sci. 2002;43:2782–2790. [PubMed] [Google Scholar]
- 29.Lammers KM, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Playford RJ, et al. The epidermal growth factor receptor (EGF-R) is present on the basolateral, but not the apical, surface of enterocytes in the human gastrointestinal tract. Gut. 1996;39:262–266. doi: 10.1136/gut.39.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnard JA, McHugh KM. Growth factors in the gastrointestinal tract. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 4th Ed. Oxford, UK: Elsevier Academic; 2006. pp. 183–246. [Google Scholar]
- 32.Jacob C, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem. 2005;280:31936–31948. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 33.Bueno L, Fioramonti J. Protease-activated receptor 2 and gut permeability: A review. Neurogastroenterol Motil. 2008;20:580–587. doi: 10.1111/j.1365-2982.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- 34.Raimondi F, et al. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G906–G913. doi: 10.1152/ajpgi.00043.2007. [DOI] [PubMed] [Google Scholar]
- 35.Papp M, et al. Haptoglobin polymorphism: A novel genetic risk factor for celiac disease development and its clinical manifestations. Clin Chem. 2008;54:697–704. doi: 10.1373/clinchem.2007.098780. [DOI] [PubMed] [Google Scholar]
- 36.Misumi Y, Tanaka Y, Ikehara Y. Biosynthesis, intracellular processing and secretion of haptoglobin in cultured rat hepatocytes. Biochem Biophys Res Commun. 1983;114:729–736. doi: 10.1016/0006-291x(83)90841-0. [DOI] [PubMed] [Google Scholar]
- 37.Pavon EJ, et al. Proteomic analysis of plasma from patients with systemic lupus erythematosus: Increased presence of haptoglobin alpha2 polypeptide chains over the alpha1 isoforms. Proteomics. 2006;6(Suppl 1):S282–S292. doi: 10.1002/pmic.200500404. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, et al. Identification of disease-associated proteins by proteomic approach in ankylosing spondylitis. Biochem Biophys Res Commun. 2007;357:531–536. doi: 10.1016/j.bbrc.2007.03.179. [DOI] [PubMed] [Google Scholar]
- 39.Yaish P, Gazit A, Gilon C, Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988;242:933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.