Abstract
Lesional neocortical epilepsy (NE) can be associated with hippocampal sclerosis or hippocampal spectroscopic abnormalities without atrophy (dual pathology). In this study, magnetic resonance spectroscopic imaging (MRSI) was used to determine the frequency of hippocampal damage/dysfunction in NE with and without structural lesion. Sixteen patients with NE [seven temporal NE (NE-T), nine extratemporal (NE-ET)] and 16 controls were studied with a 2D MRSI sequence (Repetition time/echo time (TR/TE) = 1800/135 ms) covering both hippocampi. Seven NE patients had MR visible lesions (NE-Les), nine had normal MRI (NE-no). In each hippocampus, 12 voxels were uniformly selected. In controls, mean (± SD) NAA/(Cr + Cho) values for each voxel were calculated and voxels with NAA/(Cr + Cho) ≤ (mean in controls – 2SD in controls) were defined as ‘pathological’ in patients. Eight of 16 NE patients had at least two ‘pathological’ voxel (mean 2.5, range 2–5) in one hippocampus. Four were NE-Les and four NE-no. Three (43%) NE-T patients, had evidence for hippocampal damage/dysfunction and five (56%) had NE-ET. The ipsilateral hippocampus was affected in six of eight NE patients. Evidence for unilateral hippocampal damage/dysfunction was demonstrated in 50% of the NE patients. The type of NE, i.e. NE-Les or NE-no, NE-T or NE-ET, had no influence on the occurrence of hippocampal damage/dysfunction.
Keywords: dual pathology, hippocampal abnormalities, MR spectroscopy, NE
Introduction
The association of temporal and extratemporal lesional neocortical epilepsy (NE) with hippocampal atrophy is known as ‘dual pathology’ and can be found in up to 30% of lesional NE (NE-les) [1–3]. 1H magnetic resonance spectroscopy (MRS) allows for the non-invasive measurement of the neuronal marker N-acetylaspartate (NAA). A reduction of NAA indicates either neuronal loss and/or neuronal dysfunction. Miller et al. [4] demonstrated hippocampal NAA reductions in up to 74% of their series of 19 lesional temporal (NE-T) and extratemporal NE (NE-ET) patients, the majority without hippocampal atrophy on magnetic resonance imaging (MRI). In analogy to ‘structural dual pathology’, they introduced the term ‘functional dual pathology’ to describe this finding. However, the frequency of functional dual pathology might be even higher because hippocampal metabolic abnormalities might also occur in NE with normal MRI. In structural dual pathology, the surgical outcome is better when both pathologies, i.e. the atrophied hippocampus and the extrahippocampal lesion, can be resected. This suggests that both lesions possess an intrinsic epileptogenicity [5]. As functional dual pathology may also be associated with an intrinsic epileptogenicity, its presence might be of clinical importance. Therefore, in the following study, we used MR spectroscopic imaging (MRSI) with the aim of determining the frequency of hippocampal damage/dysfunction in NE with and without structural lesion.
Patients and methods
Study population
The committee of human research at the University of California, San Francisco (UCSF) approved the study, and written informed consent was obtained from each subject according to the Declaration of Helsinki. Sixteen consecutive patients (nine women, mean age 25.4 ± 8.2 years) were enrolled from the Northern California Comprehensive Epilepsy Center, UCSF and the California Pacific Epilepsy Program, California Pacific Medical Center, San Francisco, where they underwent a pre-surgical exploration. The identification of the primary epileptogenic region was based on seizure semiology, prolonged ictal and interictal Video/Electroencephalography (EEG)/Telemetry (VET), MRI, Positron emission tomography (PET) and in some cases ictal single photon emission computed tomography (SPECT). Seven of the NE patients had evidence for seizures arising from the lateral temporal lobes (NE-T), and nine for extratemporal seizure onset (NE-ET). Ictal and interictal EEG recordings were non-lateralizing in three NE patients. In seven patients, the MRI showed an extrahippocampal cortical malformation (NE-Les), which involved the hippocampus in one (patient 11). One patient (no. 12) had a T2 signal increase in the right hippocampus; the other eight NE had completely normal MRI (NE-no) (cf. Table 1). The control population consisted of 16 healthy, age-matched volunteers (10 women, mean age 28.1 ± 8.2 years).
Table 1.
Patient characteristics
| Patient no. | Gender | Age at examination | Focus | TL category | MRI diagnosis | No. of pathological hippocampal voxels |
|---|---|---|---|---|---|---|
| 1 | M | 19 | LFP | ET | L POT subependymal heterotopia, polymicrogyria with schizenecphaly | 0 |
| 2 | M | 35 | RTP | Tplus | R TP subependymal heterotopia and polymicrogyria with schizencephaly | 0 |
| 3 | F | 15 | LT | T | no | 3 |
| 4 | M | 24 | R + LT | T | no | 0 |
| 5 | M | 17 | LF | ET | R F and L P and RP transmantle cortex dysplasia | 2 |
| 6 | F | 21 | RTP | Tplus | no | 2 |
| 7 | F | 28 | RFC | ET | no | 2 |
| 8 | F | 31 | RTO | Tplus | R TO subependymal heterotopia, polymicrogyria with schizencephaly | 1 |
| 9 | F | 28 | LFCT | Tplus | no | 0 |
| 10 | F | 27 | F | ET | no | 0 |
| 11 | F | 16 | LTO | Tplus | L TOP polymicrogyria | 2 |
| 12 | M | 18 | RO | ET | R hippocampal signal increase | 0 |
| 13 | F | 18 | LP | ET | L P polymicrogyria | 2 |
| 14 | F | 42 | FC | ET | no | 5 |
| 15 | M | 37 | LPO | ET | L TPO subependymal heterotopia, polymicrogyria with schizencephaly. Dandy–Walker syndrome | 2 |
| 16 | M | 31 | LF | ET | no | 1 |
M, male; F, female; L, left; R, right; T, temporal; P, parietal; O, occipital; F, frontal, C, central; no, normal; path, pathological. TL category: T, seizure onset confined to the temporal lobe; Tplus, EEG evidence for involvement of temporal lobe in seizure onset; ET, extratemporal seizure onset.
Structural MRI and 2D MRSI acquisition
All studies were performed on a 1.5 T Magnetom Vision™ MR system (Siemens Inc., Iselin, NJ, USA) using a standard polarized head coil. After acquisition of scout images in coronal and sagittal orientations, a T1-weighted fast low angle shot (FLASH) images with TR/TE = 500/14 ms, slice thickness 3 mm, parallel to the long axis of the hippocampus was obtained. Spectroscopic measurements were carried out with a 2D MRSI sequence (TR/TE = 1800/135 ms) using point resolved spectroscopy (PRESS) volume pre-selection (15 mm axial, 60 mm left–right, 100 mm anterior–posterior) with 24 × 24 phase-encoding steps and a 210 mm2 field of view (FOV) angulated along the long axis of the hippocampus, covering both hippocampi.
MRSI data post-processing
Post-processing included zero filling to 32 × 32 and apodization resulting in a line broadening of 1 Hz in the spatial domain. The time-domain data were zero filled to 1024 data points and apodized with a Gaussian filter resulting in a line broadening of 4 Hz in the frequency domain. A fully automated spectral-fitting software package developed in this laboratory [6,7] was used to fit the peak areas of NAA, creatine/phosphocreatine (Cr) and choline compounds (Cho). The NAA/(Cr + Cho) ratio for each voxel was calculated to correct for cerebrospinal fluid (CSF) partial-volume effects. Careful placement of the PRESS box and an optimized excitation pulse minimized possible effects of the chemical shift artifact to such a degree that its effect could be neglected for hippocampal voxels.
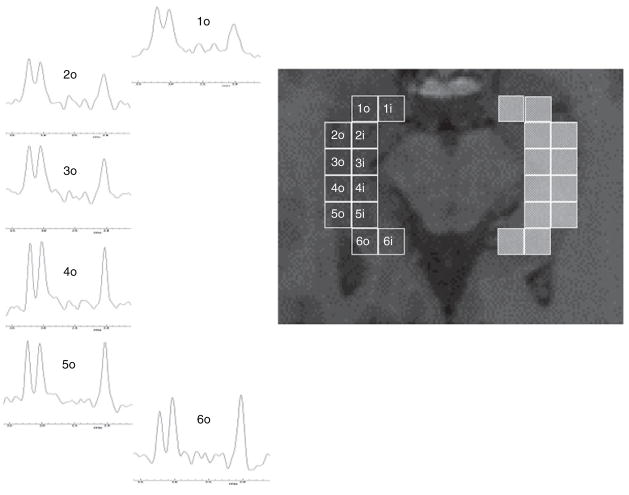
Hippocampal voxels were uniformly selected on the T1-weighted FLASH image and their position in the hippocampus indicated with a number ranging from 1 for the most anterior in the head to 6 for the most posterior in the tail of the hippocampus. From each position two voxels, i.e. an outer one and an inner one, were selected (cf. Fig. 1). Only spectra with clearly resolved peaks of NAA, Cr, and Cho and no baseline distortion were chosen for analysis. Applying these criteria, 35.2% of the voxels from position 1, 76.6% of the voxels from position 2, 95.3% of the voxels from position 3, 99.2% of the voxels from position 4 and 100% of the voxels from position 5 and 6 were included into the analysis. The percentages of voxels included at each position was the same in both groups.
Figure 1.
On the right side: schema for voxel selection. The number indicates the position, in the anterior-posterior direction, ‘o’ refers to the outer row, ‘i’ to the inner row. On the left side: distribution of ‘pathological’ voxels (red) in patient no. 14. The EEG localization in this patient did not allow a lateralization of the seizures in contrast to the hippocampal NAA/(Cr + Cho) reduction, which were restricted to the left hippocampal formation.
To identify individual voxels with abnormally low NAA/(Cr + Cho) a so-called ‘voxel-by-voxel analysis’ was used: mean and SD for each hippocampal voxel in controls were calculated. These were used to determine for each voxel position a threshold for pathologically low NAA/(Cr + Cho) values which was defined as NAA/(Cr + Cho) ≤ (mean controls – 2SD controls). In controls, one subject had one hippocampal voxel with pathologically low NAA/(Cr + Cho) pathological voxel (PV). Therefore, in patients, only hippocampi with at least two voxels with abnormally low NAA/(Cr + Cho) were considered ‘pathological’.
Statistical analysis
Mann–Whitney tests and Fisher’s exact test were used to test for differences between the subgroups of NE patients.
Results
Eight of 16 (50%) NE had at least two PV in one hippocampus. Only two NE had evidence for a structural abnormality in the hippocampus: patient 11 had a left temporo-parieto-occipital malformation involving the posterior part of the hippocampal formation. This part was correctly identified as pathological by MRS. Patient 12 had an increased signal on the T2 weighted MRI in the right anterior hippocampal formation. All voxels fulfilling the quality criteria (positions 3–6) in this hippocampus were within the normal range. There was no regional preference for PV in the hippocampus as they were equally often found in the anterior (positions 1–3, four patients) as in the posterior hippocampus (positions 4–6, two patients). Two NE had PV in both regions. PV were strictly unilateral. In six (75%) NE, the affected hippocampus was on the same, i.e. ipsilateral side, as the neocortical focus. In one patient (no. 14) with non-lateralized bilateral frontal seizure onsets, the left hippocampus was affected and in another patient (no. 6) with right temporal neocortical seizure onset, the contralateral hippocampus was affected. PV were found in four (57%) NE-Les and in four (44%) NE-no (non significant (ns) with Fisher’s exact test). The mean number of hippocampal PV in NE-no and NE-Les was not different (NS with Mann–Whitney test). Three (43%) NE-T patients had evidence for hippocampal damage/dysfunction as did five (56%) NE-ET (NS with Fisher’s exact test). The mean number of hippocampal PV was not different between NE-T and NE-ET (NS with Mann–Whitney test).
Discussion
The major finding in this study was that evidence for hippocampal damage or dysfunction was found in 50% of all NE, the majority without hippocampal structural abnormality on MRI. Reduced hippocampal NAA/(Cr + Cho) was found in 57% of the NE-Les confirming the findings of the study of Miller et al. [4] who found hippocampal NAA reductions in 74% of such patients. In contrast to that study, we included also NE-no and found evidence for hippocampal damage/dysfunction in 44% of those patients. This finding contradicts a previous study from our group, which found no evidence for hippocampal damage/dysfunction in NE-no [8]. There are two possible explanations for this discrepancy: first, the NE population in this study was not the same as in the previous study. Secondly, the two studies used different types of analyses. In the previous study, an average NAA/(Cr + Cho) value from five hippocampal voxels was calculated for each hippocampus in each subject. By averaging voxels from the whole hippocampal formation, regionally circumscribed NAA reductions might be ‘cancelled out’ by the metabolically normal reminder of the hippocampus. Furthermore, averaging hippocampal voxels does not allow to account for the hippocampal anterior–posterior NAA/(Cr + Cho) gradient [9,10]. As the analysis used in this study accounts for both of these peculiarities of hippocampal MRS, it might be more sensitive to detect hippocampal damage/dysfunction than the analysis in the previous paper.
To our knowledge, this is the first spectroscopy study reporting evidence for hippocampal damage/dysfunction in NE-no, but not the first study demonstrating hippocampal abnormalities in NE-no. Scott et al. [11] found a prolongation of the hippocampal T2 relaxation time in 28 patients with extratemporal epilepsy, 14 with normal MRI including the hippocampal formation. As in our study, the severity of hippocampal damage was similar in NE-Les and NE-no as the mean increase in T2 relaxation time was not different in the two groups. The reason for hippocampal damage/dysfunction in NE-no is unknown. However, as structural dual pathology is often associated with cortical malformations, it is tempting to speculate that hippocampal damage/dysfunction in NE-no might indicate the presence of a subtle cortical malformation not detectable by routine MRI. The application of special surface coils, high-resolution MRI, special post-processing techniques [12] or special PET tracers [13] might eventually be helpful for the non-invasive localization of such malformations in those patients.
The significance of the metabolic abnormalities in the hippocampal formation in the absence of hippocampal structural abnormalities in NE is not clear. It is possible that functional dual pathology represents a lesser degree or an early stage of structural dual pathology. In structural dual pathology, the surgical outcome is better if the atrophied hippocampus can be resected together with the neocortical lesion. This has been interpreted as evidence that both lesions possess intrinsic epileptogenicity [5,14,15]. Thus, metabolic abnormalities in functional dual pathology may indicate the development of intrinsic epileptogenicity in the hippocampus. The processes responsible for hippocampal atrophy in structural dual pathology are currently not known. There are two possibilities: first, the same factors disrupting the normal developmental process in the region of the cortical malformation could also induce more subtle malformations in the hippocampus. As a result of aberrant connections and irregular or immature cellular elements such abnormalities might be intrinsic epileptogenic. Secondly, the presence of hippocampal damage/dysfunction might be a consequence of epileptogenic activity spreading from the neocortical focus into the hippocampus. In vitro experiments have shown that repetitive propagation of epileptogenic activity from remote areas into the hippocampal formation may result in the development of independent hippocampal foci [16]. Excitotoxic processes associated with such intrinsic epileptogenicity could lead to hippocampal neuronal dysfunction and finally to hippocampal atrophy and structural dual pathology. On the contrary, it is also possible that functional dual pathology is caused by a process not associated with intrinsic epileptogenicity. An example for such a process would be deafferentiation, i.e. loss of neuronal input from the neocortical focus to the hippocampal formation [17].
This study has several limitations: (i) the study population is small. This might limit the power to detect differences between groups. (ii) All patients were referred from a tertiary center where they underwent evaluation for epilepsy surgery. Thus the findings in our study might not be representative for a more general NE population. (iii) The ultimate gold standard for the correct identification of the epileptogenic focus is the outcome after surgery. In this study, the focus identification was based on findings during non-invasive pre-surgical evaluation and only a minority of our patients had surgery. However, the ability of non-invasive techniques for seizure identification is limited and this might have influenced our ability to discriminate between NE-T and NE-ET. (iv) Spectra from the head of the hippocampus (position 1) did often not fulfill the criteria for good spectral quality and had to be rejected. Therefore, we might have missed metabolic abnormalities restricted to this region.
In conclusion, we found spectroscopic evidence for functional dual pathology in a considerable percentage of NE-Les but also of NE-no. Therefore, the concept of ‘functional dual pathology’ as introduced by Miller et al. [4] should eventually be expanded to also include neocortical epilepsy with normal MRIs. As hippocampal damage occurs in a high percentage ipsilateral to the neocortical focus, it may be useful for focus lateralization in NE. A larger patient group needs to be studied to determine the significance of functional dual pathology, particularly its relevance for surgical outcome.
Acknowledgments
The authors would like to thank Drs D.G. Vossler, R.C. Knowlton from the Swedish Epilepsy Center of the University of Washington, Seattle and Dr M.C. Salinsky from the Oregon Health Sciences University Epilepsy Center, Portland, Oregon for the referral of some of their patients for this study. This work was supported by NIH grant RO1-NS31966 (K.D.L.).
References
- 1.Levesque MF, Nakasato N, Vinters HV, Babb TL. Surgical treatment of limbic epilepsy associated with extra-hippocampal lesions: the problem of dual pathology. Journal of Neurosurgery. 1991;75:364–370. doi: 10.3171/jns.1991.75.3.0364. [DOI] [PubMed] [Google Scholar]
- 2.Raymond AA, Fish DR, Stevens JM, Cook MJ, Sisodiya SM, Shorvon SD. Association of hippocampal sclerosis with cortical dysgenesis in patients with epilepsy. Neurology. 1994;44:1841–1845. doi: 10.1212/wnl.44.10.1841. [DOI] [PubMed] [Google Scholar]
- 3.Cendes F, Cook MJ, Watson C, et al. Frequency and characteristics of dual pathology in patients with lesional epilepsy. Neurology. 1995;45:2058–2064. doi: 10.1212/wnl.45.11.2058. [DOI] [PubMed] [Google Scholar]
- 4.Miller SP, Li LM, Cendes F, et al. Medial temporal lobe neuronal damage in temporal and extratemporal lesional epilepsy. Neurology. 2000;54:1465–1470. doi: 10.1212/wnl.54.7.1465. [DOI] [PubMed] [Google Scholar]
- 5.Li LM, Cendes F, Anderman F, et al. Surgical outcome in patients with epilepsy and dual pathology. Brain. 1999;122:799–805. doi: 10.1093/brain/122.5.799. [DOI] [PubMed] [Google Scholar]
- 6.Maudsley AA, Lin E, Weiner MW. Spectroscopic imaging display and analysis. Magnetic Resonance Imaging. 1992;10:471–485. doi: 10.1016/0730-725x(92)90520-a. [DOI] [PubMed] [Google Scholar]
- 7.Soher BJ, Young K, Govindaraju V, Maudsley AA. Automated spectral analysis III: Application to in vivo proton MR spectroscopy and spectroscopic imaging. Magnetic Resonance in Medicine. 1998;40:822–831. doi: 10.1002/mrm.1910400607. [DOI] [PubMed] [Google Scholar]
- 8.Vermathen P, Ende G, Laxer KD, Knowlton RC, Matson GB, Weiner MW. Hippocampal N-acetylaspartate in neocortical epilepsy and mesial temporal lobe epilepsy. Annals of Neurology. 1997;42:194–199. doi: 10.1002/ana.410420210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermathen P, Laxer KD, Matson GB, Weiner MW. Hippocampal structures: anteroposterior N-acetylaspartate differences in patients with epilepsy and control subjects as shown with proton MR spectroscopic imaging. Radiology. 2000;214:403–410. doi: 10.1148/radiology.214.2.r00fe43403. [DOI] [PubMed] [Google Scholar]
- 10.McLean MA, Woermann FG, Simister RJ, Barker GJ, Duncan JS. In vivo short echo time 1H magnetic resonance spectroscopic imaging (MRSI) of the temporal lobes. NeuroImage. 2001;14:501–509. doi: 10.1006/nimg.2001.0827. [DOI] [PubMed] [Google Scholar]
- 11.Scott RC, Cross JH, Gadian DG, Jackson GD, Neville BGR, Connelly A. Abnormalities in hippocampi remote from seizure focus: a T2 relaxometry study. Brain. 2003;126:1968–1974. doi: 10.1093/brain/awg199. [DOI] [PubMed] [Google Scholar]
- 12.Bernasconi A, Antel SB, Collins DL, et al. Texture analysis and morphological processing of magnetic resonance imaging assist detection of focal cortical dysplasia in extra-temporal partial epilepsy. Annals of Neurology. 2001;49:770–775. [PubMed] [Google Scholar]
- 13.Juhasz C, Chugani DC, Muzik O, et al. Alpha-methyl-L-tryptophan PET detects epileptogenic cortex in children with intractable epilepsy. Neurology. 2003;60:960–968. doi: 10.1212/01.wnl.0000049468.05050.f2. [DOI] [PubMed] [Google Scholar]
- 14.Li LM, Cendes F, Watson C, et al. Surgical treatment of patients with single and dual pathology, relevance of lesion and hippocampal atrophy to seizure outcome. Neurology. 1997;48:437–444. doi: 10.1212/wnl.48.2.437. [DOI] [PubMed] [Google Scholar]
- 15.Cascino GD, Jack CR, Parisi JE, et al. Operative strategy in patients with MRI identified dual pathology and temporal lobe epilepsy. Epilepsy Research. 1993;14:175–182. doi: 10.1016/0920-1211(93)90022-y. [DOI] [PubMed] [Google Scholar]
- 16.Khalilov I, Holmes GL, Ben-Ari Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocamapal propagation of seizures. Nature Neuroscience. 2003;6:1079–1085. doi: 10.1038/nn1125. [DOI] [PubMed] [Google Scholar]
- 17.Roffman JL, Lipska BK, Bertolino A, et al. Local and downstream effects of excitotoxic lesions in the rat medial prefrontal cortex on in vivo 1H-MRS signals. Neuropsychopharmacology. 2000;22:430–439. doi: 10.1016/S0893-133X(99)00143-8. [DOI] [PubMed] [Google Scholar]