Abstract
The glucocorticoid receptor (GR) affects the transcription of genes involved in diverse processes, including energy metabolism and the immune response, through DNA-binding dependent and independent mechanisms. The DNA-binding dependent mechanism occurs by direct binding of GR to glucocorticoid response elements (GREs) at regulatory regions of target genes. The DNA-binding independent mechanism involves binding of GR to transcription factors and coactivators that, in turn, contact DNA. A small molecule that competes with GR for binding to GREs could be expected to affect the DNA-dependent pathway selectively by interfering with the protein-DNA interface. We show that a DNA-binding polyamide that targets the consensus GRE sequence binds the glucocorticoid-induced zipper (GILZ) GRE, inhibits expression of GILZ and several other known GR target genes, and reduces GR occupancy at the GILZ promoter. Genome-wide expression analysis of the effects of this polyamide on a set of glucocorticoid-induced and -repressed genes could help to elucidate the mechanism of GR regulation for these genes.
Keywords: gene regulation, glucocorticoid response element, nuclear receptor, protein-DNA interface, Py-Im polyamide
The glucocorticoid receptor (GR) is a member of the ligand-activated nuclear receptor group of transcription factors that bind with high affinity to glucocorticoids (GCs) such as cortisol and dexamethasone. GR is structurally similar to the androgen and progesterone receptors, containing a zinc-finger motif DNA-binding domain, a dimerization domain, and a ligand-binding domain (1). Ligand binding releases GR from sequestration by cytoplasmic heat shock proteins (2) and activates a series of cellular activities that lead to nuclear localization and homodimerization. Like other steroid hormone receptors, GR is known to modulate gene transcription via the binding of receptor dimers to specific palindromic sequences called glucocorticoid response elements (GREs), usually located in the cis-regulatory region of target genes—a mode of action termed transactivation. Additionally, the GR has been shown to exert its actions through an indirect non–DNA-binding mechanism, termed transrepression, in which transcriptional modulation is achieved through cross-talk between GR and other transcription factors such as NF-κB (3), activator protein-1 (AP-1) (4, 5), Sma and Mad-related protein, and members of the STAT family (6). This protein-protein cross-talk does not require the DNA-binding activity of the GR, because GR mutants that are deficient in dimerization function have been shown to lose DNA-binding ability as well as simple GRE-mediated transcription function but retain their transrepression activity (4, 7).
Because many GR target genes are immune modulators, synthetic GR agonists such as dexamethasone are among the most effective anti-inflammatory drugs available for the treatment of a variety of chronic and acute inflammatory diseases. Unfortunately, because of the functions of other GR target genes, long-term treatment with corticosteroids results in metabolic and behavioral derangements that can be treatment limiting. Although the GR targets involved in inflammatory and immune regulation have not been comprehensively defined, there is a great deal of evidence suggesting that transrepression—GR interaction with NF-κB and/or AP-1 and the subsequent suppression of their target genes—is the major mechanism by which GCs achieve their desired anti-inflammatory effect (5, 8, 9).
An understanding of the mechanisms of GR activity on target genes has been explored using a variety of approaches, including microarray analysis (10, 11); ChIP scanning (12); and modulation of GR activity using siRNA (13), genetic mutants (4), and ligands with modified structures (14). However, these methods would not be expected to differentiate explicitly between the direct and indirect DNA-binding mechanisms of GR action. A small molecule that competes with GR for binding to the consensus GRE could be expected to disrupt GR-DNA binding specifically and be used as a tool to identify GR target genes whose regulation mechanism depends on a direct protein-DNA interface. This differentiation of mechanisms of GR target gene regulation could contribute to efforts to develop more specific GR modulators that retain immunosuppressant functions while minimizing side effects resulting from other GR targets (15).
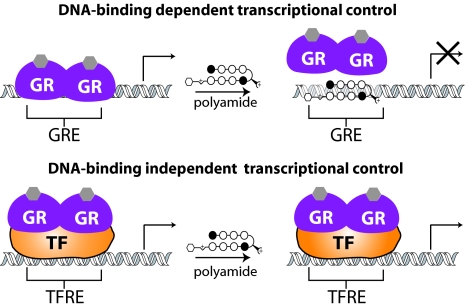
N-methylpyrrole (Py)–N-methylimidazole (Im) polyamides are a class of programmable DNA-binding ligands capable of binding sequence specifically to DNA with affinities and specificities comparable to those of natural DNA-binding proteins (16, 17). As oligomers composed of Im and Py heterocyclic rings, Py-Im polyamides achieve sequence specificity via side-by-side pairings of the heterocyclic amino acids in the minor groove of DNA: Im paired against Py distinguishes G·C from C·G, and Py paired against Py binds both A·T and T·A (18, 19). Py-Im polyamides have previously been used to modulate gene expression in cell culture via inhibition of the transcription factor-DNA interface of both hypoxia inducible factor (20, 21) and androgen receptor (22) to their respective DNA response elements. Interruption of the GR-DNA–binding interaction by polyamides represents an opportunity to regulate a distinct subset of the known binding sites for the protein. Because Py-Im polyamides can be selectively programmed to recognize the known DNA-binding sequence of the GR, there is a unique opportunity to inhibit the DNA-binding dependent activity of endogenous GR while leaving the protein-protein–mediated activity unaffected (Fig. 1).
Fig. 1.
Effect of polyamide-DNA binding on the 2 major modes of gene regulation demonstrated by the GR. (Top) Direct mechanism is dependent on GR-DNA binding at the GRE. (Bottom) Indirect mechanism is dependent on GR binding to another protein, indicated here as a general transcription factor (TF) [with binding site TF response element (TFRE)]. A sequence-specific polyamide designed to bind to the GRE but not to the TFRE would alter gene expression controlled by the DNA-binding dependent mechanism but not by the DNA-binding independent mechanism.
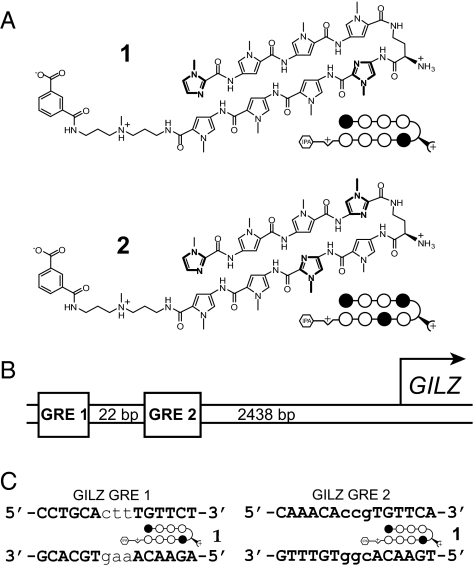
In this study, we designed a polyamide targeted to the sequence 5′-WGWWCW-3′ (where W represents either a T·A or an A·T base pair) found in the consensus GRE, with the goal of disrupting GR-GRE binding (Fig. 2A, polyamide 1). This polyamide binds the 2 known GREs found in the promoter of the well-characterized GC-induced leucine zipper (GILZ) gene, inhibits expression of GILZ and 17% of transcripts induced by dexamethasone in cultured alveolar epithelial cells (A549), and reduces GR occupancy at the GILZ promoter in vivo. A “mismatch” polyamide that targets the sequence 5′-WGWCGW-3′ (Fig. 2A, polyamide 2) is used as a control for non–GRE-binding polyamide effects. The subset of GR-regulated genes uniquely affected by the GRE-targeted polyamide may represent a set of genes regulated by GR through direct GR-GRE binding.
Fig. 2.
Polyamide design and GILZ promoter structure. (A) Structure of match polyamide 1 designed to bind 5′-WGWWCW-3′ and mismatch control polyamide 2 designed to bind 5′-WGWCGW-3′, where W represents a T·A or an A·T base pair. Ball-and-stick models of polyamides represent the structure shown, and imidazole and pyrrole monomer units are represented by filled and open circles, respectively. The isophthalic acid tail moiety is represented by a hexagon. (B) Representation of the GILZ promoter region with its 2 functional GRE sites indicated. (C) Sequences of the 2 GILZ GREs shown with polyamide 1 bound to its target site.
Results
Binding Affinities of Py-Im Polyamides to GRE1 and GRE2 of the GILZ Promoter.
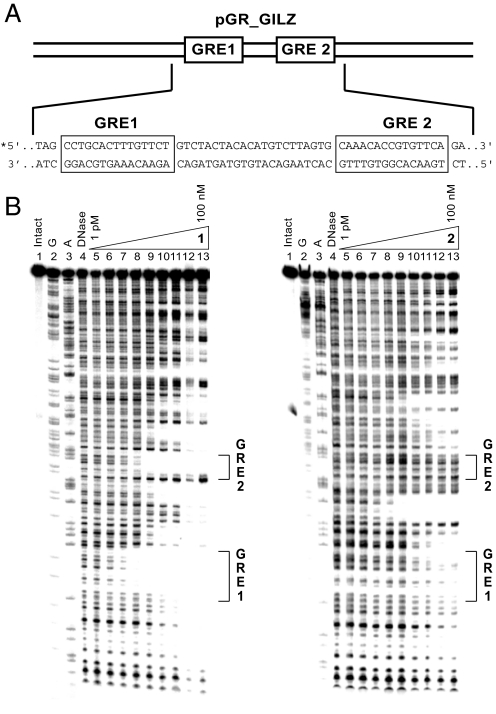
The proximal GILZ promoter contains 2 functional GREs (12) (GRE1: 5′-CCTGCActtTGTTCT-3′ and GRE2: 5′-AAACAccgTGTTCA-3′) spaced 22 bp apart ≈2,500 bp upstream of the transcription start site (Fig. 2 B and C). The DNA-binding affinity of polyamides 1 and 2 on this sequence was measured by quantitative DNase I footprint titrations using a 5′ 32P-labeled PCR fragment of pGR_GILZ, which contains a 78-bp sequence from the promoter encompassing both functional GILZ GREs (Fig. 3A). Polyamide 1 has Ka = 1.9 ± 0.8 × 1010 M−1 for the GRE1 consensus half-site 5′-TGTTCT-3′ and Ka = 8.8 ± 1.8 × 109 M−1 for the GRE2 consensus half-site 5′-TGTTCA-3′. Binding of polyamide 2, which targets the sequence 5′-WGWCGW-3′, to the GREs is not measurable by these methods (Ka ≤ 1 × 107 M−1) (Fig. 3B). Binding of polyamide 2 with Ka = 6.3 ± 0.4 × 109 M−1 is observed at the site 5′-TGTCTT-3′ located between the GREs. This is a single base pair mismatch site for polyamide 2; thus, polyamide 2 can be expected to demonstrate some binding to this site (16).
Fig. 3.
DNAse I footprinting of GILZ promoter region. (A) Sequence of the pGR_GILZ plasmid insert. (B) Storage phosphor autoradiograms from quantitative DNase I footprint titrations of polyamides 1 and 2. Lane 1, intact DNA; lane 2, G reaction; lane 3, A reaction; lane 4, DNase control; lanes 5–13, DNase I digestion products in the presence of 1, 3, 10, 30, 100, or 300 pM or 1, 3, 10, 30, or 100 nM polyamide, respectively.
EMSA.
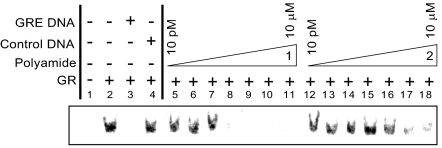
The effects of polyamides 1 and 2 on the binding of recombinant human GR to an oligo containing the GRE1 site of the GILZ promoter were measured by EMSA (Fig. 4). Incubation of the 32P-labeled GRE DNA with recombinant human GR produces a gel shift that is reduced in the presence of polyamide 1 at concentrations as low as 10 nM. Polyamide 2 has minimal effect at the same concentrations. The shift is abolished by incubation with a 100-fold excess of unlabeled GRE DNA but is unaffected by similar treatment with a scrambled control DNA, indicating a specific shift resulting from a GR-GRE–binding event.
Fig. 4.
Storage phosphor autoradiogram from EMSA of recombinant human GR binding to a 27-bp oligonucleotide duplex containing the GILZ GRE1. Lanes represent the following conditions, where * represents 32P labeling: 1, free *GRE DNA; 2, *GRE DNA + GR; 3, *GRE DNA+ GR + GRE DNA; 4, *GRE DNA + GR + scrambled DNA; and 5–18, *GRE DNA + GR + polyamide 1 (lanes 5–11) or polyamide 2 (lanes 12–18) in concentrations increasing from 10–100 pM; 1, 10, or 100 nM; and 1–10 μM, respectively. For full gel, including nonshifted free DNA bands, see Fig. S1.
Inhibition of GC-Induced GILZ Expression.
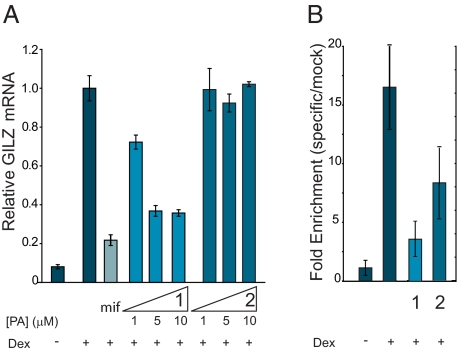
Induction of GILZ mRNA by dexamethasone in the presence of polyamides 1 and 2 in A549 cells was measured by quantitative real-time RT-PCR. Polyamide 1 inhibits the expression of dexamethasone-induced GILZ mRNA up to 65% at 5 and 10 μM, as measured in this assay (Fig. 5A). Polyamide 2 does not show a measurable effect on GILZ expression at these concentrations. The GR antagonist mifepristone was used as a control and inhibits the expression of GILZ up to 79% at 3 μM. GR occupancy at the GILZ promoter was assessed by chromatin immunoprecipitation (Fig. 5B). A 6-h dexamethasone treatment results in a 15-fold increase in GR occupancy at the GILZ promoter; treatment of the cells with polyamide 1 for 48 h before harvest reduces this occupancy, whereas treatment with the mismatch polyamide 2 shows a more modest effect. Although polyamide 2 does not bind the GRE sites, we cannot exclude the possibility that it may bind to other regions of the GILZ promoter. Although polyamide 2 affects GILZ promoter occupancy, albeit significantly less so than polyamide 1, it is somewhat surprising that polyamide 2 only minimally affects GILZ mRNA. It is not known what degree of occupancy is necessary for maximal induction of GILZ under these conditions, however. Treatment of cells by both polyamides 1 and 2 modestly affects cell proliferation and viability in a concentration- and time-dependent manner (Fig. S2).
Fig. 5.
Inhibition of dexamethasone (dex)-induced GILZ expression by polyamides 1 and 2. (A) Induction of GILZ mRNA in the presence of mifepristone (mif), polyamide 1, and polyamide 2 as measured by quantitative real-time PCR. Polyamide 1 inhibits expression of GILZ mRNA up to 65% at 5 and 10 μM, whereas polyamide 2 shows no effect. A 3-μM mif control inhibits expression up to 79%. Error bars represent SD. (B) Chromatin immunoprecipitation assays with anti-GR or mock antibody treatment expressed as fold enrichment (specific/mock) of DNA sequences at the GILZ promoter. GR occupancy at the GILZ promoter is decreased in the presence of polyamide 1 (10 μM) and to a lesser extent by polyamide 2. Error bars represent SD.
Genome-Wide Microarray Analysis.
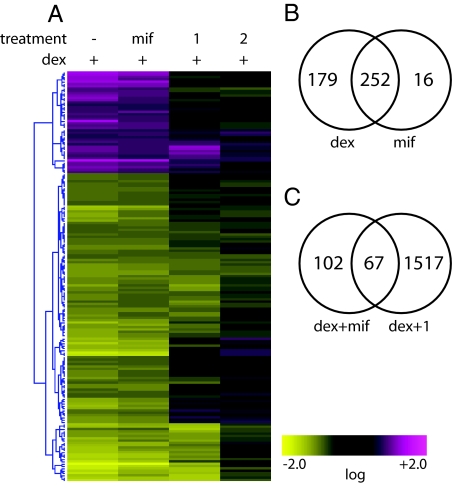
Global effects of polyamides 1 and 2 and the GR antagonist mifepristone on gene expression in dexamethasone-stimulated A549 cells were monitored with Affymetrix high-density Human Genome U133 Plus 2.0 arrays, which interrogate >50,000 transcripts. Of these transcripts, 431 were affected greater than 2-fold by dexamethasone compared with noninduced control, with 323 transcripts induced and 108 repressed. An agglomerative clustering analysis of the transcripts affected by dexamethasone demonstrates distinctive patterns of genes that are GC-responsive and GR-mediated but have a differential response to treatment by polyamides 1 and 2 (Fig. 6A) at a concentration of 5 μM. The GR antagonist mifepristone abolishes the activity of GR, and therefore was used as a control to identify those transcripts that were affected by a GR-related mechanism. Four hundred thirty-one transcripts were either induced or repressed by 2-fold or greater (P < 0.01) by dexamethasone treatment. Among these, the effects of dexamethasone were abolished by simultaneous treatment with 3 μM mifepristone for 252 transcripts, indicating that these 252 were a result of GR activity (Fig. 6B). For 76 transcripts of this set of 252, the effects of dexamethasone treatment were abolished by polyamide 1 to a greater extent than by polyamide 2, indicating a sequence-specific polyamide effect on those transcripts (Fig. 6C). This set of 76 transcripts corresponds to 67 genes, listed in Table 1. We would anticipate that the regulation of these genes by GR involves a direct GR-GRE interaction. To analyze the potential GRE sequence-specific role of polyamide treatment within the subset of GR-regulated genes, those genes that showed a similar effect from both polyamides 1 and 2 were considered to have non–GRE-specific effects and were not included among those analyzed. Many of the genes identified by the microarray as dexamethasone responsive are consistent with those previously identified in this cell line (15). A list of the effect of all treatment conditions on the GR-regulated genes is available in Table S1.
Fig. 6.
Global effects on genes interrogated by using Affymetrix high-density Human Genome U133 Plus 2.0 arrays. (A) Agglomerative clustering of transcripts induced or repressed by dexamethasone (dex) by at least 2-fold under the 4 specified conditions: no treatment control, mifepristone (mif, 3 μM), polyamide 1 (5 μM), and polyamide 2 (5 μM). Clustering was based on an error-weighted Euclidean correlation of intensity ratios for each treatment as compared with dex-induced controls. (B) Venn diagram representing genes affected ( fold change >2.0, P < 0.01) by dex and mif. (C) Venn diagram representing genes affected ( fold change > 2.0, P < 0.01) by dex and mif as well as by dex and polyamide 1. Numbers inside the intersections represent genes affected by both treatments.
Table 1.
Genes affected >2-fold by dexamethasone and mifepristone whose activity is modulated by polyamide 1 and not by polyamide 2*
| Gene | Fold induction | Gene | Fold induction | Gene | Fold induction | Gene | Fold repression |
|---|---|---|---|---|---|---|---|
| CDKN1C (N33167) | 20 | IGFBP1 (NM_000596) | 4 | 49111_at (N80935) | 3 | NR4A2 (NM_006186) | 6 |
| FKBP5 (NM_004117) | 14 | CDC42EP3 (AI754416) | 4 | TIPARP (AL556438) | 3 | IER2 (NM_004907) | 3 |
| DNAJC15 (NM_013238) | 14 | PLEKHA7 (AA758861) | 4 | EPB41L4A (AU144565) | 3 | EREG (NM_001432) | 3 |
| FGD4 (AI949549) | 11 | CEP3 (AI801777) | 4 | CEBPD (NM_005195) | 3 | IER3 (NM_003897) | 3 |
| CIDEC (NM_022094) | 8 | LOC153346 (AU157049) | 4 | EMP1 (NM_001423) | 3 | NR0B1 (NM_000475) | 3 |
| EDN3 (NM_000114) | 8 | IL6R (NM_000565) | 4 | LOC54492 (AK026748) | 3 | MAFK (BG231691) | 3 |
| PTGER4 (AA897516) | 8 | ARRB1 (BE207758) | 4 | ARRB1 (BC003636) | 3 | CYP24A1 (NM_000782) | 3 |
| METTL7A (NM_014033) | 7 | CDC42EP3 (AL136842) | 4 | SOCS1 (AB005043) | 3 | EDN1 (NM_001955) | 2 |
| FAM105A (NM_019018) | 7 | AKAP13 (NM_006738) | 3 | SCNN1G (AI985987) | 3 | NEIL3 (NM_018248) | 2 |
| ATAD4 (NM_024320) | 6 | 244650_at (AA581439) | 3 | EPB41L4B (NM_019114) | 3 | PTGS2 (AY151286) | 2 |
| GOLSYN (NM_017786) | 6 | PKP2 (NM_004572) | 3 | ARRB1 (NM_004041) | 3 | RND1 (U69563) | 2 |
| CORO2A (AL515381) | 5 | FBXL16 (AI613010) | 3 | IL6R (NM_000565) | 3 | ||
| TFCP2L1 (NM_014553) | 5 | CEBPD (AV655640) | 3 | MT1F (BF246115) | 2 | ||
| RASSF4 (N49935) | 5 | FOXO3 (AV725666) | 3 | JPH2 (AA716165) | 2 | ||
| CDH16 (NM_004062) | 4 | FLVCR2 (NM_017791) | 3 | SLC22A5 (NM_003060) | 2 | ||
| ACSL1 (NM_021122) | 4 | MT2A (NM_005953) | 3 | RHOU (AL096776) | 2 | ||
| MT1X (NM_002450) | 4 | KIAA0146 (AI363213) | 3 | MAN1C1 (NM_020379) | 2 | ||
| AKAP13 (NM_006738) | 4 | 43511_s_at (AI201594) | 3 | KIAA0232 (D86985) | 2 | ||
| ACSL1 (NM_001995 | 4 | ETNK2 (NM_018208 | 3 |
*These genes may represent glucocorticoid-responsive genes that are controlled via direct GR-binding mechanisms.
Discussion
A mechanistic understanding of cell signaling is fundamental to the development of improved medicines and diagnostics. Toward this end, the functions of individual biomolecules and their involved pathways have been explored using both biological and chemical methods. Genetic approaches include gene knockouts, dominant negative proteins, and siRNA to affect the activity of selected genes. Chemical approaches have used preexisting, designed, or discovered small molecules that perturb a particular protein or specific protein-protein interaction and have been used successfully to characterize cell signaling pathways (23–25).
The elucidation of transcription factor, coactivator, and corepressor activation; their gene targets; and their mechanisms of activity is a focus of intense research interest. Genome-wide high-throughput approaches, including mRNA microarray analysis (26), ChIP-chip (27–29), and (more recently) ChIP-sequencing (30), have been used to define the targets of transcription factors. Specific protein-DNA interactions are the interfaces where information from protein signaling is converted into programs of gene expression. Used in the context of genome-wide analysis, small molecules that perturb this interface in a predictable manner could become useful tools for understanding gene expression. Programmable DNA-binding Py-Im polyamides offer a chemical approach to perturbing protein-DNA interactions that could be used for characterization of transcription factor-DNA interactions. We have reported previously that Py-Im polyamide 1, which targets 5′-WGWWCW-3′, inhibits androgen receptor binding to its consensus sequence 5′-GGTACAnnnTGTTCT-3′ (22). As members of the same class of transcription factors, androgen receptor and GR share highly conserved DNA-binding domains, similar consensus sequences, and a transactivation mode of action. However, each acts within its individual biological context and responds to distinct chemical stimuli. The use of polyamide 1 in the context of disrupting the GR-GRE interface offers additional perspective in the mechanistic study of GR gene regulatory action and its dual transactivation/transrepression mechanisms. Because of the central role of GR in multiple inflammatory response pathways and its role as a drug target, mechanistic studies of GR-modulated gene expression are a growing field of study. However, the multiple mechanisms by which GR mediates gene expression makes identification of GR target genes a challenge (6, 15, 31). The complex transcriptional activity of the GR involves DNA-binding dependent as well as DNA-binding independent mechanisms that result in both gene activation and gene repression. In general, the most well-understood mechanism is that by which genes are activated by direct DNA binding of GR to a GRE in the regulatory region of target genes, followed by recruitment of coactivators and the general transcriptional machinery. Another well-documented, although less understood, mechanism is the transrepression mechanism, in which GR exerts its influence indirectly by binding to other proteins and transcription factors, leading to the repression of genes controlled by those proteins. Intriguingly, an increasing body of evidence indicates that much of the anti-inflammatory activity of GCs is mediated by the transrepression mechanism, a finding that has been attributed largely to the repression of key inflammatory transcription factors, including AP-1 and NF-κB (3–5). Meanwhile, many of the reported side effects of GC treatment have been attributed to a transactivation mechanism (32). Efforts to develop GC-based drugs that can dissociate anti-inflammatory and immunosuppressive effects from side effects have focused largely on establishing a method of dissociating the transrepression activity from the transactivation activity of the GR. Much of the work in this area has centered about the development of unique GR-binding ligands that display a more selective gene-regulation pattern than classical GCs. The end result of this body of effort has demonstrated that the problem is complex, because slight alterations in ligand structure and chemical reactivity can have a pronounced influence on the transcriptional regulatory activity of GR (14).
An additional complication to mechanistic study of the GR is the fact that examples have been noted in which genes are repressed through DNA-binding dependent GR action, in addition to those in which genes are activated through DNA-binding independent action. Techniques using immunoprecipitated chromatin fragments reveal a great deal of useful information about GR target sites but are not expected to distinguish between sites of direct GR-GRE interaction and sites of indirect GR-protein-DNA interaction. Our approach utilizes a DNA-binding polyamide targeting the consensus GRE that would be expected to dissociate the direct DNA-binding from the indirect DNA-binding independent GR gene regulatory mechanisms.
In this study, a sequence-specific polyamide targeted toward the consensus DNA-binding sequence of the GR was tested on a well-known GC-induced gene, GILZ, to establish its ability to disrupt GR-DNA binding and thereby regulate gene transcription. Polyamide 1, designed to target the sequence 5′-WGWWCW-3′, has been shown by DNase I footprinting to bind at subnanomolar concentrations to the right half-site of each of 2 different functional GREs located in the promoter region of the GILZ gene. Quantitative real-time PCR analysis of RNA isolated from A549 cells treated with polyamide 1 demonstrates a 60% reduction in dexamethasone-induced GILZ mRNA levels as compared with vehicle control. Chromatin immunoprecipitation indicates that in the presence of polyamide 1, the dexamethasone-induced GR occupancy of the GILZ promoter is reduced, suggesting that it is polyamide occupancy at this site that is responsible for the lowered mRNA expression levels. A control polyamide 2 targeted at a different sequence, 5′-WGWCGW-3′, shows a reduced effect on GR promoter occupancy and no effect on GILZ gene transcription.
The established ability of polyamide 1 to disrupt the GR-GRE interaction for GILZ led us to a genome-wide search for other transcriptional events that are interrupted sequence specifically. Affymetrix microarrays interrogating >50,000 transcripts were chosen to examine the global effect of polyamides 1 and 2 at a concentration of 5 μM on dexamethasone-treated cells. The GR antagonist mifepristone was used as a control to isolate which of the dexamethasone-responsive transcripts result from GR activity. These conditions isolated 252 genes considered to be genuine GR-modulated GC-affected transcripts. Both polyamides had a similar and modest effect on cell proliferation under these experimental conditions.
To tease out which of these effects is attributable to a binding event at a 5′-WGWWCW-3′ sequence corresponding to a GRE, we have eliminated genes from the list that were affected by polyamide 2, because both the footprinting and ChIP data show that it is possible that this compound may have an effect on genes whose promoters contain a 5′-WGWCGW-3′ site. Of the list of 252 GR-modulated transcripts, treatment with polyamide 1 had a unique effect on 170 transcripts over treatment with polyamide 2. This left us with a final list of 170 transcripts whose expression is confidently affected both by GR and our sequence-specific small molecule. Of this final list of confidently interrogated transcripts, we find 76 transcripts, corresponding to 67 genes, that are identified as genes whose expression was modulated by polyamide 1. We believe this list of genes to represent 67 genes that are regulated by direct GR-DNA interactions. Although little information exists in the literature linking GC response with these genes, we note that several genes with known functional GRE-binding sites are included in the list (Table 2). For genes in which the binding site has been identified, the sequence is listed, with the predicted polyamide 1 target-binding site underlined. The GREs for the genes listed in this table have been determined to have GC-induced GR occupation at the indicated location relative to the given gene. For some of these genes, functionality has been confirmed via point mutation or a luciferase-driven reporter assay. The fact that these genes contain polyamide 1 binding sites and demonstrate responsiveness to polyamide 1 treatment gives us confidence that the subset of genes identified here represents a previously undescribed list of DNA-binding dependent genes to be explored by the GR field.
Table 2.
Genes identified in our study that have previously been shown to have GR-occupied and/or functional GREs
There is still very little known about many of the genes that are affected by GCs, including which genes are activated as a result of transrepressed mechanisms or transactivated mechanisms. The lack of identified GREs to be found in the literature complicates the search for genes that are regulated by the transactivation mechanism. DNA-binding polyamides represent a unique approach to differentiating the transrepression from transactivation activity of the GR—the potential to selectively block the protein-DNA interactions of endogenous GR while leaving the protein-protein interactions unaffected. This approach is limited by the target site degeneracy of both the polyamide and GR, effects of specific polyamide-DNA binding on programs of other transcription factors, and nonspecific effects of polyamides that are independent of sequence-specific polyamide-DNA binding. Small molecules that affect specific GR-protein interactions represent a complementary approach that is as yet unexplored. It is our hope that the list of genes provided by this study serves as a guide to genes that necessitate further exploration in the search to regulate the transactivation mechanism of GC response selectively.
Materials and Methods
Unless otherwise stated, DNA oligos were purchased from Integrated DNA Technologies. Unless otherwise stated, reagents were purchased from Sigma-Aldrich.
Synthesis of Polyamides.
Polyamides 1 and 2 were synthesized by solid-phase methods on Kaiser oxime resin (Nova Biochem) following established protocols (33) and were cleaved subsequently from resin with 3,3-diamino-N-methyl-dipropylamine and purified by reverse-phase HPLC. Isophthalic acid was activated with PyBOP (Nova Biochem) and conjugated to the polyamides as previously described (34). Purities and identities of the polyamides were assessed by HPLC, UV-visible spectroscopy, and MALDI-TOF MS.
Determination of DNA-Binding Affinity and Sequence Specificity.
Plasmid pGR_GILZ was constructed by inserting a 78-bp sequence from the GILZ promoter containing GRE1 and GRE2 into pUC19 plasmid. Quantitative DNase I footprint titration experiments were performed following established protocols to measure the binding affinities of polyamides 1 and 2 on a 5′–32P-labeled fragment of pGR_GILZ. Detailed experimental protocols are reported elsewhere (35).
EMSA.
The oligonucleotide 5′-GTATAGCCTGCACTTTGTTCTGTCTAC-3′ representing a 27-bp section of the GILZ promoter containing GRE1 (underlined) was annealed to its complement and end-labeled with 32P. Aqueous solutions of polyamides 1 and 2 at the indicated concentrations were incubated with the duplex at room temperature for 2 h in 5 μL 5× buffer containing 100 mM Hepes (pH 7.9), 300 mM KCl, 25 mM MgCl2, 10 mM DTT, and 50% (vol/vol) glycerol. Recombinant human GR (3.5 mg/mL; Affinity Bioreagents) was diluted 1:20 with 50 μg/μL BSA and stored as a frozen stock solution. Fresh aliquots of this solution were used for each experiment and discarded after use. Two microliters of the diluted GR protein, 1 μL of 200 ng/μL Poly(deoxyinosinic-deoxycytidylic) acid, and 1 μL of unlabeled DNA (for control lanes) were taken up in water to a final volume of 15 μL and incubated at 4 °C for 30 min. This solution was added to the polyamide-DNA complex and incubated at room temperature for 1 h before being separated on a 6% polyacrylamide gel in 1× Tris-Borate-EDTA Buffer. Gels were visualized on a phosphorimager.
Measurement of Dexamethasone-Induced mRNA.
A549 cells [American Type Culture Collection (ATCC)] were plated in 24-well plates at a density of 20–25 × 103 cells per well (40–50 × 103 cells/mL) in F12-K medium (ATCC) supplemented with 10% (vol/vol) FBS (Irvine Scientific) and 4 mM penicillin/streptomycin. After 24 h, the medium was replaced with F12-K containing 10% (vol/vol) charcoal-stripped FBS, penicillin/streptomycin, and polyamides or mifepristone at the designated concentrations. Cells were grown for an additional 48 h and then treated with 100 nM dexamethasone for 6 h (Fig. S3). Isolation of RNA and cDNA synthesis were performed as described. Quantitative real-time RT-PCR was performed with SYBR Green PCR Master Mix (Applied Biosystems) following the manufacturer's protocol on an ABI 7300 qPCR instrument (Applied Biosystems). mRNA of the genes of interest was measured relative to β-glucuronidase as an endogenous control. Primer sequences were designed using Primer3 (36) and are available on request.
Measurement of Cell Proliferation and Viability.
A549 cells were plated in 96-well plates, 100 μL at 20 × 103 cells/mL in F12-K medium containing 10% (vol/vol) charcoal-stripped FBS and 4 mM penicillin/streptomycin. After the indicated time period, 10 μL of Cell Proliferation Reagent WST-1 (Roche) was added to each well. Reagent was allowed to incubate at 37 °C for 30 min, and absorbance was read at 450 nm on a Perkin-Elmer Victor 3 multiwell plate reader.
Chromatin Immunoprecipitation.
A549 cells were plated in 15-cm diameter plates at a density of 10 × 105 cells per plate. Media conditions, polyamide treatment, time course, and dexamethasone stimulation were identical to the conditions described previously for qPCR. On completion of the 6-h dexamethasone treatment, cells were cross-linked by treatment with 1% formaldehyde for 10 min. Chromatin was isolated using a ChIP-IT kit (Amersham) following the manufacturer's protocol. Chromatin was sheared and immunoprecipitated by overnight incubation at 4 °C with N499 anti-GR antibody (a gift from Keith Yamamoto, University of California, San Francisco, CA). A 1:1 mixture of Protein G and Protein A Agarose beads (Upstate) was used to isolate the immunoprecipitated material via centrifugation. Cross-links were reversed, and the DNA was isolated via phenol/chloroform extraction followed by ethanol precipitation. qPCR utilizing primers targeted to the GILZ promoter was used to assess enrichment of bound fragments as compared with mock-precipitated (no antibody) controls. PCR assays were monitored with SYBR Green PCR Master Mix (Applied Biosystems) on an ABI 7300 qPCR instrument. Primer sequences and a more detailed experimental protocol are available on request.
Analysis of Gene Expression with Oligonucleotide Microarrays.
A549 cells were plated in 12-well plates at a density of 40–50 × 103 cells per well. Media conditions, polyamide treatment, time course, and dexamethasone stimulation were identical to the conditions described previously for qPCR and ChIP. Mifepristone was added at the same time as polyamide was added. RNA was isolated as previously described. The RNA was submitted to the Millard and Muriel Jacobs Gene Expression Facility at the California Institute of Technology, where labeled mRNA was hybridized to Affymetrix high-density Human Genome U133 Plus 2.0 arrays according to established protocols. Gene expression was analyzed using Resolver (Rosetta Biosoftware). Data were uploaded to the Gene Expression Omnibus (GEO) repository (accession no. GSE17307).
Supplementary Material
Acknowledgments.
We thank Keith Yamamoto for his kind gift of GR antibody. Mass spectrometry analyses were performed in the Spectrometry Laboratory of the Division of Chemistry and Chemical Engineering at the California Institute of Technology, supported in part by the National Science Foundation Materials Research Science and Engineering program. Oligonucleotide microarray experiments were performed in the Millard and Muriel Jacobs Genetics and Genomics Laboratory at the California Institute of Technology. This work was supported by National Institutes of Health Grant GM051747.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE17307).
This article contains supporting information online at www.pnas.org/cgi/content/full/0909192106/DCSupplemental.
References
- 1.Giguere V, Hollenberg SM, Rosenfeld MG, Evans RM. Functional domains of the human glucocorticoid receptor. Cell. 1986;46:645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- 2.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 3.McKay LI, Cidlowski JA. Cross-talk between nuclear factor-kappa B and the steroid hormone receptors: Mechanisms of mutual antagonism. Mol Endocrinol. 1998;12:45–56. doi: 10.1210/mend.12.1.0044. [DOI] [PubMed] [Google Scholar]
- 4.Heck S, et al. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J. 1994;13:4087–4095. doi: 10.1002/j.1460-2075.1994.tb06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappa B or activator protein-1: Molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 6.Newton R, Holden NS. Separating transrepression and transactivation: A distressing divorce for the glucocorticoid receptor? Mol Pharmacol. 2007;72:799–809. doi: 10.1124/mol.107.038794. [DOI] [PubMed] [Google Scholar]
- 7.Reichardt HM, et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 8.De Bosscher K, Vanden Berghe W, Haegeman G. Mechanisms of anti-inflammatory action and of immunosuppression by glucocorticoids: Negative interference of activated glucocorticoid receptor with transcription factors. J Neuroimmunol. 2000;109:16–22. doi: 10.1016/s0165-5728(00)00297-6. [DOI] [PubMed] [Google Scholar]
- 9.Hermoso M, Cidlowski J. Putting the brake on inflammatory responses: The role of glucocorticoids. IUBMB Life. 2003;55:497–504. doi: 10.1080/15216540310001642072. [DOI] [PubMed] [Google Scholar]
- 10.Wu W, et al. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004;64:1757–1764. doi: 10.1158/0008-5472.can-03-2546. [DOI] [PubMed] [Google Scholar]
- 11.Rogatsky I, et al. Target-specific utilization of transcriptional regulatory surfaces by the glucocorticoid receptor. Proc Natl Acad Sci USA. 2003;100:13845–13850. doi: 10.1073/pnas.2336092100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang JC, et al. Chromatin immunoprecipitation (ChIP) scanning identifies primary glucocorticoid receptor target genes. Proc Natl Acad Sci USA. 2004;101:15603–15608. doi: 10.1073/pnas.0407008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Wong J, Tsai SY, Tsai MJ, O'Malley BW. Progesterone and glucocorticoid receptors recruit distinct coactivator complexes and promote distinct patterns of local chromatin modification. Mol Cell Biol. 2003;23:3763–3773. doi: 10.1128/MCB.23.11.3763-3773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang JC, et al. Novel arylpyrazole compounds selectively modulate glucocorticoid receptor regulatory activity. Genes Dev. 2006;20:689–699. doi: 10.1101/gad.1400506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Bosscher K, Haegeman G. Latest perspectives on anti-inflammatory actions of glucocorticoids. Mol Endocrinol. 2009;23:281–291. doi: 10.1210/me.2008-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dervan PB, Edelson BS. Recognition of the DNA minor groove by pyrrole-imidazole polyamides. Curr Opin Struct Biol. 2003;13:284–299. doi: 10.1016/s0959-440x(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 17.Hsu CF, et al. Completion of a programmable DNA-binding small molecule library. Tetrahedron. 2007;63:6146–6151. doi: 10.1016/j.tet.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kielkopf CL, et al. A structural basis for recognition of A. T and T.A base pairs in the minor groove of B-DNA. Science. 1998;282:111–115. doi: 10.1126/science.282.5386.111. [DOI] [PubMed] [Google Scholar]
- 19.White S, Szewczyk JW, Turner JM, Baird EE, Dervan PB. Recognition of the four Watson-Crick base pairs in the DNA minor groove by synthetic ligands. Nature. 1998;391:468–471. doi: 10.1038/35106. [DOI] [PubMed] [Google Scholar]
- 20.Olenyuk BZ, et al. Inhibition of vascular endothelial growth factor with a sequence-specific hypoxia response element antagonist. Proc Natl Acad Sci USA. 2004;101:16768–16773. doi: 10.1073/pnas.0407617101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nickols NG, Jacobs CS, Farkas ME, Dervan PB. Modulating hypoxia-inducible transcription by disrupting the HIF-1-DNA interface. ACS Cheml Biol. 2007;2:561–571. doi: 10.1021/cb700110z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nickols NG, Dervan PB. Suppression of androgen receptor-mediated gene expression by a sequence-specific DNA-binding polyamide. Proc Natl Acad Sci USA. 2007;104:10418–10423. doi: 10.1073/pnas.0704217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lampson MA, Kapoor TM. Unraveling cell division mechanisms with small-molecule inhibitors. Nat Chem Biol. 2006;2:19–27. doi: 10.1038/nchembio757. [DOI] [PubMed] [Google Scholar]
- 24.Spencer DM, Wandless TJ, Schreiber SL, Crabtree GR. Controlling signal transduction with synthetic ligands. Science. 1993;262:1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- 25.Stockwell BR. Chemical genetics: Ligand-based discovery of gene function. Nat Rev Genet. 2000;1:116–125. doi: 10.1038/35038557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young RA. Biomedical discovery with DNA arrays. Cell. 2000;102:9–15. doi: 10.1016/s0092-8674(00)00005-2. [DOI] [PubMed] [Google Scholar]
- 27.Lee TI, et al. Transcriptional regulatory networks in Saccharomyces cerevisiae. Science. 2002;298:799–804. doi: 10.1126/science.1075090. [DOI] [PubMed] [Google Scholar]
- 28.Bolton EC, et al. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007;21:2005–2017. doi: 10.1101/gad.1564207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren B, et al. Genome-wide location and function of DNA binding proteins. Science. 2000;290:2306–2309. doi: 10.1126/science.290.5500.2306. [DOI] [PubMed] [Google Scholar]
- 30.Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 31.So AYL, Chaivorapol C, Bolton EC, Li H, Yamamoto KR. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet. 2007;3:927–938. doi: 10.1371/journal.pgen.0030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen J, Miner JN. The search for safer glucocorticoid receptor ligands. Endocr Rev. 2005;26:452–464. doi: 10.1210/er.2005-0002. [DOI] [PubMed] [Google Scholar]
- 33.Belitsky JM, Nguyen DH, Wurtz NR, Dervan PB. Solid-phase synthesis of DNA binding polyamides on oxime resin. Bioorg Med Chem. 2002;10:2767–2774. doi: 10.1016/s0968-0896(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 34.Nickols NG, Jacobs CS, Farkas ME, Dervan PB. Improved nuclear localization of DNA-binding polyamides. Nucleic Acids Res. 2007;35:363–370. doi: 10.1093/nar/gkl1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trauger JW, Dervan PB. Footprinting methods for analysis of pyrrole-imidazole polyamide/DNA complexes. Methods Enzymol. 2001;340:450–466. doi: 10.1016/s0076-6879(01)40436-8. [DOI] [PubMed] [Google Scholar]
- 36.Rozen SS, Helen J. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa, NJ: Humana; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.