CD4+FoxP3+ regulatory T cells play an important regulatory mission with regard to the generation of peripheral immune responses. Recognition of this involvement has engendered enthusiasm for the development of new strategies to attempt to influence immune responses in clinically important settings. In the case of cancer or viral insult, diminution of Treg numbers and / or blocking their function provide promising approaches to enhance anti-tumor / pathogen immunity. Alternatively, augmenting Treg numbers and / or function is now envisioned as a potential strategy to down-regulate autoimmune responses and to facilitate the establishment of tolerance to allogeneic transplantation antigens. Key elements for any such proposed strategies are reagents which can effectively impact the Treg compartment. Theoretically, manipulating Treg cells in vitro as well as in vivo would elevate the likelihood of clinical success dependent on the intended purpose. Approaches to activate and expand murine and human natural Treg cells ex-vivo are now well established. Most involve the use anti-CD3mab plus anti-CD28 co-stimulation together with IL-2 and more recently, rapamycin has shown the capacity to further select for Treg cell expansion.1–6 Both animal as well as human Treg cell cultures generally respond to these types of protocols and while there has been significant variation in the overall expansion reported, the general consensus is that over a 1–2 week time interval, several hundred fold increases in recovery following anti-CD3/CD28 + IL-2 is not unreasonable.1,7,8 In addition to direct antibody ‘targeting’ of Treg cells in vitro, the use of allogeneic APC populations has also been reported to expand Treg numbers ex-vivo, and following in vivo administration, these Tregs expressed functional activity including tolerance induction.9–11 Interestingly, rapamycin treatment of myeloid derived DC diminished MHC class II and B7 expression resulting in poor allogeneic Tconv stimulation while enriching for functional Treg cells.5 A fundamental issue beginning to be understood is the precise nature of the ex-vivo expanded Treg cells including their overall functional capabilities. To date there have been few studies carefully assessing the relative regulatory capacity of in vitro expanded vs. fresh in vivo populations in individual well defined models. One study reported that anti-CD3/CD28 mAb bead driven in vitro expanded of TCR transgenic Treg cells enhanced their functional in vivo activity while another using anti-CD3/CD28mAb beads examining polyclonally activated allogeneic Tregs in a GVHD model reported that greater numbers of in vitro expanded vs. fresh Tregs were needed to induce comparable levels of suppression.12,13 The winged-helix family transcription factor FoxP3 is not only a marker for Treg cells, but is also important in programming the regulatory function of these cells.14,15 Some studies have reported a decrease in the level of FoxP3 expression following in vitro expansion of several Treg populations which may reflect epigenetic regulation.16–18 Thus, the decreased functional capacity exhibited by some ex-vivo expanded Treg cells could reflect their diminished FoxP3 levels.
In addition to in vitro manipulation of Treg cells, control of these cells in situ remains a major objective of the field. In situ reduction strategies are potentially powerful and several approaches aimed at deleting Tregs have shown at least partial success. For example, the administration of anti-IL2R abs and the infusion of IL2-DPT, i.e. diftitoxin) have induced significant diminishment of human and murine peripheral Treg levels.19–23 However, because these reagents target surface CD25 expression, such approaches can only ablate the CD4+FoxP3+ compartment in the range of 50–70% as CD25−FoxP3+ cells cannot be deleted using these strategies. Consequently, the remainder of the Treg compartment together with the rapid rebound of the non-deleted regulatory cells to normal Treg levels (several days) complicates interpretation in these types of studies.24
In contrast to in situ Treg deletion, Hunig and colleagues first reported the ability to expand Tregs in vivo by targeting CD28 in rats.25 Using a superagonistic anti-CD28 ab, Treg cells were found to be preferentially expanded over other T cell subsets, on the order of a 20x increase of lymph node Tregs within 3 days of infusion.25 Use of a murine anti-CD28 mab in an allogeneic BMT model resulted in increased numbers of donor Tregs in recipient lymph nodes associated with protection from acute GVHD.26 A number of groups have used DC based protocols to expand alloantigen and conventional antigen reactive Treg cells in situ increasing enthusiasm towards regulating transplantation responses.5,10,27 Interestingly, not only have rapamycin treated DC shown promise in this regard, but RAPA itself has also been found to promote expansion of FoxP3 Tregs which in the context of allogeneic transplants may promote transplant antigen specific Tregs.28 Still other protocols including anti-CTLA4 ab treatment blockade and the infusion of intravenous immunoglobulin have also reportedly expanded Treg cells in situ.29–31
More recently, the use of IL-2 based strategies has generated further enthusiasm for in vivo strategies to facilitate Treg expansion. Boyman and colleagues reported that the infusion of anti-IL-2 / IL-2 cytokine complexes can stimulate rapid and large scale expansion of CD4+CD25+ T cells in situ.32 A single anti-IL2mab clone (JES6-1A12) complexed to IL-2 was found to effectively target and expand CD4+CD25+ T cells in vivo.32
We have found that within a few days following the final infusion of such complexes, Treg levels rapidly return to normal, further highlighting the stringent physiological regulation of this compartment.33,34 The ability to augment Treg cell numbers in situ is clearly attractive from the perspective of transplantation tolerance induction. Studies from our laboratory have recently employed complex administration (IL2/anti-IL-2 complex = IAC) to manipulate endogenous Treg cells in recipients following MHC-matched allogeneic hematopoietic progenitor cell transplants.33 Interestingly, IAC infusion was found to target residual host Treg cells remaining following sub-lethal TBI conditioning34 resulting in their rapid and marked expansion within the first 7–10 days post-transplant. Examination of the host vs. graft (HVG) response in these reduced intensity conditioned recipients demonstrated that such immunity was efficiently blocked by this IAC infusion which was accompanied by the rapid and efficient engraftment of allogeneic T cell depleted marrow grafts.33 Thus, with respect to BMT, these observations suggested that 1) following reduced intensity conditioning and BMT, surviving host Tregs present can be stimulated and expanded by infusion of these complexes and retain in vitro suppressive function (unpublished, MG, AS, RL) and 2) in situ manipulation of Treg cells is a viable approach to regulate allo-immunity post-transplant.
An important benefit of such an in vivo approach is the circumvention of the need to isolate, expand and harvest Treg cells from cultures prior to their application in the transplant setting. A number of additional manipulations in recipients can be envisioned to strengthen such Treg mediated regulation and facilitate engraftment with the objective of alloantigen tolerance induction. For example, in vitro and in vivo studies have observed that in the presence of co-stimulatory signal blockade, Treg cells appear to retain their functional capacity.35,36 Thus, interfering with co-stimulatory signals between donor APC and host T cells (e.g. use of rapamycin, CTLA-4 blockade, etc.) to further ‘weaken’ allo-responsiveness post-transplant combined with expanding the Treg compartment may provide a heightened and more potent suppressive environment for hematopoietic engraftment and tolerance induction. It is tempting to consider that while polyclonal expansion of Tregs with IAC likely ensues in our transplant model, administration of IAC following alloantigen (i.e. post-BMT) infusion may also result in the expansion of Treg cells with allo-specific TCR. While we do not as yet know if such Tregs are generated by this protocol, an increase in Treg cells with anti-donor antigen specificity could further strengthen their ability to inhibit conventional host T cells responding to donor alloantigen via direct antigen stimulation on donor APC following transplant. Notably, the administration of cytokine / antibody complexes in non-transplant settings is also being examined. A recent study reported that the infusion of IL2 / anti-IL2 complexes both prior to airway challenge or therapeutically following airway inflammation augmented FoxP3+ as well as other regulatory T cell (i.e. TR1) populations and resulted in significant reduction in airway pathology in an experimental airway allergy model.37 Finally, an intriguing observation following 2–3 IAC infusions was the finding that CD25 levels were markedly enhanced on CD4+CD25+ T cells with minimal alteration of FoxP3 levels, which contrasts the reported diminution in some in vitro systems noted above.33 What is presently unknown however, is whether sustained in vivo IAC administration may lead to prolonged activation / expansion of Tregs and any down regulation of their FoxP3 expression and functional capability. The elevation of high affinity IL-2R expression thus provides a potential target for cytokine, i.e. IL-2 driven stimulation and the in situ expansion of Tregs. Indeed, the addition of IL-2 in vitro to IAC stimulated Tregs resulted in driving high levels of proliferation compared to freshly isolated Tregs from non-IAC treated animals.33 Such observations suggest that infusion of relatively low amounts of IL-2 in vivo following even a single pulse of IAC may be capable of driving and maintaining Treg expansion, for example during the initial phases of alloreactivity, thus providing an alternative to multiple complex injections. Interestingly, several investigations have demonstrated the capacity of IL-2 to expand human Tregs in vivo in cancer, autoimmune and lymphopenic environments and an intriguing recent study noted that low dose IL-2 infusions can expand FoxP3+ Treg cells in allogeneic HCT recipients of donor CD4+ T cell infusions.38–42 Thus, we speculate that in situ activation of Tregs under these conditions could conceivably enhance their responsiveness to low dose IL-2.
It is interesting to speculate that once Tregs have become activated, other molecules may be capable of providing ‘targets’ to expand and regulate the functioning of these cells. For example, the up-regulation of 41BB expression on Tregs in response to IL-2 enabled their effective in vitro expansion with a soluble 4-1BBL reagent.43 Recent studies examining Treg cells following allogeneic HCT proposed that IL-4 produced by NKT cells was responsible for expanding donor Treg cells in vivo post-transplant and seminal plasma has been proposed to be associated with expansion of the CD4+CD25+ Treg pool contributing to maternal immune tolerance during pregnancy.44,45 Clearly, the development of reagents which can target and stimulate Treg cells in situ will provide additional opportunities to harness this compartment for the augmentation of ‘wanted’ or suppression of ‘unwanted’ immune responses.
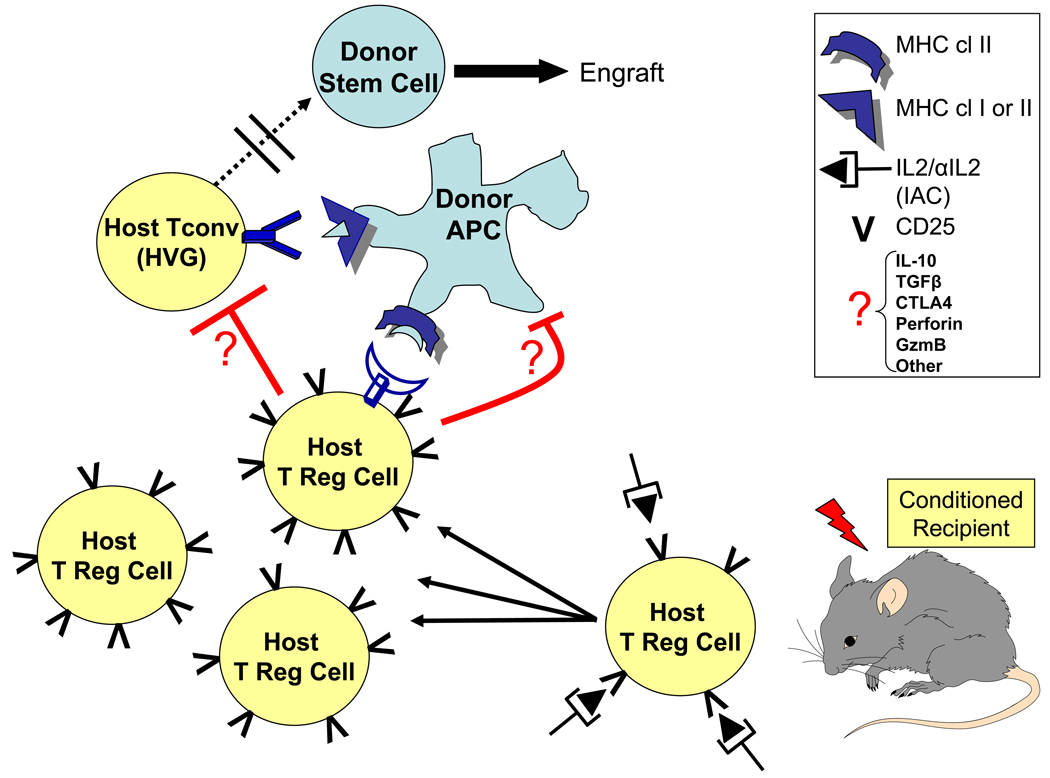
Fig. 1. Model of IAC induced suppression of host vs. graft Tconv cells and facilitation of chimerism post-allogeneic HSCT.
Following conditioning, residual host Treg cells can be activated and expanded in situ by IL2 / anti-IL2 complex (IAC). A working hypothesis for the regulation of HVG and facilitation of engraftment is the subsequent engagement of host Treg cells and host Tconv cells at the donor APC interface. Inhibition of resistance against the donor graft might proceed via direct (Treg-Tconv) and / or indirect (Treg-donor APC) pathways. The effector molecules which mediate the regulation are unknown.
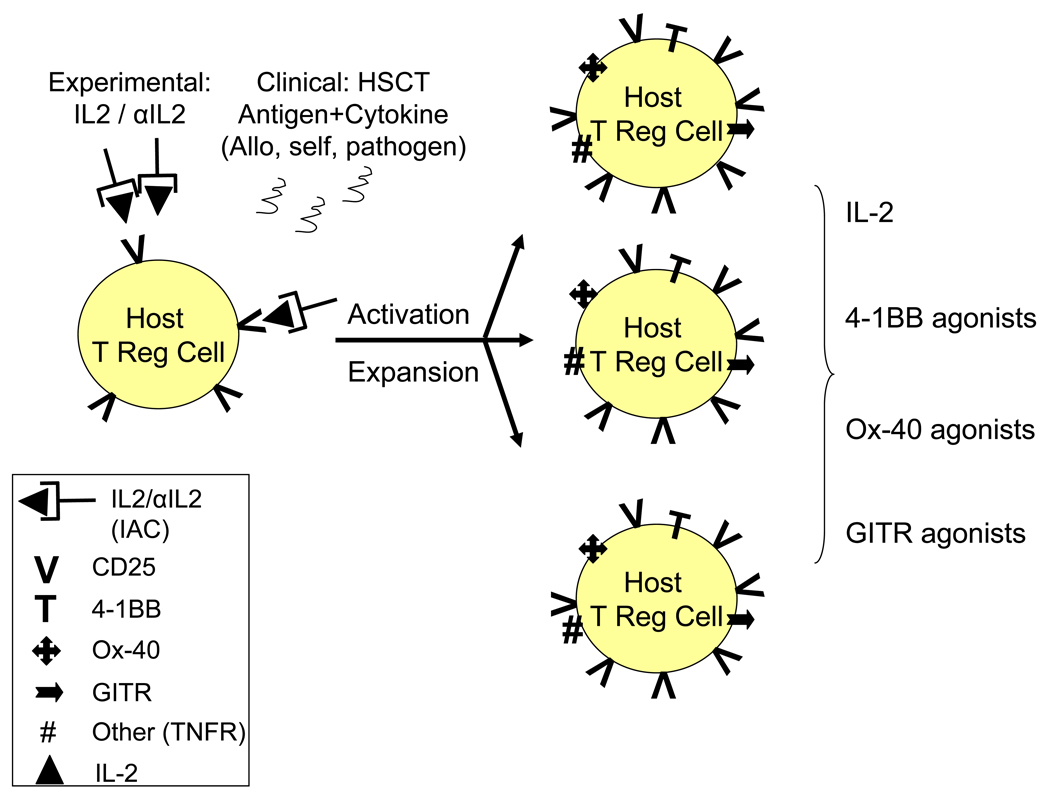
Fig. 2. Potential strategies for manipulation of Treg cells following IAC or antigen induced activation by targeting up-regulated cell surface molecules.
Treg cells can be activated and expanded following experimental treatment with IAC (IL2 / anti-IL2 complex) or via responsiveness to auto (self), allogeneic (transplant) or conventional (pathogen) antigen. Following activation, up-regulation of cell surface molecules including CD25 and TNFR family members may provide ‘targets’ for additional manipulation of different Treg populations. 4-1BB (TNFRS9), Ox-40 (TNFRS4) and GITR (TNFRS18) reported to affect Treg function are shown, but other TNF family molecules as well as molecules yet to be identified could become potential targets for manipulation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Godfrey WR, Ge YG, Spoden DJ, et al. In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood. 2004;104:453–461. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- 2.Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann P, Eder R, Kunz-Schughart LA, et al. Large-scale in vitro expansion of polyclonal human CD4+CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 4.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 5.Turnquist HR, Raimondi G, Zahorchak AF, et al. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 6.Putnam AL, Brusko TM, Lee MR, et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58:652–662. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Earle KE, Tang Q, Zhou X, et al. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clinical Immunology. 2005;115:3–9. doi: 10.1016/j.clim.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen JL, Trenado A, Vasey D, et al. CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamazaki S, Iyoda T, Tarbell K, et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joffre O, Santolaria T, Calise D, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14:88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chai JG, Coe D, Chen D, et al. In vitro expansion improves in vivo regulation by CD4+CD25+ regulatory T cells. J Immunol. 2008;180:858–869. doi: 10.4049/jimmunol.180.2.858. [DOI] [PubMed] [Google Scholar]
- 13.Sega EI, Leveson-Gower D, Nguyen VH, et al. Functional Comparison of Freshly Isolated and Ex-Vivo Expanded CD4+CD25+Foxp3+ Regulatory T Cells in Suppressing Murine Acute GvHD; ASH Annual Meeting Abstracts; 2008. p. 812. [Google Scholar]
- 14.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 15.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann P, Eder R, Boeld TJ, et al. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006;108:4260–4267. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann P, Boeld TJ, Eder R, et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39:1088–1097. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- 18.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med. 2007;204:1543–1551. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barao I, Hanash AM, Hallett W, et al. Suppression of natural killer cell-mediated bone marrow cell rejection by CD4+CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:5460–5465. doi: 10.1073/pnas.0509249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnett B, Kryczek I, Cheng P, et al. Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol. 2005;54:369–377. doi: 10.1111/j.1600-0897.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 21.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahnke K, Schonfeld K, Fondel S, et al. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer. 2007;120:2723–2733. doi: 10.1002/ijc.22617. [DOI] [PubMed] [Google Scholar]
- 23.Litzinger MT, Fernando R, Curiel TJ, et al. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 2007;110:3192–3201. doi: 10.1182/blood-2007-06-094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 25.Lin CH, Hunig T. Efficient expansion of regulatory T cells in vitro and in vivo with a CD28 superagonist. Eur J Immunol. 2003;33:626–638. doi: 10.1002/eji.200323570. [DOI] [PubMed] [Google Scholar]
- 26.Beyersdorf N, Ding X, Blank G, et al. Protection from graft-versus-host disease with a novel B7 binding site-specific mouse anti-mouse CD28 monoclonal antibody. Blood. 2008;112:4328–4336. doi: 10.1182/blood-2008-03-146662. [DOI] [PubMed] [Google Scholar]
- 27.Lan YY, Wang Z, Raimondi G, et al. "Alternatively activated" dendritic cells preferentially secrete IL-10, expand Foxp3+CD4+ T cells, and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 2006;177:5868–5877. doi: 10.4049/jimmunol.177.9.5868. [DOI] [PubMed] [Google Scholar]
- 28.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 29.Kavanagh B, O'Brien S, Lee D, et al. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112:1175–1183. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessel A, Ammuri H, Peri R, et al. Intravenous immunoglobulin therapy affects T regulatory cells by increasing their suppressive function. J Immunol. 2007;179:5571–5575. doi: 10.4049/jimmunol.179.8.5571. [DOI] [PubMed] [Google Scholar]
- 31.Ephrem A, Chamat S, Miquel C, et al. Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis. Blood. 2008;111:715–722. doi: 10.1182/blood-2007-03-079947. [DOI] [PubMed] [Google Scholar]
- 32.Boyman O, Kovar M, Rubinstein MP, et al. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 33.Shatry A, Levy RB. In situ Activation and Expansion of Host Tregs: A New Approach to Enhance Donor Chimerism and Stable Engraftment in MHC-matched Allogeneic HCT. Biology of Blood and Marrow Transplantation. 2009 doi: 10.1016/j.bbmt.2009.03.011. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayer AL, Jones M, Chirinos J, et al. Host CD4+CD25+ T cells can expand and comprise a major component of the Treg compartment after experimental HCT. Blood. 2009;113:733–743. doi: 10.1182/blood-2008-08-173179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. Journal of Experimental Medicine. 2001;193:1311–1317. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verbinnen B, Billiau AD, Vermeiren J, et al. Contribution of regulatory T cells and effector T cell deletion in tolerance induction by costimulation blockade. J Immunol. 2008;181:1034–1042. doi: 10.4049/jimmunol.181.2.1034. [DOI] [PubMed] [Google Scholar]
- 37.Wilson MS, Pesce JT, Ramalingam TR, et al. Suppression of murine allergic airway disease by IL-2:anti-IL-2 monoclonal antibody-induced regulatory T cells. J Immunol. 2008;181:6942–6954. doi: 10.4049/jimmunol.181.10.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Chua KS, Guimond M, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 40.Tang Q, Adams JY, Penaranda C, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zorn E, Nelson EA, Mohseni M, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zorn E, Mohseni M, Kim H, et al. Combined CD4+ donor lymphocyte infusion and low-dose recombinant IL-2 expand FOXP3+ regulatory T cells following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:382–388. doi: 10.1016/j.bbmt.2008.12.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elpek KG, Yolcu ES, Franke DD, et al. Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J Immunol. 2007;179:7295–7304. doi: 10.4049/jimmunol.179.11.7295. [DOI] [PubMed] [Google Scholar]
- 44.Pillai AB, George TI, Dutt S, et al. Host natural killer T cells induce an IL-4 dependent expansion of donor CD4+CD25+Foxp3+ Tregs that protects against graft-versus-host disease. Blood. 2009 doi: 10.1182/blood-2008-06-165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robertson SA, Guerin LR, Bromfield JJ, et al. Seminal Fluid Drives Expansion of the CD4+CD25+ T Regulatory Cell Pool and Induces Tolerance to Paternal Alloantigens in Mice. Biol Reprod. 2009 doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]