Abstract
Objective
To explore the structural neuroimaging correlates of visual constructive impairment in patients with mild to moderate Alzheimer disease (AD).
Background
There is considerable heterogeneity in the non-memory cognitive deficits associated with AD. Structural neuroimaging with MRI is an important diagnostic tool that is gaining acceptance as a surrogate measure of brain pathology in AD treatment trials. Most MRI measurements have focused on medial temporal lobe or global cortical atrophy, which may not reflect some important clinical features of AD.
Methods
Thirty-two patients with probable AD were stratified into two groups based on their relative performance on a visual constructive task, the copy of a modified Rey-Osterrieth figure (Rey). The two groups did not differ in basic demographic features or in neuropsychological performance, other than on the visual constructive task. MRI measurements of hippocampal volume, cortical gray matter volume, and focal cortical gray matter loss were performed in the patients and a group of 71 age-matched, normal controls.
Results
Both groups showed significant, bilateral hippocampal as well as cortical gray matter volume loss relative to controls. The more spatially impaired AD group (SAD) had more right than left cortical gray matter loss, whereas the opposite was true in the less spatially impaired group (NSAD). The SAD group had significantly less gray matter in the right inferior temporal gyrus relative to the NSAD group. Atrophy of this region was correlated with performance on the Rey task in all patients with AD.
Conclusions
Right inferotemporal atrophy may serve as a neuroimaging marker of visual constructive impairment in mild to moderate AD. Heterogeneous cortical atrophy is a common feature of AD.
A clinical diagnosis of probable AD requires the presence of memory impairment plus impairment in one other cognitive domain, such as language, executive function, or visuospatial function.1 Thus many patients with mild AD present with different cognitive deficits.2 This clinical heterogeneity has been correlated with heterogeneity in the regional distribution of AD pathology at autopsy.3-6 Functional and structural neuroimaging techniques can assess regional brain pathology, and may reflect the heterogeneity in the onset and progression of AD.7-9
Visual constructive impairment is a common finding in patients with mild to moderate AD10,11 that is associated with hypometabolism of posterior parietal and visual cortical areas.12 In some patients with incipient AD, visuospatial impairment precedes the development of amnestic symptoms.13 This suggests that prominent neocortical atrophy might be observed in regions that are needed for visuospatial processes in the absence of significant medial temporal lobe atrophy in these patients.14 Alternatively, patients with early visual constructive impairment may have a combination of hippocampal and neocortical atrophy, with the greatest degree of atrophy in regions that are important for visuospatial function. To explore these two possibilities, unbiased measurements of regional cortical gray matter loss and focused measurements of hippocampal and total cortical gray matter volume were compared in two groups of patients with probable AD who differed only in their ability to complete a visual constructive task, copying a modified version of the Rey-Osterrieth figure.
Methods
Subjects
A total of 103 subjects participated. There were 71 controls and 32 patients. All subjects were evaluated at the University of California at San Francisco Memory and Aging Center. Informed consent was obtained for all components of the study. Subjects received a general medical and neurologic evaluation. Patients with Alzheimer disease (AD) met the probable AD criteria as outlined by the National Institute of Neurologic and Communicative Disorders and Stroke-AD and Related Disorders Association.1 All patients and 22 of the controls underwent neuropsychological testing. Z-scores were calculated for all neuropsychological test values relative to a group of 49 patients with mild probable AD (mean age = 75.6, mean Mini-Mental State Examination [MMSE] = 26.7).
Patients were classified into two groups based on their performance on a design copying task that was a simplified version of the Rey-Osterrieth figure (Rey)15 (figure 1). The spatial AD group (SAD) was defined as subjects with AD who had a z-score equal to or less than -1.5 on their copy of the modified Rey and greater than or equal to -1 on all other tests. The nonspatial AD group (NSAD) was defined as subjects who had a z-score equal to or less than -1.5 on one other neuropsychological test and greater than or equal to -1 on the Rey. There was no difference in presenting complaint or first symptom between the two patient groups.
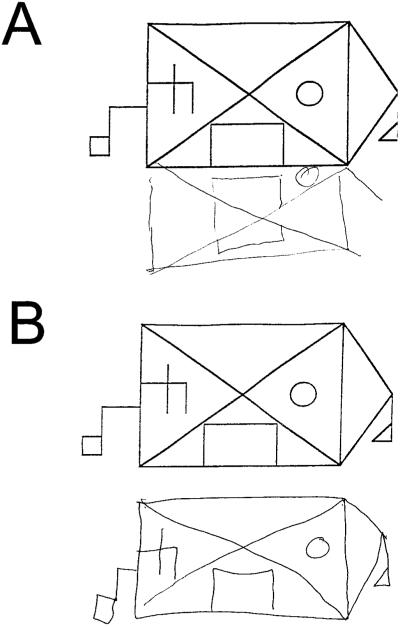
Figure 1.
Examples of modified Rey-Osterrieth copy performance in two patients with probable Alzheimer disease (AD). (A) A 54-year-old man with a Mini-Mental State Examination (MMSE) score of 24 from the spatial AD group. (B) A 59-year-old man with a MMSE score of 20 from the nonspatial AD group.
Neuropsychological tests
A neuropsychological screening battery was administered to all patients with AD and 22 controls to assess the major domains of cognitive and psychological functioning. General intellectual function was assessed using the MMSE.16 The California Verbal Learning Test-Mental Status Version (CVLT-MS)15 was used to evaluate verbal episodic memory, and nonverbal episodic memory was measured using a modified version of the Rey-Osterrieth complex figure (Rey) with a 10-minute free recall delay trial. Language assessment included the abbreviated (15-item) Boston Naming Test (BNT),17 comprehension of seven syntactically complex commands and questions, repetition of three phonemically complex phrases, semantic fluency (animals/1 minute), and phonemic fluency (words beginning with the letter D/1 minute). Visuospatial assessment included copying the Rey as well as Trial 1 of the Design Fluency subtest of the Delis-Kaplan Executive Functions Scale.15 Tests of executive functioning included a visuo-motor set-shifting and sequencing task (a modified version of the Trails B test18), backwards digit span to assess working memory, and the Stroop interference task19 to assess inhibition of an overlearned response. Ability to perform five arithmetic calculations was also assessed. Mood status was measured using the Geriatric Depression Scale.20
Group differences in neuropsychological scores were compared using a univariate analysis of variance (ANOVA) statistic with Tukey post hoc. χ2 and ANOVA statistics revealed that sex and age differences between groups were not significant; thus no covariates were used in these analyses. Statistical analysis was accomplished using the SPSS software package (version 10.0.5 for Windows, SPSS Inc., Chicago, IL).
MRI scanning
Images were obtained at the San Francisco VA Magnetic Resonance Unit. T1-weighted (magnetization-prepared rapid gradient echo [MPRAGE]) MRI scans were obtained on a 1.5-T Magnetom VISION system (Siemens Inc., Iselin, NJ). The structural MRI sequence used here is identical to that described in a previous study.21
Volumetric measurements
Volumes of the left and right hippocampus were determined by semiautomated methods as previously described22 in all patients and 26 controls. MPRAGE images were segmented into gray matter, white matter, and CSF using semiautomated methods (tissue segmentation).23 The total intracranial volume (TIV) and total cortical gray matter volume (TCV) were determined based on this tissue-segmented MRI data. To account for variations in head size between subjects, hippocampal volumes and TCV were normalized to the TIV of each subject by dividing by the TIV. Asymmetry of hippocampal volume was assessed by dividing the left hippocampal volume by the right hippocampal volume.
Voxel-based morphometry
For the VBM analysis, images were preprocessed and statistically analyzed using the SPM99 software package (http://www.fil.ion.ucl.ac.uk/spm), using standard procedures.24,25 Two additional spatial transformation procedures, nonlinear warping of subject images to the template image and modulation by the Jacobian determinate of the spatial transformation matrix, were added to improve registration to the template image and to preserve volumetric information in the results.26 These methods have been validated against traditional region of interest volumetric methods in patients with AD.27
To examine the patterns of atrophy specific to each patient group, the following contrasts were performed: 1) nonspatial AD vs controls: the areas of gray matter loss in the patients with nonspatial AD relative to the controls were examined (NSAD < controls); 2) spatial AD vs controls: the areas of gray matter loss in the patients with spatial AD relative to the controls were examined (SAD < controls); 3) spatial AD vs nonspatial AD: the areas of gray matter loss in the patients with spatial AD relative to the patients with nonspatial AD were examined (SAD < NSAD). We accepted a statistical threshold of p < 0.05 at the voxel level, corrected for multiple comparisons, for the main contrast. The multiple comparisons correction used in SPM99 is based on the theory of Gaussian fields, and has been validated for use in VBM.24
Localization of areas of significant cortical and subcortical gray matter loss was accomplished by superimposing the regions of significant atrophy on the averaged T1-weighted image used to create the template for spatial normalization and visual comparison with the cerebral atlas of Duvornoy.28 Regions of atrophy are reported in Montreal Neurologic Institute coordinates.29 Asymmetry of cortical gray matter loss was assessed by adding the total number of suprathreshold voxels in each hemisphere.
Comparisons of tissue-segmented and voxel-based results
To compare the relative degree of regional atrophy (hippocampal or right inferior temporal gyrus) with the degree of global cortical atrophy in each group, the magnitude of atrophy in the volumetric measurements was compared to the magnitude of atrophy identified in the VBM analysis using an ANOVA with Tukey post hoc of each volume or gray matter concentration (VBM analysis) normalized to that from the control group.
Correlations of MRI and neuropsychological data
Neuropsychological test results were correlated with extracted gray matter concentrations at the peak suprathreshold voxels in VBM analyses 2 and 3 using two-tailed Pearson correlations. A significance level of p < 0.05 was accepted.
Results
Neuropsychology
The demographic and neuropsychological profiles of the control and patient groups are shown in table 1. There was no significant difference in age, sex, education, or verbal fluency (animals/minute) between the two AD groups and controls. Figure 1 shows examples of performance on the modified Rey copy task by a NSAD patient and a SAD patient. The SAD group performed worse than the NSAD and control groups on this task (p < 0.001, ANOVA with Tukey post hoc; see table 1). Both groups were impaired relative to controls in recalling the figure after 10 minutes (p < 0.001), but not different from one another. There was no difference in performance on any of the other neuropsychological tests between the two patient groups (p > 0.1; see table 1). The NSAD group, but not the SAD group, was impaired relative to controls (p < 0.05) on backward digit span and a verbal fluency task (D-words/minute). Both groups performed worse than controls on all other tests (p < 0.05; see table 1).
Table 1.
Demographic and neuropsychological values for patients and controls
| Characteristics | Overall ANOVA | Control, mean (SD) | NSAD, mean (SD) | SAD, mean (SD) |
|---|---|---|---|---|
| Number | 22 | 23 | 9 | |
| Age, y | F(2,42) = 0.53 | 71.6 (8.4) | 74.4 (11.4) | 75.2 (8.9) |
| Education, y | F(2,42) = 0.56 | 16.2 (2.9) | 15.1 (3.7) | 16.4 (2.1) |
| MMSE | F(2,50) = 41.2 | 29.4 (0.8) | 24.2 (3.2)* | 24.2 (3.2)* |
| Rey Copy, max 17 | F(2,50) = 18.0 | 14.9 (1.5) | 14.1 (2.4) | 9.3 (3.5)*† |
| Rey 10 min recall, max 17 | F(2,49) = 34.9 | 10.9 (3) | 3 (3.8)* | 2.8 (3.2)* |
| CVLT 4th trial, n correct | F(2,49) = 32.2 | 8.3 (0.8) | 4.6 (1.6)* | 5.4 (2.2)* |
| CVLT 10 min recall, n correct | F(2,49) = 42.2 | 6.8 (1.9) | 1.4 (2.1)* | 2 (1.7)* |
| Design Fluency, n correct | F(2,50) = 23.5 | 10.3 (3.1) | 4.8 (2.7)* | 5.2 (2.5)* |
| Calculations, n correct | F(2,49) = 6.8 | 4.7 (0.5) | 3.81 (1)‡ | 3.78 (1.1)‡ |
| Backward digit span | F(2,51) = 4.5 | 4.8 (1.1) | 3.7 (1.1)* | 4.3 (1.4) |
| Verbal fluency, D words/min | F(2,50) = 11.5 | 15 (6) | 7.5 (4.2)* | 12 (5.1) |
| Verbal fluency, animals/min | F(2,52) = 2.4 | 12.3 (7.2) | 8.6 (4.3) | 12.3 (6.5) |
| Modified Boston Naming Test, max 15 | F(2,51) = 20.9 | 14.6 (0.7) | 10.2 (3.2)* | 11.8 (2.2)‡ |
| Stroop, n correct | F(2,40) = 40.3 | 49.7 (11.1) | 17.6 (11.4)* | 23.2 (11.3)* |
| Trails B, sq rt correct lines/min | F(2,51) = 65.4 | 5.6 (1.0) | 1.8 (1.3)* | 2.3 (1.2)* |
| Geriatric Depression Scale | F(2,48) = 1.8 | 4.5 (3.3) | 5.2 (5) | 8.1 (6.2) |
p < 0.001 vs controls.
p < 0.001 vs NSAD.
p < 0.05 vs controls.
ANOVA = analysis of variance; NSAD = non-spatial Alzheimer disease; SAD = spatial Alzheimer disease; MMSE = Mini-Mental State Examination; CVLT = California Verbal Learning Test.
Voxel-based morphometric analysis of SPM-segmented MRI scans
There was an asymmetric (left > right) distribution of cortical atrophy in the NSAD group (NSAD < controls; table 2). In order of statistical significance, the left hippocampus, right and left perisylvian cortices, left angular gyrus, left middle temporal gyrus, left dorsomedial thalamus, and right hippocampus had less gray matter than controls (see table 2).
Table 2.
Regions of atrophy in nonspatial and spatial Alzheimer disease (AD) as compared with controls and each other
| Anatomic region | BA | x, y, z* | z-Score | p Value† |
|---|---|---|---|---|
| Nonspatial AD (n = 23) vs controls (n = 71) | ||||
| L hippocampus | 28 | -28, -13, -18 | 6.92 | <0.001 |
| R insula/perisylvian cortex | 40 | 38, -22, 17 | 5.45 | 0.001 |
| L insula/perisylvian cortex | 40 | -36, -24, 15 | 5.43 | 0.001 |
| L angular gyrus | 7 | -43, 56, 50 | 5.38 | 0.001 |
| L middle temporal gyrus | 21 | -61, -20, -6 | 5.17 | 0.004 |
| L dorsomedial thalamus | NA | -4, 15, 9 | 4.88 | 0.014 |
| R hippocampus | 28 | 23, -9, -16 | 5.02 | 0.017 |
| Spatial AD (n = 9) vs controls (n = 71) | ||||
| R middle temporal gyrus | 21 | 68, -29, -13 | 5.21 | 0.003 |
| R inferior temporal gyrus | 37 | 64, -42, -14 | 5.14 | 0.004 |
| R middle occipital gyrus | 19 | 68, -20, -14 | 4.93 | 0.004 |
| Spatial AD (n = 9) vs nonspatial AD (n = 23) | ||||
| R inferior temporal gyrus‡ | 37 | 60, -46, -16 | 4.65 | 0.037 |
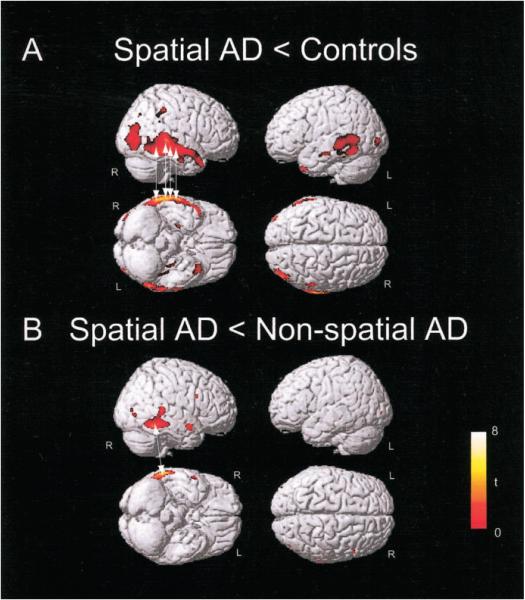
When the SAD group was compared with controls (SAD < controls), an asymmetric (right > left) distribution of gray matter loss was identified. Focal areas of gray matter loss were identified in right occipital and temporal lobe structures (figure 2A, see table 2). The right middle temporal gyrus, right inferior temporal gyrus, and right middle occipital gyrus showed the greatest relative gray matter loss. The SAD group had significantly less gray matter in the right inferior temporal gyrus as compared to the NSAD group (SAD < NSAD; see figure 2B, table 2). There were no significant differences in regional hippocampal gray matter loss identified using VBM.
Figure 2.
Regions of cortical brain atrophy in spatial Alzheimer disease (SAD) relative to controls and to non-spatial AD (NSAD). Voxels with significantly (p < 0.001, uncorrected) less gray matter in the stated comparison displayed on a normal control brain. (A) Regions of cortical gray matter loss in the SAD group (n = 9) relative to controls (n = 71). Arrows indicate the location of voxels, the atrophy of which was significantly (p < 0.05) correlated with performance on the modified Rey-Osterrieth copy task. (B) The posterior portion of the right inferior temporal gyrus had significantly less gray matter in the SAD group (n = 9) as compared to the NSAD group (n = 23). Arrow shows the voxel that was most significantly correlated with modified Rey-Osterrieth copy task performance (r = 0.632, p < 0.001; see figure 4).
Volumetric measurements of tissue-segmented MRI scans
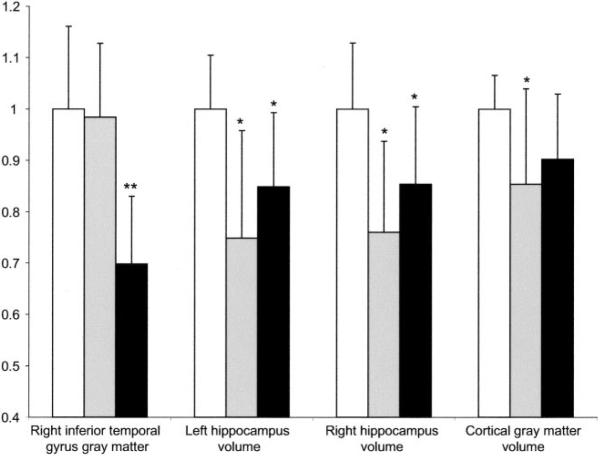
As expected, both the NSAD and AD groups had smaller hippocampi than the control group (p < 0.001, ANOVA with Tukey post hoc; figure 3). Relative to controls, left hippocampal volume was reduced by 25 ± 21% in the NSAD group and 15 ± 14% in the SAD group. There was no difference in hippocampal volumes between the two patient groups (p > 0.1). There were no asymmetries in the volumes of the hippocampi in any of the three groups (right/left hippocampal volume = 1.0). Both groups had less cortical gray matter than controls (see figure 3).
Figure 3.
Magnitude of focal and global atrophy in nonspatial and spatial Alzheimer disease (AD) groups. Gray matter concentration in the right inferior temporal gyrus (Montreal Neurologic Institute coordinates = 60, -46, -16), hippocampal and cortical gray matter volumes normalized to controls. Error bars indicate SD. □ = Control group (n = 26),  = nonspatial Alzheimer disease (NSAD) group (n = 23),■ = spatial Alzheimer disease group (n = 9). *p < 0.001 vs controls, analysis of variance with Tukey post hoc. **p < 0.001 vs controls and NSAD group.
= nonspatial Alzheimer disease (NSAD) group (n = 23),■ = spatial Alzheimer disease group (n = 9). *p < 0.001 vs controls, analysis of variance with Tukey post hoc. **p < 0.001 vs controls and NSAD group.
Comparison of tissue-segmented and voxel-based neuroimaging results
To demonstrate that both the NSAD and SAD group had global cortical and hippocampal atrophy as expected in patients with typical AD, the tissue segmented gray matter volumes and gray matter concentration at the peak suprathreshold voxel within the right inferior temporal gyrus (VBM) were normalized to control values and plotted in figure 3. The magnitude of gray matter loss in the SAD group was greater in the right inferior temporal gyrus than in the hippocampi and total cortical gray matter, whereas the opposite was true in the NSAD group.
Correlations of neuroimaging and neuropsychological results
Gray matter concentration at multiple voxels within the right inferior and middle temporal gyri was correlated with Rey-Osterrieth copy score in all 32 patients with AD (see figure 2, A and B, arrows; table 2). The most significant correlation was found in the right inferior temporal gyrus (r = 0.632, p < 0.001; Pearson, two-tailed; arrow in figure 2B, figure 4). No correlation existed between atrophy of this region and performance on delayed recall of the Rey, design fluency, MMSE, CVLT, modified Trails B, or abbreviated BNT (p > 0.1). There was no correlation between the cortical gray matter or hippocampal volume and performance on the Rey copy (p > 0.1).
Figure 4.
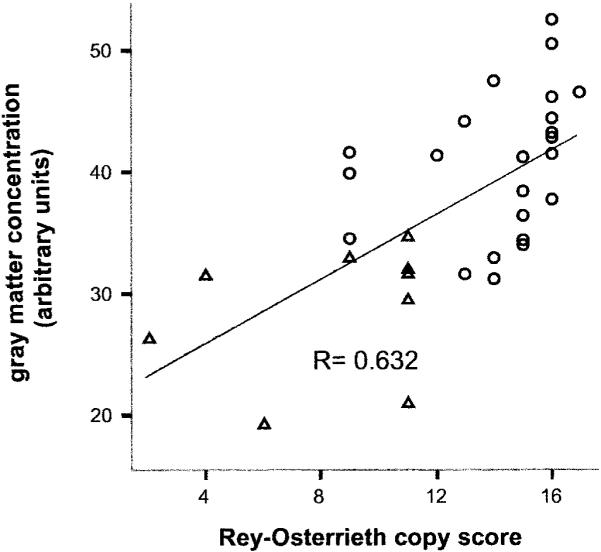
Right inferior temporal gyrus atrophy is correlated with Rey copy performance. Gray matter concentration at a single voxel (Montreal Neurologic Institute coordinates = 60, -46, -16) within the right inferior temporal gyrus is plotted against score on copy of the modified Rey-Osterrieth figure in 32 patients with Alzheimer disease (AD).Ο = Patients with nonspatial AD; △ = patients with spatial AD.
Discussion
This study demonstrates that impairment on a neuropsychological measure of visual constructive ability is strongly correlated with the degree of gray matter loss in the right inferior temporal gyrus, and not with other measures of hippocampal, regional, or global cortical atrophy, in a group of patients with probable AD. All patients showed evidence of hippocampal and generalized cortical atrophy, irrespective of the degree of right inferotemporal atrophy. Moreover, a group of patients with AD who performed significantly worse than other patients with AD on the visual constructive task showed significantly more atrophy of the right inferior temporal gyrus, but not other brain structures. These findings suggest that atrophy of the right inferior temporal gyrus may serve as a neuroimaging marker of visual constructive impairment in patients with AD.
Copying a complex two-dimensional figure requires neural processes related to visual perception, working memory, executive control, and motor sequencing. As such, damage to multiple brain regions could theoretically produce deficits on such a task. Thus, it was striking that atrophy of a single neuroanatomic region, the right inferior temporal gyrus, was correlated with this deficit in patients with probable AD. Atrophy of this region was not correlated with design fluency performance. This finding may reflect a different cognitive demand in the design fluency task, because the task requires a subject to generate new shapes as opposed to copying shapes. The generation of novel designs is in part dependent on executive function, which is likely to have different anatomic correlates in the frontal lobes.
These results are consistent with earlier cross-sectional and longitudinal FDG-PET and structural MRI studies that demonstrated asymmetric cortical hypometabolism12,30 and atrophy31 in patients with probable AD. A previous SPECT study showed relative hypoperfusion of the right parietotemporal region in some patients with AD with impaired performance on the Rey copy,32 and an analysis of sulcal variability in patients with AD demonstrated significant correlations between right temporoparietal atrophy and visuospatial impairment.9 We have extended these results by providing a more detailed localization of a specific visual constructive deficit.
Nonhuman primate studies have demonstrated that the activity of neurons in a cortical region that is homologous to the right inferior temporal gyrus, the inferotemporal cortex, is increased when the animal is asked to process low dimensional configurations of two-dimensional shapes.33 This suggests that the selective atrophy of this region in our patients may have led to difficulty in creating a mental representation of the image that was to be copied. A recent atrophy-corrected fMRI study of an angle discrimination task demonstrated an increase in blood oxygen level dependent signal in the same cortical region in patients with AD relative to controls,34 suggesting that neuronal activity may be increased in this region during such tasks. Whether this increased activity represents a compensatory mechanism or reflects an activity-dependent neurodegenerative mechanism is unclear.
Focal cortical atrophy is most commonly associated with atypical or early onset presentations of AD, such as the syndromes of posterior cortical atrophy,10 primary progressive aphasia,35 and the frontal lobe variant of AD.6 Prominent cortical atrophy, sometimes in the absence of hippocampal atrophy, is readily appreciable on MRI scans in these patients.36 Here it is demonstrated that typical patients with AD, all of whom were initially evaluated for memory or word-finding difficulties, may also display regional cortical atrophy that is measurable while the patients are still alive. If these measurements were repeated in a population of incipient AD patients with isolated visuospatial impairment,13 this pattern of atrophy might be detectable in the absence of hippocampal atrophy.
Cortical atrophy is highly correlated with histopathologic markers of AD, and accounts for a significant proportion of premortem cognitive deficits in clinical-pathologic correlations of patients with definite AD.3 The results presented here show that measurements of well-defined cortical regions of interest may improve the clinical relevance of structural MRI-based measurements in AD. In future longitudinal studies of AD, it will be of interest to correlate the change in atrophy of the right inferior temporal gyrus with changes in performance on neuropsychological measures of visual constructive ability.
Acknowledgments
A.L.B. is a fellow of the John Douglas French Foundation. Supported by NIA P01 AG19724-01A1 (B.L.M.), the State of California, and the McBean Foundation.
References
- 1.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 2.Kramer JH, Miller BL. Alzheimer's disease and its focal variants. Semin Neurol. 2000;20:447–454. doi: 10.1055/s-2000-13177. [DOI] [PubMed] [Google Scholar]
- 3.Riley KP, Snowdon DA, Markesbery WR. Alzheimer's neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 4.Harasty JA, Halliday GM, Xuereb J, et al. Cortical degeneration associated with phonologic and semantic language impairments in AD. Neurology. 2001;56:944–950. doi: 10.1212/wnl.56.7.944. [DOI] [PubMed] [Google Scholar]
- 5.Giannakopoulos P, Gold G, Duc M, et al. Neuroanatomic correlates of visual agnosia in Alzheimer's disease: a clinicopathologic study. Neurology. 1999;52:71–77. doi: 10.1212/wnl.52.1.71. [DOI] [PubMed] [Google Scholar]
- 6.Johnson JK, Head E, Kim R, et al. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56:1233–1239. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- 7.Haxby JV, Grady CL, Koss E, et al. Heterogeneous anterior-posterior metabolic patterns in dementia of the Alzheimer type. Neurology. 1988;38:1853–1863. doi: 10.1212/wnl.38.12.1853. [DOI] [PubMed] [Google Scholar]
- 8.Callen DJA, Black SE, Gao F, et al. Beyond the hippocampus: MRI volumetry confirms widespread limbic atrophy in AD. Neurology. 2001;57:1669–1674. doi: 10.1212/wnl.57.9.1669. [DOI] [PubMed] [Google Scholar]
- 9.Mega MS, Thompson PM, Cummings JL, et al. Sulcal variability in the Alzheimer's brain: correlations with cognition. Neurology. 1998;50:145–151. doi: 10.1212/wnl.50.1.145. [DOI] [PubMed] [Google Scholar]
- 10.Mendez MF, Mendez MA, Martin R, et al. Complex visual disturbances in Alzheimer's disease. Neurology. 1990;40(3 Pt 1):439–443. doi: 10.1212/wnl.40.3_part_1.439. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo M, Anderson SW, Dawson J, et al. Visual attention impairments in Alzheimer's disease. Neurology. 2000;54:1954–1959. doi: 10.1212/wnl.54.10.1954. [DOI] [PubMed] [Google Scholar]
- 12.Pietrini P, Furey ML, Graff-Radford N, et al. Preferential metabolic involvement of visual cortical areas in a subtype of Alzheimer's disease: clinical implications. Am J Psychiatry. 1996;153:1261–1268. doi: 10.1176/ajp.153.10.1261. [DOI] [PubMed] [Google Scholar]
- 13.Mapstone M, Steffenella TM, Duffy CJ. A visuospatial variant of mild cognitive impairment: getting lost between aging and AD. Neurology. 2003;60:802–808. doi: 10.1212/01.wnl.0000049471.76799.de. [DOI] [PubMed] [Google Scholar]
- 14.Thal DR, Rub U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 15.Delis DC, Lucas JA, Kopelman MD. Memory. In: Fogel BS, Schiffer RB, editors. Synopsis of neuropsychiatry. Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 169–191. [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan E, Goodglass H, Wintraub S. The Boston Naming Test. Lea and Febiger; Philadelphia: 1983. [Google Scholar]
- 18.Reitan RM. Validity of the Trailmaking Test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 19.Golden C. Stroop Color and Word Test: manual for clinical and experimental uses. Stoelting; Chicago, IL: 1978. [Google Scholar]
- 20.Yesavage JA, Brink TL, Rolse TL, et al. Development and validity of a Geriatric Depression Scale: a preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 21.Du AT, Schuff N, Zhu XP, et al. Atrophy rates of entorhinal cortex in AD and normal aging. Neurology. 2003;60:481–486. doi: 10.1212/01.wnl.0000044400.11317.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu YY, Schuff N, Du AT, et al. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging. 2002;16:305–310. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanabe JL, Amend D, Schuff N, et al. Tissue segmentation of the brain in Alzheimer disease. AJNR Am J Neuroradiol. 1997;18:115–123. [PMC free article] [PubMed] [Google Scholar]
- 24.Ashburner J, Friston KJ. Voxel-based morphometry-the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 25.Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 26.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 27.Good CD, Scahill RI, Fox NC, et al. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17:29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- 28.Duvornoy HM. Surface, three-dimensional sectional anatomy with MRI, and blood supply. Springer-Verlag Wein; New York: 1999. The human brain. [Google Scholar]
- 29.Evans AC, Collins DL, Mills SR, et al. 3D statistical neuroanatomical models from 305 MRI volumes. Proc IEEE-Nuclear Science Symposium and Medical Imaging Conference.1993. pp. 1813–1817. [Google Scholar]
- 30.Haxby JV, Grady CL, Koss E, et al. Longitudinal study of cerebral metabolic asymmetries and associated neuropsychological patterns in early dementia of the Alzheimer type. Arch Neurol. 1990;47:753–760. doi: 10.1001/archneur.1990.00530070043010. [DOI] [PubMed] [Google Scholar]
- 31.Thompson PM, Hayashi KM, de Zubicaray G, et al. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishiai S, Koyama Y, Seki K, et al. Unilateral spatial neglect in AD: significance of line bisection performance. Neurology. 2000;55:364–370. doi: 10.1212/wnl.55.3.364. [DOI] [PubMed] [Google Scholar]
- 33.Op de Beeck H, Wagemans J, Vogels R. Inferotemporal neurons represent low-dimensional configurations of parameterized shapes. Nat Neurosci. 2001;4:1244–1252. doi: 10.1038/nn767. [DOI] [PubMed] [Google Scholar]
- 34.Prvulovic D, Hubl D, Sack AT, et al. Functional imaging of visuospatial processing in Alzheimer's disease. Neuroimage. 2002;17:1403–1414. doi: 10.1006/nimg.2002.1271. [DOI] [PubMed] [Google Scholar]
- 35.Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–432. [PubMed] [Google Scholar]
- 36.Galton CJ, Patterson K, Xuereb JH, et al. Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroim-aging and pathological study of 13 cases. Brain. 2000;123(Pt 3):484–498. doi: 10.1093/brain/123.3.484. [DOI] [PubMed] [Google Scholar]