Abstract
N-acetylaspartate (NAA) is found exclusively in neurons and their processes in the adult brain. Since the regional distribution of NAA may be imaged using magnetic resonance spectroscopic imaging (1H-MRSI), a regional measure of neuronal density may be noninvasively obtained. The technique may be particularly useful in the diagnosis of diseases where neurons are selectively injured, since these diseases do not result in definitive changes on conventional imaging studies. The goal of this study was to determine whether 1H-MRSI measurement of NAA detects neuronal loss following global ischemia. 1H-MRSI was performed in rats 24 h after global ischemia was induced by bilateral carotid occlusion plus hypotension.1−H-MRSI showed that NAA was decreased by 29–74% in vulnerable regions, including the cortex, striatum, hippocampus, and, to a lesser extent, the thalamus. No change was observed in the brain stem or cerebellum. Regions where 1H-MRSI observed NAA was decreased also had histological evidence of selective neuronal necrosis and showed marked increase of lactate and alanine. These results show that 1H-MRSI detected loss of NAA in brain regions with selective neuronal loss, suggesting that 1H-MRSI measurements of NAA could detect neuronal loss in a variety of disease states where there is selective neuronal necrosis.
Keywords: N-acetylasparte, magnetic resonance spectroscopy, global ischemia
INTRODUCTION
Magnetic resonance imaging (MRI) is of little utility in establishing the diagnosis or prognosis of the hypoxic-ischemic encephalopathy that occurs after cardiac arrest (1). To detect pathology, CT and MRI rely primarily on either changes in brain water content or disruption of the blood-brain barrier. Much of the water in the brain is in nonneuronal tissue. Furthermore, reactive gliosis may replace part of the water signal from lost neurons (2). Although atrophy occurs with neuronal loss, the presence of atrophy is neither a sensitive nor a specific measure of neuronal loss, especially in the elderly. Accordingly, CT and MRI have limited utility in diagnosing neurodegenerative diseases such as Alzheimer's and other diseases where neurons die selectively, such as hypoxic ischemic encephalopathy or status epilepticus (3–5).
The amino acid N-acetylaspartate (NAA) is thought to be present largely, if not exclusively, in the brain. Interest in NAA has increased because this metabolite produces the largest single peak on proton magnetic resonance spectroscopy of the brain and because there is mounting evidence that NAA is localized only in neurons in the adult brain. NAA is absent in glial tumors in vivo as well as in glial tumor extracts (6, 7). NAA is found only in neurons and not in astrocytes or oligodendrocytes in rat primary cell cultures, although it may also be found in oligodendrocyte precursor cells (8, 9). There is NAA immunoreactivity only in neuron cell bodies and axons in the adult brain, and NAA appears to be localized to all neuronal classes (10). Axonal injury results decreased NAA in target neurons, suggesting that NAA is transynaptically transported (11). NAA is decreased in a variety of human neurodegenerative diseases associated with neuronal loss (12–14). Therefore, it has been proposed that NAA is a marker of neuronal density or viability.
Despite considerable interest in the use of NAA as a marker of neuronal viability, relatively few experiments have been performed in intact animals to test the hypothesis that brain reductions in NAA concentrations are associated with histological evidence of selective neuronal loss. There have been many previous studies that have shown that there is decreased NAA after focal ischemia, brain tumors or after striatal kainate lesions (15–18) where there is death or displacement of both neurons and glia that is easily detectable by conventional imaging. These forms of injury do not mimic the selective neuronal loss that occurs in neurodegenerative diseases. Transient global cerebral ischemia mimics the hypoxicischemic encephalopathy that follows cardiac arrest and is an excellent model with which to study this question. Previous reports have demonstrated that transient global cerebral ischemia produces relatively selective neuronal damage; neurons in the hippocampus, striatum, and cortex are considered to be particularly vulnerable (5, 19, 20). Therefore, we investigated the effects of transient global cerebral ischemia in the rat on the distribution of brain NAA and other metabolites 24 h after the ischemia insult. Specifically, we tested the hypothesis that NAA is selectively lost from vulnerable “rostral” regions such as the cortex, hippocampus, and striatum and is spared in relatively resistant “caudal” regions, including the thalamus, brain stem, and cerebellum. Three-dimensional 1H-MRSI measurements were performed on the rat brain 24 h after global ischemia to determine the regional alterations of the 1H-MRSI NAA signal. The regional distribution of NAA changes was compared with histological correlates of neuronal damage.
MATERIALS AND METHODS
Surgical Preparation
Anesthesia was induced with 5% isoflurane in 10 fasted male Wistar rats, 280–320 g. Rats were intubated, ventilated with 30% 02/70% N02, and maintained under general anesthesia with 1% isoflurane. Both femoral arteries and a single femoral vein were cannulated. Blood pressure was monitored continuously, and arterial blood gases were analyzed hourly and maintained at physiologic values. The common carotid arteries (CCAs) were isolated via a midline neck incision. Animals were positioned in a stereotaxic instrument, and a 30 Ga needle temperature probe (Omega, Stamford, CT) was inserted into frontal muscle, and the temperature maintained at 37.2 ± 0.2° with a heating pad and a thermostat-regulated heating lamp and/or cooling fan. Bitemporal needle electrodes were placed for continuous EEC monitoring. All animal procedures were in accordance with the standards set forth by the NIH and were performed under a protocol approved by the Animal Subcommittee at the Department of Veterans of Affairs, San Francisco.
Induction of Ischemia
The experimental model of simultaneous bilateral carotid artery occlusion and systemic hypotension was used to induce severe forebrain ischemia (21). Thirty seconds before induction of ischemia. isoflurane was discontinued and the animal was paralyzed with pancuronium (1.0 mg/kg, intravenous). Atraumatic vascular clips were placed bilaterally on the CCAs, and approximately 10 cc blood was withdrawn from the femoral arterial catheter until the mean arterial pressure (MAP) was 45 mmHg. The onset of ischemia was marked by EEG isoelectricity in six of the animals, for which the subsequent analysis was performed. Withdrawn blood was heparinized with 100 I.U./10 cc and stored in a 37°C water bath. After 30 min of occlusion, the carotid clips were removed and the withdrawn blood was rapidly reinfused until the MAP > 100 mmHg. Protamine, 3 mg, was infused intravenously to prevent the occurrence of intracranial hemorrhage from excessive heparinization.
Histology
This long duration of ischemia produces severe histopathologic changes within 24 h (22). To evaluate histology at the 24-h time point a second group of eight rats were operated on as previously described and killed 24 h after ischemia. Rats were perfused with 500 cc of 4% buffered formaldehyde (FA); their brains were removed and stored in 4% FA for at least 24 h before processing for paraffin embedding. Six-micron sections were taken of paraffin-embedded brains and stained with cresyl violet. Slices were taken from the following four coordinates (relative to bregma): 1) 0.2 mm post, 2) 3.8 mm post, 3) 4.2 mm post, and 4) 4.4 mm post. Damage was assessed using a 5-point scale: 0 = no damage; 1 = 1–25% ischemic neurons (shrunken with pyknotic nuclei); 2 = 26–50% ischemic neurons; 3 = 51–75% ischemic neurons; 4 = 76–100% ischemic neurons, but no infarction; 5 = as in 4, but with gross infarction. In the anterior hippocampus, CAl, CA3, and dentate pyramidal neuronal loss were scored separately. The average score for these three regions was then used to represent the hippocampal region.
Magnetic Resonance Imaging
A QUEST 4400 imaging spectrometer (Nalorac, Martinez, CA) operating at 7T was used for collection of all MR data. A single-turn surface coil (2.2 × 1.7 cm) tuned for 1H (300 MHz) was used for both spin excitation and signal detection. The surface coil was fixed to an animal carrier designed to consistently position the brain of the animal at the isocenter of the magnet. The animal was carefully positioned with relation to the surface coil so that the signal intensity in axial slices through the brain would be relatively homogeneous. 1H-MRSI and MRI were measured at 24 h after the transient ischemia.
All RF pulses used for both MRI and 1H-MRSI sequences were B1-compensated to minimize variations of signal intensities due to the use of a surface coil for excitation. A BIR-4 pulse (23) was used for excitation with an effective tip angle of 45°, which permitted TR to be reduced to shorten the total acquisition time, while minimizing T1 weighting of the data. A symmetrized B1-compensated pulse was used for spin refocusing (24). In the MRI sequence, this pulse was used in conjunction with a linear gradient to achieve slice selection. In the 1H-MRSI sequence, this refocus pulse was centered 850 Hz upfield from the water resonance and combined with balanced gradient crushers for improved water suppression. The phase of the refocus pulse was cycled by 90° on alternate phase-encoding steps to move DC artifacts from the center to the edge of the processed image data.
MR scout images were collected using a T2-weighted spin echo sequence (TE80/TR1000). A 128 × 128 image, zero-filled to 256 × 256 for display, was obtained over a 3 × 3 cm FOV with a 3-mm slice thickness. Multislice MRI data sets were collected as single-slice serial acquisitions.
Three-dimensional 1H-MRSI was measured at 300 MHz using a water-suppressed spin echo pulse sequence with an echo time of 272 ms and a pulse recycle time of 1000 ms (25). Phase-encoding gradients were employed in 16 linear steps in all three spatial dimensions over a 3 × 2 × 3 cm field of view, yielding a nominal spatial resolution of 1.9 × 1.3 × 1.9 mm3. Spectroscopic imaging data were collected as symmetric spin echoes with 512 points and a sweep width of 4000 Hz. The total acquisition time was 136 intravenous. Typically, exponential multiplication (5 Hz equivalent) was applied to time domain data. Data sets were zero-filled to 32 points in the spatial domain and filtered using a Gaussian function (e−0.56k2) to reduce Gibbs ringing artifacts. Reconstructed data were examined with spectroscopic imaging display (SID) software developed in our laboratory (26). Images of individual metabolites were created, with pixel intensity proportional to the signal integrated over a user-defined chemical shift range.
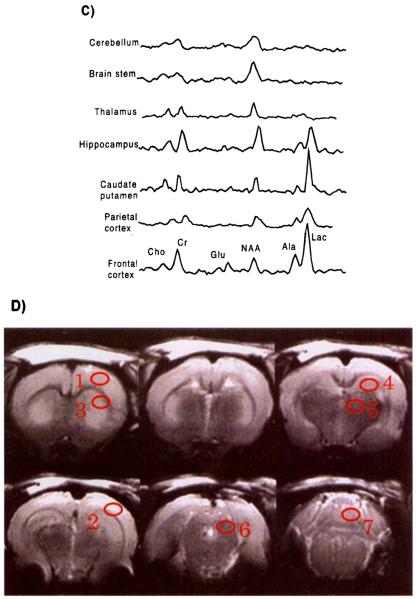
MRSI and MRI studies were conducted on 10 animals at 24 h. after induction of ischemia. In addition four control animals were studied with the same MR protocol. For all animals, spectra were selected from seven bilaterally symmetric regions of the brain, as indicated in Fig. 1D (14 total measurements for each animal). These selected regions were contained within the sensitive region of the surface coil, enabling spectra to be obtained with good signal-to-noise ratio from all regions. Metabolite integrals for total creatine at 3.03 ppm, choline at 3.24 ppm, NAA at 2.02 ppm, alanine at 1.48 ppm, and lactate at 1.34 ppm were then obtained by line-fitting with a Gaussian lineshape using NMR1 (Tripos Inc., St. Louis). Metabolite integrals at each location were then corrected for the inhomogeneous sensitivity profile of the surface coil by using average creatine integrals at each location obtained from the control animals. This compensation scheme assumes that total creatine MR visibility is not spatially dependent in the normal brain. In addition, to account for variation between studies, each data set was normalized using the NAA signal intensity obtained from the left brain stem, which is uninjured in this model.
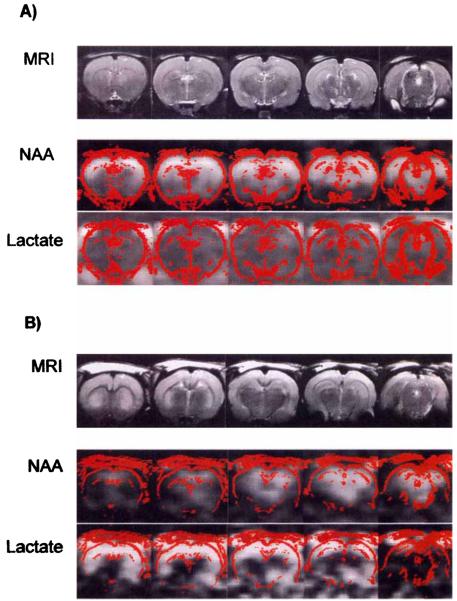
FIG. 1.
(A) Representative MRI and 1H-MRSI of control animal. T2-weighted MRIs are presented in the top row. The spatial distributions of signal intensity from the NAA and lactate/lipid spectral regions for image planes corresponding to the MRI planes are presented in the middle and bottom rows, respectively. The red outlines in the spectroscopic images represent a high-pass filter from the corresponding MRI, to provide spatial orientation. (B) Representative MRI and 1H-MRSI of the rat brain 24 h after global ischemia. T2-weighted MRIs are presented in the top row. The spatial distributions of signal intensity from the NAA and lactate/lipid spectral regions for image planes corresponding to the MRI planes are presented in the middle and bottom rows, respectively. The red outlines in the spectroscopic images represent a high-pass filter from the corresponding MRI. (C) Representative spectra from different brain regions obtained 24 h after global ischemia. Spectra were obtained from regions indicated on the MRI. Alanine and lactate are apparent in midfrontal and frontal brain regions but were not observed in caudal brain regions. (D) Coronal MRI sections illustrating regions from which representative spectra were obtained; 1 = anterior cortex, 2 = parietal cortex, 3 = caudate putamen, 4 = anterior hippocampus, 5 = thalamus, 6 = midbrain, 7 = cerebellum.
Metabolite integrals at each region in normal and postglobally ischemic animals were compared statistically using the Student's t-test for unpaired data, with a P value <0.01 considered significant.
RESULTS
Of the 10 animals studied, 6 demonstrated global ischemic injury while 4 showed evidence of focal injury and were therefore excluded from the study. Fig. 1 illustrates the MRI, NAA, and lactate images in representative control and global ischemia animals. There was a bright signal originating from the NAA peak in control brains that was substantially reduced in the rats imaged 24 h after severe ischemia. Lactate signal, on the other hand, was not present in the control brains but was increased after ischemia. MRIs were not appreciably different in the two groups, except that some ischemic brains had some diffuse increase in signal intensity in the cortex, as is evident in Fig. 1B. Note that the increased signal intensity seen at the surface is due to the surface coil reception profile. Figure 2 illustrates the mean changes in metabolite in various regions. In comparison with normal control animals, NAA signal (Fig. 2A) was decreased [P < 0.01) and lactate (Fig. 2B) was increased (P < 0.01) in the cortex, hippocampus, caudate, and thalamus, which represent the regions that are ischemic in this model. Increased alanine (Fig. 2C) was also observed where lactate was increased. There were no significant changes in NAA or lactate in the brain stem or cerebellum, regions that are supplied by the posterior circulation and are not ischemic. No significant differences were observed with creatine (Fig. 2D), and choline (Fig. 2E) was significantly different from normal controls (P < 0.05) only in the caudate-putamen region.
FIG. 2.
Mean signal integrals of metabolites (A-NAA, B-lactate, C-alanine, D-creatine, E-choline) in control (white bars) and 24-h postischemic rats (black bars) by region. Fcx,= frontal cortex; Pcx, parietal cortex; Cp, caudate-putamen; Hi, hippocampus; Thl, thalamus; Stem, brain stem; Cb, cerebellum. Error bars represent standard errors of the mean. *P < 0.05, **P < 0.01 difference between control and ischemic brain.
Figure 3 summarizes the results of scoring the neuronal injury in these regions. The most severe neuronal loss was in the cortex and caudate-putamen, the regions with the largest decrease in NAA signal. Although there was almost complete loss of neurons in CA1, there was little or no loss of neurons in the other hippocampal regions within the hippocampal voxel, resulting in a medium damage score of 2, correlating with the less severe spectroscopic depletion of NAA in that region. There was either no damage or modest damage (1-2 scores) in the thalamus, in keeping with the slight, yet still significant, changes in NAA in that region. The cerebellum and brain stem were not examined in this study, but these structures are supplied exclusively by the basilar artery and, thus, would not be expected to be ischemic in this model. Histological changes have not been observed in these regions in this model (21).
FIG. 3.
Histogram of neuronal injury scores by region. 0 = no damage; 1 = 1-25% ischemic neurons (shrunken with pyknotic nuclei); 2 = 26-50% ischemic neurons; 3 = 51- 75% ischemic neurons; 4 = 76-100% ischemic neurons, but no infarction; 5 = as in 4, but with gross infarction. Box indicates medium neuronal damage score. • indicates score from an individual animal, left or right side.
DISCUSSION
The major findings of this study were: 1) The 1H-MRSI NAA signal was reduced 24 h after global ischemia in regions where there was selective neuronal necrosis, i.e., the cortex, hippocampus, caudate, and thalamus; 2) There was increased lactate signal in these same regions; 3) There were no changes of NAA or lactate in regions where there was little or no neuronal necrosis, i.e., the brain stem or cerebellum; 4) 1H-MRSI changes were detected in regions where there were no easily discernible MRI changes; thus, 1H-MRSI may be more useful than conventional MRI imaging in detecting neuronal loss in some disease states.
Reduced NAA
The finding of reduced NAA in this study is best explained by decreased production of NAA in neurons and loss of NAA from damaged neurons. These data confirm that there is relatively selective neuronal necrosis in regions where the NAA signal is reduced. Immunohistochemical and tissue-culture data suggest that NAA is produced exclusively by neurons in the adult brain (8-10). Furthermore, acetyl-CoA-l-aspartate-N-acetyltransferase, the synthetic enzyme for NAA, is localized in mitochondria (27). A wealth of data support the hypothesis that the action of excitatory amino acids underlies, at least in part, ischemic neuronal injury and that mitochondrial dysfunction may be an important mechanism by which excitotoxicity is expressed (28). Thus, decreased production of NAA by the mitochondrial enzyme acetyl-CoA-l-aspartate-N-acetyltransferase after ischemia due either to mitochondrial dysfunction or neuronal death is the most likely explanation for decreased NAA after global ischemia.
It is less likely that increased catabolism is the primary event that leads to decreased NAA after global ischemia. Acetoaspartase, the degradative enzyme for NAA, has recently been identified as the enzyme deficiency in Canavan's disease (29). The observation that the pathology in Canavan's disease includes abnormal astrocytes and dysmyelination strongly suggests that acetoaspartase is glial. In vivo immunohistochemical and glial cell culture measurements of NAA suggest that NAA concentrations are very low in glia (9, 10). Thus, NAA may be stored in neurons, released, then rapidly taken up and degraded in glia. While the primary event in global ischemia is selective neuronal injury resulting in decreased NAA production, glia are relatively spared (4). Thus, degradation of NAA in glia may be unaffected by ischemia, contributing to the decline in whole-brain NAA levels.
Increased Lactate
The second finding of this study is the presence of increased lactate signal 24 h after global ischemia. Although lactic acidosis is an ubiquitous feature of ischemia, its presence after restoration of blood flow after global ischemia is surprising since there is rapid restoration of oxidative metabolism and high energy intermediates (30). Thus, lactic acidosis may be the result of other mechanisms in the postischemic brain.
Mitochondrial damage induced by excitatory amino acids is one possible mechanism. The excitatory amino acid glutamate is released during ischemia, resulting in elevations of neuronal intracellular calcium via activation of receptor-gated calcium channels (31, 32). Increased intracellular calcium may be toxic to neurons via several mechanisms, including the disruption of oxidative respiration via mitochondrial damage and direct inhibition of oxidative enzymes (33, 34). Increased brain lactate has been observed in other excitotoxic diseases (35). Another explanation for the increased lactate signal after ischemia is that alkalosis is produced by the “luxury perfusion” seen after ischemia. Alkalosis could also increase neuronal lactate activation by increasing the activity of phosphofructokinase (36). Whatever the mechanism resulting in increased lactate after global ischemia, the increased lactate signal is an additional means for localizing abnormal tissue with 1H-MRSI. However, since lactic acid may also be produced in other pathological conditions such as brain neoplasia (37), the specificity of the finding of increased lactate may be limited.
Comparison with Other Imaging Techniques
1H-MRSI detects alterations in metabolites other than water. Since these metabolites are much less plentiful than water, the signal-to-noise ratio and spatial resolution of 1H-MRSI are less than with conventional imaging techniques. Thus, 1H-MRSI may be best suited to diseases in which there are diffuse changes in brain metabolites, while conventional MRI is better suited for detecting focal structural lesions such as stroke or neoplasia. This study demonstrates that decreases in NAA signal parallel regional histologic measures of diffuse neuronal loss in the absence of specific MRI changes. Thus, the 1H-MRSI NAA signal may be a sensitive way of detecting neuronal loss in patients after global ischemia, as was illustrated by a case report of decreased 1H-MRSI NAA in a patient with severe hypoxic-ischemic encephalopathy (38). These data also support the utility of 1H-MRSI in other diseases where there is diffuse selective neuronal loss, such as hypoglycemic coma, status epilepticus, and neurodegenerative diseases such as Alzheimer's and Huntington's diseases and HIV dementia (12–14, 39–43).
Limitations
While these data support the hypothesis that 1H-MRSI NAA measurements may be a means of imaging neuronal loss, there are a number of limitations to this conclusion: 1) Changes in the relaxation time of NAA itself may also change the amplitude of the 1H-MRSI NAA signal; 2) There are other N-acetyl-containing compounds in the brain, including N-acetylaspartylglutamate, that may contribute to the NAA signal; 3) The signal to noise ratio of MRSI data is frequently relatively low, and there is in addition some variability of NAA concentrations in the normal brain, which may limit the sensitivity of 1H-MRSI NAA measurements in detecting mild degrees of neuronal loss (44); 4) The metabolic role of NAA in the brain is not known, and factors other than neuronal viability may also determine brain NAA concentration. Some neurotropic drugs have been reported to alter brain NAA concentrations by as much as 20% (45). Furthermore, metabolic changes may decrease brain NAA, albeit modestly, perhaps via depletion of aspartate stores (46).
The image contrast in the MRI acquisition performed in this study was primarily sensitive to T2-weighting, although some T1-weighting will also be present. MRI tissue contrast is known to decrease at higher field strengths, and the MRI findings may differ at field strengths lower than the 7 Tesla used in this study. Nevertheless, our findings are in agreement with human studies performed at 0.02 T (1).
This study compared changes of metabolites with ischemia in different regions of the brain. Three factors affect the sensitivity to observe these changes in a spatially dependent manner: 1) the sensitivity profile of the surface-coil used for signal detection, 2) changes in field inhomogeneity in different regions of the brain, for example, broader linewidths can be seen in Fig. 1C for the cerebellum and brain stem, and 3) the limited spatial resolution relative to the size of each brain region, as well as out-of-voxel contamination (Gibbs ringing) associated with the MRSI data. However, comparison of the standard deviations of the resultant data, for example, in the creatine and choline data shown in Fig. 2, would indicate that in this study there is no significant difference between the different brain regions.
Further work will be required to address these limitations. 1) Technical improvements in 1H-MRSI, such as shortened echo times and development of quantitative techniques, will lessen the effect of relaxation time changes on metabolite signal amplitude (47). Furthermore, little is known about the effect of many disease states on NAA relaxation times. 2) Extract studies of rat brain suggest that NAA contributes about 80% of the 1H-MRSI NAA signal (48), but further study is required to determine what the other components of the signal are and whether they change after ischemia. 3) While there is some variability in elderly controls, significant differences in NAA/choline and NAA/Cr ratios in Alzheimer's patients and elderly controls have been reported (40). 4) More studies in animal and human disease states are required to determine the specificity of NAA changes for neuronal loss. A better understanding of NAA role and regulation in the normal brain would facilitate our understanding of its alterations in disease states.
Despite these limitations, our data support the hypothesis that 1H-MRSI measurements of NAA and other metabolites may be useful in detecting selective neuronal loss in hypoxic-ischemic encephalopathy. 1H-MRSI NAA measurements may be useful in detecting neuronal loss in diseases in which there is selective neuronal loss without MRI and CT correlates.
ACKNOWLEDGMENT
The authors thank Dr. Jingquan Lan (Department of Neurology, University of California and DVA Medical Center) for assisting in the histological procedures.
This work was supported by PHS grants CA48815 (A.A.M.) and DK33293 (M.W.W.), the Veterans Administration Medical Research Service (M.W.W., S.H.G.), and Rehabilitation Research and Development Service (S.H.G.).
REFERENCES
- 1.Roine RO, Raininko R, Erkinjuntti T, Ylikoski A, Kaste M. Magnetic resonance imaging findings associated with cardiac arrest. Stroke. 1993;24:1005–1014. doi: 10.1161/01.str.24.7.1005. [DOI] [PubMed] [Google Scholar]
- 2.Schechter R, Yen SH, Terry RD. Fibrous Astrocytes in senile dementia of the Alzheimer type. J. Neuropathol. Exp. Neurol. 1981;40:95–101. doi: 10.1097/00005072-198103000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Smith ML, Auer RN, Siesjo BK. The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia. Acta Neuropathol. 1984;64:319–332. doi: 10.1007/BF00690397. [DOI] [PubMed] [Google Scholar]
- 4.Auer RN, Siesjo BK. Biological differences between ischemia, hypoglycemia, and epilepsy. Ann. Neurol. 1988;24:699–707. doi: 10.1002/ana.410240602. [DOI] [PubMed] [Google Scholar]
- 5.Brierley JB, Meldrum BS, Brown AW. The threshold and neuropathology of cerebral “anoxic-ischemic” cell change. Arch. Neurol. 1973;29:367–374. doi: 10.1001/archneur.1973.00490300029003. [DOI] [PubMed] [Google Scholar]
- 6.Nadler JV, Cooper JR. Metabolism of the aspartyl moiety of N-acetyl-l-aspartic acid in the rat brain. J. Neurochem. 1972;19:2091–2105. doi: 10.1111/j.1471-4159.1972.tb05119.x. [DOI] [PubMed] [Google Scholar]
- 7.Peeling J, Sutherland G. High-resolution 1H NMR spectroscopy studies of extracts of human cerebral neoplasms. Magn. Reson. Med. 1992;24:123–136. doi: 10.1002/mrm.1910240113. [DOI] [PubMed] [Google Scholar]
- 8.Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J. Neurosci. 1993;13:981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urenjak J, Williams SR, Gadian DG, Noble M. Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro. J. Neurochem. 1992;59:55–61. doi: 10.1111/j.1471-4159.1992.tb08875.x. [DOI] [PubMed] [Google Scholar]
- 10.Moffett JR, Namboodiri MA, Neale JH. Enhanced carbodiimide fixation for immunohistochemistry: application to the comparative distributions of N-acetylaspartylglutamate and N-acetylaspartate immunoreactivities in rat brain. J. Histochem. Cytochem. 1993;41:559–570. doi: 10.1177/41.4.8450195. [DOI] [PubMed] [Google Scholar]
- 11.Rango M, Spagnoli D, Tomei G, Bamonti F, Scarlato G, Zetta L. Central nervous system trans-synaptic effects of acute axonal injury: a 1H magnetic resonance spectroscopy study. Magn. Reson. Med. 1995;33:595–600. doi: 10.1002/mrm.1910330503. [DOI] [PubMed] [Google Scholar]
- 12.Dunlop DS, Mc Hale DM, Lajtha A. Decreased brain N-acetylaspartate in Huntington's disease. Brain Res. 1992;580:44–48. doi: 10.1016/0006-8993(92)90925-y. [DOI] [PubMed] [Google Scholar]
- 13.Meyerhoff DJ, MacKay S, Bachman L, Poole N, Dillon WP, Weiner MW, Fein G. Reduced brain N-acetylaspartate suggests neuronal loss in cognitively impaired human immunodeficiency virus-sero-positive individuals: in vivo 1H magnetic resonance spectroscopic imaging. Neurology. 1993;43:509–515. doi: 10.1212/wnl.43.3_part_1.509. [DOI] [PubMed] [Google Scholar]
- 14.Klunk WE, Panchalingam K, Moossy J, McClure RJ, Pettegrew JW. N-acetyl-l-aspartate and other amino acid metabolites in Alzheimer's disease brain: a preliminary proton nuclear magnetic resonance study. Neurology. 1992;42:1578–1585. doi: 10.1212/wnl.42.8.1578. [DOI] [PubMed] [Google Scholar]
- 15.Guimaraes AR, Schwartz P, Prakash MR, Carr CA, Berger UV, Jenkins BG, Coyle JT, Gonzalez RG. Quantitative in vivo H-1 nuclear magnetic resonance spectroscopic imaging of neuronal loss in rat brain. Neuroscience. 1995;69:1095–1101. doi: 10.1016/0306-4522(95)00300-8. [DOI] [PubMed] [Google Scholar]
- 16.Sager TN, Laursen H, Hansen AJ. Changes in N-acetyl-aspartate content during focal and global brain ischemia of the rat. J. Cereb. Blood Flow Metab. 1995;15:639–646. doi: 10.1038/jcbfm.1995.79. [DOI] [PubMed] [Google Scholar]
- 17.Mathews VP, Barker PB, Blackband SJ, Chatham JC, Bryan RN. Cerebral metabolites in patients with acute and subacute strokes: concentrations determined by quantitative proton MR spectroscopy. AJR Am. J. Roentgenol. 1995;165:633–638. doi: 10.2214/ajr.165.3.7645484. [DOI] [PubMed] [Google Scholar]
- 18.Barker PB, Blackband SJ, Chatham JC, Soher BJ, Samphilipo MA, Magee CA, Hilton JD, Strandberg JD, Anderson JH. Quantitative proton spectroscopy and histology of a canine brain tumor model. Magn. Reson. Med. 1993;30:458–464. doi: 10.1002/mrm.1910300408. [DOI] [PubMed] [Google Scholar]
- 19.Kirino T, Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984;62:201–208. doi: 10.1007/BF00691853. [DOI] [PubMed] [Google Scholar]
- 20.Swan JH, Evans MC, Meldrum BS. Long-term development of selective neuronal loss and the mechanism of protection by 2-amino-7-phosphonoheptanoate in a rat model of incomplete forebrain ischaemia. J. Cereb. Blood Flow Metab. 1988;8:64–78. doi: 10.1038/jcbfm.1988.9. [DOI] [PubMed] [Google Scholar]
- 21.Nordstrom CH, Siesjo BK. Effects of phenobarbital in cerebral ischemia. Part I: cerebral energy metabolism during pronounced incomplete ischemia. Stroke. 1978;9:327–335. doi: 10.1161/01.str.9.4.327. [DOI] [PubMed] [Google Scholar]
- 22.Gaspary HL, Simon RP, Graham SH. BW10O3C87 and NBQX but not CGS19755 reduce glutamate release and cerebral ischemic necrosis. Eur. J. Pharmacol. 1994;262:197–203. doi: 10.1016/0014-2999(94)90733-1. [DOI] [PubMed] [Google Scholar]
- 23.Staewen RS, Johnson AJ, Ross BD, Parrish T, Merkle H, Garwood M. 3-D FLASH imaging using a single surface coil and a new adiabatic pulse. Invest. Radiol. 1990;25:559–567. doi: 10.1097/00004424-199005000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Conolly S, Glover G, Nishimura D, Macovski A. A reduced power selective adiabatic spin-echo pulse sequence. Magn. Reson. Med. 1991;18:28–38. doi: 10.1002/mrm.1910180105. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez EJ, Maudsley AA, Higuchi T, Weiner MW. Three-dimensional 1H spectroscopic imaging of cerebral metabolites in the rat using surface coils. Magn. Reson. Imaging. 1992;10:965–974. doi: 10.1016/0730-725x(92)90451-5. [DOI] [PubMed] [Google Scholar]
- 26.Maudsley AA, Lin E, Weiner MW. Spectroscopic imaging display and analysis. Magn. Reson. Imaging. 1992;10:471–485. doi: 10.1016/0730-725x(92)90520-a. [DOI] [PubMed] [Google Scholar]
- 27.Alonso E, Garcia-Perez A, Bueso J, Rubio V. N-acetyl-l-glutamate in brain: assay, levels, and regional and subcellular distribution. Neurochem. Res. 1991;16:787–794. doi: 10.1007/BF00965688. [DOI] [PubMed] [Google Scholar]
- 28.Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxicischemic neuronal death. Annu. Rev. Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 29.Matalon R, Kaul R, Casanova J, Michals K, Johnson A, Rapin I, Gashkoff P, Deanching M, SSIEM Award Aspartoacylase deficiency: the enzyme defect in Canavan disease. J. Inherit. Metab. Dis. 1989;12(S):329–331. doi: 10.1007/BF03335413. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu H, Graham SH, Chang LH, Mintorovitch J, James TL, Faden AI, Weinstein PR. Relationship between extracellular neurotransmitter amino acids and energy metabolism during cerebral ischemia in rats monitored by microdialysis and in vivo magnetic resonance spectroscopy. Brain Res. 1993;605:33–42. doi: 10.1016/0006-8993(93)91353-t. [DOI] [PubMed] [Google Scholar]
- 31.Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J. Neurochem. 1984;43:1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- 32.Choi DW. Ionic dependence of glutamate neurotoxicity. J. Neurosci. 1987;7:369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon RP, Griffiths T, Evans MC, Swan JH, Meldrum BS. Calcium overload in selectively vulnerable neurons of the hippocampus during and after ischemia: an electron microscopy study in the rat. J. Cereb. Blood Flow Metab. 1984;4:350–361. doi: 10.1038/jcbfm.1984.52. [DOI] [PubMed] [Google Scholar]
- 34.Vlessis AA, Widener LL, Bartos D. Effect of peroxide, sodium, and calcium on brain mitochondrial respiration in vitro: potential role in cerebral ischemia and reperfusion. J. Neurochem. 1990;54:1412–1418. doi: 10.1111/j.1471-4159.1990.tb01977.x. [DOI] [PubMed] [Google Scholar]
- 35.Beal MF, Hyman BT, Koroshetz W. Do defects in mitochondrial energy metabolism underlie the pathology of neurodegenerative diseases? Trends Neurosci. 1993;16:125–131. doi: 10.1016/0166-2236(93)90117-5. [DOI] [PubMed] [Google Scholar]
- 36.Hugg JW, Duijn JH, Matson GB, Maudsley AA, Tsuruda JS, Gelinas DF, Weiner MW. Elevated lactate and alkalosis in chronic human brain infarction observed by 1H and 31P MR spectroscopic imaging. J. Cereb. Blood Flow Metab. 1992;12:734–744. doi: 10.1038/jcbfm.1992.104. [DOI] [PubMed] [Google Scholar]
- 37.Remy C, Arus C, Ziegler A, Lai ES, Moreno A, Le Fur Y, Decorps M. In vivo, ex vivo, and in vitro one- and two-dimensional nuclear magnetic resonance spectroscopy of an intracerebral glioma in rat brain: assignment of resonances. J. Neurochem. 1994;62:166–179. doi: 10.1046/j.1471-4159.1994.62010166.x. [DOI] [PubMed] [Google Scholar]
- 38.Graham SH, Meyerhoff D, Bayne L, Weiner MW. Magnetic resonance spectroscopy of N-acetylasparate in hypoxic-ischemic encephalopathy. Ann. Neurol. 1994;35:490–494. doi: 10.1002/ana.410350420. [DOI] [PubMed] [Google Scholar]
- 39.Longo R, Giorgini A, Magnaldi S, Pascazio L, Ricci C. Alzheimer's disease histologically proven studied by MRI and MRS: two cases. Magn. Reson. Imaging. 1993;11:1209–1215. doi: 10.1016/0730-725x(93)90249-d. [DOI] [PubMed] [Google Scholar]
- 40.Meyerhoff DJ, MacKay S, Constans JM, Norman D, Van Dyke C, Fein G, Weiner MW. Axonal injury and membrane alterations in Alzheimer's disease suggested by in vivo proton magnetic resonance spectroscopic imaging. Ann. Neurol. 1994;36:40–47. doi: 10.1002/ana.410360110. [DOI] [PubMed] [Google Scholar]
- 41.Ebisu T, Rooney WD, Graham SH, Weiner MW, Maudsley AA. N-acetylaspartate as an in vivo marker of neuronal viability in kainate-induced status epilepticus: 1-H magnetic resonance spectroscopic imaging. J. Cerebr. Blood Flow Metabol. 1994;14:373–382. doi: 10.1038/jcbfm.1994.48. [DOI] [PubMed] [Google Scholar]
- 42.Cendes F, Andermann F, Preul MC, Arnold DL. Lateralization of temporal lobe epilepsy based on regional metabolic abnormalities in proton magnetic resonance spectroscopic images. Ann. Neurol. 1994;35:211–216. doi: 10.1002/ana.410350213. [DOI] [PubMed] [Google Scholar]
- 43.Hugg JW, Laxer KD, Matson GB, Maudsley AA, Weiner MW. Neuron loss localizes human temporal lobe epilepsy by in vivo proton magnetic resonance spectroscopic imaging. Ann. Neurol. 1993;34:788–794. doi: 10.1002/ana.410340606. [DOI] [PubMed] [Google Scholar]
- 44.Tallan HH, Moore S, Stein SH. N-acetyl-l-aspartic acid in brain. J. Biochem. 1956;219:257–264. [PubMed] [Google Scholar]
- 45.Mcintosh JC, Cooper JR. Function of N-acetyl aspartic acid in brain: Effect of certain drugs. Nature. 1964;203:658. doi: 10.1038/203658a0. [DOI] [PubMed] [Google Scholar]
- 46.deGraaf AA, Deutz NE, Bosman DK, Chamuleau RA, de Haan JG, Bovee WM. The use of in vivo proton NMR to study the effects of hyperammonemia in the rat cerebral cortex. NMR Biomed. 1991;4:31–37. doi: 10.1002/nbm.1940040106. [DOI] [PubMed] [Google Scholar]
- 47.Husted CA, Duijn JH, Matson GB, Maudsley AA, Weiner MW. Molar quantitation of in vivo proton metabolites in human brain with 3D magnetic resonance spectroscopic imaging. Magn. Reson. Imaging. 1994;12:661–667. doi: 10.1016/0730-725x(94)92461-9. [DOI] [PubMed] [Google Scholar]
- 48.Burri R, Bigler P, Straehl P, Posse S, Colombo JP, Herschkowitz N. Brain development: 1H magnetic resonance spectroscopy of rat brain extracts compared with chromatographic methods. Neurochem. Res. 1990;15:1009–1016. doi: 10.1007/BF00965747. [DOI] [PubMed] [Google Scholar]